Understanding the Mechanistic Pathways of N2 Reduction to Ammonia on (110) Facets of Transition Metal Carbides
Abstract
1. Introduction
2. Materials and Methods
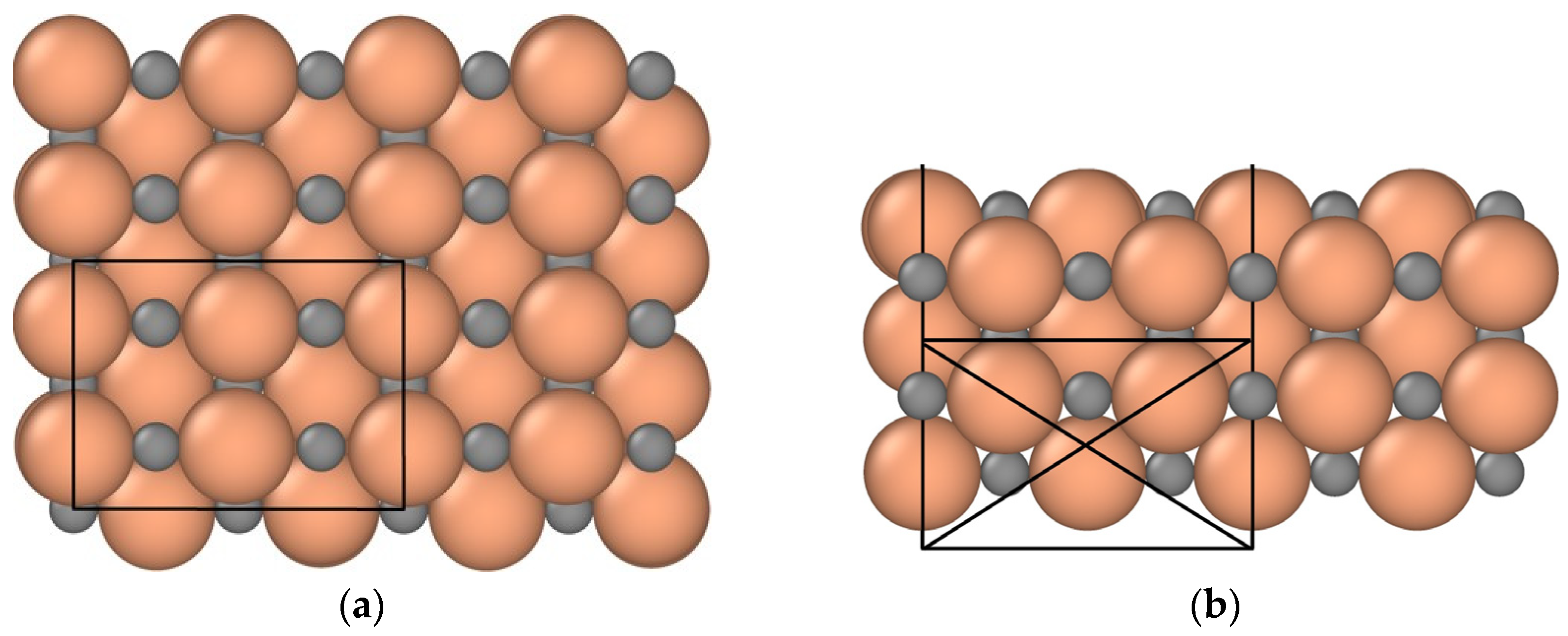
2.1. Modeling Parameters
2.2. Electrochemical Modeling and Reaction Pathways
3. Results
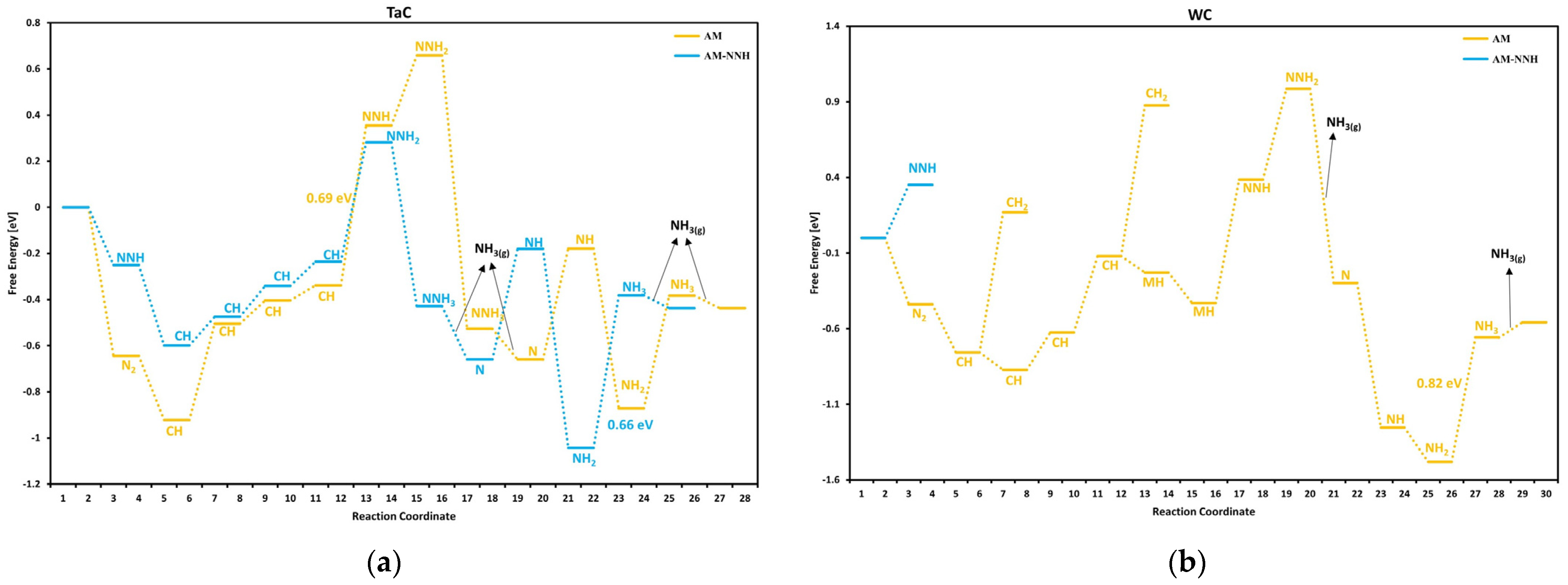
3.1. Mechanism of Nitrogen Reduction Reaction
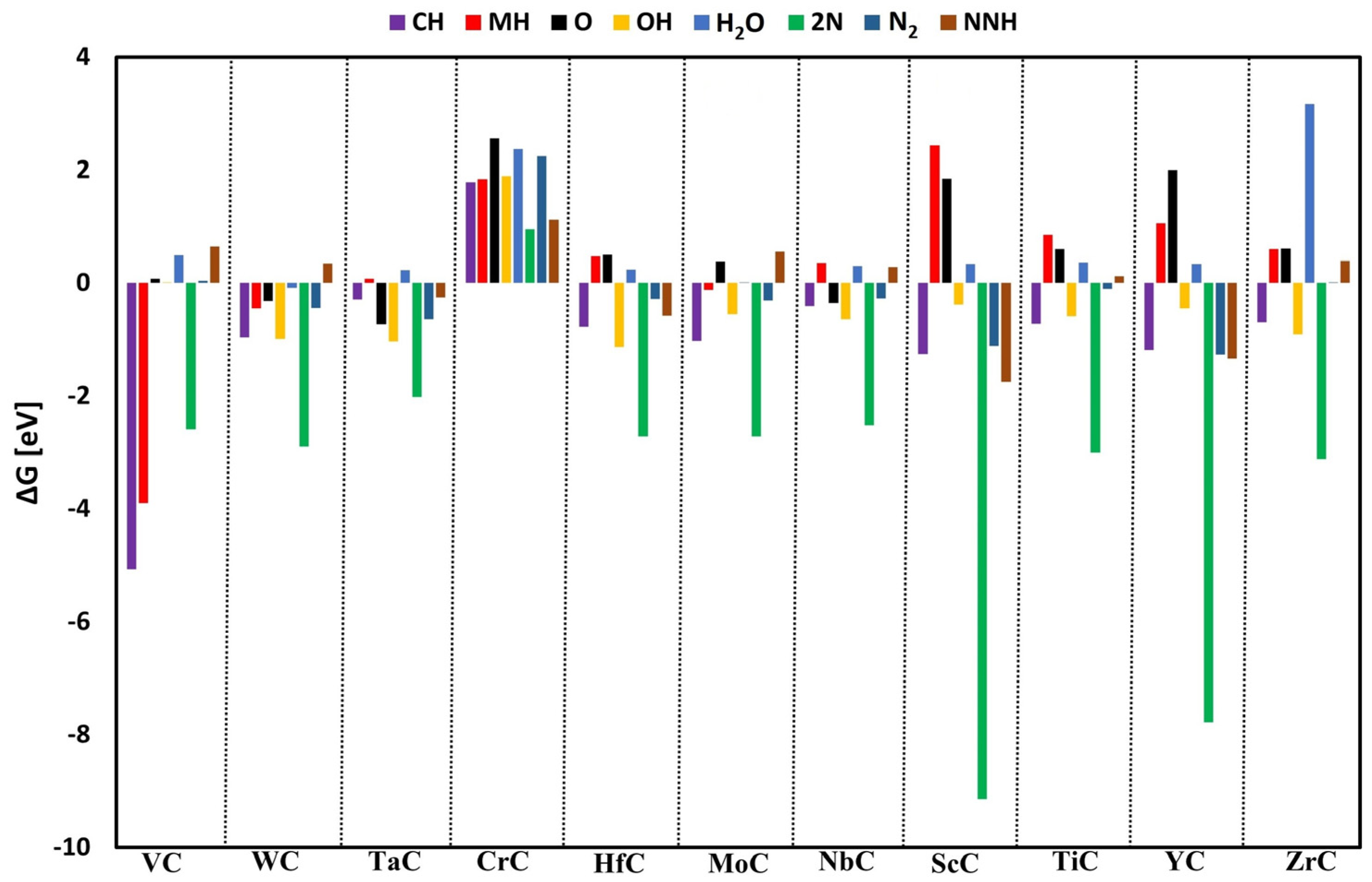
3.2. Surface Poisoning
3.3. Catalytic Activity
3.4. Exploring the Scaling Relations and Formation of Volcano Plots
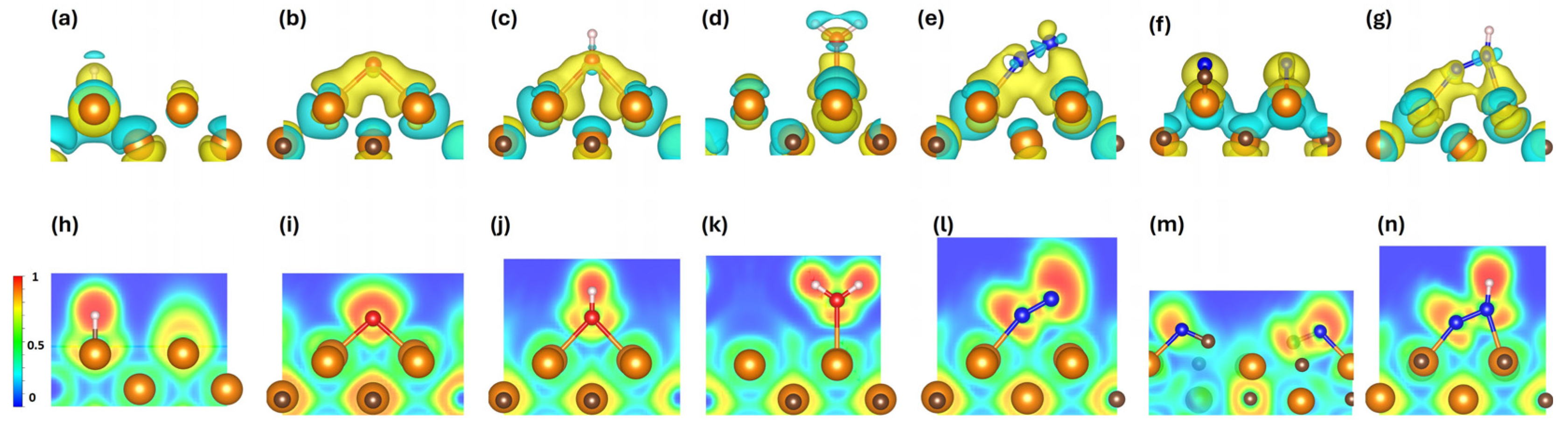
3.5. Bader Charge Analysis and Charge Iso-Surfaces
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, D.; Zhang, Q.; Meng, F.L.; Zhong, H.X.; Shi, M.M.; Zhang, Y.; Yan, J.M.; Jiang, Q.; Zhang, X.B. Electrochemical reduction of N2 under ambient conditions for artificial N2 fixation and renewable energy storage using N2/NH3 cycle. Adv. Mater. 2017, 29, 1604799. [Google Scholar] [CrossRef]
