Abstract
Lithium-conducting NASICON materials have emerged as a promising alternative to organic liquid electrolytes for high-energy-density Li-metal batteries, owing to their superior ionic conductivity and excellent air stability. However, their practical application is hindered by poor sintering characteristics and high grain boundary resistance. In this investigation, Li1.3Al0.3−xYxTi1.7(PO4)3 (LAYTP-x, x = 0.00, 0.01, 0.03, 0.05, and 0.07) were successfully synthesized via conventional solid-state reaction to explore the impact of Y3+ on both ionic conductivity and chemical stability. The structural, morphological, and transport properties of the samples were comprehensively characterized in order to identify the optimal doping concentration. All samples exhibited a NASICON structure with a uniform distribution of Y elements within the electrolyte. Due to its highest relative density (95.8%), the LAYTP-0.03 electrolyte demonstrated the highest total conductivity of 2.03 × 10−4 S cm−1 with a relatively low activation energy of 0.33 eV, making it suitable for solid-state batteries. When paired with the NCM811 cathode, the Li/LAYTP-0.03/NCM811 cell exhibited outstanding electrochemical performance: a high capacity of 155 mAh/g was achieved at 0.2C after 50 cycles with a Coulombic efficiency of approximately 100%, indicating highly reversible lithium plating/stripping facilitated by the LAYTP-0.03 electrolyte. These results suggest that the LAYTP-0.03 ceramic electrolyte could be a promising alternative for developing safe solid-state Li-metal batteries.
1. Introduction
New energy electric vehicles are smarter and cheaper than fuel vehicles, but the battery is still a big problem, such as battery life, density, weight, price, and safety issues. The current power batteries are roughly the following types, which are ternary lithium batteries, lithium iron phosphate batteries, lithium cobalt acid batteries, nickel-metal hydride batteries, solid-state batteries, etc. [1,2]. Among them, the rapid advancement of portable electronics and stationary energy storage systems has established lithium-ion batteries (LIBs) as the predominant power source due to their high energy densities and low environmental impact [3,4,5,6]. However, the limited thermal stability of polyolefin membranes and flammable organic electrolytes hinder the large-scale development of lithium-ion batteries with high energy and safety standards [7,8]. Compared with liquid batteries, solid-state batteries (SSBs) have some advantages such as long driving range, low cost, high safety, and longer life. At present, the range of most liquid batteries is only between 400 km and 600 km, and the range of solid-state batteries can easily break through 1000 km, and the charging speed is more than three times faster than that of liquid batteries [9,10,11,12]. As one of the key materials of SSBs, solid-solid electrolytes (SSEs) demonstrated stable ion transport, a wide electrochemical window, and uniform lithium-ion deposition, thereby promoting battery system stability [13,14]. Furthermore, the solid characteristics of solid electrolytes effectively inhibit side reactions and dendrite growth in metal-negative electrodes while eliminating vaporization, flammability, and explosion risks associated with organic liquid electrolyte lithium-ion batteries, significantly enhancing overall battery safety [15].
With further development and optimization, solid-state electrolytes (SSEs) can be categorized into oxide, polymer, and sulfide electrolytes [14,16]. Among these, oxide-based ceramics have demonstrated high mechanical strength, wide electrochemical windows, and high ionic conductivity, making them easier to prepare on a large scale to meet the requirements of large-size batteries [5,17]. One typical oxide electrolyte is the NASICON-type Li1.3Al0.3Ti1.7(PO4)3 (LATP) electrolyte, which has garnered widespread attention due to its stability under ambient conditions, low density, high ionic conductivity, and stability at high voltages when paired with cathodes such as Ni-rich NMC [18,19,20]. Despite LATP grains exhibiting a high Li+ conductivity of 10−4 S cm−1, the overall ionic conductivity is still insufficient for the development and application of solid-state batteries [21]. Currently, various strategies have been employed to enhance the lithium-ion conductivity of LATP solid electrolyte for all-solid-state lithium batteries through methods such as adding sintering aids [22,23] or employing special sintering techniques [24,25,26] and elements doping [13,27,28].
Element doping is an effective method for enhancing ionic conductivity by adjusting lattice parameters or controlling bottleneck size [29]. Specifically, heteroatom doping with trivalent atoms can lead to the production of highly dense ceramics in LATP crystals. Yttrium (Y) doping has been found to alter the structural phase transition temperatures in various inorganic compounds by influencing structural distortion levels. For instance, Y3+ has been shown to stabilize the α phase of Li1.15Y0.15Zr1.85(PO4)3 (LYZP), resulting in a significant increase in total Li-ion conductivity (7 × 10−5 S cm−1) [30]. Additionally, the substitution of Al3+ with trivalent ions such as Y3+, Gd3+, and Sc3+ has been reported to improve the ionic conductivity of LATP by enhancing bond effects at grain boundaries and generating non-lithium conductive phases [31]. Using sol-gel-vacuum drying and solid-phase sintering, Zhao et al. [32] obtained an LAYTP system with excellent electrochemical properties, and the ionic conductivity was as high as 7.8 × 10−4 S cm−1. In addition to aliovalent doping, microstructure, density, and grain size also play crucial roles in determining ionic conductivity. So, further research is needed to establish the relationships among density, grain size, and conductivity. In this study, we synthesized a series of Y-doped LATP samples Li1.3Al0.3−xYxTi1.7(PO4)3 (LAYTP-x, x = 0.00–0.07) using a traditional solid-state reaction method. A high relative density and excellent electrochemical properties were recorded, and the structure-property relationships and possible mechanism of Y doping in LATP are additionally discussed.
