Properties and Applications of Supersaturated Metastable Alloys Obtained via Electrodeposition
Abstract
1. Introduction
1.1. Supersaturation and Metastability
1.2. Supersaturated Alloys
1.3. Electrodeposition and Electroless Deposition
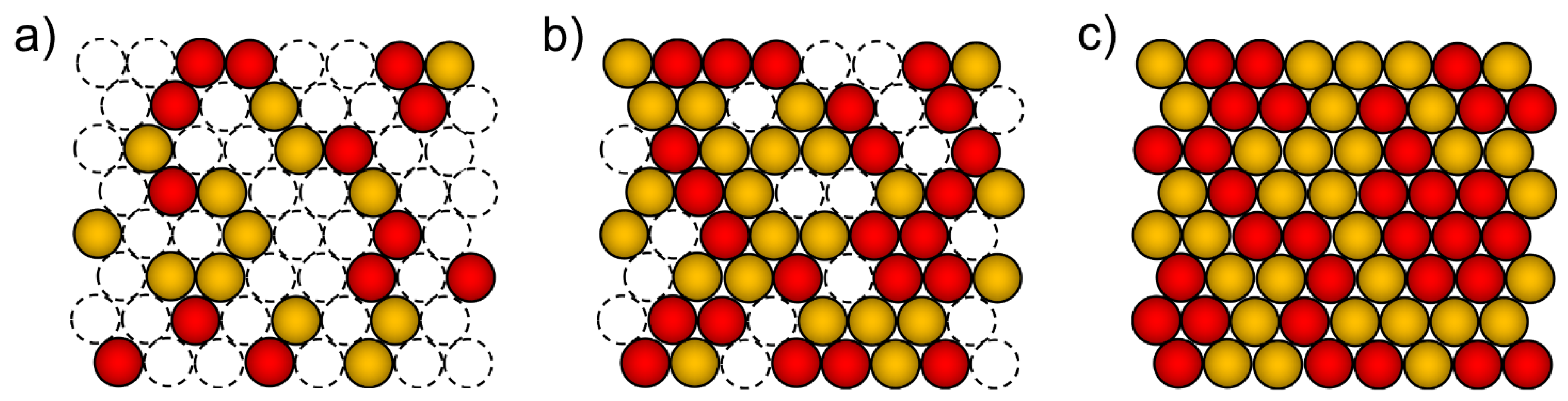
2. Electrodeposition of Metastable Alloys
3. Properties and Applications of Some Electrodeposited Supersaturated Alloys
3.1. Ni-P and Co-P
3.2. Fe-P
3.3. Fe-Zn
3.4. Co-Cu
3.5. Cu-Ag
3.6. Ag-Ni
3.7. Other Alloys
4. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Balakrishna Bhat, T.; Arunachalam, V.S. Strengthening Mechanisms in Alloys. Proc. Indian Acad. Sci. Sect. C Eng. Sci. 1980, 3, 275–296. [Google Scholar] [CrossRef]
- Dieter, G.E.; Bacon, D. Mechanical Metallurgy; McGraw-Hill: New York, NY, USA, 1976; Volume 3. [Google Scholar]
- Haasen, P. Mechanical Properties of Solid Solutions. In Fundamental Aspects of Structural Alloy Design; Springer: Berlin/Heidelberg, Germany, 1977; pp. 3–25. [Google Scholar]
- Borgioli, F. From Austenitic Stainless Steel to Expanded Austenite-S Phase: Formation, Characteristics and Properties of an Elusive Metastable Phase. Metals 2020, 10, 187. [Google Scholar] [CrossRef]
- Borgioli, F. The “Expanded” Phases in the Low-Temperature Treated Stainless Steels: A Review. Metals 2022, 12, 331. [Google Scholar] [CrossRef]
- Wang, W.-H.; Dong, C.; Shek, C.H. Bulk Metallic Glasses. Mater. Sci. Eng. R Rep. 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of Metallic Supercooled Liquid and Bulk Amorphous Alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and Properties of High-Entropy Alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Shojaei, Z.; Khayati, G.R.; Darezereshki, E. Review of electrodeposition methods for the preparation of high-entropy alloys. Int. J. Miner. Metall. Mater. 2022, 29, 1683–1696. [Google Scholar] [CrossRef]
- Xu, Z.F.; Wang, Y.; Gao, X.M.; Peng, L.Y.; Qiao, Q.; Xiao, J.J.; Guo, F.Y.; Wang, R.G.; Yu, J.K. Electrochemical Deposition and Corrosion Resistance Characterization of FeCoNiCr High-Entropy Alloy Coatings. Coatings 2023, 13, 1167. [Google Scholar] [CrossRef]
- Serban, B.A.; Olaru, M.T.; Badea, I.C.; Mitrica, D.; Burada, M.; Anasiei, I.; Ghita, M.; Tudor, A.I.; Matei, C.A.; Popescu, A.M.J.; et al. Non-Aqueous Electrodeposition and Characterization of AlCrCuFeNi High Entropy Alloy Thin Films. Materials 2022, 15, 6007. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, C.L.P.; Janardhana, R.K.S.K.; Reddy, K.M.; Murapaka, C.; Joardar, J.; Sarada, B.; Tamboli, R.R.; Hu, Y.X.; Zhang, Y.M.; Wang, X.D.; et al. An advancement in the synthesis of unique soft magnetic CoCuFeNiZn high entropy alloy thin films. Sci. Rep. 2021, 11, 8836. [Google Scholar] [CrossRef]
- Dehestani, M.; Sharafi, S.; Khayati, G.R. The effect of pulse current density on the microstructure, magnetic, mechanical, and corrosion properties of high-entropy alloy coating Fe-Co-Ni-Mo-W, achieved through electro co-deposition. Intermetallics 2022, 147, 107610. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, S.F.; Zuo, L.H.; Li, J.K.; Guo, J.X.; Wang, P.; Sun, J.F.; Dai, L. Recent advances of high-entropy electrocatalysts for water electrolysis by electrodeposition technology: A short review. Rare Met. 2024, 43, 2371–2390. [Google Scholar] [CrossRef]
