The Nature of High-Temperature Peaks of Thermally Stimulated Luminescence in NaCl:Li and KCl:Na Crystals
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. High-Temperature TSL of NaCl and NaCl:Li Crystals
3.2. Spectra of High-Temperature TSL of NaCl:Li Crystals
3.3. Spectra of High-Temperature TSL of KCl:Na and LiF:Mg, Ti (TLD-100) Crystals
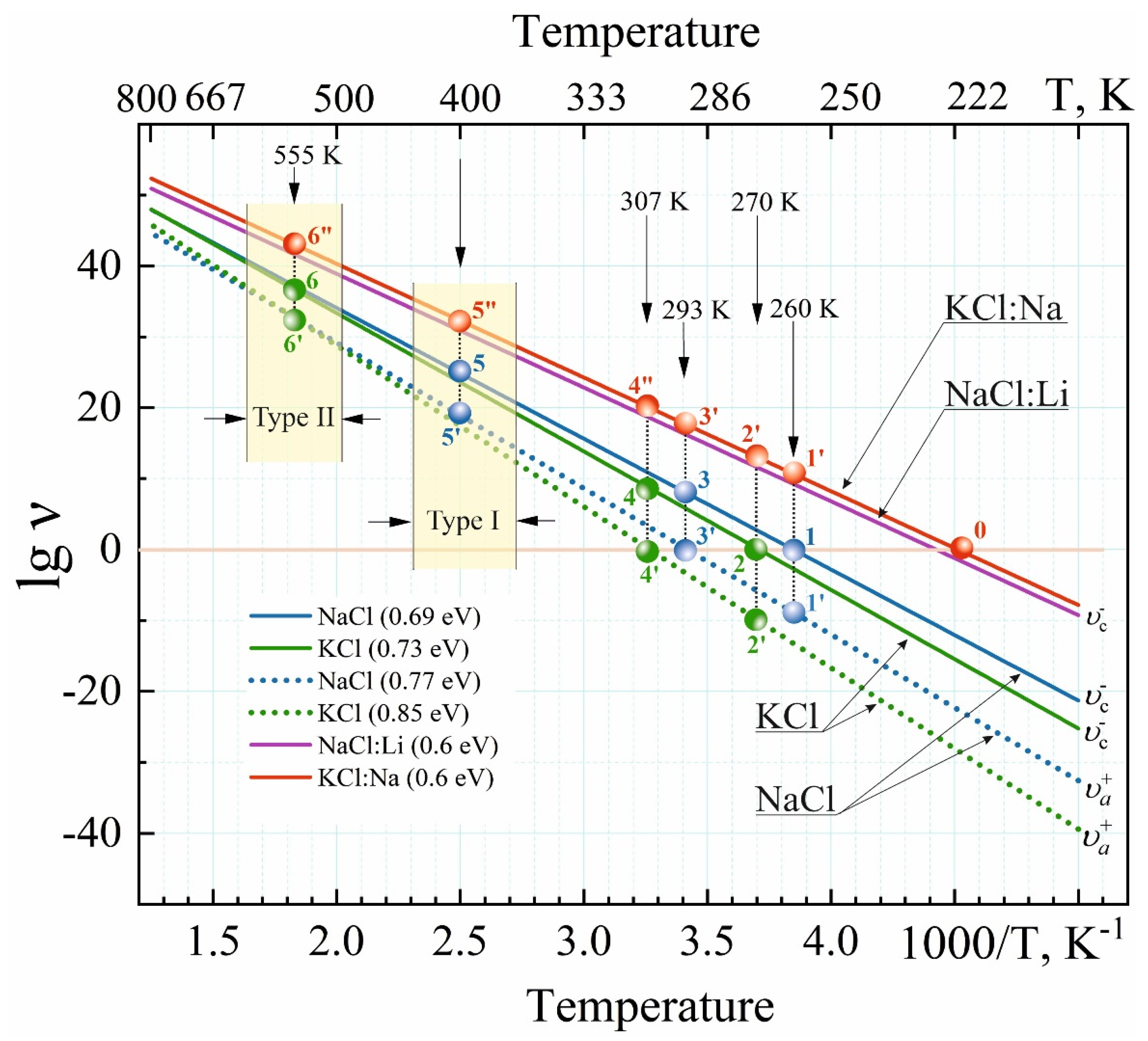
3.4. Temperature Dependence of Vacancy Frequency Jumps in NaCl and KCl Crystals
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stoneham, A.M.; Itoh, N. Materials Modification by Electronic Excitation. Appl. Surf. Sci. 2000, 155, 186–193. [Google Scholar] [CrossRef]
- Lushchik, C.; Lushchik, A. Evolution of Anion and Cation Excitons in Alkali Halide Crystals. Phys. Solid State 2018, 60, 1487–1505. [Google Scholar] [CrossRef]
- Song, K.S.; Williams, R.T. Self-Trapped Excitons; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-85236-7. [Google Scholar]
- Takizawa, Y.; Kamada, K.; Yoshino, M.; Kim, K.J.; Kutsuzawa, N.; Yamaji, A.; Kurosawa, S.; Yokota, Y.; Sato, H.; Toyoda, S.; et al. Growth of Thallium-Doped CsI/CsCl/KCl Eutectics and Their Scintillation Properties. Opt. Mater. X 2022, 15, 100159. [Google Scholar] [CrossRef]
- Miyazaki, K.; Nakauchi, D.; Kato, T.; Kawaguchi, N.; Yanagida, T. Development of Tl-Doped KI Single Crystal Scintillators. Radiat. Phys. Chem. 2023, 207, 110820. [Google Scholar] [CrossRef]
- Syrotych, Y.; Gorbenko, V.; Pejchal, J.; Kucerkova, R.; Mares, J.A.; Nikl, M.; Kamada, K.; Yoshikawa, A.; Fedorov, A.; Sidleskiy, O.; et al. Two- and Three-Layered Composite Scintillators Based on the Ce3+-Doped GAGG and TbAG Garnets for the Simultaneous Registration of Various Types of Ionizing Radiation. Cryst. Growth Des. 2024, 24, 6954–6964. [Google Scholar] [CrossRef]