2. Experimental Details
Generally, the preparation process of ceramic solid electrolyte must first be weighed according to the experimental formula, and then through the mixing, roasting, secondary mixing, sintering, and other processes, and finally obtain the electrolyte powder or ceramic sheet. In this work, Li1.3Al0.3−xYxTi1.7(PO4)3 (LAYTP-x, x = 0.00, 0.01, 0.03, 0.05 and 0.07) solid-electrolyte ceramic electrolytes were prepared by conventional solid-state reaction method using Li2CO3 ( 99%, Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China), Al2O3 (99%, Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China), TiO2 (99.8%, Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China), NH4H2PO4 (99.95%, Shanghai McLean Chemical Reagent Co., Ltd, Shanghai, China), and Y2O3 (99.95%, Shanghai Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). All raw materials were accurately weighed according to the stoichiometric ratio, of which 10 wt% Li2CO3 is weighed to make up for the loss of lithium during the sintering process. Then, the weighed raw material was transferred to the agate ball mill tank, and anhydrous ethanol was used as the solvent for ball milling at 300 r/min for 4 h. After drying, the mixed powders were calcined at 800 °C for 4 h. After calcination, the mixture was milled again for 3 h. After drying, a certain amount of LATP powder was weighed, and 5 wt% PVA was added, and the powder was pressed into a round sheet with a diameter of 15 mm and a thickness of about 1.2 mm by a tablet press. After that, the green ceramic disc was kept at 600 °C for 2 h to remove the added PVA impurities, and then was sintered at 950 °C for 6 h to obtain white regular LATP ceramic sheets. In addition, after polishing the LATP ceramic sheet, a layer of Ag electrodes was fired on each side for subsequent electrochemical testing.
The apparent density of the sintered pellets was obtained from the weight and the dimensions (diameter and thickness) measured using a screw gauge. The thickness was averaged from several measurements at different locations to consider possible inhomogeneity of the pellet shape. The crystal structure of the samples was determined by X-ray diffractometer (XRD) analysis (Bruker D8) using Cu radiation (λ: Kα1 = 1.54060 Å, Kα2 = 1.54440 Å). The surface morphologies of electrolytes were observed by using field emission scanning electron microscopy (FESEM) (SU-8010, HITACH Co., Ltd., Tokyo, Japan). Electrochemical impedance spectroscopy (EIS) of the electrolytes was measured on an electrochemical workstation (CHI660E, Shanghai Chenhua Instrument Co., Ltd., Shanghai, China) from 10−1 Hz to 106 Hz. The ionic conductivity (σ, S·cm−1) of the electrolyte was calculated using Equation (1): σ = L/(Rb × A), in which L (cm) is the thickness of the electrolytes determined by a digital micrometer, the area of the electrode (cm2), and Rb the value of bulk resistance corresponding to the intercept of low-frequency signal in the Nyquist plot with real axis by EIS, respectively.
NCM811 (NCM811 = LiNi0.8Co0.1Mn0.1O2) cathodes and Li metal anodes were employed to assemble the Li–NCM811 batteries. NCM811 as the active material, Super P (carbon black) as the conducting agent, and poly(vinylidene fluoride) (PVDF–LiTFSI) as the binder were added to N-methylpyrrolidone (NMP) at a ratio of 8:1:1. The active material load was controlled to 2–3 mg/cm2. The Li/LAYTP-0.03/NCM811 are assembled in an Ar-filled glove box (O2 < 5 ppm, H2O < 5 ppm), where LAYTP-0.03 pellet acts as the separator and electrolyte. The Celgard 2400 soaked by a liquid electrolyte (~5 μL 1M LiPF6/EC-DMC(1:1)) was sandwiched between LATP pellet and Li metal to prevent the reaction between Li and LATP in CR2032.