- Bian, H.W.; Wang, R.; Zhang, K.Z.; Zheng, H.L.; Wen, M.J.; Li, Z.M.; Li, Z.H.; Wang, G.X.; Xie, G.W.; Liu, X.; et al. Facile electrodeposition synthesis and super performance of nano-porous Ni-Fe-Cu-Co-W high entropy alloy electrocatalyst. Surf. Coat. Technol. 2023, 459, 129407. [Google Scholar] [CrossRef]
- Brenner, A. Electrodeposition of Alloys: Principles and Practice; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 1483223116. [Google Scholar]
- Zargarnezhad, H.; Dolati, A. A 3D Continuum-Kinetic Monte Carlo Simulation Study of Early Stages of Nucleation and Growth in Ni Electrodeposition. Electrochim. Acta 2017, 236, 1–9. [Google Scholar] [CrossRef]
- Treeratanaphitak, T.; Pritzker, M.D.; Abukhdeir, N.M. Kinetic Monte Carlo Simulation of Electrodeposition Using the Embedded-Atom Method. Electrochim. Acta 2014, 121, 407–414. [Google Scholar] [CrossRef]
- Ataalite, H.; Dardouri, M.; Arbaoui, A.; Fathi, A.; Hasnaoui, A.; Sbiaai, K. Kinetic Monte Carlo Simulation of Polycrystalline Silver Metal Electrodeposition: Scaling of Roughness and Effects of Deposition Parameters. Phys. Chem. Chem. Phys. 2023, 25, 4216–4229. [Google Scholar] [CrossRef] [PubMed]
- Ataalite, H.; Dardouri, M.; Hasnaoui, A.; Sbiaai, K. Morphological Surface Study of Silver Electrodeposition by Kinetic Monte Carlo-Embedded Atom Method. Phys. Status Solidi B 2022, 259, 2100559. [Google Scholar] [CrossRef]
- Onabuta, Y.; Kunimoto, M.; Wang, S.; Fukunaka, Y.; Nakai, H.; Homma, T. Multiscale Simulation of Irregular Shape Evolution during the Initial Stage of Zn Electrodeposition on a Negative Electrode Surface. J. Phys. Chem. C 2022, 126, 5224–5232. [Google Scholar] [CrossRef]
- Cheimarios, N.; To, D.; Kokkoris, G.; Memos, G.; Boudouvis, A.G. Monte Carlo and Kinetic Monte Carlo Models for Deposition Processes: A Review of Recent Works. Front. Phys. 2021, 9, 631918. [Google Scholar] [CrossRef]
- Mascaró, F.V.; McCrum, I.T.; Koper, M.T.M.; Rost, M.J. Nucleation and Growth of Dendritic Islands during Platinum Oxidation-Reduction Cycling. J. Electrochem. Soc. 2022, 169, 112506. [Google Scholar] [CrossRef]
- Zhu, J.Z.; Liu, Z.K.; Vaithyanathan, V.; Chen, L.Q. Linking phase-field model to CALPHAD: Application to precipitate shape evolution in Ni-base alloys. Scr. Mater. 2002, 46, 401–406. [Google Scholar] [CrossRef]
- Schwen, D.; Jiang, C.; Aagesen, L.K. A sublattice phase-field model for direct CALPHAD database coupling. Comput. Mater. Sci. 2021, 195, 110466. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Y.; Zhang, X.; Lu, X.-G.; Wang, C.; Zhang, Y. Microscopic Phase-Field Simulation of γ’ Precipitation in Ni-Based Binary Alloys Coupled with CALPHAD Method. Crystals 2022, 12, 971. [Google Scholar] [CrossRef]
- Okamoto, H.; Okamoto, H. Phase Diagrams for Binary Alloys; ASM international: Materials Park, OH, USA, 2000; Volume 44. [Google Scholar]
- Mallory, G.O.; Hajdu, J.B. Electroless Plating: Fundamentals and Applications; William Andrew: Norwich, NY, USA, 1990; ISBN 0936569077. [Google Scholar]