- Howalt, J.G.; Vegge, T. Electrochemical ammonia production on molybdenum nitride nanoclusters. Phys. Chem. Chem. Phys. 2013, 15, 20957–20965. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, X.; Ren, X.; Ma, Y.; Shi, X.; Tian, Z.; Asiri, A.M.; Chen, L.; Tang, B.; Sun, X. Electrochemical ammonia synthesis via nitrogen reduction reaction on a MoS2 catalyst: Theoretical and experimental studies. Adv. Mater. 2018, 30, 1800191. [Google Scholar] [CrossRef]
- Liu, C.; Li, Q.; Zhang, J.; Jin, Y.; MacFarlane, D.R.; Sun, C. Theoretical evaluation of possible 2D boron monolayer in N2 electrochemical conversion into ammonia. J. Phys. Chem. C 2018, 122, 25268–25273. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, T.; Wang, H.; Zhao, R.; Chen, H.; Wang, T.; Wei, P.; Luo, Y.; Zhang, Y.; Sun, X. Boron nanosheet: An elemental two-dimensional (2D) material for ambient electrocatalytic N2-to-NH3 fixation in neutral media. ACS Catal. 2019, 9, 4609–4615. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, L.; Xie, X.Y.; Li, X.; Ma, Y.; Liu, Q.; Fang, W.-H.; Shi, X.; Cui, G.; Sun, X. Ti3C2Tx (T = F, OH) MXene nanosheets: Conductive 2D catalysts for ambient electrohydrogenation of N2 to NH3. J. Mater. Chem. A 2018, 6, 24031–24035. [Google Scholar] [CrossRef]
