Abstract
The solid form of the drug can directly affect the physicochemical properties, bioavailability, safety, and efficacy of the drug, and its types mainly include amorphous state, single-component polycrystalline, hydrate, solvate, salt, and cocrystal. Polymorphic drugs are solid drugs whose active ingredients exist in a specific crystalline state. Polymorphic drugs are solid drugs whose active ingredients exist in a specific crystalline state. Drug polymorphism refers to the presence of two or more different crystalline states of the drug. Pharmaceutical cocrystal is a new type of solid form that can improve the stability, solubility, and bioavailability of active pharmaceutical ingredients and many other physicochemical properties. The determination of the crystalline form of a drug and its content is of great significance in ensuring the quality of the polymorphic drug and its safety. In this paper, the quantitative analysis methods of polymorphs and pharmaceutical cocrystals are reviewed, the advantages and disadvantages of various methods are analyzed mainly from three types of techniques, namely, X-ray diffraction, spectroscopy, and thermal analysis, and the specific applications of various methods are commented on through examples. The analytical methods that can effectively determine the content of polymorphic drugs are comprehensively mastered to provide a reference for the establishment of quality standards for polymorphic drugs.
1. Introduction
The solid state of the drug can directly affect the physicochemical properties, bioavailability, safety, and efficacy of the drug, and its types mainly include amorphous state, single-component polycrystalline, hydrate, solvate, salt, and cocrystal [1].
In scientific research and clinical medicine, the determination of the optimal solid form of a drug candidate plays an important role in the efficacy of the drug. In recent years, many newly marketed chemical drugs have low solubility and poor permeability. About 60–70% of the chemicals in these medications are linked to BCS classes II (low solubility/high permeability) and IV (poor solubility/low permeability) [2,3]. Due to low water solubility, many active pharmaceutical ingredients (APIs) have not been developed into formulations, which results in low bioavailability of the drug [4]. Different parts of the gastrointestinal tract have different pH values. Thus, the oral administration of drugs with different solubilities in gastrointestinal fluids at different pH values often leads to nonlinear and variable absorption, making it impossible to evaluate the efficacy and safety of the drug correctly. Therefore, limited drug solubility is a major challenge in developing oral dosage forms [5].
Researchers have developed a number of methods to improve the solubility of APIs, including pre-drug formation, inclusion complexes with cyclodextrins, solid dispersions, salt formation, the addition of co-solvents, polycrystallization, nanoparticles, and cocrystallization. Each of these techniques has its advantages and disadvantages, but in many cases, success depends on the specific physicochemical properties of the API and the polymer [6].
Polymorphic drugs are solid drugs, especially solid chemical drugs, in which the pharmacodynamic components exist in a specific crystalline state. A polymorphic drug is a drug product made from a substance in a dominant drug crystalline state, i.e., a chemical drug ingredient with multiple crystalline solid states of matter [7].
Drug polymorphism refers to the existence of two or more different crystalline states of a drug. Drugs that are solid chemical substances can create a range of distinct crystalline solid material states because of variations in their symmetry laws and molecular arrangement forms. The same substance with different polymorphism solid states is usually called a “polycrystalline phenomenon” or “homogeneous heterocrystalline phenomenon” [8]. Since the different crystalline forms of drugs can seriously affect the clinical therapeutic effects of some drugs, the toxic side effects of drugs, the quality of drugs, etc., the study of the different crystalline forms of solid chemical drugs and their quantitative methods has become one of the non-negligible research contents in the process of drug research and development.
Pharmaceutical cocrystal is a new solid form that provides a basis for optimal solid form selection [9]. The 2018 Guidance for Industry: Regulatory Classification of Drug Cocrystals issued by the FDA defines cocrystals as crystalline materials composed of two or more different molecules, typically an “active pharmaceutical ingredient (API)” and cocrystal formers (“conformers”), in the same crystal lattice [10]. When two or more distinct molecules or ionic compounds with the same stoichiometric ratio are present in the same cell, the initial covalent link between the molecules will not be disrupted during the formation of the cocrystal (Figure 1). Therefore, a drug cocrystal can be thought of as a type of supramolecular self-assembly, which is formed by thermodynamic, kinetic, and intermolecular contact forces into supramolecular lattices and then by a sequence of stacking, assembly, and arranged into a three-dimensional crystal structure. Drug cocrystal does not affect the pharmacological properties of drugs but can improve some physicochemical properties of drugs, such as melting point [11], tablet compression [12], stability [13], solubility [14], and bioavailability [15], through cocrystal technology. Due to bonding through non-covalent bonding forms, cocrystals require more attention in terms of the stability of their state of matter at high temperatures, at high humidity, and in light conditions.
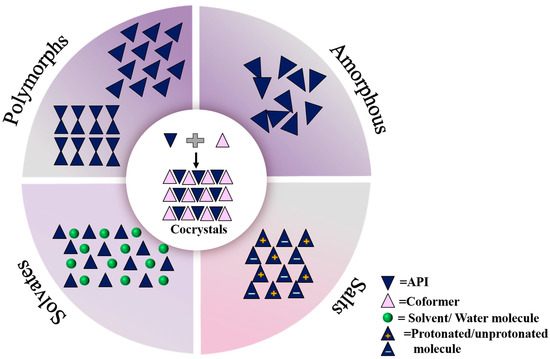
Figure 1.
Active pharmaceutical ingredients in different solid forms.
The existence of a solid drug form is one of the important factors affecting the quality of drugs. Only qualitative analysis of the crystalline form of raw materials or preparations can no longer meet the requirements. In order to determine the content of effective, crystalline form in APIs or preparations and to ensure the therapeutic efficacy of drugs, it is necessary to establish appropriate quantitative methods for polymorphic drugs, especially to find a suitable method for the quantitative determination of polymorphism and cocrystal drugs and their quality control is of great significance.
The methodology for quantitative analysis of polymorphic drugs relies heavily on the crystallographic, spectroscopic, and thermodynamic differences between different crystalline forms to be established. Different physicochemical analysis techniques can characterize the different features and properties of drugs. The focus of research at the particle level is the crystal structure, and techniques like thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), and X-ray powder diffraction (PXRD) are frequently employed. Attenuated total reflection infrared spectroscopy (ATR-FTIR), diffuse reflectance Fourier-transform infrared spectroscopy (DRIFTS), near-infrared spectroscopy (NIRS), Raman spectroscopy, solid-state nuclear magnetic resonance (NMR), terahertz (THz) spectroscopy, and other vibrational spectra can be used to characterize the conformation of molecules at the molecular level, as different drug lattice structures frequently result in different conformations [16].
There is no strict distinction between the quantitative determination of drug polymorphism and drug cocrystals, and the quantitative analytical methods usually applicable to drug crystallites are equally applicable to drug cocrystals. Although there have been reports on the quantitative analysis of solid drugs using a variety of methods [17,18,19], they are not comprehensive and novel enough. In this paper, a variety of quantitative methods are discussed comprehensively, and mainly focus on the practical application of various quantitative methods in the past 10 years, and innovatively introduce the dynamic vapor adsorption and other methods that have been applied to the quantitative analysis of solid crystalline drugs in recent years, which fills the gap pertaining to the inadequacy of the quantitative methods in the existing literature, and provides researchers with a more adequate basis for the quantitative analysis of solid drugs.
In this paper, the application of analytical methods in the quantitative analysis of solid form drugs and their research progress, mainly from the diffraction method, spectroscopic method, and three types of techniques of thermal analysis (Figure 2), focusing on the PXRD method, infrared spectroscopy, Raman spectroscopy, NMR method, and DSC method, are reviewed, the advantages and disadvantages of the various techniques are analyzed, and the specific applications of the various methods are commented on through examples.
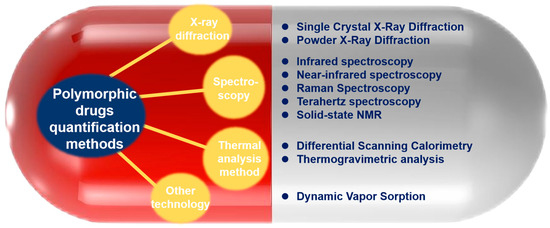
Figure 2.
Methods for quantitative analysis of polymorphic drugs.
2. Quantitative Analysis Methods
2.1. X-Ray Diffraction
Principle of X-ray diffraction quantitative analysis: The basic principle is that the electron scattered by X-ray irradiation on the crystal radiates electromagnetic waves of the same frequency as the incident wave [20]. Due to the interference between scattered waves, the waves superimpose on each other in some directions, and diffraction lines can be observed in this direction, while in other directions, the waves cancel each other, and no diffraction lines are produced [21]. The diffraction line intensity of each phase increases with the increase in its phase content, and the content of the phase can be determined from the calculation of the intensity value. The diffraction intensity of each phase in a multiphase specimen increases with the content of that phase, but it does not necessarily have a linear proportional relationship due to the influence of various factors [22]. When quantitative analysis of the physical phase is performed, high precision is required for intensity testing and analysis.
Single-crystal X-ray diffraction (SXRD) is the most accurate and authoritative method to identify the crystalline substance state of a drug, which belongs to the absolute crystallographic identification method. The spatial arrangement of atoms (molecules, ions), structural symmetry, and interaction forces within the crystal structure can be determined by SXRD to successfully identify the crystalline state of a drug [23]. The object of SXRD analysis is only a single crystal, and the principle is to make use of the diffraction effect produced by X-rays on the crystals (Figure 3), and the analytical data represent the results of a certain crystalline type of the pure product. The SXRD method can reveal the cause of the crystalline form of the test article and give various quantitative data on the crystallography of the crystalline substance. By analyzing the single-crystal X-ray diffraction of different crystalline drugs, we can obtain (1) quantitative crystallographic data of different crystalline substances; (2) using the quantitative data of crystalline substances obtained by single-crystal structural analysis, we can obtain the exclusive powder X-ray diffraction patterns and data of pure products of each crystalline substance by theoretical calculation method, which can be used as the control powder diffraction patterns of 100% crystalline purity of various crystalline drugs; (3) using the theoretically calculated powder diffraction patterns of pure products of various crystalline substances; and (4) using the theoretically calculated powder diffraction patterns of crystalline pure products as the basis for crystalline detection, and by analyzing crystalline samples screened or prepared under different experimental conditions to determine the types of crystalline substances and crystalline purity. At present, there are fewer examples of applying the SXRD method alone for the quantitative study of crystalline drugs, and it is usually combined with the results of the PXRD method or other measurement methods for comprehensive evaluation.
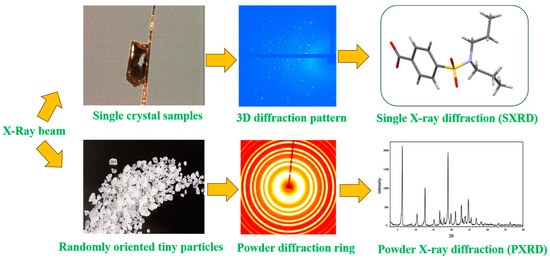
Figure 3.
Comparison of test principles for single-crystal X-ray and powder X-ray diffraction.
The external standard method, internal standard method, and K-value method are the three categories of the quantitative analysis of material composition using an X-ray diffractometer. The K-value approach is an enhancement of the internal standard method that does away with the laborious process of producing standard curves by combining the benefits of the internal and external standard methods. The K-value method simplifies the analytical procedure, the calculations are simpler, the results are more reliable, and the content of the physical phase in the sample can be quantitatively determined [24]. However, since it is necessary to prepare a pure sample of the phase to be measured that is contained in the sample to be tested and to prepare a series of experimental samples with a known mass fraction of the internal standard, it is easy to introduce accidental errors, and thus, the experimental results have a large error.
Table 1 below briefly describes several commonly used quantitative methods.

Table 1.
Principles, formulas, advantages, and disadvantages of quantitative analysis methods for powder X-ray diffraction.
Powder X-Ray Diffraction (PXRD)
PXRD, or powder X-ray diffraction, is a potent method for analyzing pharmacological solids. It offers distinct benefits in the quantitative study of mixes and is extensively employed in the identification of crystalline solid phases. PXRD is a relatively early-developed method for the analysis of drug crystals and is a plot that characterizes the diffraction effect of the test article on X-rays, i.e., the position of the diffraction peak (d or 2θ value) about the diffraction intensity (Figure 3). The PXRD plot of many different peak parameters can be used for this analysis, and peak high strength, peak-to-high-intensity ratio, and peak area are the most common parameters in a univariate analysis [25].
The volume or weight of a substance engaged in diffraction is proportionate to the diffraction intensity it produces, according to theory, which forms the basis of the PXRD method for quantitative analysis. Thus, the volume or mass fraction of a phase involved in diffraction in a mixture can be determined from the magnitude of the diffraction intensity, and thus, the content of a phase in the mixture can be determined [26]. Polycrystalline or powdered solid crystals are the samples that are used. By primarily comparing their diffraction data with the diffraction database of the International Center for Diffraction Data, it is utilized to identify new chemicals and examine them qualitatively. PXRD can also be used for quantitative analysis and crystallinity determination and has the advantages of high accuracy, good resolution, no damage to the sample, low detection cost, short time consumption, and simple operation, and is widely used in polycrystalline-type research [27]. In addition, in situ variable temperature X-ray diffraction analysis (VT-XRD) can also be used for variable temperature and relative humidity studies by PXRD, which allows the real-time monitoring of the structural change characteristics of the material under different heat treatment conditions, and is widely used in the study of phase transitions of materials, recrystallization processes, and the thermal stability of pharmaceutical hydrates. Both low- and high-temperature phases can be used, which helps to directly identify the crystalline phase as a function of temperature.