- Lewis, D.B.; Marshall, G.W. Investigation into the Structure of Electrodeposited Nickel-Phosphorus Alloy Deposits. Surf. Coat. Technol. 1996, 78, 150–156. [Google Scholar] [CrossRef]
- Ishida, K.; Nishizawa, T. The Co-P (Cobalt-Phosphorus) System. Bull. Alloy Phase Diagr. 1990, 11, 555–560. [Google Scholar] [CrossRef]
- Brenner, A.; Couch, D.E.; Williams, E.K. Electrodeposition of Alloys of Phosphorus with Nickel or Cobalt. J. Res. Nat. Bur. Stand 1950, 44, 109. [Google Scholar] [CrossRef]
- Brüning, R.; Brown, D.A.; Bera, H.; Jakse, N. Molecular Dynamics Simulations of Amorphous Ni–P Alloy Formation by Rapid Quenching and Atomic Deposition. J. Phys. Condens. Matter 2020, 32, 154001. [Google Scholar] [CrossRef]
- Sha, W.; Wu, X.; Keong, K.G. Electroless Copper and Nickel-Phosphorus Plating: Processing, Characterisation and Modelling; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 0857090968. [Google Scholar]
- Pillai, A.M.; Rajendra, A.; Sharma, A.K. Electrodeposited Nickel–Phosphorous (Ni–P) Alloy Coating: An in-Depth Study of Its Preparation, Properties, and Structural Transitions. J. Coat. Technol. Res. 2012, 9, 785–797. [Google Scholar] [CrossRef]
- Li, G.; Gao, Y.P.; Liu, R.P. Binary Ni–P Bulk Amorphous Glass Prepared by Electrodeposition Method. J. Non. Cryst. Solids 2007, 353, 4199–4202. [Google Scholar] [CrossRef]
- Hu, C.-C.; Bai, A. Influences of the Phosphorus Content on Physicochemical Properties of Nickel–Phosphorus Deposits. Mater. Chem. Phys. 2003, 77, 215–225. [Google Scholar] [CrossRef]
- Hu, C.-C.; Bai, A. Composition Control of Electroplated Nickel–Phosphorus Deposits. Surf. Coat. Technol. 2001, 137, 181–187. [Google Scholar] [CrossRef]
- Huang, H.-C.; Chung, S.-T.; Pan, S.-J.; Tsai, W.-T.; Lin, C.-S. Microstructure Evolution and Hardening Mechanisms of Ni–P Electrodeposits. Surf. Coat. Technol. 2010, 205, 2097–2103. [Google Scholar] [CrossRef]
- Mahalingam, T.; Raja, M.; Thanikaikarasan, S.; Sanjeeviraja, C.; Velumani, S.; Moon, H.; Kim, Y.D. Electrochemical Deposition and Characterization of Ni–P Alloy Thin Films. Mater. Charact. 2007, 58, 800–804. [Google Scholar] [CrossRef]
- Hou, K.-H.; Jeng, M.-C.; Ger, M.-D. A Study on the Wear Resistance Characteristics of Pulse Electroforming Ni–P Alloy Coatings as Plated. Wear 2007, 262, 833–844. [Google Scholar] [CrossRef]
- Seo, M.H.; Kim, J.S.; Hwang, W.S.; Kim, D.J.; Hwang, S.S.; Chun, B.S. Characteristics of Ni–P Alloy Electrodeposited from a Sulfamate Bath. Surf. Coat. Technol. 2004, 176, 135–140. [Google Scholar] [CrossRef]
- Lin, C.S.; Lee, C.Y.; Chen, F.J.; Chien, C.T.; Lin, P.L.; Chung, W.C. Electrodeposition of Nickel-Phosphorus Alloy from Sulfamate Baths with Improved Current Efficiency. J. Electrochem. Soc. 2006, 153, C387. [Google Scholar] [CrossRef]
- Chang, L.; Chen, C.-H.; Fang, H. Electrodeposition of Ni–P Alloys From a Sulfamate Electrolyte: Relationship Between Bath PH and Structural Characteristics. J. Electrochem. Soc. 2007, 155, D57. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, F.; Chang, L.; Zheng, J.; Zhang, Z.; Zhang, J.; Cao, C. Electrodeposition of High Corrosion Resistance Cu/Ni–P Coating on AZ91D Magnesium Alloy. Appl. Surf. Sci. 2011, 257, 9213–9220. [Google Scholar] [CrossRef]