- Azofra, L.M.; Li, N.; MacFarlane, D.R.; Sun, C. Promising prospects for 2D d2–d4 M3C2 transition metal carbides (MXenes) in N2 capture and conversion into ammonia. Energy Environ. Sci. 2016, 9, 2545–2549. [Google Scholar] [CrossRef]
- Azofra, L.M.; Sun, C.; Cavallo, L.; MacFarlane, D.R. Feasibility of N2 binding and reduction to ammonia on Fe-deposited MoS2 2D sheets: A DFT study. Chem.–A Eur. J. 2017, 23, 8275–8279. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, G.F.; Ding, L.; Chen, X.; Ding, L.X.; Wang, H. Efficient electrocatalytic N2 fixation with MXene under ambient conditions. Joule 2019, 3, 279–289. [Google Scholar] [CrossRef]
- Schlögl, R. Catalytic synthesis of ammonia—A “never-ending story”? Angew. Chem. Int. Ed. 2003, 42, 2004–2008. [Google Scholar] [CrossRef]
- Koebel, M.; Madia, G.; Elsener, M. Selective catalytic reduction of NO and NO2 at low temperatures. Catal. Today 2002, 73, 239–247. [Google Scholar] [CrossRef]
- Le, Y.-Q.; Gu, J.; Tian, W.Q. Nitrogen-fixation catalyst based on graphene: Every part counts. Chem. Commun. 2014, 50, 13319–13322. [Google Scholar] [CrossRef]
- Chirik, P.J. One electron at a time. Nat. Chem. 2009, 1, 520–522. [Google Scholar] [CrossRef]
- Van der Ham, C.J.; Koper, M.T.; Hetterscheid, D.G. Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev. 2014, 43, 5183–5191. [Google Scholar] [CrossRef] [PubMed]
- Kadac-Czapska, K.; Knez, E.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Grembecka, M. Microplastics derived from food packaging waste—Their origin and health risks. Materials 2023, 16, 674. [Google Scholar] [CrossRef]
- Paal, Z.; Ertl, G.; Lee, S. Interactions of potassium, oxygen and nitrogen with polycrystalline iron surfaces. Appl. Surf. Sci. 1981, 8, 231–249. [Google Scholar] [CrossRef]
- Ertl, G.; Weiss, M.; Lee, S. The role of potassium in the catalytic synthesis of ammonia. Chem. Phys. Lett. 1979, 60, 391–394. [Google Scholar] [CrossRef]
- Spencer, N.; Schoonnaker, R.; Somorjai, G. Structure Sensitivity in the Iron Single Crystal Catalysed Synthesis of Ammonia; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 1981.