There are a variety of PXRD quantitative analysis methods in use, but they can be broadly classified into two categories: methods involving the use of internal or external standards or methods using the complete diffraction curve (full-spectrum fitting method). The latter can be subdivided into the Rietveld method, the full powder spectral decomposition method, and the least-squares best-fit summation method. Pre-registered pure standards are used in the full-mode summation approach, while calibrated crystallographic data are used in the Rietveld method.
Alexander et al. [28] first proposed the basic parameters used for the quantification of powder mixtures in 1948 and derived the mathematical relationship between the peak intensity and the crystalline content of the corresponding component in the sample. This method is used for quantification in two basic ways: the single-peak method and the full-spectrum method.
The single-peak method establishes the correspondence between the intensity of the characteristic peaks and each crystalline component of the drug under test. Lou [29] et al. used powder X-ray diffraction to quantify chlorosartan potassium polymorphs and established a method for the determination of chlorosartan potassium polymorphs. It can be applied to API quality control as well as the quantitative examination of mixes of crystalline types I and III of chlorosartan potassium polymorphs. The typical peak area ratios of crystalline type I at 11.13 °2θ and crystalline type III at 5.64 °2θ were employed as the quantitative parameters, and the calibration curves were linear in the range of 1–50% (w/w). The measurement findings showed a precision ranging from 0.6 to 4.9%, with a quantification limit of 2.02% (w/w). This is a sensitive and accurate new method for quantifying the crystalline type III content in the polycrystalline form of chlorosartan potassium using data obtained by PXRD. The method proved to be an efficient and practical method for the quantitative determination of polycrystalline mixtures.
The PXRD single-peak quantitative analysis method is simple to model and fast to operate. However, the morphology, particle size, and orientation of the specimen may affect the accuracy of the results of the analysis. Therefore, the operating conditions should be strictly controlled in practical applications to obtain more reliable data.
The full-spectrum method establishes the correspondence between the intensity of the full-spectrum peaks and the components of each crystalline form of the drug under test. The full-spectrum method developed in recent years is increasingly used for the quantification of drug polymorphs. Chambi [30] et al. developed a method for the quantification of mebendazole (MBZ) crystals in A- and C-type suspensions using the Rietveld method (RM) and the Topas program for solid form quantification. The authors evaluated the crystalline A- and crystalline C-type content in MBZ using the conditions in the PXRD-RM (FSM + PO) method, which uses Rietveld refinement, and the final results are shown in Figure 4, which, together with the quality of fit metrics therein, indicate that the experimental and computed PXRD plots are in general agreement. And the experiment was repeated for six groups, all of which obtained similar results with a standard quality of fit. And the F-Sndecor statistical test method was used to model and analyze the two groups of data, PXRD: FSM + PO and RBM + PO, and mode obtained the same quantitative phase results and, therefore, proved that the method can be used for the quantitative control of polycrystalline forms in MBZ, which can effectively avoid the impact of the solid form of the APIs due to the change in their solid form.
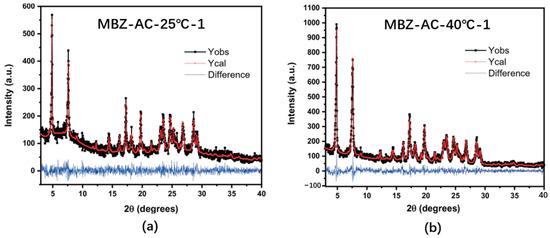
Figure 4.
Experimental and calculated PXRD patterns obtained via RM for samples (a) MBZ-AC-25 °C-1 and (b) MBZ-AC-40 °C-1. The Red and black lines represent the calculated and the observed patterns, respectively, and the blue line illustrates the difference between the observed and calculated patterns. [30].
Examples of studies using PXRD quantitative analysis in the last decade are listed in Table 2 below. Among them, the internal standard method requires the addition of a reference phase to the sample to be measured and the plotting of a series of gradient working curves, which are not suitable for samples with complex spectral lines. Although the external standard method does not require the addition of the reference phase, it is not possible to analyze some samples for which the pure phase is difficult to obtain, and the K-value method simplifies the analytical procedure, and the reliability of the results is better, but due to the necessity of preparing pure samples containing the substance phase to be measured in the samples to be tested and a series of experimental samples with known mass fractions of the internal standard, it is easy to introduce the accidental error, and thus, the experimental results have a larger error. Although the full-spectrum fitting method does not require the addition of a reference phase to draw a series of gradient working curves, it requires complex mathematical calculations, such as the joint equation and the least-squares method. In summary, there are various methods for the quantitative analysis of X-ray diffraction, each with its own advantages and disadvantages, and it is necessary to select the quantitative analysis method suitable for their samples to minimize the error in practical application.

Table 2.
List of solid drugs quantified by the PXRD method.
2.2. Spectroscopy
2.2.1. Infrared Spectroscopy (IR)
IR is infrared spectrophotometry because infrared spectroscopy is easy to operate, the amount of samples is small, the analysis is fast, and the identification of substances is reliable, so infrared spectroscopy has become more widely used currently in the qualitative analysis of substances [38,39]. However, IR can also be applied to quantitative analysis [40,41]. Quantitative infrared spectroscopy [42] provides a more intuitive and comprehensive view of the sample during the analysis process, detects changes in the sample promptly, and also allows the sample to continue the normal reaction process and be measured without damaging the sample. The principle is to determine the molecular structure of substances and identify compounds based on the difference in the internal intermolecular forces and strengths of different crystalline forms and to determine the differences in substances through the movement of absorption peak positions, changes in absorption intensity, and the increase or decrease in the number of absorption peaks during the detection process, so that the qualitative and quantitative detection of crystalline APIs or solid formulations is achieved [43]. The commonly used method is the relative peak intensity method. However, traditional infrared spectroscopy has low sensitivity and large quantitative analysis errors; therefore, infrared spectroscopy is currently less used in the quantitative analysis of polymorphic drugs and is usually studied in combination with other measurement methods.
Attenuated Total Reflectance Infrared Spectroscopy (ATR-FTIR)
A practical technique such as Fourier-transform infrared spectroscopy is used to evaluate and study molecular interactions both qualitatively and quantitatively (FTIR). In addition, it has a wide frequency measurement range, fast measurement speed, high wavenumber accuracy, low noise, and high luminous flux. With the aid of several auxiliary techniques, including photoacoustic spectroscopy, diffuse reflectance spectrum, reflection and absorption spectrum, and emission spectrum, among others, it can be used in a wide range of fields.
Attenuated total reflection spectrum (ATR for short) is also called internal reflection spectrum. The principle of attenuated total reflection spectrum can be simply summarized as follows: when the incident angle is greater than the critical angle, the incident light will be refracted back to the total reflection crystal after penetrating into the light-sparing medium (sample) to a certain depth. The light entering the sample is absorbed in the frequency range at which the sample absorbs light, and the intensity is attenuated; in the frequency range where the sample does not absorb light, the light is fully reflected. ATR is a prominent FTIR sampling instrument. Owing to its user-friendliness and rapid analysis speed, it significantly reduces the analysis time and generally obviates the necessity for sample preparation [44]. The selection of optimal ATR crystals and accessories can further enhance the success rate of FTIR sampling and streamline sample handling [45]. The ATR spectrum is the infrared spectrum of a sample placed on a crystal, reflecting the molecular motion of the chemical bonds in the part of the sample through which the light passes, i.e., the chemical characteristics of the surface or interface. Attenuated total reflection has a unique advantage for polymorphic drugs—avoiding the risk of transcrystallization caused by the conventional potassium bromide compression method.
A versatile non-destructive analysis tool enables qualitative and quantitative analyses with small sample volumes, without the need for pretreatment. When the method is employed for quantitative investigations, there are disadvantages too, like restrictions in cases where the chosen absorption bands and those brought on by the presence of additional components somewhat overlap. However, the shortcomings of ATR-FTIR can be addressed, and its strengths can be increased by combining it with a chemometric method like partial least squares (PLS). This combination offers a great statistical and mathematical approach to correlate the concentration of particular components in complex mixtures with spectrum information. When there are specific instances of overlap, when components interact molecularly, or when absorbance rises disproportionately with concentration, this assessment is helpful [46].
Kapourani et al. [47] assessed the application of artificial neural networks (ANNs) in the creation of novel stoichiometric models. These models have the capacity to concurrently identify and quantify the proportion of both pure crystalline and amorphous drugs within a drug that forms a substantial amorphous solid dispersion (ASD). Attenuated total reflectance Fourier-transform infrared spectroscopy (ATR- FTIR) was used to select riviroxaban (RIV, drug), and Sol Plus® (SOL, matrix carrier) was used to prepare a suitable ASD model system by adding different ratios of crystalline and amorphous drugs to the amorphous solid dispersion (ASD), and calibration and test sets were prepared, and 24 full factorial designs of experiments were used to examine the artificial neural network structure and study parameters, selection of spectral regions, and data preprocessing. The results showed that the RMSEp (root mean square error of prediction error) of the test samples indicated improved prediction performance for both crystalline drugs (RMSEp = 0.86) and amorphous drugs (RMSEp = 2.14).
Figure 5 depicts the ATR-FTIR spectra of the fabricated ternary samples, which contain crystalline and amorphous RIV as well as ASD in diverse weight ratios. In every instance, the spectral analysis revealed that an augmentation in the concentration of both crystalline and amorphous RIV brought about an increase in the intensity of their specific ATR-FTIR peaks.
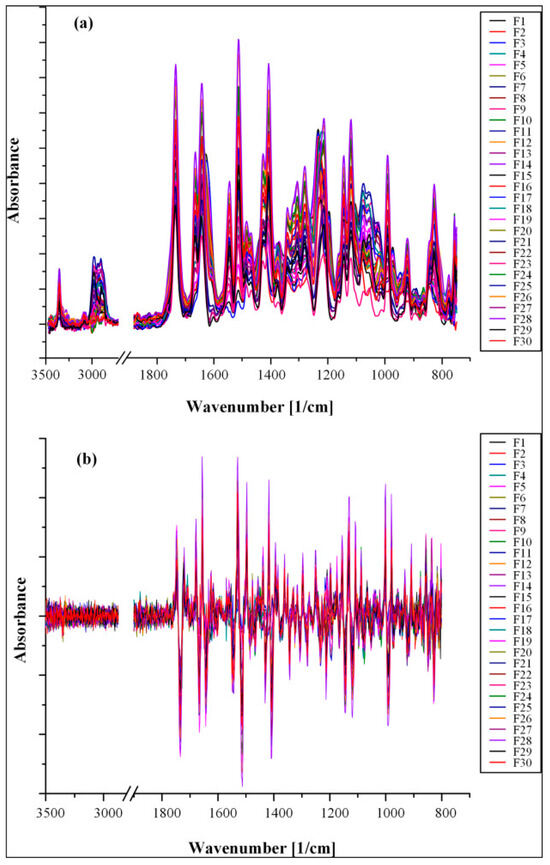
Figure 5.
Untransformed (a) and second-derivative (b) ATR-FTIR spectra of samples comprising crystalline RIV, amorphous RIV, and ASDs [47].
The superiority of artificial neural networks compared to traditional regression techniques (e.g., partial least squares and principal component regression) suggests that, in cases where there is a high degree of structural similarity between the compounds under study (i.e., crystalline and amorphous states of the same compounds), it is necessary to implement more powerful or complex regression techniques, such as artificial neural networks.
Diffuse Reflectance Fourier-Transform Infrared Spectroscopy (DRIFTS)
Diffuse reflectance Fourier-transform infrared spectroscopy (DRIFTS) is an infrared spectroscopic method for the direct determination of solid powder samples [48] and has been applied to the determination of active ingredients in pharmaceutical preparations [49] and trace elements in environmental samples [50]. When using DRIFTS, the sample preparation process is not affected by thermal and mechanical energy because the sample is dispersed in a non-absorbing medium. In addition, DRIFTS is insensitive to the effect of particle size, and the instrument is more versatile, which makes DRIFTS highly suitable for the quantification of crystalline forms, especially for the study of the transformation law between crystalline forms and the amount of transformation.
In 1978, Fuller and Griffiths developed a diffuse reflection battery that was the first to achieve an acceptable f/N ratio at a resolution of 2–4 cm−1. Subsequently, several companies introduced commercial diffuse reflection attachments that modified the original optical geometry employed by Fuller and Griffiths [51]. DRIFTS enables quantitative analyses by converting the diffuse reflectance spectrum into the Kubelka–Munk (K-M) functional form [52,53], and its expression is written as follows:
where is the diffuse reflectance, is the reflectance of the sample relative to a non-absorbent standard, a is the absorbance, c is the analyte concentration, and s is the scattering coefficient. When diffuse reflectance infrared spectroscopy is used for quantitative analysis, the absorbance intensity of the measured diffuse reflectance infrared spectra does not conform to Lambert–Beer’s law, i.e., it is not linearly related to the content (concentration) of the sample components due to the presence of specularly reflected light. Therefore, the diffuse reflectance should be expressed as a K-M function to reduce or eliminate any wavelength-dependent specular reflection effects. The K-M function is proportional to the sample concentration, c, when the sample size is uniform, and the specular reflectance effect on the sample surface is very small, s being a constant so that the sample concentration is in the low range. The method of diluting the sample to be tested by adding the sample to a diffuse reflecting medium (usually potassium halide) powder for sufficient grinding can effectively eliminate specular reflection and reduce absorption peak saturation. In general, the finer the particles are ground, the lower the concentration of the sample, and the more homogeneous the sample is when mixed with potassium bromide, the more the intensity of the bands in the measured diffuse reflectance spectra, and it has a good linear relationship with the concentration of the component to be measured. Systematic studies have been performed to evaluate the effects of particle size [54], powder sample preparation method [55], presence of absorbing substrates [56,57], and filling pressure [58] on the diffuse reflectance IR spectra. Another element that has an impact on the quantity of specular reflection is the optical geometry utilized, as was illustrated in a sequence of articles released by Brimmer et al. All studies by researchers [59,60] have emphasized the importance of controlling particle size and obtaining a homogeneous sample mixture in an absorbent-free matrix, as well as maintaining reproducible filling densities when attempting quantitative diffuse reflectance analysis.