- Vijayan, S.; Luo, N.; Aindow, M. Microstructural Stability and Phase Transformations in Electrodeposited Cobalt-Phosphorus Coatings. J. Alloys Compd. 2017, 719, 142–150. [Google Scholar] [CrossRef]
- Bernasconi, R.; Allievi, F.; Sadeghi, H.; Magagnin, L. Codeposition of Nickel–Phosphorus Alloys Reinforced with Boron Carbide Microparticles: Direct and Pulse Plating. Trans. IMF 2017, 95, 52–59. [Google Scholar] [CrossRef]
- Wang, F.; Itoh, K.; Watanabe, T. Relationship between the Crystallographic Structure of Electrodeposited Fe-P Alloy Film and Its Thermal Equilibrium Phase Diagram. Mater. Trans. 2003, 44, 127–132. [Google Scholar] [CrossRef]
- Sridharan, K.; Sheppard, K. Crystallization of Amorphous Iron-Nickel-Phosphorus Alloys Prepared by Electrodeposition. J. Mater. Process. Technol. 1997, 68, 109–116. [Google Scholar] [CrossRef]
- Schlesinger, M.; Paunovic, M. Modern Electroplating, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 1118063147. [Google Scholar]
- Kuznetsov, A.N.; Cherstiouk, O.V.; Zaikovskii, V.I.; Rudina, N.A.; Kvon, R.I.; Oshchepkov, A.G. Electrodeposited Ni-P Electrodes: An Effect of Amorphous Structure on the Electrochemical Behavior and Electrocatalytic Activity in the Hydrogen Oxidation Reaction in Alkaline Media. J. Electroanal. Chem. 2023, 944, 117676. [Google Scholar] [CrossRef]
- Armyanov, S.; Vitkova, S.; Blajiev, O. Internal Stress and Magnetic Properties of Electrodeposited Amorphous Fe–P Alloys. J. Appl. Electrochem. 1997, 27, 185–191. [Google Scholar] [CrossRef]
- Mita, K.; Ikeda, T.; Maeda, M. Phase Diagram Study of Fe-Zn Intermetallics. J. Phase Equilibria 2001, 22, 122–125. [Google Scholar] [CrossRef]
- Hara, T.; Adaniya, T.; Sagiyama, M.; Honma, T.; Tonouchi, A.; Watanabe, T.; Ohmura, M. The Phase Composition and Wor Kability of Electrodeposited Fe-Zn Alloy. Trans. Iron Steel Inst. Jpn. 1983, 23, 954–958. [Google Scholar] [CrossRef]
- Kondo, K.; Hinotani, S.; Ohmori, Y. Crystal Structure and Morphology of Electrodeposited Zinc-Iron Binary Alloys. J. Appl. Electrochem. 1988, 18, 154–161. [Google Scholar] [CrossRef]
- Gu, M.; Marder, A.R. Morphological Changes of Electrodeposited Zn and Zn-Fe Coatings during Heating. J. Mater. Sci. 1991, 26, 4588–4598. [Google Scholar] [CrossRef]
- Ieffa, S.; Bernasconi, R.; Nobili, L.; Cavallotti, P.L.; Magagnin, L. Direct and Pulse Plating of Metastable Zn–Ni Alloys. Trans. IMF 2014, 92, 321–324. [Google Scholar] [CrossRef]
- Drewien, C.A.; Marder, A.R. Effect of Annealing upon Decomposition of Electrodeposited Iron-Zinc Alloy Coatings. J. Mater. Sci. 1994, 29, 965–972. [Google Scholar] [CrossRef]
- Drewien, C.A.; Goldstein, J.I.; Marder, A.R. η to G Phase Transformation in Electrodeposited Iron-Zinc Alloy Coatings. Metall. Mater. Trans. A 1994, 25, 1119–1125. [Google Scholar] [CrossRef]
- Kanamura, T.; Morita, J.; Nakayama, M.; Arai, K.; Ogawa, Y. Properties of Iron-Zinc Alloy-Electroplated Galvannealed Steel Sheet. Nippon. Steel Technol. Rep. 1994, 63, 23–26. [Google Scholar]