- Ertl, G.; Lee, S.; Weiss, M. Kinetics of nitrogen adsorption on Fe (111). Surf. Sci. 1982, 114, 515–526. [Google Scholar] [CrossRef]
- Ertl, G.; Lee, S.; Weiss, M. Adsorption of nitrogen on potassium promoted Fe (111) and (100) surfaces. Surf. Sci. 1982, 114, 527–545. [Google Scholar] [CrossRef]
- Bragulla, S.C.H.; von Seggern, A.R.; Lorenz, J.; Harms, C.; Wark, M.; Friedrich, K.A. Improving the Ca-assisted Urea-Glass Method for the Synthesis of Zirconium Nitride as Potential Electrocatalyst for the Nitrogen Reduction Reaction. ChemCatChem 2024, e202400613. [Google Scholar] [CrossRef]
- Ješić, D.; Pomeroy, B.; Kamal, K.M.; Kovačič, Ž.; Huš, M.; Likozar, B. Photo-and Photoelectrocatalysis in Nitrogen Reduction Reactions to Ammonia: Interfaces, Mechanisms, and Modeling Simulations. Adv. Energy Sustain. Res. 2024, 2400083. [Google Scholar] [CrossRef]
- Guo, P.; Yin, F.; Ni, Z.; Li, G.; Chen, B. MIL-101 (Fe) supported Au-Cu nanoalloy for enhanced electrochemical nitrogen fixation in gas–liquid-solid three-phase reactor. Chem. Eng. J. 2024, 494, 153134. [Google Scholar] [CrossRef]
- Macfarlane, D.R.; Skúlason, E.; Hosono, H.; Shao, M. Preface to Special Issue on Sustainable Ammonia Synthesis. ChemSusChem 2024, 17, e20230139. [Google Scholar] [CrossRef] [PubMed]
- Hanifpour, F.; Canales, C.P.; Fridriksson, E.G.; Sveinbjörnsson, A.; Tryggvason, T.K.; Yang, J.; Arthur, C.; Jónsdóttir, S.; Garden, A.L.; Ólafsson, S.; et al. Operando quantification of ammonia produced from computationally-derived transition metal nitride electro-catalysts. J. Catal. 2022, 413, 956–967. [Google Scholar] [CrossRef]
- Hanifpour, F.; Sveinbjörnsson, A.; Canales, C.P.; Skúlason, E.; Flosadóttir, H.D. Preparation of nafion membranes for reproducible ammonia quantification in nitrogen reduction reaction experiments. Angew. Chem. 2020, 132, 23138–23142. [Google Scholar] [CrossRef]
- He, T.; Chen, M.; Sun, H.; Mei, J.; Noufal, M.; Liu, Q.; Santiago, A.R.P. Chemical insights into the interfacial phenomena of low-dimensional heterointerfaces. Chem 2024, 10, 1974–1993. [Google Scholar] [CrossRef]
- He, T.; Santiago AR, P.; Kong, Y.; Ahsan, M.A.; Luque, R.; Du, A.; Pan, H. Atomically dispersed heteronuclear dual-atom catalysts: A new rising star in atomic catalysis. Small 2022, 18, 2106091. [Google Scholar] [CrossRef]
- Iqbal, A.; Sku, E.; Abghoui, Y. Catalytic Nitrogen Reduction on the Transition Metal Carbonitride (110) Facet: DFT Predictions and Mechanistic Insights. J. Phys. Chem. C 2024, 128, 10300–10307. [Google Scholar] [CrossRef]
- Iqbal, A.; Skúlason, E.; Abghoui, Y. Are (100) facets of transition metal carbonitrides suitable as electrocatalysts for nitrogen reduction to ammonia at ambient conditions? Int. J. Hydrogen Energy 2024, 64, 744–753. [Google Scholar] [CrossRef]
- Iqbal, A.; Skulason, E.; Abghoui, Y. Electrochemical Nitrogen Reduction to Ammonia at Ambient Condition on the (111) Facets of Transition Metal Carbonitrides. ChemPhysChem 2024, 25, e202300991. [Google Scholar] [CrossRef]