In recent years, with the emergence of a wide variety of diffuse reflectance accessories suitable for use with various infrared instruments, as well as the rapid development of computer software technology, the refinement of mathematical treatments (e.g., derivative spectroscopy), and the application of a number of chemometric methods (e.g., the application of partial least squares (PLS), artificial neural networks (ANNs), etc.), multivariate calibration models such as PLS have the advantage of enabling quantitative analysis of multi-component mixtures with overlapping bands, using mixtures for calibration curves, modeling interactions between components, and using multiple wavelength combinations for calibration curves. The field of application of diffuse reflectance infrared spectroscopy is rapidly expanding and is becoming a routine method of qualitative and quantitative analyses.
2.2.2. Near-Infrared Spectroscopy (NIRS)
NIRS is a fast, non-destructive method [61,62]. Solid drugs can be measured directly without pretreatment by integrating the sphere mode. Compared with other detection techniques, it is possible to perform overall determination without breaking non-covalent bonds, and it may be suitable for the quantitative determination of cocrystals. NIRS mainly reflects the multiplicity and ensemble frequency absorption of hydrogen-containing groups in molecules and can qualitatively and quantitatively analyze the active ingredients that are directly or indirectly related to these groups. NIRS quantitative analysis mainly applies chemometric methods by extracting the NIRS correlation information between specimen spectra and specimen content, establishing relevant mathematical calibration models, and predicting the content or properties of unknown components through the resulting mathematical models [63]. Compared with DRIFTS, NIRS spectral peaks contain less information, have lower resolution, and wider information bands, and sometimes peak overlap occurs. To increase the resolution of adjacent peaks, the NIRS spectra often need to be preprocessed before data calculation. The significant advantages of the NIRS method are the fast analysis speed and the ability to identify not only the different crystalline forms of active ingredients but also to detect the purity of crystalline forms in APIs and preparations [64].
The raw spectrum data were processed using a variety of chemometric and statistical techniques to retrieve information important to pharmacology. Several parameters were assessed using calibration models produced by principal component analysis, partial least-squares regression analysis, and multiple regression analysis (MLR). The sample’s characteristics and the quantity of components that must be simultaneously examined and assessed indicate which calibration method should be used [65].
LIU et al. [66] employed the NIR solid-state analysis technique in conjunction with chemometrics to develop a quantitative model for the low content of CFZ and Mono-CFZ (cagliflozin monohydrate) in cagliflozin (CFZ) tablets. The NIR spectra of the three solid forms of CFZ are presented in Figure 6. Various preprocessing methods were utilized to preprocess the NIR data within the range of 10000–4000 cm−1 for establishing PLSR calibration models to quantitatively determine CFZ and Mono-CFZ in the two ternary mixture samples. In comparison with other methods, the model established through sg1 + WT preprocessing exhibited the largest R2 value (0.9986) and an appropriate selection of N(3). Consequently, this model was chosen as the optimal calibration model, with a calibration curve of Y = 0.0480 + 0.9928 X, demonstrating good performance in the CFZ content range of 0.00000–10.0000% w/w%. It is observable from the figure that the raw NIR spectra of each calibration sample are highly similar to one another and contain a significant number of noise peaks, thereby rendering it challenging to establish a relationship between the peak intensity or peak area and the CFZ content. sg1 + WT preprocessing removes the interference of baseline shifts or smoothing of the background and noise, allowing the peak intensity to increase with the increase in the CFZ content. This method can be used to monitor CFZ and Mono-CFZ content during the production, storage, and transportation of CFZ tablets. The quantitative analysis of the content of impurity crystals in the production of pharmaceuticals is performed to ensure the quality of pharmaceuticals.
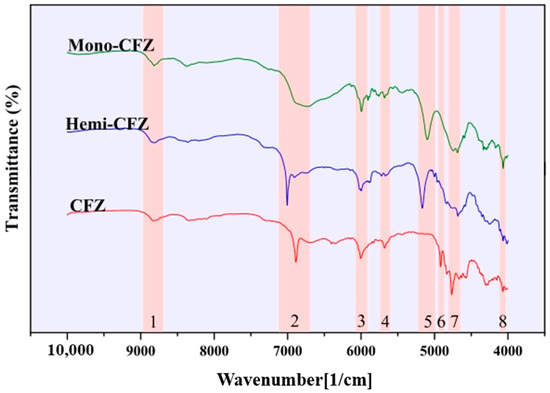
Figure 6.
NIR spectra of three solid forms of CFZ [66].
Korang-Yeboah et al. [67]. successfully assessed AWS and CWS in formulations with low API levels (4.4 and 10%). Chemometric models were created and validated using PLSR and PCR. Warnecke et al. [68] quantified crystalline lactose monohydrate in amorphous lactose using near-infrared spectroscopy. A partial least-squares (PLS) regression model was created, and the complete concentration range model’s root mean square error of cross-validation (RMSECV) was found to be 0.87% (w/w). The RMSECV of NIR was 0.20% (w/w) after the calibration of samples comprising 0–10% (w/w) of crystalline material, and the detection limit was 0.43% (w/w). In addition, NIRS can also be used for the quantitative analysis of drug cocrystals. Wood et al. [69] utilized near-infrared spectroscopy to forecast the concentrations of two drug cocrystals, namely, the 1:1 ibuprofen–nicotinamide (IBU-NIC) and the 1:1 carbamazepine–nicotinamide (CBZ-NIC). Partial least-squares (PLS) regression models were constructed for the two cocrystal pairs by employing a standard set of samples. The RMSEPs (%) for the two cocrystals were 0.95 and 3.53, respectively. The outcomes demonstrated that near-infrared spectroscopy can be effectively employed to precisely distinguish the cocrystallized forms of individual components as well as two distinct drug cocrystal pairs.
2.2.3. Raman Spectroscopy (Raman)
Raman spectroscopy is a technique that collects and analyzes scattered light by shining a monochromatic light source onto a specimen, and the interaction between the photons and the molecular vibration of the specimen causes the wavelength of the scattered light to deviate from the wavelength of the incident light [70]. Raman is a fast and effective process analysis technique that can be applied to analyze the vibrational and rotational information of drug molecule structures and to identify the solid form of drugs [71,72]. Similar to IR, Raman spectroscopy is capable of reflecting the vibrational details of groups within the molecular structure of a compound. The distinction lies in the fact that IR is induced by alterations in the molecular dipole distance, whereas Raman is generated as a result of changes in molecular polarizability. General infrared absorption is not obvious for non-polar groups. Raman absorption is obvious. Vibrations that are difficult to reflect in the infrared spectrum are strong in the Raman spectrum. In addition, the linear range of quantitative analysis is widened so that multiple elements or compounds with unique content can be detected simultaneously [73]. Raman spectroscopy is sensitive to the environment at the molecular level, so different crystalline forms or differences between crystalline and amorphous states of solid drugs can be easily seen in Raman spectroscopy. The lack of specialized sample preparation and the sensitivity to changes in the crystalline form of solid drug samples make Raman spectroscopy one of the ideal methods for crystallographic analysis.
In the field of crystal analysis, it is known that the Raman intensity exhibits an inverse proportionality to the fourth power of the laser wavelength. Moreover, the Raman signal is comparatively weaker than that obtained from UV or visible excitation. These aspects represent certain drawbacks associated with Raman spectroscopy [74]. If the sample, especially an organic substance, fluoresces under the excitation of the incident laser beam, the Raman scattering signal may be overwhelmed by a large fluorescent background [75]. Raman spectroscopy also requires that the purity and homogeneity of the crystals meet certain requirements for results. Otherwise, the accuracy of the test will be reduced [76]. In order to overcome these shortcomings, many Raman spectroscopic techniques have emerged, such as dispersive and Fourier-transform Raman spectroscopy, confocal microscopic Raman spectroscopy, resonance Raman, surface-enhanced Raman, spin Raman, and so on [77]. At present, it is widely used in the fields of polymorphic drugs and cocrystal, especially for the quantitative study of drug cocrystals.
Raman can be used to quantify the content of cocrystals and monomers in solid formulations. Koide et al. [78] used Raman for the quantification of carbamazepine–butanedioic acid cocrystal, carbamazepine, and butanedioic acid in tablets using the partial least-squares method combined with spectral pretreatment.
Barmpalexis et al. [79] utilized Raman spectroscopy for the independent quantification of carbamazepine–nicotinamide (CBZ/NIC) and ibuprofen–nicotinamide cocrystals (IBU/NIC) in Soluplus®-based formulations. The spectral peaks of the mixtures of IBU/NIC and CBZ/NIC cocrystals with SOL in ratios ranging from 90/10 to 1/99 w/w were analyzed and modeled using feed-forward, back-propagation artificial neural networks (ANNs) and partial least-squares (PLS) regression analysis. Figure 7 presents the Raman spectra of IBU/NIC (Figure 7A) and CBZ/NIC (Figure 7B) cocrystal mixtures containing SOL at various weight ratios.
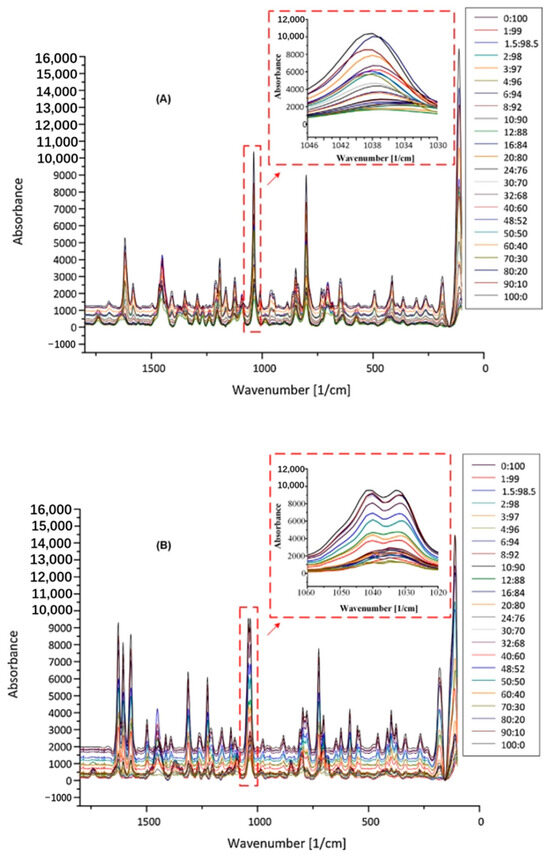
Figure 7.
Raman spectra of IBU/NIC (A) and CBZ/NIC (B) mixtures with SOL at different ratios of cocrystals to polymer weight [79].
Spectral analysis showed that the increase in the concentration of IBU/NIC and CBZ/NIC led to an increase in the intensity of the cocrystal peaks. A better ANN fit was obtained on Raman spectra (root mean square error of prediction, RMSEp, of 0.43 and 0.34 for IBU/NIC-SOL and CBZ/NIC-SOL, respectively) using DOSC preprocessing. A comparison of the ANN fitting results with PLS regression (RMSEp of 0.94 for IBU/NIC-SOL and 7.29 for CBZ/NIC-SOL) demonstrated the advantage of ANN fitting, which can be attributed to its inherent nonlinear predictive ability. A quantitative method for determining the cocrystal content of cocrystal HME-based formulations was developed.
Transmission Raman spectroscopy (TRS) is a sensitive tool for the detection of polycrystalline phenotypes in pharmaceutical tablets, and an increasing number of researchers are using TRS for the quantitative analysis of drug crystals. Motoki et al. [80] devised a method to monitor the metastable cocrystal polycrystalline phenotypes of caffeine–glutaric acid cocrystal crystals within model tablets by means of transmission low-frequency Raman spectroscopy. It was thereby confirmed that transmission low-frequency Raman spectroscopy is applicable for the quantitative monitoring of cocrystal polycrystalline phenotypes.
Chemometrics has received further attention for more accurate quantitative analysis of drug crystals. For example, Feng [81] used carbamazepine TEM tablets containing 20% (w/w) carbamazepine (Type I), 74.0% (w/w) filler (mannitol and microcrystalline cellulose), cross-linked sodium carboxymethyl cellulose, silica, and 6% (w/w) magnesium stearate, and the sensitivity of multi-crystal detection was examined. Quantitative models were created and optimized using multiple regression and data preprocessing, and multivariate detection limits were calculated based on statistical hypothesis testing. The absolute prediction error of the transmission spectroscopy model for the independent validation set was 0.241% (w/w), and the detection limit of the method was estimated to be 1.31% (w/w).
Furthermore, a novel transmission low-frequency Raman spectroscopy approach was employed to measure the polycrystalline form of active pharmaceutical ingredients (APIs) in pharmaceutical tablets [82]. A new transmission geometry for low-frequency Raman spectroscopy was devised. From the results of the partial least-squares (PLS) modeling of the crystalline III content of carbamazepine and the predicted values in the tablets, it was discovered that the correlation coefficient of the transmission mode (R2 = 0.98) exceeded that of the backscattering mode (R2 = 0.97).
Raman spectroscopy has a good application prospect because it requires less handling of the test specimen, avoids the transcrystallization process to a certain extent, and allows testing under certain humidity conditions with a faster testing speed. However, in some cases, the Raman spectra of different crystalline forms may be similar, so it is not easy to find independent and obvious characteristic peaks [83].
2.2.4. Terahertz (THz) Spectroscopy
Electromagnetic waves in the frequency range of 0.1–10 THz are collectively referred to as terahertz waves. THz radiation is a new type of far-infrared coherent radiation source, and terahertz time-domain spectroscopy (THz-TDS) is a new type of spectroscopic technology that utilizes THz spectral pulses to study the physicochemical properties of matter [84].