3. Results and Discussion
Figure 1a depicts the XRD patterns of LAYTP-x (x = 0.00, 0.01, 0.03, 0.05, 0.07) ceramic pellets sintered at 950 °C. It is evident from the data that the diffraction peaks of all samples correspond to NASICON-type LTP (PDF#35–0754) [23,33]. The peak positions closely align with theoretical values, exhibiting only minor shifts. Furthermore, the peak intensity and width remain consistent across all samples, indicating that the addition of Y3+ does not impact the structural composition of LATP and Nasicon-structure samples have been successfully synthesized. Impurity phases such as LiTiPO5 are observed in the XRD patterns, likely resulting from either LATP phase decomposition during sintering [23,34,35,36]. In addition, other small impurity peaks could belong to YPO4, and the amount of this impurity increases with the increase in the addition of Y3+. The formation of YPO4 in the Y doping LATP systems can be due to the fact that Y cannot fully enter the LATP lattice because the ionic radii of Y3+ (0.93 Å) are larger than that of Al3+ (0.535 Å) as well as Ti4+ (0.60 Å). Similar phenomena have been reported in other literature [32,37,38]. To further validate the crystallographic evolution of LATP-x ceramics, lattice parameters were calculated and presented in Figure 1b. Notably, it was observed that lattice parameters (a) and (c) varied with increasing x values; this suggests that Y enters the LATP lattice and reduces lattice distortion in LATP-based ceramics—an effect attributed to Al3+ replacement by Y3+, potentially influencing electrical properties accordingly.
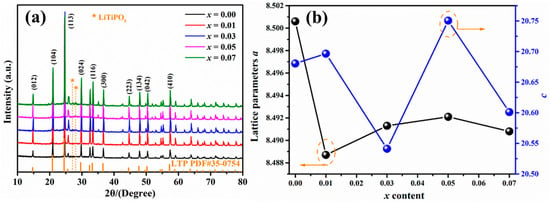
Figure 1.
(a) XRD patterns of LAYTP-x ceramic pellets sintered at 950 °C and (b) detailed information on the response of the variation of lattice parameters as a function of x (The black line points to a, and the blue line points to c).
Figure 2a–f display SEM images and relative density histograms of LATP and LAYTP-x (x = 0.01, 0.03, 0.05, 0.07). With the increase in the Y3+ additive, the surface SEM image reveals a denser grain structure with more rectangular grains for the LAYTP-x samples. The grain size initially increased and then exhibited more regular growth, leading to a reduction in grain boundaries and defects, ultimately enhancing the density and electrochemical performance of LATP ceramics [39,40]. As depicted in Figure 2f and Table 1, the relative density of LAYTP increases from 90.3% for LATP to 95.8% for LAYTP-0.03 due to larger grain size; however, further increase in Y3+ content (x = 0.05 and 0.07) resulted in a slight decrease in relative density to 92.5% and 93%, possibly attributed to excessive formation of secondary LiTiPO5 or YPO4 phases across grain boundaries. Despite this decline, as indicated by results presented in Table 1, all samples exhibited high relative density ρrd (>92%), suggesting that the LAYTP-x ceramics were well sintered, which is a prerequisite to achieving high conductivity because tightly connected grains support the continuous transport path for lithium ions. The energy-dispersive X-ray spectroscopy (EDS) elemental mapping results of each element in LAYTP-0.03 are shown in Figure 2(c1–c4), revealing a uniform distribution of Y elements within the electrolyte area.
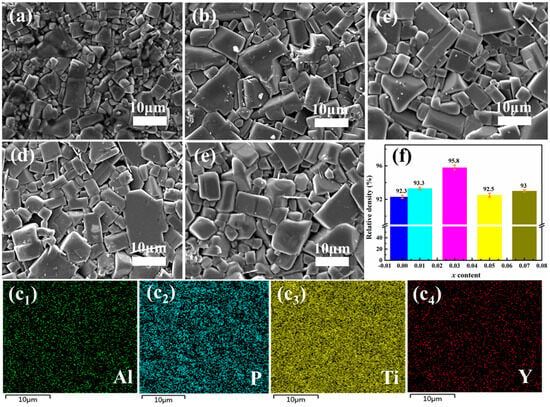
Figure 2.
SEM images of (a) LATP, (b) LAYTP-0.01, (c) LAYTP-0.03, (d) LAYTP-0.05, and (e) LAYTP-0.07. (f) Histogram showing the average relative density. (c1–c4) Mapping images of Al, P, Ti, and Y elements in LAYTP-0.03.

Table 1.
Lattice parameters a and c, relative density (ρrd), total conductivity (σtotal), and activation energy (Ea) of Li1.3Al0.3−xYxTi1.7(PO4)3 ceramic solid electrolytes.