- Ellingsson, V.; Iqbal, A.; Skúlason, E.; Abghoui, Y. Nitrogen reduction reaction to ammonia on transition metal carbide catalysts. ChemSusChem 2023, 16, e202300947. [Google Scholar] [CrossRef] [PubMed]
- Abghoui, Y.; Iqbal, A.; Skúlason, E. The role of overlayered nitride electro-materials for N2 reduction to ammonia. Front. Catal. 2023, 2, 1096824. [Google Scholar] [CrossRef]
- Höskuldsson, Á.B.; Dang, T.; Sakai, Y.; Ishikawa, A.; Skúlason, E. High-throughput computational screening of doped transition metal oxides as catalysts for nitrogen reduction. Cell Rep. Phys. Sci. 2023, 4, 101595. [Google Scholar] [CrossRef]
- Tayyebi, E.; Abghoui, Y.; Skulason, E. Elucidating the mechanism of electrochemical N2 reduction at the Ru (0001) electrode. ACS Catal. 2019, 9, 11137–11145. [Google Scholar] [CrossRef]
- Abghoui, Y.; Sigtryggsson, S.B.; Skúlason, E. Biomimetic nitrogen fixation catalyzed by transition metal sulfide surfaces in an electrolytic cell. ChemSusChem 2019, 12, 4265–4273. [Google Scholar] [CrossRef]
- Abghoui, Y.; Garden, A.L.; Howalt, J.G.; Vegge, T.; Skúlason, E. Electroreduction of N2 to ammonia at ambient conditions on mononitrides of Zr, Nb, Cr, and V: A DFT guide for experiments. ACS Catal. 2016, 6, 635–646. [Google Scholar] [CrossRef]
- Abghoui, Y.; Skúlason, E. Electrochemical synthesis of ammonia via Mars-van Krevelen mechanism on the (111) facets of group III–VII transition metal mononitrides. Catal. Today 2017, 286, 78–84. [Google Scholar] [CrossRef]
- Dahl, S.; Logadottir, A.; Egeberg, R.C.; Larsen, J.H.; Chorkendorff, I.; Törnqvist, E.; Nørskov, J.K. Role of steps in N2 activation on Ru (0001). Phys. Rev. Lett. 1999, 83, 1814. [Google Scholar] [CrossRef]
- Skulason, E.; Bligaard, T.; Gudmundsdóttir, S.; Studt, F.; Rossmeisl, J.; Abild-Pedersen, F.; Vegge, T.; Jónsson, H.; Nørskov, J.K. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction. Phys. Chem. Chem. Phys. 2012, 14, 1235–1245. [Google Scholar] [CrossRef]
- Jacobsen, C.J.; Dahl, S.; Hansen, P.L.; Törnqvist, E.; Jensen, L.; Topsøe, H.; Prip, D.V.; Møenshaug, P.B.; Chorkendorff, I. Structure sensitivity of supported ruthenium catalysts for ammonia synthesis. J. Mol. Catal. A Chem. 2000, 163, 19–26. [Google Scholar] [CrossRef]
- Ham, D.J.; Lee, J.S. Transition metal carbides and nitrides as electrode materials for low temperature fuel cells. Energies 2009, 2, 873–899. [Google Scholar] [CrossRef]
- Hwu, H.H.; Chen, J.G. Surface chemistry of transition metal carbides. Chem. Rev. 2005, 105, 185–212. [Google Scholar] [CrossRef]
- Kelly, T.G.; Chen, J.G. Metal overlayer on metal carbide substrate: Unique bimetallic properties for catalysis and electrocatalysis. Chem. Soc. Rev. 2012, 41, 8021–8034. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.V.; Chen, J.G. Monolayer platinum supported on tungsten carbides as low-cost electrocatalysts: Opportunities and limitations. Energy Environ. Sci. 2011, 4, 3900–3912. [Google Scholar] [CrossRef]
- Esposito, D.V.; Hunt, S.T.; Stottlemyer, A.L.; Dobson, K.D.; McCandless, B.E.; Birkmire, R.W.; Chen, J.G. Low-cost hydrogen-evolution catalysts based on monolayer platinum on tungsten monocarbide substrates. Angew. Chem. Int. Ed. 2010, 51, 9859–9862. [Google Scholar] [CrossRef]