Unlike NMR spectroscopy, terahertz is used to analyze the crystalline shape of a sample by measuring the energy required for different lattice jumps versus the jump energy provided by the corresponding electromagnetic wave [85]. Compared with other detection methods, THz spectroscopy has unique advantages: (1) it is more sensitive to temperature changes and can be used for the analysis of heating processes or the detection of high-temperature materials; (2) THz time-domain spectroscopy technique generates femtosecond pulses, and its detection is time-resolved, which can be used for the study of dynamic processes in amorphous body systems; (3) the energy of THz radiation is low, which can be used for non-destructive testing and avoid specimens in the measurement process; (4) THz signal contains material amplitude and phase information, and the absorption coefficient and refractive index of the material can be obtained without using the Kramer–Kronig relationship [86]; the crystalline shape and physical and chemical properties of the sample can be analyzed based on absorption coefficient and refractive index [87]; and (5) the THz-TDS signal-to-noise ratio is high due to the use of electromagnetic pulses for measurement.
Infrared spectra and THz spectra are produced in different ways; the former is mainly caused by intra- or intermolecular hydrogen bonding, and the latter is mainly caused by lattice vibrations and hydrogen bonding vibrations in lower-energy states [88]. THz spectroscopy is more sensitive to differences in intermolecular binding than IR, making it suitable for the analysis of drug crystalline forms [89,90]. In recent years, terahertz technology is mainly used in the field of materials and chemistry, and its application in drug analysis is shown in Figure 8. The application of terahertz technology to the field of polymorphic drugs has only been developed in recent years, and it can only be achieved in the laboratory at present [91]. THz spectroscopy combined with chemometrics provides a rapid non-destructive quantitative analysis of polymorphic drugs, which is also useful for the quantitative analysis of crystalline forms in the pharmaceutical industry.
Figure 8.
Application of terahertz technology in drug analysis.
THz-TDS technology can be used for quantitative analysis [92,93]. To measure the crystallinity of microcrystalline cellulose (MCC) samples, Vieira [94] et al. used partial least-squares (PLS) univariate and multivariate regression of frequency domain signals.
Silva et al. [95] put forward an analytical method founded on terahertz time-domain spectroscopy (THz-TDS) and partial least-squares (PLS) regression modeling for the quantification of mebendazole (MBZ) polymorphs, specifically the crystalline types A, B, and C, in pharmaceutical raw materials. Figure 9a shows the terahertz spectra of MBZ polycrystalline types in the useful range (0.4–4.8 terahertz). The spectral distributions of the different crystalline forms are easily distinguishable, showing clearly distinct absorption bands. Figure 9b shows the terahertz spectra of the sample mixtures used in this study.
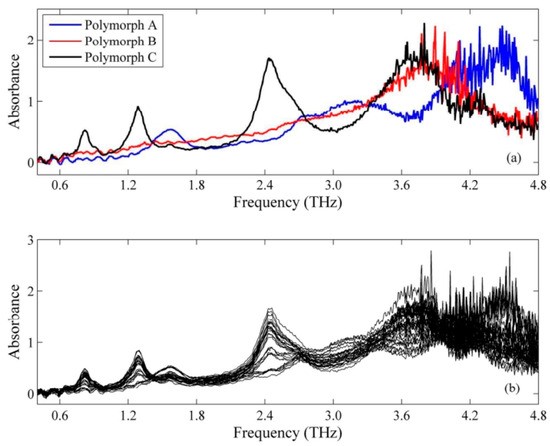
Figure 9.
Raw THz spectra of MBZ polymorphs (a) and mixtures (b) [95].
PLS regression models were constructed by utilizing preprocessed terahertz spectra within distinct spectral ranges. Among the preprocessing techniques and spectral ranges that were assessed, the first-order derivatives generated by Savitzky–Golay (SG) filters, with second-order polynomials and 51-point windows, yielded the most favorable outcomes in the range of 0.4–4.4 terahertz (as depicted in Figure 10). The PLS model merely demands two latent variables to account for over 98% of the data variance.
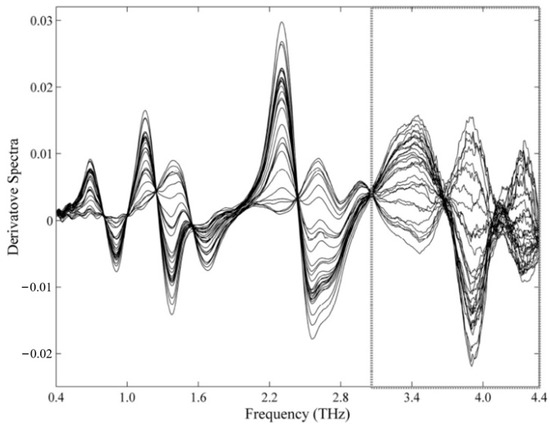
Figure 10.
Differential THz spectra of MBZ mixtures (SG, second-order polynomial, 51 window points). Removed spectral regions [95].
RMSEP values of 1.5% w/w, 1.2% w/w, and 1.8% w/w were utilized to distinguish the metamizole A, B, and C polymorphisms. The limits of detection (LOD) for the optimal PLS regression model was 2.7–4.3% w/w, 2.9–4.0% w/w, and 2.4–3.1% w/w, respectively. This analytical performance surpassed the methods reported in the literature that employed near-infrared (NIR) and mid-infrared (MIR) spectroscopy. The principal merit of NIR spectroscopy lies in its direct access to retinal information. As a result, the method developed proved to be an effective method for determining polycrystalline CBM crystals in raw materials. The method can be used as a practical analytical tool for the quality control of pharmaceutical raw materials.
2.2.5. Solid-State Nuclear Magnetic Resonance (SS-NMR)
Solid-state nuclear magnetic resonance (SS-NMR) is an analytical technique for the study of solid-state samples, allowing for the analysis of polycrystalline miscible crystals and the determination of certain characteristic crystalline patterns [96]. SS-NMR provides structural information at the atomic level and usually reflects the local electronic environment and nuclear spacing of the nucleus in the form of chemical shifts [97]. SS-NMR can simultaneously determine the physical and chemical properties of active pharmaceutical ingredients and excipients in pharmaceutical formulations. SS-NMR also has high selectivity because the nuclei of APIs often have chemical shifts that are different from those of general excipients [98].
The signal intensity at the magnetic equivalent position is proportional to the corresponding spin number, so NMR measurements provide accurate information about the compositional content of the sample and can be used for quantitative determination of crystal form [99].
Proton decoupling, magic angle rotation (MAS), and cross-polarization (CP) were used to produce high-resolution 13C SS-NMR spectra. The quantitative carbon spectroscopy cross-polarized magic angle spinning (CP-MAS) solid-state NMR method has been widely used to quantify polymorphs and percent morphological transformation in APIs and solid dosages [100]. 13C-CP-MAS NMR can quantify the relative amounts of API polymorphs, hydrates, solvates, salts, and amorphous forms in physical mixtures [101]. The benefit of 13C SS-NMR is that it is a non-destructive test technique that yields material structure information [102]. When individual component reference spectra are unavailable, the integrated intensity of two independent lines in the spectrum can be compared to quantitatively estimate defects, amorphous content, or mixed NMR phases.
Tinmanee et al. [103] identified the characteristic peaks of gabapentin crystalline type II and crystalline type III by 13C ss-NMR characterization (Figure 11) and developed a quantitative method using 13C-CP-MAS NMR intensities. The chemical shift peak areas at 28.2 and 39.1 ppm were selected for quantifying crystalline form II, while those at 37.6 and 41.0 ppm were employed for crystalline form III measurements. The natural logarithm of the peak area for each crystalline form correlates with the contact time (τ). The extrapolated peak area at τ = 0 was determined for each form. Subsequently, standard curves were constructed based on the natural logarithm of the percentage of peak area (for crystalline form II and crystalline form III, respectively) at τ = 0 for the corresponding crystalline forms. Method specificity, accuracy, sensitivity, and reproducibility were good.
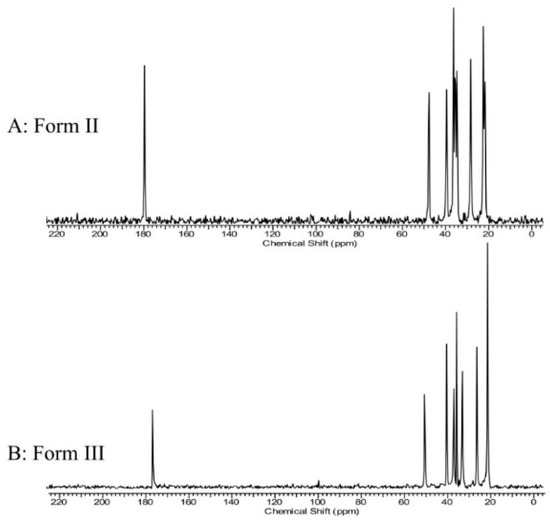
Figure 11.
13C CP/MAS NMR spectra (0–220 ppm) of gabapentin form II (A) and gabapentin form III (B) [103].
Currently, there are fewer examples of applying this method for the quantitative analysis of drug cocrystals. Compared to other spectroscopic methods, the determination of high-resolution ss-NMR usually undergoes high-speed rotation, which may lead to phase transitions of substable crystalline forms during the test. In addition, the technique requires strict control of the sample temperature during the experiment and a long detection time. Therefore, the application of this technique for crystal quantification has some limitations.
Examples of quantitative analysis of crystalline drugs using spectroscopic methods are shown in Table 3 below.

Table 3.
List of quantitative crystalline drug analysis using spectroscopic methods.
2.3. Thermal Analysis Method
2.3.1. Differential Scanning Calorimetry (DSC)
In recent years, with the continuous development of DSC technology, DSC has not only been limited to traditional qualitative studies but also has been gradually applied to quantitative analysis such as drug purity [123] and polymorph studies [124,125]. Since thermal effects occur between crystalline forms or melting, there is a proportional relationship between the corresponding heat change and the crystalline content. Therefore, the DSC method can be used to quantify the content of drug crystalline forms. The DSC method not only can quantify the cocrystal content of a drug but also can determine the crystallinity of the drug with the premise of 100% crystallinity of solid drug, which requires a small amount of sample and is easy to operate, but the damage to the sample is irreversible, so it is not suitable for the quantitative analysis of difficult to obtain or valuable samples. The thermodynamic differences in crystallinity are strongly influenced by the sample size, thickness, sample disk position, and thermocouple during measurement; in addition, the thermodynamic differences in different samples themselves often lead to large differences in the detection limits and quantification limits of different sample quantification methods.
Previous research has proven that DSC-based melting point and heat function measurements are accurate in determining API purity [126,127]. DSC at low heating rates determines the API melting range, and van’t Hoff can determine the molar percentage of impurities [128].
where ΔHf is the molar enthalpy in J mol−1, F is the fraction of melting corresponding to Tm, R is the gas constant (8.3143 J K−1 mol−1), x2 is the molar fraction of impurities, and Tm and T0 are the melting points of impurities and main materials, respectively, in Kelvin.
This van’t Hoff-based DSC method has excellent accuracy and precision in identifying impurity phase compositions of <3% (w/w) [128,129]. Furthermore, in comparison to other traditional purity determination techniques, the procedure is rapid and reliable and does not require impurity standards. Trace polycrystalline impurities have been successfully analyzed through the development of DSC-based purity assessment methods [130].
In a previous study, we analyzed the conditions for preparing voriconazole cocrystals by rapidly removing the solvent and determining the purity of the product using DSC detection of melting point, heat function, and heat absorption peak shape [131]. The combination of saccharin and the theophylline–sugar extract cocrystal resulted in a low cocrystal mixture with a low melting point of 198 °C, according to the results of the analysis [132]. Enantiomeric polymorphs, which consist of a 1:1 cocrystal of acetamide and ethylmalonic acid [133], exhibit a comparable solid-state phase transition at 77 °C. Consequently, differential scanning calorimetry (DSC) represents a practical approach for ascertaining the purity of cocrystal crystals and for detecting and quantifying cocrystal enantiomeric polymorphs.
Bruni [134] et al. present an accurate thermodynamic model that elaborates on the qualitative and quantitative analyses of DSC measurements of the zatoprofen/4,40-bipyridine system producing cocrystal samples of two distinct compositions. The model yields quantitative predictions (enthalpy of reaction and composition of the cocrystal) that are in good agreement with the experimental data while adequately accounting for the qualitative components of the intricate experimental context. FT-IR and NMR spectroscopy data, along with X-ray diffraction measurements, indicate cocrystal production and composition. The so-called Thaman triangle’s scientific underpinnings are strengthened and rationalized by the quantitative treatment of DSC measurements, which also makes the model broadly applicable. Bruni et al. [135] also prepared binary mixtures of nateglinide and benzamide with different compositions and analyzed them by differential scanning calorimetry (DSC). Cocrystal mixtures and cocrystal compositions 2N:3B were obtained by a combination of Taman and least-squares methods; two straight lines were verified to have physical significance for model prediction, and the data were checked for self-consistency and reliability of results. He also used DSC technology to detect and quantify the presence of polymorphic impurities in dexetoprofen trometamol samples [136]. With the method proposed here, polymorphic impurities can be detected even when only a very low percentage (0.3%) is present, while they can be reliably quantified at levels above 2%.
Liu et al. [137] developed a useful analytical method for quantifying ETV-A in the binary mixture of ETV-H and ETV-A of ETV (entecavir): the integral enthalpy of the phase transition in the DSC thermoanalytical plots was chosen to quantify the amount of ETV-A in the binary mixture. ETV-H showed a characteristic thermal absorption peak in the DSC single-cycle plot that was not present in ETV-A, but was absent in the DSC plot. The thermal cycle DSC curves of the 11 binary ETV mixtures of ETV-A mixtures to quantify the amount of ETV-A are shown in Figure 12, and the DSC plot of ETV-H is shown in Figure 12; it can be seen that, as the ETV-A content increases, the heat of the inherent thermal absorption peak decreases. The inherent thermal absorption peak heat divided by the weight of the sample is the enthalpy of crystallization transition. The enthalpy of this thermal absorption peak was therefore chosen to measure the amount of ETV-A in the binary mixture.