Figure 3a presents the Nyquist plots obtained from EIS measurements to verify the resistance of LAYTP-x pellets in relation to the formation of grain boundaries and crystallinity. The impedance spectra selected are representative of the prepared materials, displaying one semicircle and one tail in the high- and low-frequency spectrum regions, which were effectively fitted by the equivalent circuit depicted in Figure 3a. The straight line is accurately described by a constant phase element (CPE), reflecting charge accumulation on blocking electrodes [13]. CPE is commonly utilized instead of a capacitor due to factors such as electrode porosity and rough interfaces between electrodes and ceramic electrolytes [23,41]. The semicircle corresponds to grain boundary resistance (Rgb) and can be characterized by a parallel connection of resistor Rgb and constant phase element CPEgb. The bulk resistance (Rb) is determined by the distance between the origin of the axes and the intercept with the Z axis at high frequencies [23,35]. A comparison of measured resistance values revealed that Rgb was approximately 10 times higher than Rb. This implies that manipulation of the microstructure has the potential to enhance overall conductivity, for example, by increasing grain size and minimizing the number of grain boundaries. The low-frequency intercept of the semicircle with the Z axis represents total resistance (Rt). It is noteworthy that the total resistance of the LATP solid electrolytes decreased significantly with the addition of Y3+ from 0 to 0.03 and then slightly increased from 0.03 to 0.07. The lowest total resistance was 7.28 × 102 Ω in LAYTP-0.03 due to its high relative density ρrd (95.8%).
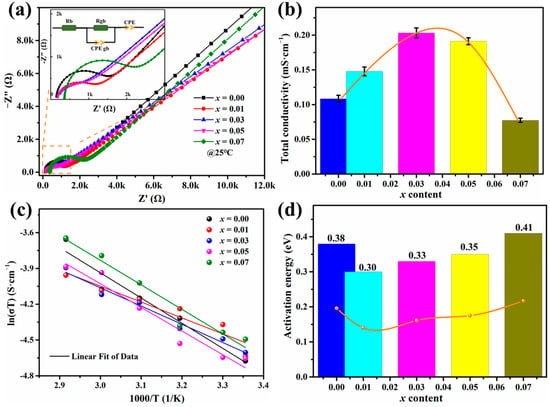
Figure 3.
(a) Nyquist plots measured at room temperature for LAYTP-x ceramic pellets, together with equivalent circuit for fitting Nyquist plots. (b) Ionic conductivities of LAYTP-x pellets at room temperature. (c) Arrhenius plots of the total lithium-ionic conductivity of LAYTP-x electrolyte samples. (d) Detailed information on the response of the activation energy as a function of x.
Figure 3b and Table 1 present the data on ionic conductivity for LAYTP-x pellets. According to the data, the mathematical model of ion conductivity and Y ion doping amount can be concluded as σT = −0.0098x3 + 0.0617x2 − 0.0735x + 0.1293. Specifically, total ionic conductivity increased at x = 0.01–0.03 and then decreased with Y3+ addition, reaching a peak at an addition amount of Y3+ at 0.03, resulting in an ionic conductivity value of 2.03 × 10−4 S cm−1, which is about 1.87 times as high as that of un-doped electrolyte and even comparable to that reported by many other groups (Table 2), indicating the LAYTP-0.03 could prove a most advantageous in terms of electrochemical performance. The increase in relative density led to a twofold increase in conductivity compared to unmodified LATP solid electrolytes. Therefore, the sintering of LATP was significantly improved by adding Y3+, which enhanced its relative density and consequently its ionic conductivity. Furthermore, as an impurity with extremely low ionic conductivity, LiTiPO5, and YPO4 will hinder lithium-ion transmission across grain boundaries and thus induce grain boundary ionic resistivity [38]. On the basis of the above discussions, we conclude the following: (i) ions are successfully added to the LATP sample lattice; (ii) an appropriate amount of Y ion doping (0.03) can increase the grain size, and a proper amount of impurity phases (LiTiPO5 or YPO4) is necessary to increase the ceramic density, thereby increasing the ionic conductivity; (iii) as the non-lithium-ion conductor, the excessive interfacial phases (0.05–0.07) not only inhibited the growth of grain, but also were not conducive to ion conduction, thus reducing the ionic conductivity of ceramic electrolyte.

Table 2.
Comparison of the total conductivities for LATP electrolytes, measured at room temperature.
The electrochemical impedance spectroscopy (EIS) curves of LAYTP electrolyte pellets were examined over a temperature range of 25 to 70 °C. The activation energy (Ea) is a crucial property of LATP electrolytes, reflecting the lithium-ion migration rate and free sites, and can be determined using the Arrhenius Equation (2): σtotal = Aexp (−Ea/kT), where A is the pre-exponential factor, k is the Boltzmann constant, and T is the ambient temperature [35,47]. The temperature dependence of ionic conductivity and corresponding activation energy values calculated from the Arrhenius plots for pristine and doped samples are depicted in Figure 3c. For LATP, LATP-0.01, LATP-0.03, LATP-0.05, and LATP-0.07, the activation energy barriers are computed as 0.38 eV, 0.30 eV, 0.33 eV, 0.35 eV, and 0.41 eV, respectively (Table 1). These findings indicate that optimal levels of Y3+ doping result in enhanced ion conductivity with lower activation energies; suggesting that lower energy barriers facilitate improved charge ion diffusion rates [13].
