Abstract
Dye-sensitized solar cells (DSSCs) have attracted renewed research interest as a potential low-cost substitute for conventional silicon photovoltaics. This work aims to improve the photovoltaic performance of the DSSCs by incorporating multi-walled carbon nanotubes (MWCNTs) into the BaTiO3 photoelectrode. The pure BaTiO3 and BaTiO3/MWCNT nanocomposites were sensitized with N719 dye and fabricated into solar cell devices for testing. The structural characterization confirmed the successful formation of the nanocomposite with an optimal dispersion at 6% of MWCNT incorporation, beyond which agglomeration effects manifested. The optical analysis verified the modulation of defect states and bandgap engineering induced by the MWCNT network. The morphological studies revealed irregular nanoparticle clusters with embedded nanotubes. Solar cell testing under AM1.5G-simulated sunlight demonstrated a peak power conversion efficiency of 4.044% for 6% of MWCNT doping, constituting a 6-fold increment versus pure BaTiO3 (0.693%). It originated from the simultaneous enhancements in the open-circuit voltage and short-circuit current enabled by the favorable band structure alterations and percolation-assisted charge transport. However, further increasing MWCNT content deteriorated the device metrics, owing to emerging limitations like trapping. The rational integration of multi-walled carbon nanotubes with lead-free ferroelectric metal oxides can contribute to the development of emerging organic-inorganic hybrid solar platforms.
1. Introduction
The consumption of energy around the globe has been continuously climbing for nearly half a century, and the global energy demand is estimated to reach 17 terawatts (TW) by 2040 [1]. The combustion of fossil fuels, which can include coal, oil, and gas, is the source of the great majority of this energy [2,3]. These fuels contribute to the emission of greenhouse gasses such as carbon dioxide and other gasses that trap heat in the atmosphere, which in turn contributes to the global warming and climate change [4,5].
Renewable energy sources, such as solar, wind, hydro, and geothermal energy, provide alternatives to fossil fuels that are both more environmentally friendly and more sustainable [6,7,8]. Solar energy, with its plentiful supply and relatively simple collection methods, stands out as one of the most promising of these sources [9,10,11]. Solar cells, commonly referred to as photovoltaic (PV) cells, can convert sunlight into electricity, which enables the solar energy to be harvested and used. Currently, there are several types of solar cells available, with some being more promising than others [12].
Photovoltaic technologies like perovskite and dye-sensitized solar cells (DSSCs) have garnered substantial research attention in the recent years as viable alternatives to conventional silicon-based devices [13]. Hybrid organic–inorganic lead halide perovskites are particularly promising due to their exceptional light-harvesting capacity coupled with low-cost solution-based fabrication methods, leading to photoconversion efficiencies rivalling commercial modules. Additionally, the tandem architectures integrating crystalline silicon with perovskite layers have demonstrated efficiency increments by synergistically combining the benefits of both systems [14].
However, concerns around lead toxicity, long-term operational stability, and sourcing adequate ruthenium supplies for conventional dyes have shifted focus to sustainable lead-free substitutes like dye-sensitized cells. By incorporating light-absorbing photosensitizers and charge-transporting metal oxide layers, DSSCs provide an economical pathway to solar energy conversion without demanding high-temperature processes. But the attainment of commercially adequate efficiency and durability remains a key bottleneck. Optimizing the nanostructured metal oxide photoelectrode component is, therefore, critical.
Titanium dioxide (TiO2) and zinc oxide (ZnO) have been extensively utilized as DSSC photoanodes due to their strong UV light absorption and photon capture. However, challenges remain in improving their electron dynamics, operational lifetimes, and fabrication scalability. Hence, alternative materials such as tin oxide [15], iron oxide [16], copper oxide [17], and bismuth ferrite [18] have been explored.
Among these alternatives, barium titanate (BaTiO3) has recently elicited interest due to several favorable attributes [19,20]. BaTiO3 offers a high dielectric constant and refractive index, enabling strong optical absorption and photon capture in the visible range, surpassing the predominantly UV response of TiO2. The band energetics of BaTiO3 are also suitable for effective dye sensitization and charge injection. Moreover, BaTiO3 exhibits inherently suppressed electron-hole recombination rates upon photoexcitation, allowing for more efficient charge carrier transport within the photoanode [21] and directly enabling improvements in energy conversion efficiency [22]. In contrast, TiO2 suffers from higher recombination losses, hindering its charge collection efficiency.