- Salas-Juárez, C.J.; Ugalde-Valdés, M.A.; Guzmán-Mendoza, J.; Nolasco-Altamirano, D.; Martínez-Gil, M.; Gómez-Dominguez, C.E.; Guarin, C.A.; Melendrez, R.; Rivera-Montalvo, T. Persistent Luminescence of Commercial TLD-100 Dosimeter: Using Shallow Traps for Radiation Dosimetry. Radiat. Meas. 2023, 167, 106997. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Meng, F.; Jiang, H.; Yao, S.; Zhang, X.; Liu, J.; Sun, X.; Wang, L.; Mahadevan, C.K. Insight into the Structural, Mechanical and Optical Properties of NaCl:Tb Crystals for the WLED and TLD Applications. Ceram. Int. 2023, 49, 28274–28282. [Google Scholar] [CrossRef]
- Aboelezz, E.; Pogue, B.W. Review of Nanomaterial Advances for Ionizing Radiation Dosimetry. Appl. Phys. Rev. 2023, 10, 21312. [Google Scholar] [CrossRef]
- Salah, N.; Sahare, P.D.; Rupasov, A.A. Thermoluminescence of Nanocrystalline LiF:Mg, Cu, P. J. Lumin. 2007, 124, 357–364. [Google Scholar] [CrossRef]
- Horowitz, Y.S. Linearisation of the Dose Response of Composite Peak 5 in LiF:Mg,Ti by Post-Irradiation Photon Excitation. Phys. Med. Biol. 2024, 69, 212001. [Google Scholar] [CrossRef]
- Khoshakhlagh, M.; Islamian, J.; Abedi, S.; Mahmoudian, B. Development of Scintillators in Nuclear Medicine. World J. Nucl. Med. 2015, 14, 156–159. [Google Scholar] [CrossRef]
- Watanabe, K.; Kawabata, Y.; Yamazaki, A.; Uritani, A.; Iguchi, T.; Fukuda, K.; Yanagida, T. Development of an Optical Fiber Type Detector Using a Eu:LiCaAlF6 Scintillator for Neutron Monitoring in Boron Neutron Capture Therapy. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2015, 802, 1–4. [Google Scholar] [CrossRef]
- Horowitz, Y.S.; Oster, L.; Eliyahu, I. The Saga of the Thermoluminescence (TL) Mechanisms and Dosimetric Characteristics of LiF:Mg,Ti (TLD-100). J. Lumin. 2019, 214, 116527. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Kim, G.; Ye, S.-J. Estimating 10B and 14N Doses Using a Pair of LiF-Based TLD-600 and TLD-700 as an Alternative to Gold Activation Method. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2023, 1050, 168141. [Google Scholar] [CrossRef]