In order to demonstrate the feasibility of the LAYTP electrolyte, Li/LAYTP-0.03/NCM811 solid-state batteries were assembled. A schematic of the solid-state batteries is shown in Figure 4a, with LAYTP-0.03 serving as both electrolyte and separator. The cell exhibits discharge-specific capacities of 155, 130, 112, and 94 mAh g−1 at current densities of 0.1, 0.3, 0.5, and 1C, respectively (Figure 4b). When the current density is reduced to 0.2C, the average reversible capacity can be restored to about 150 mAh g−1, demonstrating excellent rate capability. The high capacity of 155 mAh g−1 achieved at a current density of 0.2C after 50 cycles at a temperature of 25 °C indicates superior electrochemical performance due to the high ion conductivity (Figure 4c). These results indicate that the introduction of Y3+ effectively inhibits the reaction between the active materials. Moreover, the corresponding Coulombic efficiencies of the Li/LAYTP-0.03/NCM811 cell approach 100% during 50 cycles, revealing a highly reversible lithium plating/stripping enabled by the LAYTP-0.03 electrolyte. Figure 4d described the smooth charge-discharge profiles of Li/LAYTP-0.03/NCM811 cells at different cycles, illustrating that no side reactions occurred in the cell [48]. Such an outstanding electrochemical performance verifies that the NCM811 cathode and lithium metal have good compatibility with the LAYTP-0.03 ceramic electrolyte, which proves the possibility of the LAYTP-0.03 ceramic electrolyte applied in lithium-metal batteries.
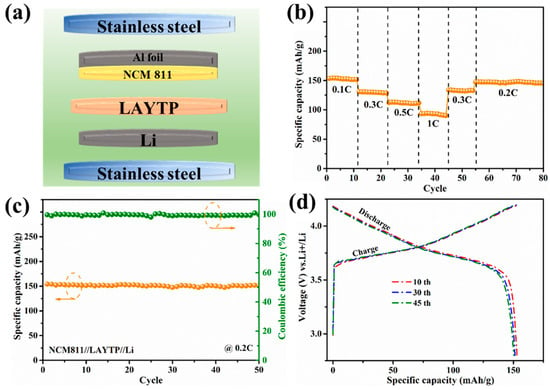
Figure 4.
(a) Schematic of the hybrid Li/LAYTP-0.03/NCM811 full-cell configuration. (b) Rate capability, (c) Cycle performance (The orange line points to specific capacity, and the green line points to Coulombic efficiency), and (d) Galvanostatic charge/discharge performance at 0.2C and 25 °C.
4. Conclusions
In this work, we present the successful fabrication of enhanced performance Li1.3Al0.3−xYxTi1.7(PO4)3 (LAYTP-x, x = 0~0.07) solid electrolyte by solid-state synthesis. The NASICON structure of LATP is well maintained in LAYTP-x. With Y3+ substitution increasing, the grain size of LAYTP-0.03 generally exhibits an upward trend and Y elements are uniformly distributed in the electrolyte. The highest total ion conductivity of 2.03 × 10−4 S·cm−1 was obtained in LAYTP-0.03, with a relative density of 95.8%, and activation energy as low as 0.33 eV for the total conductivity. The discharge capacity and capacity retention rate of Li/LAYTP-0.03/NCM811 are higher: a stable cycling performance with a discharge capacity of 155 mAh·g−1 and a Coulombic efficiency of approximately 100% was obtained at 0.2C for the full cell fabricated with LATP ceramics. These results indicate that anion doping strategies can create dense conductive interfaces, providing inspiration for researchers to solve the problem of low grain boundary conductivity and promoting the development of solid-state batteries. Finally, the next focus of research should focus on the interface problem between electrolyte and electrode, and the main methods include electrolyte interface modification, adding polymer electrolyte excess layer, and so on.
Author Contributions
Conceptualization, Z.Y. and F.Q.; methodology, Q.S.; software, L.Y.; valdtion, G.C. and X.Y.; data curation, Z.Y.; writing—original draft preparation, Z.Y.; writing—review and editing, F.Q., P.T. and K.Z.; funding acquisition, Z.Y. and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Research of Jiangsu Higher Education Institutions of China (No. 23KJB430038), the Natural Science Foundation of Jiangsu Province (BK20221099), the Research and Innovation Team Building Project of Wuxi Institute of Technology (ZKTD04); the Natural Science Research Project of Wuxi Institute of Technology (BT2023-06).