- Xu, Y.; Wang, W.; Yu, F.; Wang, Y.; Qi, M.; Zhao, Y.; Wang, Y. Effects of Pulse Frequency and Current Density on Microstructure and Properties of Biodegradable Fe-Zn Alloy. J. Mater. Res. Technol. 2022, 18, 44–58. [Google Scholar] [CrossRef]
- Yuasa, M.; Kajikawa, K.; Hakamada, M.; Mabuchi, M. Saturation Magnetization in Supersaturated Solid Solution of Co–Cu Alloy. Appl. Phys. Lett. 2009, 95, 162502. [Google Scholar] [CrossRef]
- Pratama, K.; Barrirero, J.; Mücklich, F.; Motz, C. Microstructure Evolution and Mechanical Stability of Supersaturated Solid Solution Co-Rich Nanocrystalline Co-Cu Produced by Pulsed Electrodeposition. Materials 2020, 13, 2616. [Google Scholar] [CrossRef] [PubMed]
- Kreye, H.; Hornbogen, E. Recrystallisation of Supersaturated Copper-Cobalt Solid Solutions. J. Mater. Sci. 1970, 5, 89–95. [Google Scholar] [CrossRef]
- Berkowitz, A.E.; Mitchell, J.R.; Carey, M.J.; Young, A.P.; Rao, D.; Starr, A.; Zhang, S.; Spada, F.E.; Parker, F.T.; Hutten, A. Giant Magnetoresistance in Heterogeneous Cu–Co and Ag–Co Alloy Films. J. Appl. Phys. 1993, 73, 5320–5325. [Google Scholar] [CrossRef]
- Liu, K.; Nagodawithana, K.; Searson, P.C.; Chien, C.L. Perpendicular Giant Magnetoresistance of Multilayered Co/Cu Nanowires. Phys. Rev. B 1995, 51, 7381. [Google Scholar] [CrossRef]
- Ram, S.; Frankwicz, P.S. Granular GMR Sensors of Co–Cu and Co–Ag Nanoparticles Synthesized through a Chemical Route Using NaBH4. Phys. Status Solidi 2001, 188, 1129–1140. [Google Scholar] [CrossRef]
- Prieto, A.G.; Fdez-Gubieda, M.L.; Meneghini, C.; Garcia-Arribas, A.; Mobilio, S. Microstructural and Magnetic Evolution upon Annealing of Giant Magnetoresistance Melt-Spun Co-Cu Granular Alloys. Phys. Rev. B 2003, 67, 224415. [Google Scholar] [CrossRef]
- Pratama, K.; Tian, C.; Sharma, A.; Watroba, M.; Gubicza, J.; Dilasari, B.; Schwiedrzik, J.; Michler, J. Pulsed Electrodeposition of Homogenous and Heterogeneous Solid Solution Layered Structure in High Strength Nanocrystalline CoCu Alloys. Surf. Coat. Technol. 2024, 480, 130613. [Google Scholar] [CrossRef]
- Giancoli, D.C. Physics for Scientists and Engineers with Modern Physics; Pearson Education: London, UK, 2008; Volume 2, ISBN 0131495089. [Google Scholar]
- Kim, M.J.; Lee, H.J.; Yong, S.H.; Kwon, O.J.; Kim, S.-K.; Kim, J.J. Facile Formation of Cu-Ag Film by Electrodeposition for the Oxidation-Resistive Metal Interconnect. J. Electrochem. Soc. 2012, 159, D253. [Google Scholar] [CrossRef]
- Kim, M.J.; Yong, S.H.; Ko, H.S.; Lim, T.; Park, K.J.; Kwon, O.J.; Kim, J.J. Superfilling of Cu-Ag Using Electrodeposition in Cyanide-Based Electrolyte. J. Electrochem. Soc. 2012, 159, D656–D658. [Google Scholar] [CrossRef]
- Volov, I.; Swanson, E.; O’Brien, B.; Novak, S.W.; Boom, R.V.D.; Dunn, K.; West, A.C. Pulse-Plating of Copper-Silver Alloys for Interconnect Applications. J. Electrochem. Soc. 2012, 159, D677–D683. [Google Scholar] [CrossRef]
- Strehle, S.; Menzel, S.; Wendrock, H.; Acker, J.; Gemming, T.; Wetzig, K. Thermo-Mechanical Behavior and Microstructural Evolution of Electrochemically Deposited Low-Alloyed Cu(Ag) Thin Films. Microelectron. Eng. 2004, 76, 205–211. [Google Scholar] [CrossRef]