- Behmadi, M.; Mohammadi, S.; Ravari, M.E.; Mohammadi, A.; Loushab, M.E.; Toossi, M.T.B.; Ghergherehchi, M. Neutron Dosimetry with a Pair of TLDs for the Elekta Precise Medical Linac and the Evaluation of Optimum Moderator Thickness for the Conversion of Fast to Thermal Neutrons. Nucl. Eng. Technol. 2024, 56, 753–761. [Google Scholar] [CrossRef]
- Baker, M.D.; Hanusa, C.R.H.; Paltoo, K.; Danzig, E.; Belanger, J. Bonding in Sodium Chloride Nanotubes: A New Analysis via Madelung Constants and Cohesive Energies. J. Phys. Chem. C 2013, 117, 25742–25747. [Google Scholar] [CrossRef]
- Fernandez-Lima, F.A.; Henkes, A.V.; da Silveira, E.F.; Nascimento, M.A.C. Alkali Halide Nanotubes: Structure and Stability. J. Phys. Chem. C Nanomater. Interfaces 2012, 116, 4965–4969. [Google Scholar] [CrossRef]
- Froborg, F.; Duffy, A. Annual Modulation in Direct Dark Matter Searches. J. Phys. G Nucl. Part. Phys. 2020, 47, 94002. [Google Scholar] [CrossRef]
- Hailey, C.J.; Craig, W.W.; Harrison, F.A.; Hong, J.; Mori, K.; Koglin, J.; Yu, H.T.; Ziock, K.P. Development of the Gaseous Antiparticle Spectrometer for Space-Based Antimatter Detection. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2004, 214, 122–125. [Google Scholar] [CrossRef]
- Shunkeyev, K.; Tilep, A.; Sagimbayeva, S.; Ubaev, Z.; Lushchik, A. The Effect of Instability of KCl:Na Single Crystals. Crystals 2023, 13, 364. [Google Scholar] [CrossRef]
- Shunkeyev, K.; Tilep, A.; Sagimbayeva, S.; Lushchik, A.; Ubaev, Z.; Myasnikova, L.; Zhanturina, N.; Aimaganbetova, Z. The Enhancement of Exciton-like Luminescence in KCl Single Crystals under Local and Uniaxial Elastic Lattice Deformation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2022, 528, 20–26. [Google Scholar] [CrossRef]