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
This work was supported by the Natural Science Research of Jiangsu Higher Education Institutions of China (No. 23KJB430038), the Natural Science Foundation of Jiangsu Province (BK20221099), the Research and Innovation Team Building Project of Wuxi Institute of Technology (ZKTD04); the Natural Science Research Project of Wuxi Institute of Technology (BT2023-06).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, Z.; Zhou, Y.; Li, Y.; Li, T. Selective lithium recovery and regeneration of ternary cathode from spent lithium-ion batteries: Mixed HCl-H2SO4 leaching-spray pyrolysis approach. J. Energy Chem. 2024, 98, 284–293. [Google Scholar] [CrossRef]
- Gu, X.; Wu, Q.; Cai, Y.; Wu, Y.; Jiang, Q.; Li, Y.; Tian, H.; Yao, X.; Su, Z. Enhanced ionic conductivity of composite solid electrolyte by defective Li1.3Al0.3Ti1.7(PO4-y)3 for solid-state Li-ion batteries. Ceram. Int. 2024, 50, 10137–10143. [Google Scholar] [CrossRef]
- Wang, J.; Ou, T.; Gao, L.; Zeng, L.; Sun, H.; Hu, Y.; Pei, X.; Tan, Y. Rational Design of Vinylene Carbonate-Inspired 1,3-Dimethyl-1H-imidazol-2(3H)-one Additives to Stabilize High-Voltage Lithium Metal Batteries. ACS Nano 2024, 18, 5930–5939. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J. Prospects of organic electrode materials for practical lithium batteries. Nat. Rev. Chem. 2020, 4, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Sabato, A.G.; Nuñez Eroles, M.; Anelli, S.; Sierra, C.D.; Gonzalez-Rosillo, J.C.; Torrell, M.; Pesce, A.; Accardo, G.; Casas-Cabanas, M.; López-Aranguren, P.; et al. 3D printing of self-supported solid electrolytes made of glass-derived Li1.5Al0.5Ge1.5P3O12 for all-solid-state lithium-metal batteries. J. Mater. Chem. A 2023, 11, 13677–13686. [Google Scholar] [CrossRef]
- Nandihalli, N. A Review of Nanocarbon-Based Anode Materials for Lithium-Ion Batteries. Crystals 2024, 14, 800. [Google Scholar] [CrossRef]
- Jie, Y.; Ren, X.; Cao, R.; Cai, W.; Jiao, S. Advanced Liquid Electrolytes for Rechargeable Li Metal Batteries. Adv. Funct. Mater. 2020, 30, 1910777. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Tan, J.; Liu, Z.; Bao, C.; Li, A.; Liu, Q.; Li, B. Incombustible solid polymer electrolytes: A critical review and perspective. J. Energy Chem. 2024, 93, 264–281. [Google Scholar] [CrossRef]
- Yang, H.; Jing, M.; Wang, L.; Xu, H.; Yan, X.; He, X. PDOL-Based Solid Electrolyte Toward Practical Application: Opportunities and Challenges. Nano-Micro Lett. 2024, 16, 109–141. [Google Scholar] [CrossRef]
- Zou, J.; Gao, X.; Zhou, X.; Yang, J.; Tang, J.; Kou, H.; Chang, R.; Zhang, Y. Al and Ta co-doped LLZO as active filler with enhanced Li+ conductivity for PVDF-HFP composite solid-state electrolyte. Nanotechnology 2023, 34, 155402. [Google Scholar] [CrossRef]
- Huang, M.; Lan, L.; Shen, P.; Liang, Z.; Wang, F.; Zhong, Y.; Wu, C.; Kong, F.; Hu, Q. Preparation and Study of Poly(Vinylidene Fluoride-Co-Hexafluoropropylene)-Based Composite Solid Electrolytes. Crystals 2024, 14, 982. [Google Scholar] [CrossRef]
- Kang, J.; Guo, X.; Gu, R.; Hao, H.; Tang, Y.; Wang, J.; Jin, L.; Li, H.; Wei, X. Enhanced electrochemical performance of Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte by anion doping. Nano Res. 2024, 17, 1465–1472. [Google Scholar] [CrossRef]
- Zhu, Y.; Gonzalez-Rosillo, J.C.; Balaish, M.; Hood, Z.D.; Kim, K.J.; Rupp, J.L.M. Lithium-film ceramics for solid-state lithionic devices. Nat. Rev. Mater. 2020, 6, 313–331. [Google Scholar] [CrossRef]
- Sabrina, Q.; Majid, N.; Sugawara, R.U. Hiroshi, Electrospinning of Bacterial Cellulose Modified with Acetyl Groups for Polymer Electrolyte Li-Ion Batteries. J. Electron. Mater. 2024, 53, 1896–1902. [Google Scholar] [CrossRef]