- Strehle, S.; Menzel, S.; Bartha, J.W.; Wetzig, K. Electroplating of Cu(Ag) Thin Films for Interconnect Applications. Microelectron. Eng. 2010, 87, 180–186. [Google Scholar] [CrossRef]
- Bernasconi, R.; Hart, J.L.; Lang, A.C.; Magagnin, L.; Nobili, L.; Taheri, M.L. Structural Properties of Electrodeposited Cu-Ag Alloys. Electrochim. Acta 2017, 251, 475–481. [Google Scholar] [CrossRef]
- Lee, K.H.; Kong, W.; Han, M.; Park, D.J.; Ahn, J.H.; Han, S.Z.; Park, Y.-B.; Lee, K.H.; Choe, S. Properties of Nanocrystalline CuAg Foil Prepared via Electrodeposition. J. Alloys Compd. 2021, 881, 160522. [Google Scholar] [CrossRef]
- Gambino, J.P. Improved Reliability of Copper Interconnects Using Alloying. In Proceedings of the 2010 17th IEEE International Symposium on the Physical and Failure Analysis of Integrated Circuits, Singapore, 5–9 July 2010; IEEE: New York, NY, USA, 2010; pp. 1–7. [Google Scholar]
- Swingler, J. Performance and Arcing Characteristics of Ag/Ni Contact Materials under DC Resistive Load Conditions. IET Sci. Meas. Technol. 2011, 5, 37–45. [Google Scholar] [CrossRef]
- Proux, O.; Mimault, J.; Revenant-Brizard, C.; Regnard, J.R.; Mevel, B. Structural Evolution of NiAg Heterogeneous Alloys upon Annealing. J. Phys. Condens. Matter 1999, 11, 147. [Google Scholar] [CrossRef]
- Barthelemy, A.; Cros, V.; Duvail, J.L.; Fert, A.; Morel, R.; Parent, F.; Petroff, F.; Steren, L.B. Giant Magnetoresistance in Magnetic Nanostructures. Nanostructured Mater. 1995, 6, 217–226. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Y.; Chen, X.; Wen, R.; Yue, G.-H.; Peng, D.-L. Facile Synthesis of Near-Monodisperse Ag@Ni Core–Shell Nanoparticles and Their Application for Catalytic Generation of Hydrogen. Nanotechnology 2011, 22, 195604. [Google Scholar] [CrossRef]
- Greeley, J.; Nørskov, J.K. Large-Scale, Density Functional Theory-Based Screening of Alloys for Hydrogen Evolution. Surf. Sci. 2007, 601, 1590–1598. [Google Scholar] [CrossRef]
- Tyler, E.H.; Clinton, J.R.; Luo, H.L. Electrical Resistivity of Metastable Ag-Ni Alloys. Solid State Commun. 1973, 13, 1409–1411. [Google Scholar] [CrossRef]
- Cavallotti, P.L.; Nobili, L.; Vicenzo, A. Phase Structure of Electrodeposited Alloys. Electrochim. Acta 2005, 50, 4557–4565. [Google Scholar] [CrossRef]
- Foster, D.G.; Shapir, Y.; Jorné, J. Scaling of Roughness in Silver Electrodeposition. J. Electrochem. Soc. 2003, 150, C375. [Google Scholar] [CrossRef]
- Gómez, E.; García-Torres, J.; Vallés, E. Study and Preparation of Silver Electrodeposits at Negative Potentials. J. Electroanal. Chem. 2006, 594, 89–95. [Google Scholar] [CrossRef]
- Liang, D.; Liu, Z.; Hilty, R.D.; Zangari, G. Electrodeposition of Ag–Ni Films from Thiourea Complexing Solutions. Electrochim. Acta 2012, 82, 82–89. [Google Scholar] [CrossRef]
- Xie, B.-G.; Sun, J.-J.; Lin, Z.-B.; Chen, G.-N. Electrodeposition of Mirror-Bright Silver in Cyanide-Free Bath Containing Uracil as Complexing Agent without a Separate Strike Plating Process. J. Electrochem. Soc. 2008, 156, D79. [Google Scholar] [CrossRef]
- Dhanapal, K.; Vasumathi, M.; Santhi, K.; Narayanan, V.; Stephen, A. Double Dumbbell Shaped AgNi Alloy by Pulsed Electrodeposition. In Proceedings of the AIP Conference Proceedings, Shymkent, Kazakhstan, 11–13 September 2014; American Institute of Physics: College Park, MD, USA, 2014; Volume 1576, pp. 95–97. [Google Scholar]