- Shunkeyev, K.; Aimaganbetova, Z.; Myasnikova, L.; Maratova, A.; Ubaev, Z. Mechanisms of Radiation Defect Formation in KCl Crystals under the Influence of Local and Plastic Deformation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2021, 509, 7–11. [Google Scholar] [CrossRef]
- Kohzuki, Y.; Ohgaku, T. Deformation Luminescence of X-Ray-Irradiated KCl:Eu2+ Single Crystals by Compression. J. Lumin. 2023, 253, 119469. [Google Scholar] [CrossRef]
- Ramirez, M.; Bausa, L.; Biernacki, S.W.; Kaminska, A.; Suchocki, A.; Grinberg, M. Influence of Hydrostatic Pressure on Radiative Transition Probability of the Intrashell 4f Transitions in Yb3+ Ions in Lithium Niobate Crystals. Phys. Rev. B 2005, 72, 224104. [Google Scholar] [CrossRef]
- Elango, A.; Sagimbaeva, S.; Sarmukhanov, E.; Savikhina, T.; Shunkeev, K. Effect of Uniaxial Stress on Luminescence of X- and VUV-Irradiated NaCl and NaBr Crystals. Radiat. Meas. 2001, 33, 823–827. [Google Scholar] [CrossRef]
- Shunkeyev, K.; Lushchik, A.; Myasnikova, L.; Sagimbaeva, S.; Ubaev, Z.; Aimaganbetova, Z. Deformation-Stimulated Ex Luminescence in a RbI Single Crystal. Low Temp. Phys. 2019, 45, 1127–1130. [Google Scholar] [CrossRef]
- Grinberg, M. Excited States Dynamics under High Pressure in Lanthanide-Doped Solids. J. Lumin. 2011, 131, 433–437. [Google Scholar] [CrossRef]
- Mahlik, S.; Malinowski, M.; Grinberg, M. High Pressure and Time Resolved Luminescence Spectra of Gd3Ga5O12:Pr3+ Crystal. Opt. Mater. 2011, 33, 1525–1529. [Google Scholar] [CrossRef]
- Mahlik, S. High-Pressure Study of Phosphors Emission. In Phosphor Handbook: Experimental Methods for Phosphor Evaluation and Characterization; CRC Press: Boca Raton, FL, USA, 2022; pp. 419–545. [Google Scholar]