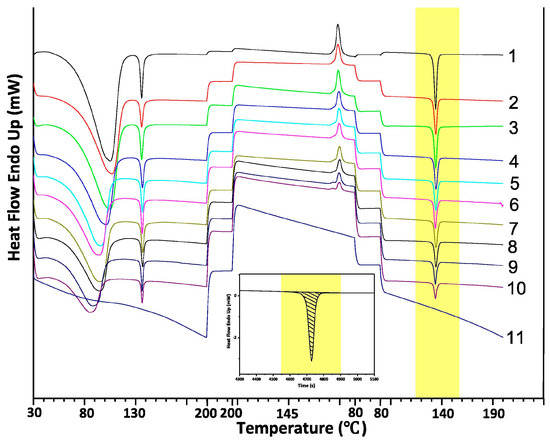
Figure 12.
The DSC thermal cycle of 11 binary mixture samples of the solid form of ETV. The embedded figure is a schematic of the thermal integration of a single endothermic peak for 0% ETV-A content [137].
The standard curve derived from the DSC technique was represented by the equation Y = 100.455 + 7.255X, with an R2 value of 0.997. The content range of ETV-A in the binary mixture was from 0 to 100% (w/w). The limit of detection (LOD) for ETV-A was determined to be 0.909%, and the limit of quantification (LOQ) was 2.755%. The findings indicated that the DSC technique is both precise and accurate and is applicable for the determination of ETV-A content within binary mixtures.
In drug cocrystal studies, sometimes only one thermal analysis method may be applied to obtain only one-sided analytical results, which cannot fully investigate the thermodynamic properties of the sample. Therefore, the use of multiple thermal analysis techniques and their combinations with other analytical techniques are receiving increasing attention. The combination of DSC and PXRD methods allows for the quantitative analysis of cocrystal crystals. Tong et al. [138] quantified the relative content of salmeterol xenonate powder using a combination of DSC, HPLC, and PXRD. The results of the methodological investigation showed that the method has good accuracy and reproducibility. In addition, Lefort et al. [139] quantified amorphous forms in algal sugar preparations using combined ss-NMR and DSC.
2.3.2. Thermogravimetric Analysis (TGA)
Thermal gravimetric analysis (TGA) is a thermal analysis technique that uses software to regulate temperature in order to determine a substance’s mass as a function of temperature. Thermogravimetric analysis (TGA), a thermoanalytical approach, determines a substance’s mass alteration (encompassing mass change [either gain or loss] and mass change rate) in relation to temperature or time within a controlled environment [140]. Three variants of TGA are as follows: Dynamic TGA, wherein the sample is subjected to a continuous temperature rise, typically progressing linearly with time; isothermal/static TGA, which holds the sample under specific conditions/temperatures for an adequately extended period, during which the sample’s weight variation is gauged; and mass TGA, which heats the sample to a constant weight in each sequence while elevating the temperature. TGA is capable of probing the physical phenomena associated with material transformation, such as crystal melting, evaporation, sublimation, and adsorption, and can be categorized into dynamic heating and static temperature control modalities. TGA is usually used to determine the total volatile content of a sample, as it can only measure changes in the mass of a sample, especially decreases.
The TGA technique is particularly useful when the drug cocrystal contains volatile components because it can determine the stoichiometry of volatile components by weight loss and is suitable for the identification of crystalline substances containing different amounts and types of crystalline solvents (or water) in the test article. However, TGA only provides information about the content of volatile components and cannot identify their elements. When TGA is used in combination with mass spectrometry (MS) and Fourier-transform infrared spectroscopy (FTIR), volatile components can be identified and quantified to achieve qualitative and quantitative analyses of volatile components in the test sample [141].
With TG methods, it can be difficult to interpret an increase or decrease in mass; TG-DTA or TG-DSC techniques allow scientists to better understand thermal phenomena (crystallization, melting, cocrystallization), as the difference between mass and heat flow can be measured simultaneously. Meloxicam–salicylic acid, norfloxacin–riboflavin, norfloxacin–glucagon, and ciprofloxacin–pyridine carboxylic acid cocrystallization systems have been characterized by TG-DTA or TG-DSC techniques, and thermal events have been attributed with some certainty [142,143,144,145]. The study of the meloxicam–salicylic acid cocrystal system by fugitive gas analysis by TG-FTIR was also reported to reveal the formation of ketones during cocrystal degradation [146].
Guizani [147] et al. suggest a quick quantitative thermal analysis technique that combines chemometrics and thermogravimetric analysis (TGA) to examine the composition of synthetic mixed cellulose fibers. Thermogravimetric analysis combined with partial least-squares regression (TGA-PLSR) serves as effective means for investigating the proportion of biopolymer in novel man-made cellulose–lignin and cellulose–chitosan blended fibers. It exhibits a cross-validation error of 1.94 weight percent within the range of 0 to 100 weight percent, and the thermal analysis approach can estimate the polyester content in cellulose–polyester blends with good precision in a reasonably short amount of time (~1 h) and without the need for chemicals. It is applicable to many different types of composites.
2.4. Dynamic Vapor Sorption (DVS)
Formulation processes (e.g., micronization, pulverization, lyophilization, spray drying, milling, and tableting) result in varying degrees of amorphous forms in the crystals, and the resulting amorphous forms are mainly adsorbed on the surface of the crystals, affecting their surface properties, which can seriously affect the post-processing of the product and the stability of the drug. This requires the quantification, monitoring, and control of amorphous forms. DVS technology can be used to quantify trace amorphous forms in drug crystals, where trace amorphous quantification specifically refers to the quantification of amorphous forms with <10% amorphous content.
Amorphous solids absorb relatively more water than their crystalline counterparts. This is due to the fact that, in amorphous solids, both surface adsorption and native adsorption occur. Consequently, the quantity of water absorbed is contingent upon the accessible surface area. Figure 13 depicts in a schematic manner the adsorption (occurring on the surface) and desorption (taking place in the bulk) of water vapor on both crystalline and amorphous solids.
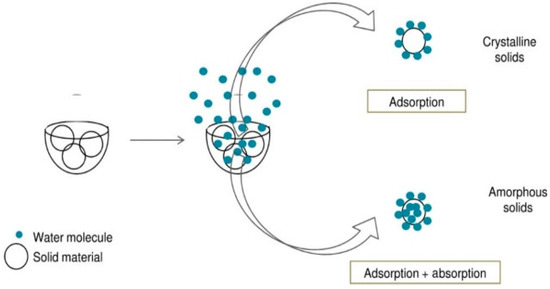
Figure 13.
Schematic diagram of adsorption and desorption of water vapor in crystalline and amorphous solids [148].
The degree of water adsorption and desorption is associated with the amorphous content within the sample. DVS can ascertain the amorphous content by computing either the maximum quantity of water absorbed by the sample or the amount of water taken up by the amorphous substance present in the sample.
DVS is used for the analysis of amorphous quantification by three main methods, which are summarized below:
Method 1: Equilibrium Moisture Uptake Method
The principle is to determine the amount of equilibrium moisture absorption weight gain of the crystalline form, amorphous form, and the mixture of the two; in a wide range of amorphous proportions, the equilibrium moisture absorption weight gain and the amorphous content are linearly related. The limit of detection of the equilibrium hygroscopic method is limited by the amount of hygroscopic weight gain of the sample under the selected conditions; in other words, the greater the change in mass, the higher the accuracy of the equilibrium hygroscopic method for quantitative analysis [149].
Method 2: Water/Solvent Uptake Method
The principle is to compare the weight change before and after amorphous transcrystallization under constant humidity. The difference in weight between before and after moisture absorption and the amorphous content shows a linear relationship. It is suitable for cases where amorphous forms can recrystallize to form crystals under a specific solvent atmosphere and humidity [150].
Method 3: Residual Weight Method
The principle is that the difference in adsorption and desorption at low humidity is linearly related to the amorphous content. Before amorphous transcrystallization, there are surface adsorption and main body absorption under DVS conditions, and when transcrystallization forms hydrate or solvent compound crystals with a measured chemical ratio, the desorption process is carried out, and the solvent (or water) with a measured chemical ratio that enters into the lattice is removed, so the adsorption and desorption difference under low humidity is only correlated with the amorphous content, and therefore, it can be used to perform the quantification [151]. For amorphous content under selected test conditions, compounds or hydrates of stoichiometric chemical ratios must be able to form, so the application is relatively limited.
Three methods for amorphous quantification by DVS are presented, of which Methods 2 and 3 are more accurate, but each has its own applicability. Sheokand [148] gives a decision tree for the specific choice of which method to use (Figure 14).
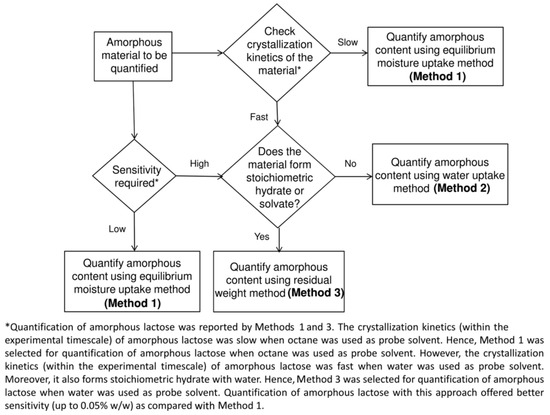
Figure 14.
DVS amorphous quantitative method selection decision tree [148].
Bagwan [152] et al. developed an analytical method based on dynamic vapor sorption (DVS) to determine the surface amorphous content of terbutaline sulfate crystals due to grinding. The calibration curve showed a linear slope of 0.999 in the plot concentration range of 0–16.36% w/w of amorphous content versus normalized weight change in surface area due to moisture absorption.
3. Advantages and Disadvantages of Solid-State Drug Quantification Methods
There are various methods for the quantitative analysis of solid drugs, and a comparison of each analytical method is shown in Table 4. For a particular system, it may sometimes be difficult to judge which method is better, so the establishment of a good quantitative method needs to take into account its sensitivity, specificity, accuracy, durability, limit of detection, limit of quantification, the difficulty of the method, and the characteristics of the specimen to be analyzed. Solid samples inherently have problems such as poor homogeneity, making the quantitative methods for drug polymorphs often unable to achieve the accuracy and precision of chromatographic methods. Usually, a method with a limit of quantification of 1% and a relative standard deviation of about 5% is already a better quantitative method.

Table 4.
Comparison of methods for quantitative analysis of polymorphic drugs.
The PXRD method is preferred for the quantification of the crystalline form of a drug. Although the quantitative results of other methods sometimes appear to be better, they are generally used only as an adjunct to the PXRD method. Spectroscopic methods are mostly treated with chemometrics due to the complexity of the data and the high spectral specificity of Raman, IR, and SS-NMR of amorphous phases. Therefore, spectroscopic methods are superior to diffraction methods in quantifying crystallinity. The non-destructive nature of spectroscopic methods makes this method superior to thermodynamic methods. Of course, in practical applications, the joint quantification of two or more methods is advocated to make up for the shortcomings of each method.
4. Conclusions and Prospect
The quantitative technology of polymorphic drugs is developing day by day, and the examples of PXRD used for quantification are becoming increasingly frequent, with the appearance and updating of various software, and the full-spectrum method will be one of the trends of future development; among the spectroscopic methods, with the improvement in the sensitivity of FT-Raman and NIRS as well as the advantages of NIRS that does not need sample preparation and has good exclusivity, it makes the application of this method in quantification a great prospect. The increase in the precision of thermal analysis instruments and the expansion of the range of heating rate enable this method to separate the characteristic peaks of different crystalline forms, thus expanding its application to the quantification of crystalline forms, but the irreversible destruction of the sample caused by its heating process limits the application of this method to a certain extent; SS-NMR has not yet fully utilized its potential in the molecular level analysis of solid-state drugs due to its high cost. The popularization of various instruments has made it possible to apply this technique in combination, which significantly improves the selectivity of quantitative methods. In conclusion, in order to ensure the quality of polymorphic drugs and their safety, the development of quantitative methods for the analysis of drug crystals and cocrystals and their optimization will remain an essential research hotspot in the future R&D and production of polymorphic drugs.