These characteristics are advantageous for achieving significant optical absorption and effective photon capture in photoelectrodes [23,24]. Moreover, the bandgap energy of BaTiO3 is very suitable for effective light absorption and efficient charge carrier dynamics when used as a photoelectrode in a dye-sensitized solar cell [25,26]. However, the intrinsically low electron mobility and conductivity of BaTiO3 result in lower conversion efficiencies compared to TiO2, necessitating strategies like conductive network incorporation to enhance its performance. TiO2 benefits from high electron mobility and efficient charge injection from dye molecules, making it a widely used and established photoanode material. Additionally, the noted stability and corrosion resistance of BaTiO3, even in acidic conditions, helps maintain the structural integrity of the active layer over long-term device operation [27], presenting advantages for durable high-efficiency dye-sensitized solar cells.
Taken together, these attributes make BaTiO3 a promising candidate to integrate into the photoanode architecture for dye-sensitized solar cells. In pursuing low-cost and high-efficiency photovoltaic materials, DSSCs have been fabricated using a range of synthesis strategies for the metal oxide photoanode component. The reported approaches include precipitation [28], hydrothermal [29], and sol–gel synthesis [30] to produce nanostructured morphologies. Furthermore, various techniques have aimed to optically and electronically optimize DSSC photoanodes, whether through elemental doping [31,32], the addition of quantum dots [33,34], or carbon nanotubes [35,36,37], the addition of co-catalysts, or other routes [38]. These efforts attempt to maximize the light-harvesting capability, charge transport kinetics, surface area, and stability in the resulting photoelectrode architecture.
The choice of photosensitizer dye also plays a crucial role in determining the overall efficiency of DSSCs. The N719 dye (di-tetrabutylammonium cis-bis(isothiocyanato)bis(2,2-bipyridyl-4,4-dicarboxylato)ruthenium(II)) has become a widely used benchmark owing to its superior performance characteristics. N719 exhibits strong absorption across the visible spectrum, enabling the effective harvesting of incident solar radiation. The carboxylate groups in its molecular structure facilitate robust anchoring to the metal oxide surface, ensuring high dye loading and stability against desorption. Furthermore, N719 enables efficient electron injection into the photoanode, a key step in the energy conversion process. The combination of a broad spectral response, excellent stability, and efficient charge transfer has made N719 one of the most used dyes for evaluating novel DSSC materials and architectures [39].
The present study aims to evaluate the impact of incorporating multi-walled carbon nanotubes (MWCNT) into the BaTiO3 photoelectrode of DSSCs. MWCNTs can improve the electrical conductivity of the photoanode, resulting in better electron transit to the external circuit [35]. Moreover, MWCNTs can increase the surface area of the photoanode, providing more sites for dye molecules to adsorb, thereby increasing the light absorption capacity [40,41]. MWCNTs can also function as a barrier layer, preventing the diffusion of electrolyte species into the photoanode, thereby enhancing the solar cell’s endurance and performance [42,43].
In this work, we have synthesized pure BaTiO3 and BaTiO3/MWCNT composites by the sol–gel method. The samples obtained were characterized by different techniques such as DSC/TGA, XRD, FTIR, and UV–visible. This study focuses on evaluating the impact of varying concentrations of MWCNTs on the optical, structural, morphological, and photovoltaic characteristics of BaTiO3 photoelectrode-based solar cells.
2. Materials and Methods
2.1. Materials
Barium nitrate (99%), citric acid (99%), 2-propanol, tert-butanol, glacial acetic acid, acetonitrile, acetylacetone, and triton X-100 were obtained from Merk (Merck Millipore, Darmstadt, Germany), and used without any further purification. A multi-walled carbon nanotube (98%, 6–13 nm × 2.5–20 µm), chloroplatinic (8% H2O) and titanium (IV) isopropoxide were supplied by Sigma-Aldrich (Sigma Aldrich, Burlington, MA, USA). Di-tetrabutylammonium cis-bis(isothiocyanato)bis(2,2-bipyridyl-4,4dicarboxylato)ruthenium (II) (N719 dye, 95%), platinum paste (Platisol T/SP) and iodolyte HI-30 were supplied by Solaronix (Solaronix SA, Au-bonne, Switzerland). Indium tin oxide (ITO)-coated glass was supplied by Kaivo Optoelectronics (ITO-P001, <10 Ω/sq, Kaivo Optoelectronic, Zhuhai, China).