- Ikezawa, M.; Kojima, T. Luminescencs of Alkali Halide Crystals Induced by UV-Light at Low Temperature. J. Phys. Soc. Jpn. 1969, 27, 1551–1563. [Google Scholar] [CrossRef]
- Lushchik, C.; Lushchik, A. Decay of Electronic Excitations with Defect Formation in Solids; Nauka: Moscow, Russia, 1989; ISBN 978-5-02-014026-4. [Google Scholar]
- Lushchik, C.; Kolk, J.; Lushchik, A.; Lushchik, N.; Taiirov, M.; Vasilchenko, E. Decay of Excitons into Long-Lived F,H and α,I Pairs in KCl. Phys. Status Solidi B 1982, 114, 103–111. [Google Scholar] [CrossRef]
- Vasilchenko, E.; Sarmukhanov, E.; Shunkeev, K.; Elango, A. Electronic Excitations Localized in KBr and KI Crystals near Vacancy Defects of Different Sizes. Phys. Status Solidi B-Basic Solid State Phys. 1992, 174, 155–163. [Google Scholar] [CrossRef]
- Lushchik, A.; Lushchik, C.; Lushchik, N.; Frorip, A.; Nikiforova, O. Formation and Decay of Electronic Excitations Localized near Divacancies in Alkali Halides. Phys. Status Solidi B 1991, 168, 413–423. [Google Scholar] [CrossRef]
- Shunkeyev, K.; Sagimbayeva, S.; Myasnikova, L.; Istlyaup, A.; Kenzhebayeva, A. High-Temperature Thermally Stimulated Luminescence of the NaCl and NaCl-Li Crystals. Eurasian J. Phys. Funct. Mater. 2024, 8, 71–78. [Google Scholar] [CrossRef]
- Schoemaker, D. g and Hyperfine Components of VK Centers. Phys. Rev. B 1973, 7, 786–801. [Google Scholar] [CrossRef]
- Castner, T.G.; Känzig, W. The Electronic Structure of V-Centers. J. Phys. Chem. Solids 1957, 3, 178–195. [Google Scholar] [CrossRef]
- Lushchik, C.H.B. Chapter 8—Creation of Frenkel Defect Pairs by Excitons in Alkali Halides. In Modern Problems in Condensed Matter Sciences; Johnson, R.A., Orlov, A.N., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; Volume 13, pp. 473–525. [Google Scholar]
- Lushchik, C.; Kolk, J.; Lushchik, A.; Lushchik, N. Radiational Creation of Frenkel Defects in KCl-Tl. Phys. Status Solidi A 1984, 86, 219–227. [Google Scholar] [CrossRef]