- Kimmel, Y.C.; Yang, L.; Kelly, T.G.; Rykov, S.A.; Chen, J.G. Theoretical prediction and experimental verification of low loading of platinum on titanium carbide as low-cost and stable electrocatalysts. J. Catal. 2014, 312, 216–220. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Höskuldsson, Á.B.; Tayyebi, E.; Skúlason, E. Computational examination of the kinetics of electrochemical nitrogen reduction and hydrogen evolution on a tungsten electrode. J. Catal. 2021, 404, 362–370. [Google Scholar] [CrossRef]
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Gudmundsdóttir, S.; Tang, W.; Henkelman, G.; Jónsson, H.; Skúlason, E. Local density of states analysis using Bader decomposition for N2 and CO2 adsorbed on Pt (110)-(1 × 2) electrodes. J. Chem. Phys. 2012, 137, 164705. [Google Scholar] [CrossRef]
- Bagger, A.; Arán-Ais, R.M.; Halldin Stenlid, J.; Campos dos Santos, E.; Arnarson, L.; Degn Jensen, K.; Escudero-Escribano, M.; Cuenya, B.R.; Rossmeisl, J. Ab initio cyclic voltammetry on Cu (111), Cu (100) and Cu (110) in acidic, neutral and alkaline solutions. ChemPhysChem 2019, 20, 3096–3105. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Studt, F.; Abild-Pedersen, F.; Bligaard, T. Fundamental Concepts in Heterogeneous Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Rossmeisl, J.; Qu, Z.-W.; Zhu, H.; Kroes, G.-J.; Nørskov, J. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 2007, 607, 83–89. [Google Scholar] [CrossRef]
- Fan, K.; Ying, Y.; Li, X.; Luo, X.; Huang, H. Theoretical investigation of V3C2 MXene as prospective high-capacity anode material for metal-ion (Li, Na, K, and Ca) batteries. J. Phys. Chem. C 2019, 123, 18207–18214. [Google Scholar] [CrossRef]
- Che, M.; Védrine, J.C. Characterization of Solid Materials and Heterogeneous Catalysts: From Structure to Surface Reactivity; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]

| ΔQ(e) | H | O | OH | H2O | N2 | 2N | NNH |
|---|---|---|---|---|---|---|---|
| TaC | 0.02 | −1.0 | −0.38 | 0.02 | −0.74 | −2.77 | −0.87 |
| WC | 0.09 | −0.84 | −0.49 | 0.06 | −0.19 | −2.62 | −0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, A.; Skúlason, E.; Abghoui, Y. Understanding the Mechanistic Pathways of N2 Reduction to Ammonia on (110) Facets of Transition Metal Carbides. Crystals 2024, 14, 770. https://doi.org/10.3390/cryst14090770
Iqbal A, Skúlason E, Abghoui Y. Understanding the Mechanistic Pathways of N2 Reduction to Ammonia on (110) Facets of Transition Metal Carbides. Crystals. 2024; 14(9):770. https://doi.org/10.3390/cryst14090770
Chicago/Turabian StyleIqbal, Atef, Egill Skúlason, and Younes Abghoui. 2024. "Understanding the Mechanistic Pathways of N2 Reduction to Ammonia on (110) Facets of Transition Metal Carbides" Crystals 14, no. 9: 770. https://doi.org/10.3390/cryst14090770
APA StyleIqbal, A., Skúlason, E., & Abghoui, Y. (2024). Understanding the Mechanistic Pathways of N2 Reduction to Ammonia on (110) Facets of Transition Metal Carbides. Crystals, 14(9), 770. https://doi.org/10.3390/cryst14090770