Author Contributions
Conceptualization, writing—original draft, data curation, formal analysis, investigation, methodology, and writing—review and editing, Y.T.; data curation, methodology, formal analysis, investigation, and writing—original draft, Y.G.; validation and investigation, B.Z.; investigation and data curation, K.H.; writing—review and editing, Y.X.; data curation and funding acquisition, L.Z.; supervision, project administration, resources, and writing—review and editing, S.Y.; funding acquisition and supervision, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the support provided by the Fundamental Research Funds for the Central Universities (no. 2022-RW350-02); Open project of Key Laboratory in Xinjiang Uygur Autonomous Region of China (2023D04035); CAMS Innovation Fund for Medical Sciences (No. 2021-I2M-1-029); and 2021 Tengzhou Talent Project.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Wang, L.D.S.; Dong, W. Research advances of polymorphism in pharmaceutical cocrystals. Chemcial Ind. Eng. 2018, 35, 29–37. [Google Scholar]
- Fong, S.Y.K.; Ibisogly, A.; Bauer-Brandl, A. Solubility enhancement of BCS Class II drug by solid phospholipid dispersions: Spray drying versus freeze-drying. Int. J. Pharm. 2015, 496, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Chandel, A.K.S. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraja, K.; Khanam, J. Enhancement of carvedilol solubility by solid dispersion technique using cyclodextrins, water soluble polymers and hydroxyl acid. J. Pharm. Biomed. Anal. 2014, 96, 10–20. [Google Scholar] [CrossRef]
- Hisada, N.; Takano, R.; Takata, N.; Shiraki, K.; Ueto, T.; Tanida, S.; Kataoka, M.; Yamashita, S. Characterizing the dissolution profiles of supersaturable salts, cocrystals, and solvates to enhance in vivo oral absorption. Eur. J. Pharm. Biopharm. 2016, 103, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Kumar, S.; Sachan, A.K.; Yadav, K. Techniques For Increasing Solubility: A Review Of Conventional And New Strategies. Asian J. Pharm. Res. Dev. 2022, 10, 144–153. [Google Scholar]
- McCrone, W.C. Physics and Chemistry of the Organic Solid State; Inderscience Publishers: Geneva, Switzerland, 1965; Volume 2, pp. 725–767. [Google Scholar]
- Shi, Q.; Chen, H.; Wang, Y.; Xu, J.; Liu, Z.; Zhang, C. Recent advances in drug polymorphs: Aspects of pharmaceutical properties and selective crystallization. Int. J. Pharm. 2022, 611, 121320. [Google Scholar] [CrossRef]
- Wong, S.N.; Chen, Y.C.S.; Xuan, B.; Sun, C.C.; Chow, S.F. Cocrystal engineering of pharmaceutical solids: Therapeutic potential and challenges. CrystEngComm 2021, 23, 7005–7038. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guide for industry. Regulatory Classification of Pharmaceutical Co-Crystals; Food and Drug Administration: Silver Spring, MD, USA, 2018. [Google Scholar]
- Kozakiewicz-Latała, M.; Junak, A.; Złocińska, A.; Pudło, W.; Prusik, K.; Szymczyk-Ziółkowska, P.; Karolewicz, B.; Nartowski, K.P. Adjusting the melting point of an Active Pharmaceutical Ingredient (API) via cocrystal formation enables processing of high melting drugs via combined hot melt and materials extrusion (HME and ME). Addit. Manuf. 2022, 60, 103196. [Google Scholar] [CrossRef]
- Bhatt, J.A.; Bahl, D.; Morris, K.; Stevens, L.L.; Haware, R.V. Structure-mechanics and improved tableting performance of the drug-drug cocrystal metformin:salicylic acid. Eur. J. Pharm. Biopharm. 2020, 153, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Chettri, A.; Subba, A.; Singh, G.P.; Bag, P.P. Pharmaceutical co-crystals: A green way to enhance drug stability and solubility for improved therapeutic efficacy. J. Pharm. Pharmacol. 2024, 76, 1–12. [Google Scholar] [CrossRef]
- Xia, M.; Jiang, Y.; Cheng, Y.; Dai, W.; Rong, X.; Zhu, B.; Mei, X. Rucaparib cocrystal: Improved solubility and bioavailability over camsylate. Int. J. Pharm. 2023, 631, 122461. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, J.; Zhou, F.; Yang, J.; Jiang, L.; Chen, Q.; Zhou, Y.; Zhang, J. A potential cocrystal strategy to tailor in-vitro dissolution and improve Caco-2 permeability and oral bioavailability of berberine. Int. J. Pharm. 2024, 666, 124789. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Moldovan, A.A.; Maloney, A.G.P.; Roberts, K.J. Exploring the CSD Drug Subset: An Analysis of Lattice Energies and Constituent Intermolecular Interactions for the Crystal Structures of Pharmaceuticals. J. Pharm. Sci. 2023, 112, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Paiva, E.M.; da Silva, V.H.; Poppi, R.J.; Pereira, C.F.; Rohwedder, J.J.R. Comparison of macro and micro Raman measurement for reliable quantitative analysis of pharmaceutical polymorphs. J. Pharm. Biomed. Anal. 2018, 157, 107–115. [Google Scholar] [CrossRef]
- Singh, K.; Bajaj, N.; Kashyap, M.; Bandyopadhyay, A.; Sengupta, A. Applications of multi-parameter sensing in pharmaceutical, agriculture and mineral industries using THz spectroscopy and Low-Wavenumber Raman spectroscopy. Opt. Laser Technol. 2024, 177, 111020. [Google Scholar] [CrossRef]
- Shang, Z.; Liu, M.; Hu, W.; Deng, T.; Su, X.; Hou, B.; Wang, J.; Gong, J. Construction and application of a qualitative and quantitative analysis system of three boscalid polymorphs based on solid-state analytical methods and chemometric tools. CrystEngComm 2022, 24, 3096–3108. [Google Scholar] [CrossRef]
- Yao, Z.; Guochuan, F.; Zhen, W. Principle of X-ray diffraction and phase analysis of doped graphene. J. Hebei North Univ. 2018, 34, 10–14. [Google Scholar]
- Xin, L. Application of wavelength dispersive X-ray fluorescence spectrometer and polycrystalline X-ray diffraction spectrometry identification of imports of copper and copper materials. Chin. J. Inorg. Anal. Chem. 2018, 8, 21–25. [Google Scholar]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-ray Diffraction Techniques for Mineral Characterization: A Review for Engineers of the Fundamentals, Applications, and Research Directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Yan, X.; Jing, W.; Qiu, Y.; Yong, W.; Ying, B.; Hong, H. Quantitative Analyzing Methods for Polymorphism of Pharmaceuticals. Petrochem. Technol. 2015, 44, 11–18+10. [Google Scholar]
- Feng, P.Z.Y.; Ting, Z.; Si, X.; Jia, S.; Kang, S. Quantitative Analysis of Magnetite Spinel Content in Copper Flash Converting Slag by X-ray Diffraction (XRD). Chin. J. Inorg. Anal. Chem. 2023, 1–10. [Google Scholar] [CrossRef]
- Sundaram, M.; Natarajan, S.; Dikundwar, A.G.; Bhutani, H. Quantification of solid-state impurity with powder X-ray diffraction using laboratory source. Powder Diffr. 2020, 35, 226–232. [Google Scholar] [CrossRef]
- Mei, M.E.; Yu, L.I.; Yang, W. Application of X-ray Powder Diffraction to Quantitative Analysis of Pharmaceutical Polymorphism. Chin. J. Mod. Appl. Pharm. 2017, 34, 1356–1360. [Google Scholar]
- Qiu, J.-B.; Li, G.; Sheng, Y.; Zhu, M.-R. Quantification of febuxostat polymorphs using powder X-ray diffraction technique. J. Pharm. Biomed. Anal. 2015, 107, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.; Klug, H.P. Basic aspects of X-ray absorption in quantitative diffraction analysis of powder mixtures. Anal. Chem. 1948, 20, 886–889. [Google Scholar] [CrossRef]
- Lou, Y.; Zuo, L. Quantification of losartan potassium polymorphs using powder X-ray diffraction. J. AOAC Int. 2021, 104, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Chambi, J.T.; Prado, L.D.; de Carvalho Patricio, B.F.; Ceballos, M.; Bianco, I.; Fandaruff, C.; Rocha, H.V.A.; Kuznetsov, A.; Cuffini, S.L. Quantitative analysis and evaluation of solid-state stability of mebendazole Forms A and C suspensions by powder X-ray diffraction using the Rietveld method. Int. J. Pharm. 2024, 650, 123721. [Google Scholar] [CrossRef]
- Siddiqui, A.; Rahman, Z.; Korang-Yeboah, M.; Khan, M.A. Development and validation of X-ray diffraction method for quantitative determination of crystallinity in warfarin sodium products. Int. J. Pharm. 2015, 493, 1–6. [Google Scholar] [CrossRef]
- Kommavarapu, P.; Maruthapillai, A.; Palanisamy, K. Identification and quantitative determination of eletriptan hydrobromide polymorphs: Thermal, diffractometric and spectrometric studies. J. Taibah Univ. Sci. 2018, 9, 586–593. [Google Scholar] [CrossRef][Green Version]
- Beyer, A.; Grohganz, H.; Löbmann, K.; Rades, T.; Leopold, C.S. Multivariate Quantification of the Solid State Phase Composition of Co-Amorphous Naproxen-Indomethacin. Molecules 2015, 20, 19571–19587. [Google Scholar] [CrossRef]
- Otsuka, Y.; Ito, A.; Matsumur, S.; Takeuchi, M.; Pal, S.; Tanak, H. Quantification of Pharmaceutical Compounds Based on Powder X-Ray Diffraction with Chemometrics. Chem. Pharm. Bull. 2016, 64, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Hu, X.; Zhou, X.; Gu, J. Determination of minor quantities of linezolid polymorphs in a drug substance and tablet formulation by powder X-ray diffraction technique. Powder Diffr. 2017, 32, 78–85. [Google Scholar] [CrossRef]
- Zappi, A.; Maini, L.; Galimberti, G.; Caliandro, R.; Melucci, D. Quantifying API polymorphs in formulations using X-ray powder diffraction and multivariate standard addition method combined with net analyte signal analysis. Eur. J. Pharm. Sci. 2019, 130, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sadul, S.K.; Perumal, A.; Somanath, T.; Kalaivani. Development and Validation of Method for the Estimation of Tacrolimus Crystalline API in Tacrolimus Extended Release Capsules 0.5mg, 1mg and 5mg by Power X-ray diffractometer (PXRD). Res. J. Pharm. Tech. 2020. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Biomolecular and bioanalytical applications of infrared spectroscopy – A review. Anal. Chim. Acta 2020, 1133, 150–177. [Google Scholar] [PubMed]
- Ozaki, Y. Infrared Spectroscopy—Mid-infrared, Near-infrared, and Far-infrared/Terahertz Spectroscopy. Anal. Sci. 2021, 37, 1193–1212. [Google Scholar] [CrossRef]
- Liu, X.; Qing, H.; Ding, J.; Zhu, Q.; He, A.; Yue, .S.; Li, X. Using barium fluoride fine particles as stationary phase for TLC/FTIR analysis. Spectrosc. Spectr. Anal. 2011, 31, 1767–1771. [Google Scholar]
- Haas, J.; Mizaikoff, B. Advances in Mid-Infrared Spectroscopy for Chemical Analysis. Annu. Rev. Anal. Chem. 2016, 9, 45–68. [Google Scholar] [CrossRef]
- Yang, Y.C.; Li, R.X.; Liu, G.Y. Study on the Thermo—oxidative Degradation Behavior of ABS Resin Based on the TGA —FTIR Hyphenated Technique. J. Instrum. Anal. 2010, 29, 777. [Google Scholar]
- Lin, C.; Ling, C.; Ya-Shan, Z.; Han, S.; Rong, L. Advances in the research of domestic Drug Polymorphism. Strait Pharm. J. 2020, 32, 1–4. [Google Scholar]
- Gauglitz, G.; Moore, D.S. Handbook of Spectroscopy; Wiley-Vch: Weinheim, Germany, 2014; Volume 1. [Google Scholar]
- Suárez, L.; García, R.; Riera, F.A.; Diez, M.A. ATR-FTIR spectroscopy for the determination of Na4EDTA in detergent aqueous solutions. Talanta. 2013, 115, 652–656. [Google Scholar] [CrossRef]
- Carolei, L.; Gutz, I.G. Simultaneous determination of three surfactants and water in shampoo and liquid soap by ATR-FTIR. Talanta. 2005, 66, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Kapourani, A.; Valkanioti, V.; Kontogiannopoulos, K.N.; Barmpalexis, P. Determination of the physical state of a drug in amorphous solid dispersions using artificial neural networks and ATR-FTIR spectroscopy. Int. J. Pharm. X 2020, 2, 100064. [Google Scholar] [CrossRef]
- Hongo, T.; Fukuda, S. TRANSMISSION ELECTRON MICROSCOPY AND FOURIER-TRANSFORM INFRARED SPECTROSCOPY STUDIES OF CHRYSOTILE DISSOLUTION USING A FLOW-THROUGH METHOD. Clay Sci. 2023, 27, 41–48. [Google Scholar]
- Zhang, J.; He, X.; Wan, R. Application of Diffuse Reflectance Fourier Transform Infrared Spectroscopy in Quantitative Analysis. Phys. Test. Chem. Anal. Part B Chem. Anal 2015, 51, 1347–1362. [Google Scholar]
- Xin, H.; An-Xin, L.; Jin, Z. Simultaneous analysis of SO42- and NO3- in soil by diffuse reflectance Fourier transform infrared spectroscopy. Chin. J. Anal. Lab. 2015, 34, 1428–1431. [Google Scholar]
- Yang, P.W.; Mantsch, H.H.; Baudais, F. A critical evaluation of three types of diffuse reflectance infrared accessories. Appl. Spectrosc. 1986, 40, 974–978. [Google Scholar] [CrossRef]