- Aboltin, D.E.; Grabovskis, V.J.; Kangro, A.R.; Lushchik, C.; O’Konnel-Bronin, A.A.; Vitol, I.K.; Zirap, V.E. Thermally Stimulated and Tunneling Luminescence and Frenkel Defect Recombination in KCl and KBr at 4.2 to 77 K. Phys. Status Solidi A 1978, 47, 667–675. [Google Scholar] [CrossRef]
- Elango, A.A.; Nurakhmetov, T.N. Structure and Generation Mechanism of Br Centres in X-Rayed KBr. Phys. Status Solidi B 1976, 78, 529–536. [Google Scholar] [CrossRef]
- Manam, J.; Gabtya, R.K.; Ratnam, V.V. Effect of Post-Irradiation Deformation on Thermoluminescence Spectra of KCl:Ca and NaCl:Ca Crystals. Phys. Status Solidi A 1983, 79, 167–171. [Google Scholar] [CrossRef]
- Manam, J.; Ratnam, V.V. Effect of Post-Irradiation Deformation on the Thermally Stimulated Luminescence Spectra of KCl:Na Crystals. Nucl. Tracks Radiat. Meas. 1985, 10, 97–100. [Google Scholar] [CrossRef]
- Manam, J.; Ratnam, V.V. V3 and FA Centers in KCl:Na Crystals. A Thermoluminescence Study. Phys. Status Solidi B 1984, 121, 393–399. [Google Scholar] [CrossRef]
- Shunkeyev, K.; Myasnikova, L.; Barmina, A.; Zhanturina, N.; Sagimbaeva, S.; Aimaganbetova, Z.; Sergeyev, D. The Thermostimulated Luminescence of Radiation Defects in KCl, KBr and KI Crystals at Elastic and Plastic Deformation. J. Phys. Conf. Ser. 2017, 830, 12138. [Google Scholar] [CrossRef]
- Shunkeyev, K.; Sergeyev, D.; Drozdowski, W.; Brylev, K.; Myasnikova, L.; Barmina, A.; Zhanturina, N.; Sagimbaeva, S.; Aimaganbetova, Z. The Deformation Stimulated Luminescence in KCl, KBr and KI Crystals. J. Phys. Conf. Ser. 2017, 830, 12139. [Google Scholar] [CrossRef]
- Itoh, N. Interstitial and Trapped-Hole Centers in Alkali Halides. Cryst. Lattice Defects 1972, 3, 115–143. [Google Scholar]
- Rodriguez-Lazcano, Y.; Correcher, V.; Garcia-Guinea, J. Luminescence Emission of Natural NaCl. Radiat. Phys. Chem. 2012, 81, 126–130. [Google Scholar] [CrossRef]