- Yang, X.; Adair, K.R.; Gao, X.; Sun, X. Recent advances and perspectives on thin electrolytes for high-energy-density solid-state lithium batteries. Energy Environ. Sci. 2021, 14, 643–671. [Google Scholar] [CrossRef]
- Miao, X.; Wang, H.; Sun, R.; Wang, C.; Zhang, Z.; Li, Z.; Yin, L. Interface engineering of inorganic solid-state electrolytes for high-performance lithium metal batteries. Energy Environ. Sci. 2020, 13, 3780–3822. [Google Scholar] [CrossRef]
- Yang, K.; Chen, L.; Ma, J.; He, Y.B.; Kang, F. Progress and perspective of Li1+xAlxTi2-x(PO4)3 ceramic electrolyte in lithium batteries. InfoMat 2021, 3, 1195–1217. [Google Scholar] [CrossRef]
- Schwietert, T.; Vasileiadis, A.; Wagemaker, M. First-Principles Prediction of the Electrochemical Stability and Reaction Mechanisms of Solid-State Electrolytes. JACS Au 2021, 1, 1488–1496. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, J.; Fan, L.; Zhang, J.; Li, X. Recent advances in Li1+xAlxTi2-x(PO4)3 solid-state electrolyte for safe lithium batteries. Energy Storage Mater. 2019, 19, 379–400. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, Q.; Hou, G.; Cheng, J.; Dai, L.; Li, J.; Ci, L. Boosting fast interfacial Li+ transport in solid-state Li metal batteries via ultrathin Al buffer layer. Nano Res. 2022, 16, 6825–6832. [Google Scholar] [CrossRef]
- Zou, K.; Cai, Z.; Ke, X.; Wang, K.; Tan, X.; Luo, D.; Huang, F.; Wang, C.; Cheng, J.; Xiao, R. Electrochemical properties of LATP ceramic electrolyte doped with LiBiO3 sintering additive and its derived sandwich structure composite solid electrolyte. Ionics 2023, 29, 2665–2678. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, Y.; Zhao, X. Effect of TeO2 sintering aid on the microstructure and electrical properties of Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte. J. Alloys Compd. 2022, 927, 167019. [Google Scholar] [CrossRef]
- Rosenberger, A.; Gao, Y.; Stanciu, L. Field-assisted sintering of Li1+xAlxTi2-x(PO4)3 solid-state electrolyte. Solid State Ion. 2015, 278, 217–221. [Google Scholar] [CrossRef]
- Li, X.; Zhuang, Z.; Chai, J.; Shao, R.; Wang, J.; Jiang, Z.; Zhu, S.; Gu, H.; Zhang, J.; Ma, Z. Atomically Strained Metal Sites for Highly Efficient and Selective Photooxidation. Nano Lett. 2023, 7, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Na, S.; Park, H.G.; Park, K. Synergistic Effect of Calcination and Sintering on the Reduction of Grain Boundary Resistance of LATP Solid Electrolyte. ACS Appl. Mater. Interfaces 2023, 15, 26985–26992. [Google Scholar] [CrossRef]
- Stenina, I.; Pyrkova, A.; Yaroslavtsev, A. NASICON-Type Li1+xAlxZryTi2−x−y(PO4)3 Solid Electrolytes: Effect of Al, Zr Co-Doping and Synthesis Method. Batteries 2023, 9, 59. [Google Scholar] [CrossRef]
- Sradhasagar, S.; Mallick, S.; Rath, A.; Pati, S.; Roy, A. Role of Fe3+ doping vis-à-vis secondary phases on the electrical transport of LiTi2(PO4)3 solid electrolyte. Mater. Today Commun. 2023, 35, 105621. [Google Scholar] [CrossRef]
- Zhu, J.; Xiang, Y.; Zhao, J.; Wang, H.; Li, Y.; Zheng, B.; He, H.; Zhang, Z.; Huang, J.; Yang, Y. Insights into the local structure, microstructure and ionic conductivity of silicon doped NASICON-type solid electrolyte Li1+xAlxTi2-x(PO4)3. Energy Storage Mater. 2022, 44, 190–196. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Liu, K.; Wang, C.A. High Li+ conduction in NASICON-type Li1+xYxZr2−x(PO4)3 at room temperature. J. Power Sources 2013, 240, 50–53. [Google Scholar] [CrossRef]
- Kothari, D.H.; Kanchan, D.K. Inter-grain Li+ conduction in Sc and Y doped LATP compounds. Phys. B Condens. Matter 2022, 627, 413599. [Google Scholar] [CrossRef]
- Zhao, E.; Guo, Y.; Xu, G.; Yuan, L.; Liu, J.; Li, X.; Yang, L.; Ma, J.; Li, Y.; Fan, S. High ionic conductivity Y doped Li1+xAlxTi2-x(PO4)3 solid electrolyte. J. Alloys Compd. 2019, 782, 384–391. [Google Scholar] [CrossRef]