- Kubelka, P. Ein Beitrag zur Optik der Farbanstriche (Contribution to the optic of paint). Z. Fur. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Braga, J.W.B.; Poppi, R.J. Figures of merit for the determination of the polymorphic purity of carbamazepine by infrared spectroscopy and multivariate calibration. J. Pharm. Sci. 2004, 93, 2124–2134. [Google Scholar] [CrossRef] [PubMed]
- Hartauer, K.J.; Miller, E.S.; Guillory, J.K. Diffuse reflectance infrared Fourier transform spectroscopy for the quantitative analysis of mixtures of polymorphs. Int. J. Pharm. 1992, 85, 163–174. [Google Scholar] [CrossRef]
- Hamadeh, I.M.; Yeboah, S.A.; Trumbull, K.A.; Griffiths, P.R. Preparation of calibration standards for quantitative diffuse reflectance infrared spectrometry. Appl. Spectrosc. 1984, 38, 486–491. [Google Scholar] [CrossRef]
- Brimmer, P.J.; Griffiths, P.R. Effect of absorbing matrixes on diffuse reflectance infrared spectra. Anal. Chem. 1986, 58, 2179–2184. [Google Scholar] [CrossRef]
- Olinger, J.M.; Griffiths, P.R. Quantitative effects of an absorbing matrix on near-infrared diffuse reflectance spectra. Anal. Chem. 1988, 60, 2427–2435. [Google Scholar] [CrossRef]
- Yeboah, S.A.; Wang, S.-H.; Griffiths, P.R. Effect of pressure on diffuse reflectance infrared spectra of compressed powders. Appl. Spectrosc. 1984, 38, 259–264. [Google Scholar] [CrossRef]
- Brimmer, P.J.; Griffiths, P.R. Angular dependence of diffuse reflectance infrared spectra. Part III: Linearity of Kubelka-Munk plots. Appl. Spectrosc. 1988, 42, 242–247. [Google Scholar] [CrossRef]
- Brimmer, P.J.; Griffiths, P.R. Angular dependence of diffuse reflectance infrared spectra. Part II: Effect of polarization. Appl. Spectrosc. 1987, 41, 791–797. [Google Scholar] [CrossRef]
- Manley, M. Near-infrared spectroscopy and hyperspectral imaging: Non-destructive analysis of biological materials. Chem. Soc. Rev. 2014, 43, 8200–8214. [Google Scholar] [CrossRef] [PubMed]
- Munawar, A.A.; Zulfahrizal; Meilina, H.; Pawelzik, E. Near infrared spectroscopy as a fast and non-destructive technique for total acidity prediction of intact mango: Comparison among regression approaches. Comput. Electron. Agric. 2022, 193, 106657. [Google Scholar] [CrossRef]
- Si, L.; Ni, H.; Pan, D.; Zhang, X.; Xu, F.; Wu, Y.; Bao, L.; Wang, Z.; Xiao, W.; Wu, Y. Nondestructive qualitative and quantitative analysis of Yaobitong capsule using near-infrared spectroscopy in tandem with chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119517. [Google Scholar] [CrossRef]
- Heinz, A.; Strachan, C.J.; Gordon, K.C.; Rades, T. Analysis of solid-state transformations of pharmaceutical compounds using vibrational spectroscopy. J. Pharm. Pharmacol. 2009, 61, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-P.; Chen, P.; Dai, J.-W.; Liu, D.; Li, J.-Y.; Xu, Y.-P.; Chu, X.-L. Recent advances of chemometric calibration methods in modern spectroscopy: Algorithms, strategy, and related issues. TrAC Trends Anal. Chem. 2022, 153, 116648. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Wang, Q.; Song, P.; Li, H.; Wu, S.; Gong, J. Quantitative analysis of low content polymorphic impurities in canagliflozin tablets by PXRD, NIR, ATR-FITR and Raman solid-state analysis techniques combined with stoichiometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 293, 122458. [Google Scholar] [CrossRef]
- Korang-Yeboah, M.; Akhtar, S.; Siddiqui, A.; Rahman, Z.; Khan, M.A. Application of NIR chemometric methods for quantification of the crystalline fraction of warfarin sodium in drug product. Drug Dev. Ind. Pharm. 2016, 42, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, S.; Wu, J.X.; Rinnan, Å.; Allesø, M.; van den Berg, F.; Jepsen, P.U.; Engelsen, S.B. Quantifying crystalline α-lactose monohydrate in amorphous lactose using terahertz time domain spectroscopy and near infrared spectroscopy. Vib. Spectrosc. 2019, 102, 39–46. [Google Scholar] [CrossRef]
- Wood, C.; Alwati, A.; Halsey, S.; Gough, T.; Brown, E.; Kelly, A.; Paradkar, A. Near infra red spectroscopy as a multivariate process analytical tool for predicting pharmaceutical co-crystal concentration. J. Pharm. Biomed. Anal. 2016, 129, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Nishizawa, J.-I.; Shibata, J.; Ito, M. Quantitative evaluation of mefenamic acid polymorphs by terahertz-chemometrics. J. Pharm. Sci. 2010, 99, 4048–4053. [Google Scholar] [CrossRef]
- Ren, J.; Mao, S.; Lin, J.; Xu, Y.; Zhu, Q.; Xu, N. Research Progress of Raman Spectroscopy and Raman Imaging in Pharmaceutical Analysis. Curr. Pharm. Des. 2022, 28, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- El-Gizawy, S.A.; Osman, M.A.; Arafa, M.F.; El Maghraby, G.M. Aerosil as a novel co-crystal co-former for improving the dissolution rate of hydrochlorothiazide. Int. J. Pharm. 2015, 478, 773–778. [Google Scholar] [CrossRef]
- Hertrampf, A.; Sousa, R.; Menezes, J.; Herd, T. Semi-quantitative prediction of a multiple API solid dosage form with a combination of vibrational spectroscopy methods. J. Pharm. Biomed. Anal. 2016, 124, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.R.; Hooper, D.C.; Zhang, L.; Wolverson, D.; Valev, V.K. Raman Techniques: Fundamentals and Frontiers. Nanoscale Res. Lett. 2019, 14, 231. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Cheng, H.; Deng, Y.; Frost, R.L. The molecular structure of chloritoid: A mid-infrared and near-infrared spectroscopic study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lin, B.; Yang, X.; Zhao, B.; Zhang, H.; Dong, Q.; Zhong, L.; Zhang, S.; Zhang, M.; Xu, X.; et al. Review of the Application of Raman Spectroscopy in Qualitative and Quantitative Analysis of Drug Polymorphism. Curr. Top. Med. Chem. 2023, 23, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Shipp, D.W.; Sinjab, F.; Notingher, I. Raman spectroscopy: Techniques and applications in the life sciences. Adv. Opt. Photonics 2017, 9, 315–428. [Google Scholar] [CrossRef]
- Koide, T.; Takeuchi, Y.; Otaki, T.; Yamamoto, K.; Shimamura, R.; Ohashi, R.; Inoue, M.; Fukami, T.; Izutsu, K.-I. Quantification of a cocrystal and its dissociated compounds in solid dosage form using transmission Raman spectroscopy. J. Pharm. Biomed. Anal. 2020, 177, 112886. [Google Scholar] [CrossRef]
- Barmpalexis, P.; Karagianni, A.; Nikolakakis, I.; Kachrimanis, K. Artificial neural networks (ANNs) and partial least squares (PLS) regression in the quantitative analysis of cocrystal formulations by Raman and ATR-FTIR spectroscopy. J. Pharm. Biomed. Anal. 2018, 158, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Osada, T.; Hisada, H.; Koide, T.; Fukami, T.; Roy, A.; Carriere, J. Quantitative Monitoring of Cocrystal Polymorphisms in Model Tablets Using Transmission Low-Frequency Raman Spectroscopy. J. Pharm. Sci. 2023, 112, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Bondi, R.W.; Anderson, C.A.; Drennen, J.K.; Igne, B. Investigation of the sensitivity of transmission Raman spectroscopy for polymorph detection in pharmaceutical tablets. Appl. Spectrosc. 2017, 71, 1856–1867. [Google Scholar] [CrossRef]
- Inoue, M.; Hisada, H.; Koide, T.; Fukami, T.; Roy, A.; Carriere, J.; Heyler, R. Transmission low-frequency Raman spectroscopy for quantification of crystalline polymorphs in pharmaceutical tablets. Anal. Chem. 2019, 91, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Tozuka, Y.; Ito, A.; Seki, H.; Oguchi, T.; Yamamoto, K. Characterization and quantitation of clarithromycin polymorphs by powder X-ray diffractometry and solid-state NMR spectroscopy. Chem. Pharm. Bull. 2002, 50, 1128–1130. [Google Scholar] [CrossRef][Green Version]
- Bawuah, P.; Zeitler, J.A. Advances in terahertz time-domain spectroscopy of pharmaceutical solids: A review. TrAC Trends Anal. Chem. 2021, 139, 116272. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, P.-H.; Yang, D.-Z.; Bi, Y.-C.; Su, B.; Zhang, B.-X.; Wang, F.-Q.; Lu, Y.; Du, G.-H. Purity and Uncertainty Study of CRM Betulin by DSC. Nat. Prod. Bioprospecting 2020, 10, 317–324. [Google Scholar] [CrossRef]
- Withayachumnankul, W.; Ferguson, B.; Rainsford, T.; Mickan, S.; Abbott, D. Simple material parameter estimation via terahertz time-domain spectroscopy. Electron. Lett. 2005, 41, 800–801. [Google Scholar] [CrossRef]
- Hong, Z.; Min, G.; Wen, W.; Qing, L.; Xiao, Y. Application of Teraherz Time Domain Spectroscopy in Chemistry and Biology. Chem. Bull. 2005, 2, 87–93. [Google Scholar]
- Neu, J.; Schmuttenmaer, C.A. Tutorial: An introduction to terahertz time domain spectroscopy (THz-TDS). J. Appl. Phys. 2018, 124, 231101. [Google Scholar] [CrossRef]
- He, M.; Guo, S. Research on application of terahertz technology in drugs. J. Electron. Meas. Instrum 2012, 26, 663–672. [Google Scholar] [CrossRef]
- Jiang, Y.; Shen, Y.; Wu, P. Application of ATR-FTIR spectroscopy in polymer film study. Prog. Chem. 2007, 19, 173. [Google Scholar]
- Huang, S.; Deng, H.; Wei, X.; Zhang, J. Progress in application of terahertz time-domain spectroscopy for pharmaceutical analyses. Front. Bioeng. Biotechnol. 2023, 11, 1219042. [Google Scholar] [CrossRef] [PubMed]
- Xiao-Jing, M.; Hong-Wei, Z.; Bin, D.; Gui-Feng, L. THz spectra of hypoxanthine and inosine. Acta Phys. Sin. 2008, 57, 3429–3434. [Google Scholar] [CrossRef]
- Liu, J.; Fan, L. Qualitative and quantitative determination of potassium aluminum sulfate dodecahydrate in potato starch based on terahertz spectroscopy. Microw. Opt. Technol. Lett. 2020, 62, 525–530. [Google Scholar] [CrossRef]
- Vieira, F.S.; Pasquini, C. Determination of cellulose crystallinity by terahertz-time domain spectroscopy. Anal. Chem. 2014, 86, 3780–3786. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, V.H.; Vieira, F.S.; Rohwedder, J.J.R.; Pasquini, C.; Pereira, C.F. Multivariate quantification of mebendazole polymorphs by terahertz time domain spectroscopy (THZ-TDS). Anal. 2017, 142, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, V.D.; Lankalapalli, S.; Guntaka, P.C.R. Posaconazole-amino acid cocrystals for improving solubility and oral bioavailability while maintaining antifungal activity and low In vivo toxicity. J. Drug Deliv. Sci. Technol. 2022, 74, 103491. [Google Scholar] [CrossRef]
- Li-xin, L.; Feng, D.; Guang-jin, H. Quantitative Cross Polarization Magic-Angle Spinning NMR Spectroscopy in Solids. Chin. J. Magn. Reson. 2020, 37, 1–15. [Google Scholar]
- Ting, C.; Qi, L.; Qi, L.; Shi, Y.; Yang, L. Application of Solid-state Nuclear Magnetic Resonance Technology in the Research of Crystalline Drugs. Her. Med. 2022, 41, 655–663. [Google Scholar]
- Stephenson, G.A.; Forbes, R.A.; Reutzel-Edens, S.M. Characterization of the solid state: Quantitative issues. Adv. Drug Deliv. Rev. 2001, 48, 67–90. [Google Scholar] [CrossRef]
- Li, M.; Xu, W.; Su, Y. Solid-state NMR spectroscopy in pharmaceutical sciences. TrAC Trends Anal. Chem. 2021, 135, 116152. [Google Scholar] [CrossRef]
- Achanta, P.S.; Niemitz, M.; Friesen, J.B.; Tadjimukhamedov, F.K.; Bzhelyansky, A.; Giancaspro, G.I.; Chen, S.-N.; Pauli, G.F. Pharmaceutical analysis by NMR can accommodate strict impurity thresholds: The case of choline. J. Pharm. Biomed. Anal. 2022, 214, 114709. [Google Scholar] [CrossRef] [PubMed]
- Calvo, N.L.; Maggio, R.M.; Kaufman, T.S. Chemometrics-assisted solid-state characterization of pharmaceutically relevant materials. Polymorphic substances. J. Pharm. Biomed. Anal. 2018, 147, 518–537. [Google Scholar] [CrossRef]
- Tinmanee, R.; Larsen, S.C.; Morris, K.R.; Kirsch, L.E. Quantification of gabapentin polymorphs in gabapentin/excipient mixtures using solid state 13 C NMR spectroscopy and X-ray powder diffraction. J. Pharm. Biomed. Anal. 2017, 146, 29–36. [Google Scholar] [CrossRef]
- da Silva, V.H.; Gonçalves, J.L.; Vasconcelos, F.V.C.; Pimentel, M.F.; Pereira, C.F. Quantitative analysis of mebendazole polymorphs in pharmaceutical raw materials using near-infrared spectroscopy. J. Pharm. Biomed. Anal. 2015, 115, 587–593. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Xu, K.; Zou, H.; Li, H.; Liang, B. Qualitative and simultaneous quantitative analysis of cimetidine polymorphs by ultraviolet–visible and shortwave near-infrared diffuse reflectance spectroscopy and multivariate calibration models. J. Pharm. Biomed. Anal. 2015, 104, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Antonio, M.; Maggio, R.M. Assessment of mefenamic acid polymorphs in commercial tablets using chemometric coupled to MIR and NIR spectroscopies. Prediction of dissolution performance. J. Pharm. Biomed. Anal. 2018, 149, 603–611. [Google Scholar] [CrossRef] [PubMed]