- Azim, M.K.M.; Sani, S.F.A.; Daar, E.; Khandaker, M.U.; Almugren, K.S.; Alkallas, F.H.; Bradley, D.A. Luminescence Properties of Natural Dead Sea Salt Pellet Dosimetry upon Thermal Stimulation. Radiat. Phys. Chem. 2020, 176, 108964. [Google Scholar] [CrossRef]
- Lushchik, A.; Lushchik, C.; Nagirnyi, V.; Shablonin, E.; Vasil’chenko, E. Low-Temperature Creation of Frenkel Defects via Hot Electron-Hole Recombination in Highly Pure NaCl Single Crystals. Low Temp. Phys. 2016, 42, 547–551. [Google Scholar] [CrossRef]
- Shunkeyev, K.; Ubaev, Z.; Lushchik, A.; Myasnikova, L. Radiation Defects in NaCl matrix Reduced Lattice Symmetry Caused by Light Cation Doping and Elastic Uniaxial Deformation. Lith. J. Phys. 2021, 61, 151–160. [Google Scholar] [CrossRef]
- Reddy, C.G.; Reddy, K.N. Effect of Plastic Deformation on the Thermally Stimulated Luminescence of NaCl:Y Crystals. Mater. Chem. Phys. 1991, 27, 325–329. [Google Scholar] [CrossRef]
- Lushchik, A.C.; Frorip, A.G. Thermalized and Hot Interstitial Halogen Ions in Alkali Halides. Phys. Status Solidi B 1990, 161, 525–535. [Google Scholar] [CrossRef]
- Beniere, M.; Chemla, M.; Beniere, F. Vacancy Pairs and Correlation Effects in KCl and NaCl Single Crystals. J. Phys. Chem. Solids 1976, 37, 525–538. [Google Scholar] [CrossRef]

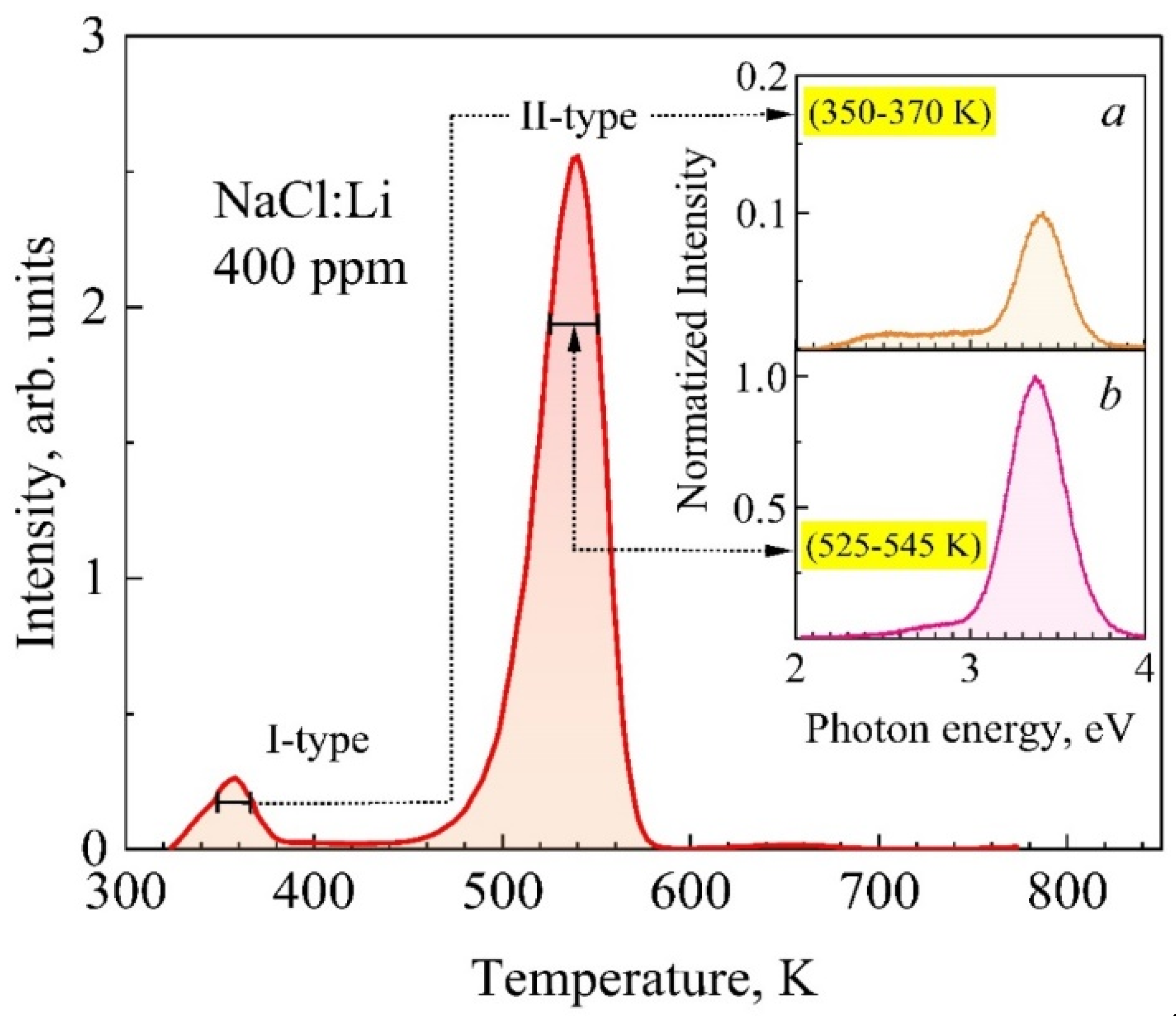
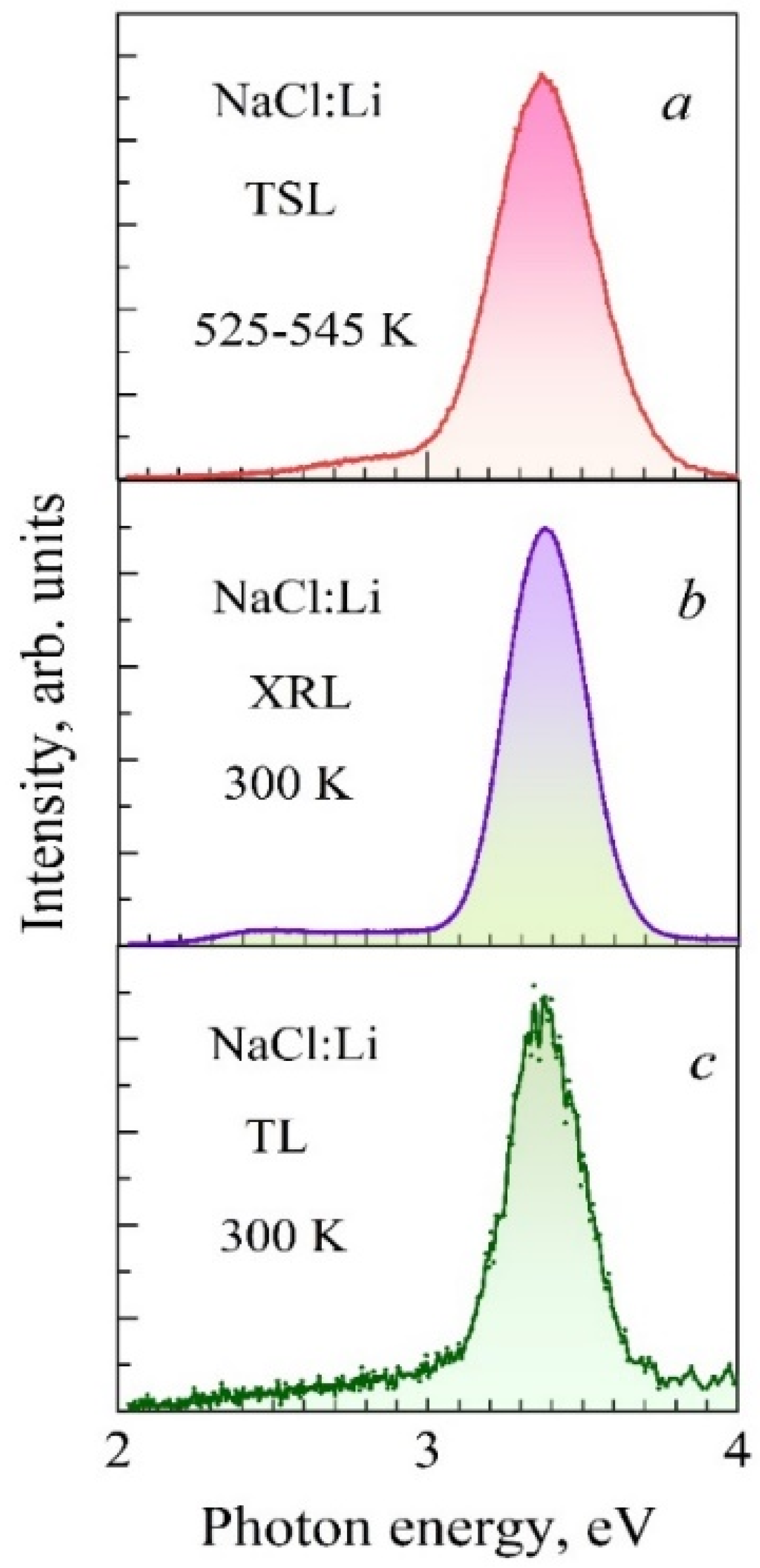
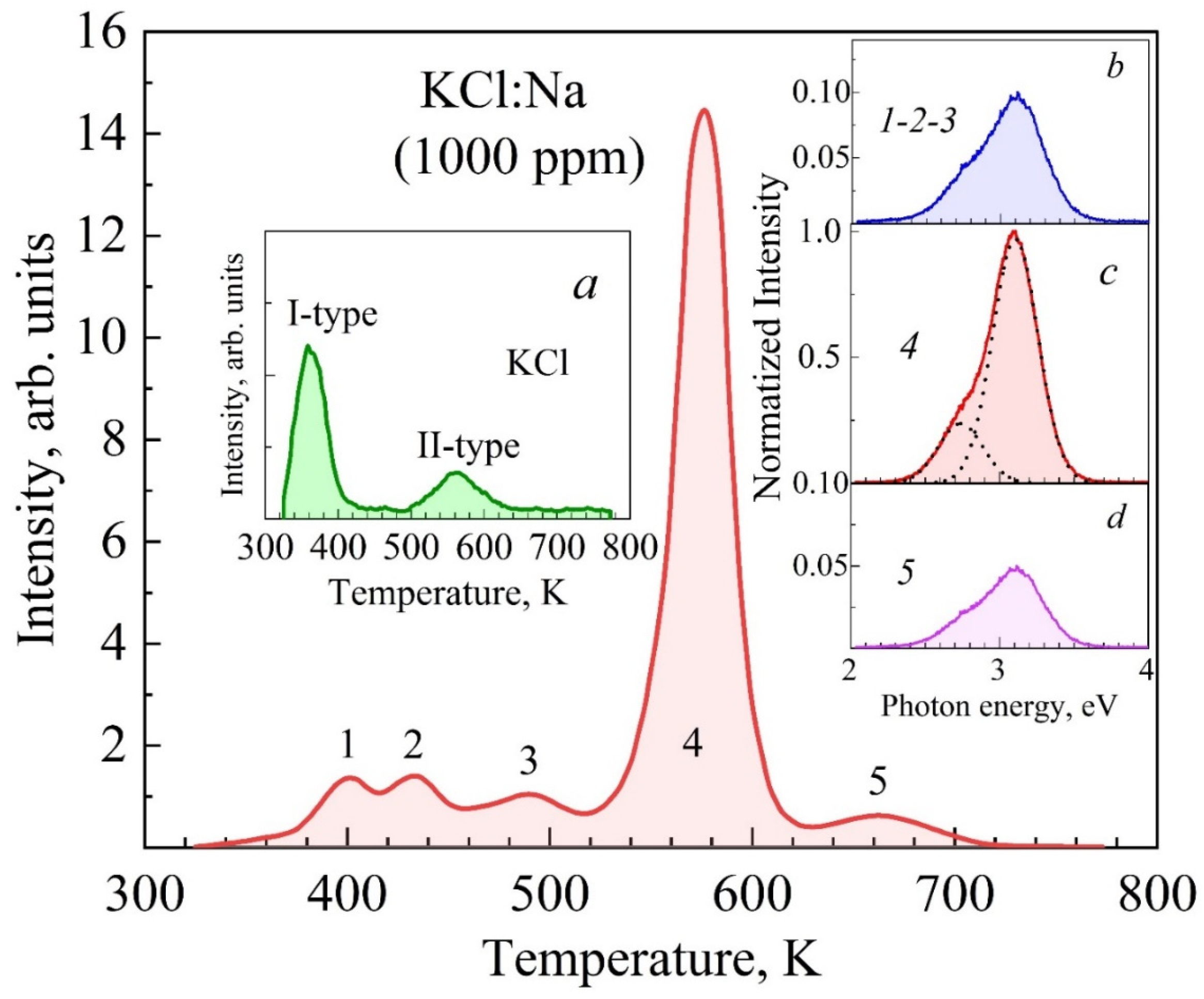
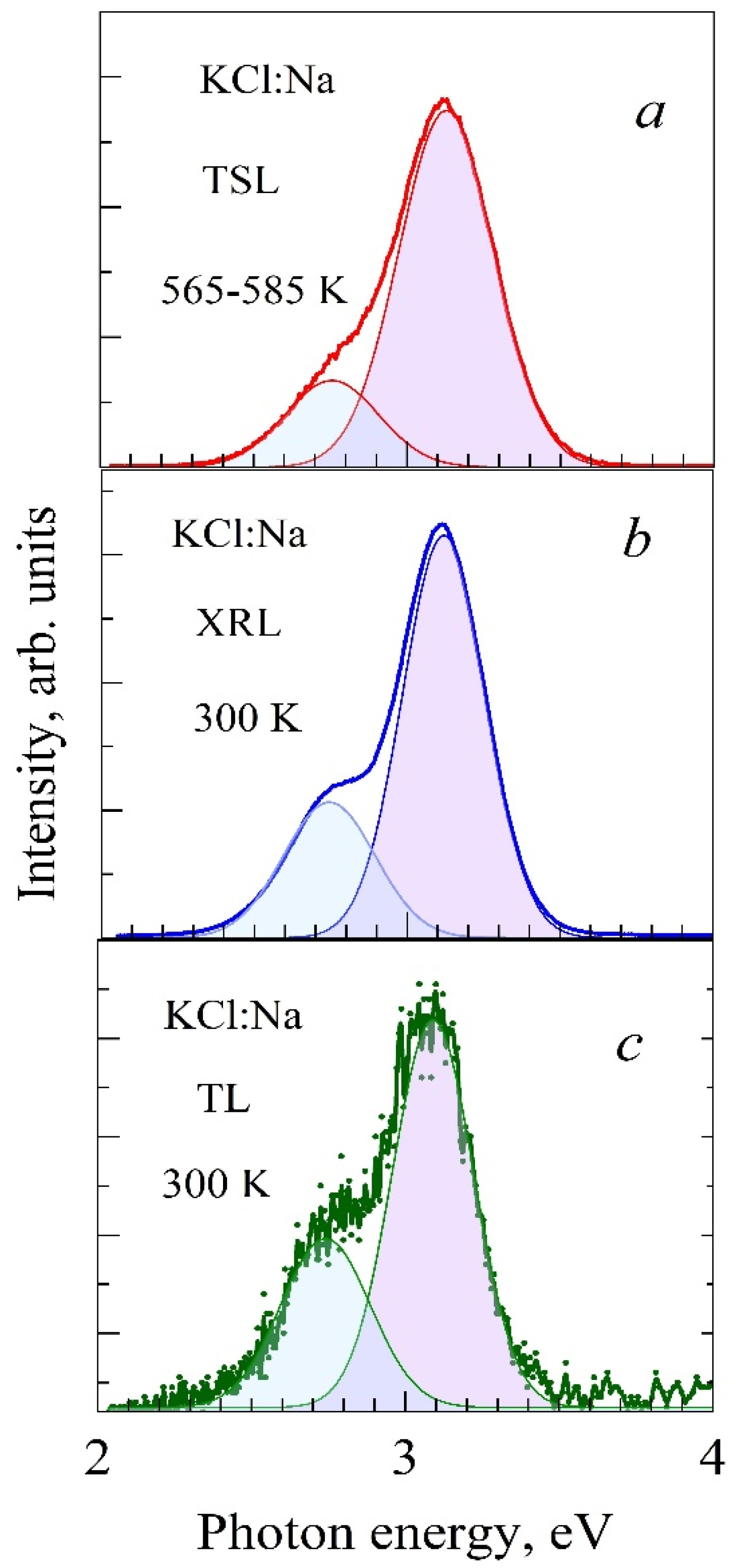
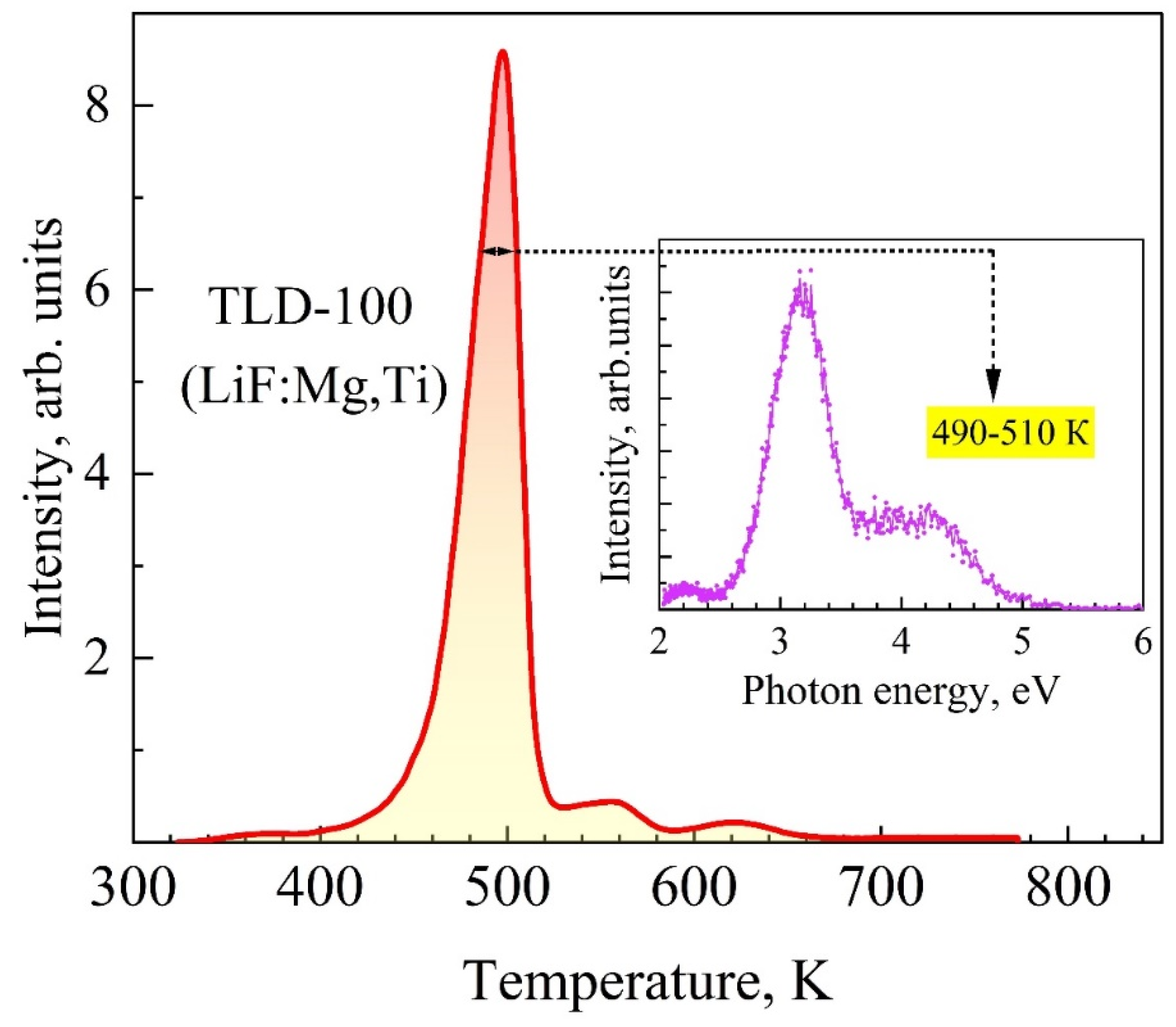
| Crystals | S, Arb. Units | , eV Type-I TSL | , eV Type-II TSL | |
|---|---|---|---|---|
| NaCl | Sa = 2.3 | 1 | 0.71 | 1.3 |
| NaCl:Li (100 ppm) | Sb = 16.5 | 7.2 | 0.82 | 1.16 |
| NaCl:Li (400 ppm) | Sc = 580 | 252 | 0.87 | 1.11 |
| Crystals | S, Arb. Units | Ea, eV Type-I TSL | Ea, eV Type-II TSL | |