2.2. BaTiO3 Synthesis
For the synthesis of barium titanate particles, 6.536 g of barium nitrate was dissolved in 40 milliliters of 2-propanol for 12 h. Subsequently, 1.253 g of citric acid was dissolved in 20 mL of 2-propanol for 30 min. The two solutions were then mixed and stirred for an additional 30 min. At this stage, 7.39 mL of titanium (IV) isopropoxide was promptly added to the mixture, followed by the addition of 2.4 mL of ultrapure water. The mixture was stirred for 10 min, then dried for 12 h at 60 °C. The obtained powder was ground and calcined at 700 °C for 2 h, with a heating rate of 5 °C/min. This calcination temperature was selected based on the results of differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) (Figure 1a). The obtained sample was labeled as BaTiO3.
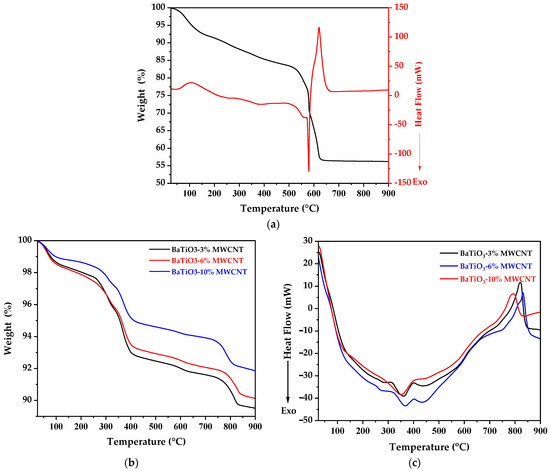
Figure 1.
(a) Thermogravimetric analysis and differential scanning calorimetry of (a) pure BaTiO3 and (b,c) BaTiO3/MWCNT nanocomposites.
2.3. BaTiO3/MWCNT Synthesis
In order to synthesize BaTiO3/MWCNT nanocomposites, the surface of 0.3 g of MWCNTs was modified through acid treatment. This involved immersing the MWCNTs in a solution of ultrapure water and nitric acid (3:1, v/v), in an ultrasonic bath for 30 min. The resulting sample was washed to achieve a neutral pH and dried at 50 °C for 12 h. Next, 1.4 g of barium titanate was mixed with 9 mL of a 1:2 dilution of glacial acetic acid and water, along with varying percentages of MWCNTs (3%, 6%, and 10% w/w). The mixture was then stirred for 2 h, dried at 50 °C for 12 h, and calcined at 700 °C for 2 h with a temperature increase of 5 °C/min. The obtained samples were labeled as BaTiO3-3%MWCNT, BaTiO3-6%MWCNT and BaTiO3-10%MWCNT according to the MWCNT content.
2.4. Solar Cell Fabrication
The fabrication of a pure BaTiO3 working electrode and a BaTiO3/MWCNT composite working electrode was performed according to the modified process described by Tiburcio et al. [18]. Initially, 1 cm2 ITO glasses were cleaned by being repeatedly washed with ultrapure water and 2-propanol using an ultrasonic bath, and then with acetone. To deposit the photoactive layer, pure BaTiO3 and BaTiO3/MWCNT composites were prepared by first mixing 60 mg of either the pure or composite sample with 0.2 mL of acetic acid to obtain a homogeneous paste. Next, 0.1 mL of Triton X surfactant was added to the paste, followed by the addition and mixing-in of 5 microliters of 2-propanol to further homogenize each paste. These final pastes were then screen printed (250 μm polystyrene mesh) onto ITO substrates and calcined at 400 °C for 30 min to produce the photoactive layers.
The ITO counter electrode was platinized by screen printing 10 microliters of platinum paste (Platisol T/SP), followed by calcination at 400 °C for 30 min.
The resulting films were then immersed in a solution of 0.3 mM dye N719 in tert-butanol and acetonitrile (1:1) for 24 h. The resulting photoelectrode and platinum counter electrode were sandwiched together, and 10 microliters of electrolyte iodine were added for testing.