- Shen, S.-P.; Tang, G.; Li, H.-J.; Zhang, L.; Zheng, J.-C.; Luo, Y.; Yue, J.-P.; Shi, Y.; Chen, Z. Low-temperature fabrication of NASICON-type LATP with superior ionic conductivity. Ceram. Int. 2022, 48, 36961–36967. [Google Scholar] [CrossRef]
- Fan, M.; Deng, X.; Zheng, A.; Yuan, S. Solvothermal synthesis high lithium ionic conductivity of Gd-doped Li1+xAlxTi2-x(PO4)3 solid electrolyte. Funct. Mater. Lett. 2021, 14, 2140002. [Google Scholar] [CrossRef]
- Kang, J.; Gu, R.; Guo, X.; Li, J.; Sun, H.; Zhang, L.; Jing, R.; Jin, L.; Wei, X. Effect of SnO–P2O5–MgO glass addition on the ionic conductivity of Li1+xAlxTi2-x(PO4)3 solid electrolyte. Ceram. Int. 2022, 48, 157–163. [Google Scholar] [CrossRef]
- Slubowska, W.; Montagne, L.; Lafon, O.; Mear, F.; Kwatek, K. B2O3-Doped LATP Glass-Ceramics Studied by X-ray Diffractometry and MAS NMR Spectroscopy Methods. Nanomaterials 2021, 11, 390. [Google Scholar] [CrossRef]
- Kothari, D.H.; Kanchan, D.K. Effect of doping of trivalent cations Ga3+, Sc3+, Y3+ in Li1+xAlxTi2-x(PO4)3 (LATP) system on Li + ion conductivity. Phys. B Condens. Matter 2016, 501, 90–94. [Google Scholar] [CrossRef]
- Xu, H.; Wang, S.; Wilson, H.; Zhao, F.; Manthiram, A. Y-Doped NASICON-type LiZr2(PO4)3 Solid Electrolytes for Lithium-Metal Batteries. Chem. Mater. 2017, 29, 7206–7212. [Google Scholar] [CrossRef]
- Yao, Z.; Zhu, K.; Zhang, J.; Li, J.; Li, X.; Wang, J.; Yan, K.; Liu, J. LiF-Assisted Synthesis of Perovskite-Type Li0.35La0.55TiO3 Solid Electrolyte for Rechargeable Lithium-Metal Batteries. J. Electron. Mater. 2022, 51, 736–744. [Google Scholar] [CrossRef]
- Hou, G.; Ma, X.; Sun, Q.; Ai, Q.; Xu, X.; Chen, L.; Li, D.; Chen, J.; Zhong, H.; Li, Y.; et al. Lithium Dendrite Suppression and Enhanced Interfacial Compatibility Enabled by an Ex Situ SEI on Li Anode for LAGP-Based All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2018, 10, 18610–18618. [Google Scholar] [CrossRef]
- Li, Z.; Li, A.; Zhang, H.; Lin, R.; Yang, Y. Interfacial Engineering for Stabilizing Polymer Electrolytes with 4V Cathodes in Lithium Metal Batteries at Elevated Temperature. Nano Energy 2020, 72, 104655. [Google Scholar] [CrossRef]
- Zhang, M.M.; Liu, J.A.; He, W. Preparation, Characterization and Conductivity Studies of Li1.3M0.3Ti1.7(PO4)3 (M = Al, Cr and Fe) Glass–Ceramics. Adv. Mater. Res. 2013, 602–604, 548–552. [Google Scholar] [CrossRef]
- Wu, X.M.; Chen, S.; Mai, F.R.; Zhao, J.H.; He, Z.Q. Influence of the annealing technique on the properties of Li ion-conductive Li1+xAlxTi2-x(PO4)3 films. Ionics 2013, 19, 589–593. [Google Scholar] [CrossRef]
- Kwatek, K.; Nowiński, J.L. Electrical properties of LiTi2(PO4)3 and Li1+xAlxTi2-x(PO4)3 solid electrolytes containing ionic liquid. Solid State Ion. 2016, 302, 54–60. [Google Scholar] [CrossRef]
- Ma, F.; Zhao, E.; Zhu, S.; Yan, W.; Sun, D.; Jin, Y.; Nan, C. Preparation and evaluation of high lithium ion conductivity Li1.3Al0.3Ti1.7P3O12 solid electrolyte obtained using a new solution method. Solid State Ion. 2016, 295, 7–12. [Google Scholar] [CrossRef]
- Kunshina, G.B.; Gromov, O.G.; Lokshin, E.P.; Kalinnikov, V.T. Sol-gel synthesis of Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte. Russ. J. Inorg. Chem. 2014, 59, 424–430. [Google Scholar] [CrossRef]
- Yao, Z.; Zhu, K.; Zhang, J.; Li, X.; Chen, J.; Wang, J.; Yan, K.; Liu, J. Co-precipitation synthesis and electrochemical properties of NASICON-type Li1+xAlxTi2-x(PO4)3 solid electrolytes. J. Mater. Sci. Mater. Electron. 2021, 32, 24834–24844. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, N.; Sun, Z.; Wu, W.; Kou, W.; Cui, G.; Zhao, K.; Wang, J. Thin laminar composite solid electrolyte with high ionic conductivity and mechanical strength towards advanced all-solid-state lithium–sulfur battery. J. Mater. Chem. A 2020, 8, 23344–23353. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).