|---|---|---|---|---|
| KCl | Sa = 2.1 | 1 | 0.71 | 1.6 |
| KCl:Na (1000 ppm) | Sb = 720 | 343 | 1.3; 1.35; 1.5 | 2.2; 2.4 |
| Crystals | , s−1 | ||||||
|---|---|---|---|---|---|---|---|
| = 1, K | , eV | = 1, K | , eV | ||||
| NaCl | 4.91 × 1012 | 260 | 0.69 | 1.64 | 293 | 0.77 | 1.38 |
| KCl | 4.25 × 1012 | 270 | 0.73 | 2.4 | 307 | 0.85 | 3.2 |
| Crystals | 293–307 K | Type-I TSL (400 K) | Type-II TSL (555 K) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| , s−1 | , s−1 | , (×104) s−1 | , (×104) s−1 | , (×106) s−1 | , (×106) s−1 | ||||
| NaCl | 33 | 1 | 33 | 4.82 | 0.37 | 13 | 13.5 | 2 | 7 |
| KCl | 46 | 1 | 46 | 2.9 | 0.19 | 15 | 11.5 | 2 | 6 |
| NaCl:Li | 1154 | 1 | 1154 | 67.4 | 0.37 | 182 | 87.8 | 2 | 44 |
| KCl:Na | 2135 | 1 | 2135 | 124.7 | 0.19 | 656 | 162.6 | 2 | 81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shunkeyev, K.; Sagimbayeva, S.; Kenzhebayeva, A.; Serikkaliyeva, Z. The Nature of High-Temperature Peaks of Thermally Stimulated Luminescence in NaCl:Li and KCl:Na Crystals. Crystals 2025, 15, 67. https://doi.org/10.3390/cryst15010067
Shunkeyev K, Sagimbayeva S, Kenzhebayeva A, Serikkaliyeva Z. The Nature of High-Temperature Peaks of Thermally Stimulated Luminescence in NaCl:Li and KCl:Na Crystals. Crystals. 2025; 15(1):67. https://doi.org/10.3390/cryst15010067
Chicago/Turabian StyleShunkeyev, Kuanyshbek, Shynar Sagimbayeva, Adelya Kenzhebayeva, and Zarina Serikkaliyeva. 2025. "The Nature of High-Temperature Peaks of Thermally Stimulated Luminescence in NaCl:Li and KCl:Na Crystals" Crystals 15, no. 1: 67. https://doi.org/10.3390/cryst15010067
APA StyleShunkeyev, K., Sagimbayeva, S., Kenzhebayeva, A., & Serikkaliyeva, Z. (2025). The Nature of High-Temperature Peaks of Thermally Stimulated Luminescence in NaCl:Li and KCl:Na Crystals. Crystals, 15(1), 67. https://doi.org/10.3390/cryst15010067