- Santhi, K.; Karthick, S.N.; Kim, H.-J.; Nidhin, M.; Narayanan, V.; Stephen, A. Microstructure Analysis of the Ferromagnetic Ag–Ni System Synthesized by Pulsed Electrodeposition. Appl. Surf. Sci. 2012, 258, 3126–3132. [Google Scholar] [CrossRef]
- Eom, H.; Jeon, B.; Kim, D.; Yoo, B. Electrodeposition of Silver-Nickel Thin Films with a Galvanostatic Method. Mater. Trans. 2010, 51, 1842–1846. [Google Scholar] [CrossRef]
- Tang, M.H.; Hahn, C.; Klobuchar, A.J.; Ng, J.W.D.; Wellendorff, J.; Bligaard, T.; Jaramillo, T.F. Nickel–Silver Alloy Electrocatalysts for Hydrogen Evolution and Oxidation in an Alkaline Electrolyte. Phys. Chem. Chem. Phys. 2014, 16, 19250–19257. [Google Scholar] [CrossRef] [PubMed]
- Yamachika, N.; Musha, Y.; Sasano, J.; Senda, K.; Kato, M.; Okinaka, Y.; Osaka, T. Electrodeposition of Amorphous Au–Ni Alloy Film. Electrochim. Acta 2008, 53, 4520–4527. [Google Scholar] [CrossRef]
- Rouya, E.; Stafford, G.R.; Bertocci, U.; Mallett, J.J.; Schad, R.; Begley, M.R.; Kelly, R.G.; Reed, M.L.; Zangari, G. Electrodeposition of Metastable Au–Ni Alloys. J. Electrochem. Soc. 2010, 157, D396. [Google Scholar] [CrossRef]
- Dolati, A.; Ghorbani, M.; Ahmadi, M.R. An Electrochemical Study of Au–Ni Alloy Electrodeposition from Cyanide–Citrate Electrolytes. J. Electroanal. Chem. 2005, 577, 1–8. [Google Scholar] [CrossRef]
- Li, J.C.; Nan, S.H.; Jiang, Q. Study of the Electrodeposition of Al–Mn Amorphous Alloys from Molten Salts. Surf. Coat. Technol. 1998, 106, 135–139. [Google Scholar] [CrossRef]
- Tsuda, T.; Hussey, C.L.; Stafford, G.R. Electrodeposition of Al–Mo alloys from the Lewis acidic aluminum chloride-1-ethyl-3-methylimidazolium chloride molten salt. J. Electrochem. Soc. 2004, 151, C379–C384. [Google Scholar] [CrossRef]
- Tsuda, T.; Hussey, C.L. Electrochemistry of Vanadium (II) and the Electrodeposition of Aluminum-Vanadium Alloys in the Aluminum Chloride-1-Ethyl-3-Methylimidazolium Chloride Molten Salt. J. Min. Metall. Sect. B Metall. 2003, 39, 3–22. [Google Scholar] [CrossRef]
- Barthel, T.; Klimanek, P.; Raub, C.J.; Zielonka, A. Microstructure Formation in Electrodeposition of Highly Supersaturated Cu-Pb and Ag-Pb Layers. In Materials Science Forum; Trans Tech Publications: Zurich, Switzerland, 1996; Volume 228, pp. 475–480. [Google Scholar]
- Revathy, T.A.; Santhi, K.; Narayanan, V.; Stephen, A. Synthesis and Characterization of Fe-Ag Alloy by Pulsed Electrodeposition. Chem. Sci. Trans. 2013, 2, S153. [Google Scholar]
- Noce, R.D.; Gomes, O.D.M.; De Magalhaes, S.D.; Wolf, W.; Guimarães, R.B.; De Castro, A.C.; Pires, M.J.M.; Macedo, W.A.A.; Givord, D.; Barthem, V.M.T.S. Magnetic Properties of Fe–Cu Alloys Prepared by Pulsed Electrodeposition. J. Appl. Phys. 2009, 106, 093907. [Google Scholar] [CrossRef]
- Mallett, J.J.; Svedberg, E.B.; Sayan, S.; Shapiro, A.J.; Wielunski, L.; Madey, T.E.; Egelhoff, W.F.; Moffat, T.P. Compositional Control in Electrodeposition of FePt Films. Electrochem. Solid-State Lett. 2004, 7, C121. [Google Scholar] [CrossRef]