- da Silva, V.H.; Soares-Sobrinho, J.L.; Pereira, C.F.; Rinnan, Å. Evaluation of chemometric approaches for polymorphs quantification in tablets using near-infrared hyperspectral images. Eur. J. Pharm. Biopharm. 2019, 134, 20–28. [Google Scholar] [CrossRef]
- Liu, M.; Fu, R.; Liu, J.; Song, P.; Li, H.; Dong, W.; Sun, Z. Quantitative Analysis of Amorphous Form in Indomethacin by Near Infrared Spectroscopy Combined with Partial Least Squares Regression Analysis. Molecules 2024, 29, 5290. [Google Scholar] [CrossRef]
- Guo, C.; Luo, X.; Zhou, X.; Shi, B.; Wang, J.; Zhao, J.; Zhang, X. Quantitative analysis of binary polymorphs mixtures of fusidic acid by diffuse reflectance FTIR spectroscopy, diffuse reflectance FT-NIR spectroscopy, Raman spectroscopy and multivariate calibration. J. Pharm. Biomed. Anal. 2017, 140, 130–136. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, J.; Zhang, B.; Zhang, L.; Yang, D.; Yang, S.; Fang, L.; Lu, Y.; Du, G. Quality control of naringenin-carbamazepine drug-drug cocrystal: Quantitative analytical method construction of ATR-FTIR and Raman combined with chemometrics. Microchem. J. 2024, 202, 110774. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, B.; Gong, L.; Hu, K.; Yang, S.; Lu, Y. Quantitative analysis of pyrazinamide polymorphs in ternary mixtures by ATR-FTIR and Raman spectroscopy with multivariate calibration. Vib. Spectrosc. 2024, 130, 103625. [Google Scholar] [CrossRef]
- Mah, P.T.; Fraser, S.J.; Reish, M.E.; Rades, T.; Gordon, K.C.; Strachan, C.J. Use of low-frequency Raman spectroscopy and chemometrics for the quantification of crystallinity in amorphous griseofulvin tablets. Vib. Spectrosc. 2015, 77, 10–16. [Google Scholar] [CrossRef]
- Lipiäinen, T.; Fraser-Miller, S.J.; Gordon, K.C.; Strachan, C.J. Direct comparison of low- and mid-frequency Raman spectroscopy for quantitative solid-state pharmaceutical analysis. J. Pharm. Biomed. Anal. 2018, 149, 343–350. [Google Scholar] [CrossRef]
- Karimi-Jafari, M.; Soto, R.; Albadarin, A.B.; Croker, D.; Walker, G. In-line Raman spectroscopy and chemometrics for monitoring cocrystallisation using hot melt extrusion. Int. J. Pharm. 2021, 601, 120555. [Google Scholar] [CrossRef] [PubMed]
- Rimsha, G.; Shahbaz, M.; Majeed, M.I.; Nawaz, H.; Rashid, N.; Akram, M.W.; Shabbir, I.; Kainat, K.; Amir, A.; Sultan, E.; et al. Raman Spectroscopy for the Quantitative Analysis of Solid Dosage Forms of the Active Pharmaceutical Ingredient of Febuxostat. ACS Omega 2023, 8, 41451–41457. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, T.; Maunu, S.L. Quantitation of a polymorphic mixture of an active pharmaceutical ingredient with solid state 13C CPMAS NMR spectroscopy. Int. J. Pharm. 2010, 394, 18–25. [Google Scholar] [CrossRef]
- Maruyoshi, K.; Iuga, D.; Watts, A.E.; Hughes, C.E.; Harris, K.D.M.; Brown, S.P. Assessing the Detection Limit of a Minority Solid-State Form of a Pharmaceutical by 1H Double-Quantum Magic-Angle Spinning Nuclear Magnetic Resonance Spectroscopy. J. Pharm. Sci. 2017, 106, 3372–3377. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, D.A.; Wijesekara, A.V.; Carnahan, S.L.; Hung, I.; Lubach, J.W.; Nagapudi, K.; Rossini, A. Rapid Characterization of Formulated Pharmaceuticals Using Fast MAS 1H Solid-State NMR Spectroscopy. J. Mol. Pharm. 2019, 16, 3121–3132. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.T.A.; Aspers, R.L.E.G.; Uusi-Penttilä, M.; Kentgens, A.P.M. Rapid Quantification of Pharmaceuticals via 1H Solid-State NMR Spectroscopy. Anal. Chem. 2022, 94, 16667–16674. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, X.; Xu, W.; Troup, G.M.; McNevin, M.J.; Nie, H.; Su, Y. Quantifying Pharmaceutical Formulations from Proton Detected Solid-State NMR under Ultrafast Magic Angle Spinning. J. Pharm. Sci. 2020, 109, 3045–3053. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-L.; Reddy, G.N.M.; Nishiyama, Y. Selective detection of active pharmaceutical ingredients in tablet formulations using solid-state NMR spectroscopy. Solid State Nucl. Magn. Reson. 2020, 106, 101651. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, L.; Bassanetti, I.; Mileo, V.; Amadei, F.; Sartori, A.; Venturi, L. Quantitation of Commercially Available API Solid Forms by Application of the NMR-qSRC Approach: An Optimization Strategy Based on In Silico Simulations. Anal. Chem. 2021, 93, 9049–9055. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Jin, J.; Xiong, J.; Yang, Z.; Gao, L.; Ma, Y.; Liu, B.-F.; Liu, X.; Chen, Y.; Zhang, G. Polymorphs of DP-VPA solid solutions and their physicochemical properties. J. Pharm. Sci. 2020, 109, 2156–2165. [Google Scholar] [CrossRef]
- Wardhana, Y.W.; Hardian, A.; Chaerunisa, A.Y.; Suendo, V.; Soewandhi, S.N. Kinetic estimation of solid state transition during isothermal and grinding processes among efavirenz polymorphs. Heliyon 2020, 6, e03876. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T. An extrapolation scheme for solid-state NMR chemical shift calculations. Chem. Phys. Lett. 2017, 677, 99–106. [Google Scholar] [CrossRef]
- Staub, H.; Perron, W. New method of purity determination by means of calorimetric differential thermal analysis. Anal. Chem. 1974, 46, 128–130. [Google Scholar] [CrossRef]
- Faroongsarng, D.; Kadejinda, W.; Sunthornpit, A. Thermal behavior of a pharmaceutical solid acetaminophen doped with p-aminophenol. AAPS PharmSciTech 2000, 1, 62–68. [Google Scholar] [CrossRef]
- Araújo, A.A.S.; Bezerra, M.D.S.; Storpirtis, S.; Matos, J.D.R. Determination of the melting temperature, heat of fusion, and purity analysis of different samples of zidovudine (AZT) using DSC. Braz. J. Pharm. Sci. 2010, 46, 37–43. [Google Scholar] [CrossRef]
- Kestens, V.; Roebben, G.; Linsinger, T. Development and validation of a differential scanning calorimetry purity determination method for polycyclic aromatic hydrocarbons. Accredit. Qual. Assur. 2010, 15, 269–281. [Google Scholar] [CrossRef]
- Tong, H.H.; Shekunov, B.Y.; York, P.; Chow, A.H. Thermal analysis of trace levels of polymorphic impurity in salmeterol xinafoate samples. Pharm. Res. 2003, 20, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.N.; Chan, S.W.S.; Peng, X.; Xuan, B.; Lee, H.W.; Tong, H.H.; Chow, S.F. Effects of the glass-forming ability and annealing conditions on cocrystallization behaviors via rapid solvent removal: A case study of voriconazole. Pharmaceutics 2020, 12, 1209. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Feng, Y.; Zhang, P.; Wei, H.; Dang, L. Selective crystal growth of theophylline-saccharin cocrystal on self-assembled monolayer from incongruent system. Cryst. Growth Des. 2015, 15, 4918–4924. [Google Scholar] [CrossRef]
- Aitipamula, S.; Wong, A.B.; Chow, P.S.; Tan, R.B. Polymorphism and phase transformations of a cocrystal of nicotinamide and pimelic acid. CrystEngComm 2012, 14, 8193–8198. [Google Scholar] [CrossRef]
- Bruni, G.; Maggi, L.; Monteforte, F.; Ferrara, C.; Capsoni, D.; Berbenni, V.; Milanese, C.; Girella, A.; Friuli, V.; Mustarelli, P. Zaltoprofen/4, 4′-Bipyridine: A Case Study to Demonstrate the Potential of Differential Scanning Calorimetry (DSC) in the Pharmaceutical Field. J. Pharm. Sci. 2021, 110, 3690–3701. [Google Scholar] [CrossRef]
- Bruni, G.; Maggi, L.; Mustarelli, P.; Sakaj, M.; Friuli, V.; Ferrara, C.; Berbenni, V.; Girella, A.; Milanese, C.; Marini, A. Enhancing the pharmaceutical behavior of nateglinide by cocrystallization: Physicochemical assessment of cocrystal formation and informed use of differential scanning calorimetry for its quantitative characterization. J. Pharm. Sci. 2019, 108, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Bruni, G.; Capsoni, D.; Milanese, C.; Cardini, A. Polymorphic quantification of dexketoprofen trometamol by differential scanning calorimetry. J. Therm. Anal. Calorim. 2022, 148, 1949–1958. [Google Scholar] [CrossRef]
- Liu, M.; Shi, P.; Wang, G.; Wang, G.; Song, P.; Liu, Y.; Wu, S.; Gong, J. Quantitative analysis of binary mixtures of entecavir using solid-state analytical techniques with chemometric methods. Arab. J. Chem. 2021, 14, 103360. [Google Scholar] [CrossRef]
- Tong, H.H.; Shekunov, B.Y.; Chan, J.P.; Mok, C.K.; Hung, H.C.; Chow, A.H. An improved thermoanalytical approach to quantifying trace levels of polymorphic impurity in drug powders. Int. J. Pharm. 2005, 295, 191–199. [Google Scholar] [CrossRef]
- Lefort, R.; De Gusseme, A.; Willart, J.-F.; Danede, F.; Descamps, M. Solid state NMR and DSC methods for quantifying the amorphous content in solid dosage forms: An application to ball-milling of trehalose. Int. J. Pharm. 2004, 280, 209–219. [Google Scholar] [CrossRef]
- Pindelska, E.; Sokal, A.; Kolodziejski, W. Pharmaceutical cocrystals, salts and polymorphs: Advanced characterization techniques. Adv. Drug Deliv. Rev. 2017, 117, 111–146. [Google Scholar] [CrossRef]
- Jing, X.; Xialin, D.; Lan, H.; Xiang, W. Research Progress in Characterization Methods of Pharmaceutical Cocrystals. Chin. J. Pharmacovigil. 2021, 18, 995. [Google Scholar]
- Ferreira, P.O.; de Moura, A.; de Almeida, A.C.; Santos, É.C.D.; Kogawa, A.C.; Caires, F.J. Mechanochemical synthesis, thermoanalytical study and characterization of new multicomponent solid forms of norfloxacin with saccharin. J. Therm. Anal. Calorim. 2022, 1–13. [Google Scholar] [CrossRef]
- Fernandes, R.P.; Nascimento, A.L.C.S.D.; Carvalho, A.C.S.; Teixeira, J.A.; Ionashiro, M.; Caires, F.J. Mechanochemical synthesis, characterization, and thermal behavior of meloxicam cocrystals with salicylic acid, fumaric acid, and malic acid. J. Therm. Anal. Calorim. 2019, 138, 765–777. [Google Scholar] [CrossRef]
- Ferreira, L.T.; Alarcon, R.T.; Perpétuo, G.L.; Bannach, G. Investigation and characterization by TG/DTG–DTA and DSC of the fusion of Riboflavin, and its interaction with the antibiotic norfloxacin in the screening of cocrystal. J. Therm. Anal. Calorim. 2019, 136, 581–588. [Google Scholar] [CrossRef]
- Torquetti, C.; Ferreira, P.O.; de Almeida, A.C.; Fernandes, R.P.; Caires, F.J. Thermal study and characterization of new cocrystals of ciprofloxacin with picolinic acid. J. Therm. Anal. Calorim. 2021, 1–8. [Google Scholar] [CrossRef]
- Liu, H.; Tong, H.H.; Zhou, Z. Feasibility of thermal methods on screening, characterization and physicochemical evaluation of pharmaceutical cocrystals. J. Therm. Anal. Calorim. 2022, 1–17. [Google Scholar] [CrossRef]
- Guizani, C.; Trogen, M.; Zahra, H.; Pitkänen, L.; Moriam, K.; Rissanen, M.; Mäkelä, M.; Sixta, H.; Hummel, M. Fast and quantitative compositional analysis of hybrid cellulose-based regenerated fibers using thermogravimetric analysis and chemometrics. Cellulose 2021, 28, 6797–6812. [Google Scholar] [CrossRef] [PubMed]
- Sheokand, S.; Modi, S.R.; Bansal, A.K. Dynamic Vapor Sorption as a Tool for Characterization and Quantification of Amorphous Content in Predominantly Crystalline Materials. J. Pharm. Sci. 2014, 103, 3364–3376. [Google Scholar] [CrossRef]
- Young, P.M.; Chiou, H.; Tee, T.; Traini, D.; Chan, H.-K.; Thielmann, F.; Burnett, D. The Use of Organic Vapor Sorption to Determine Low Levels of Amorphous Content in Processed Pharmaceutical Powders. Drug Dev. Ind. Pharm. 2008, 33, 91–97. [Google Scholar] [CrossRef]
- Hancock, G.Z.B.C. The Relationship Between the Glass Transition Temperature and the Water Content of Amorphous Pharmaceutical Solids. Pharm. Res. 1994, 11, 471–477. [Google Scholar] [CrossRef]
- Buckton, P.D.G. The use of gravimetric studies to assess the degree of crystallinity of predominantly crystalline powders. Int. J. Pharm. 1995, 123, 265–271. [Google Scholar] [CrossRef]
- Bagwan, N.U.S.; Sheokand, S.; Kaur, A.; Dubey, G.; Puri, V.; Bharatam, P.V.; Bansal, A.K. Role of surface molecular environment and amorphous content in moisture sorption behavior of milled Terbutaline Sulphate. Eur. J. Pharm. Sci. 2021, 161, 105782. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).