- Bernasconi, R.; Nova, A.; Pané, S.; Magagnin, L. Electrodeposition of Equiatomic FePt Permanent Magnets from Non-Aqueous Electrolytes Based on Ethylene Glycol. J. Electrochem. Soc. 2022, 169, 072506. [Google Scholar] [CrossRef]
- Ying, Y.; Wang, H.; Zheng, J.; Yu, J.; Li, W.; Qiao, L.; Cai, W.; Che, S. Preparation, Microstructure, and Magnetic Properties of Electrodeposited Nanocrystalline L10 FePt Films. J. Supercond. Nov. Magn. 2020, 33, 3563–3570. [Google Scholar] [CrossRef]
- Krasikov, A.V.; Kastsova, A.G.; Markov, M.A.; Bykova, A.D.; Kravchenko, I.N.; Galinovskii, A.L. Electrochemical Synthesis of Amorphous Layers from a Nonequilibrium Co–W Alloy as a Precursor for Nanocomposite Coating Formation. Russ. Metall. 2022, 2022, 666–673. [Google Scholar] [CrossRef]
- Krasikov, A.V.; Merkulova, M.V.; Mikhailov, M.S.; Vasilyeva, E.A.; Petrov, S.N.; Drozdova, N.F.; Fedoseev, M.L. Formation of a Composite Coating by Crystallization of a Supersaturated Solid Solution in the Ni–W System. Trans. Indian Inst. Met. 2024, 1–8. [Google Scholar] [CrossRef]
- Esquivel, J.; Gupta, R.K. Review—Corrosion Resistant Metastable Al Alloys: An Overview of Corrosion Mechanisms. J. Electrochem. Soc. 2020, 167, 081504. [Google Scholar] [CrossRef]
- Sziráki, L.; Kuzmann, E.; El-Sharif, M.; Chisholm, C.U.; Stichleutner, S.; Lak, G.B.; Süvegh, K.; Tatár, E.; Homonnay, Z.; Vértes, A. Electrodeposition of Novel Sn–Ni–Fe Ternary Alloys with Amorphous Structure. Appl. Surf. Sci. 2010, 256, 7713–7716. [Google Scholar] [CrossRef]



| Alloy | Type of Electrolyte | Application | References |
|---|---|---|---|
| Au-Ni | Acidic cyanide-citrate | Contacts and switches, substrate protection | [93,94,95] |
| Al-Mn | Molten salts | Corrosion protection, protective coatings | [96] |
| Al-Mo | Ionic liquid | Corrosion protection | [97] |
| Al-V | Ionic liquid | Corrosion protection | [98] |
| Cu-Pb | Alkaline citrate | Metal bearing coating | [99] |
| Ag-Pb | Alkaline cyanide | Metal bearing coating | [99] |
| Fe-Ag | Acidic perchlorate | Magnetic applications | [100] |
| Fe-Cu | Sulfate | Magnetic applications | [101] |
| Fe-Pt | Acidic chloride; acidic sulfate; non-aqueous | Magnetic properties | [102,103,104] |
| Co-W | Citrate | Wear protection | [105] |
| Ni-W | Citrate | Wear protection | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernasconi, R.; Nobili, L.; Magagnin, L. Properties and Applications of Supersaturated Metastable Alloys Obtained via Electrodeposition. Crystals 2024, 14, 761. https://doi.org/10.3390/cryst14090761
Bernasconi R, Nobili L, Magagnin L. Properties and Applications of Supersaturated Metastable Alloys Obtained via Electrodeposition. Crystals. 2024; 14(9):761. https://doi.org/10.3390/cryst14090761
Chicago/Turabian StyleBernasconi, Roberto, Luca Nobili, and Luca Magagnin. 2024. "Properties and Applications of Supersaturated Metastable Alloys Obtained via Electrodeposition" Crystals 14, no. 9: 761. https://doi.org/10.3390/cryst14090761
APA StyleBernasconi, R., Nobili, L., & Magagnin, L. (2024). Properties and Applications of Supersaturated Metastable Alloys Obtained via Electrodeposition. Crystals, 14(9), 761. https://doi.org/10.3390/cryst14090761