2.5. Characterization of the Samples
Thermal analysis of the precursor powder was carried out using simultaneous differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) on a SDT 650 module (TA Instruments, New Castle, DE, USA). The sample was heated from ambient temperature to 900 °C at 20 °C/min under nitrogen atmosphere, allowing for quantification of both heat flow changes and mass loss as a function of temperature. X-ray diffraction analysis of the pure BaTiO3 and BaTiO3/MWCNT composite samples was performed with a PANalytical Aeris Research diffractometer (Malvern Panalytical Ltd., Almelo, The Netherlands) equipped with a copper Kα radiation source and nickel filter. The structural refinement and quantification of crystallite dimensions were carried out via the Rietveld method using HighScore Plus software V4.9. The vibrational spectroscopy of the samples was performed using a Maya 2000 Pro Raman microscope (Ocean Optics Inc., Orlando, FL, USA) with a 532 nm excitation laser. Diffuse reflectance spectroscopy in the UV–visible range utilized an Evolution 220 spectrometer (Thermo Scientific Co., New York, NY, USA). Infrared transmission spectra were collected on a Bruker Invenio R Fourier transform infrared (FTIR) spectrometer (Bruker, Ettlingen, Germany) equipped with an ATR (attenuated total reflectance) system. Photoluminescence was measured using a FluoroMax Plus spectrofluorometer system (Horiba Scientific, Irvine, CA, USA). Morphological analysis was done via field emission scanning electron microscopy (FE-SEM) on a Thermo Scientific Quattro S microscope (Thermo Fisher, Eindhoven, The Netherlands). Photovoltaic testing of fabricated solar cells at room temperature utilized a Sciencetech INC system (North York, ON, Canada) with a Keithley source meter under simulated solar illumination from an AAA class simulator at 100 mW/cm2 calibrated versus a reference silicon device. Current-voltage measurements were used to evaluate photovoltaic performance.
2.6. Photovoltaic Performance of DSSCs
The electrical properties of DSSCs were evaluated through the characterization of photocurrent density–voltage (J-V) under a simulated solar irradiance. Using the established equations below, the fill factor (FF) and the total conversion efficiency (η) of the DSSCs were calculated as follows:
where, Vmax and Jmax correspond to the voltage and current density, respectively, at the maximum power point of the DSSCs. The open-circuit voltage (VOC) and short-circuit current density (JSC) were generated by the DSSC under illumination, while Pin denotes the power of the incident light intensity (100 mW/cm2) from the solar simulator.
3. Results
3.1. Thermal Analysis
The thermogravimetric analysis and differential scanning calorimetry results for the prepared BaTiO3 powders, dried at 60 °C for 12 h, are shown in Figure 1a. The first endothermic peak observed at 105 °C corresponds to the evaporation of physically adsorbed water and volatile organic solvents, resulting in a 5% mass loss at the center of the peak and 10% after the process was completed. Two exothermic reactions were located between 200 and 500 °C, which could be attributed to the evaporation of molecular trapped water and the decomposition of nitrates, resulting in a mass loss of 17%. An exothermal peak was observed at 579 °C, followed by an endothermal peak at 619 °C, which represents the decomposition of citric acid and the decomposition of barium carbonate, respectively, resulting in the production of CO2 and the formation of BaTiO3, leading to a 44% mass loss at 680 °C when the crystallization process was completed. The formation enthalpy of BaTiO3 was precisely determined through differential scanning calorimetry (DSC) analysis using the TRIOS software version 5.1. The result showed a value of 426.24 J/g with a start temperature of 585 °C, a finish temperature of 675 °C, and a peak temperature of 620 °C. This corresponds to the completion of the tetragonal phase crystallization process of BaTiO3. The calcination temperature for complete crystallization was carefully selected as 700 °C for all the synthesized samples.
The thermogravimetric profiling of the sequentially synthesized BaTiO3/MWCNT nanocomposites (Figure 1b) verifies higher weight loss with decreasing MWCNT content. The initial mass decline is attributed to the elimination of acid treatment residues, which induces MWCNT oxidation and the grafting of carboxyl/hydroxyl groups, as previously reported. The subsequent loss corresponds to the removal of the acetates adsorbed during acetic acid processing on both nanotube and inorganic constituents. The minimal decomposition after 400 °C signifies the production of thermally robust composites, following the deliberate extraction of introduced surface moieties.
The as-prepared BaTiO3-10%MWCNT sample exhibited a total 8.2% loss by 900 °C, contrasting with 10.5% for the BaTiO3-3%MWCNT counterpart. This connects to heightened surface elaborations and associated impurities enabled by the dedicated MWCNT functionalization steps prior to integration with BaTiO3. The two-phase synthesis facilitates composites with thermal stability spanning 400–900 °C, underscoring their suitability for demanding electronic applications involving oxides.
Conversely, exothermal values from differential scanning calorimetry (Figure 1c) signify the progressive suppression of the tetragonal–cubic transition upon heating with more incorporated MWCNT fillers [44]. This implies the MWCNTs increasingly hinder TiO6 octahedral rearrangements that disable the ferroelectric phase change. Interfacial effects between the BaTiO3 grains and MWCNTs are credited for this structure-stabilizing impact.
The opposing trends verify a sequential nanocomposite formation mechanism—tailored surface chemistry produces highly functionalized MWCNTs that first anchor to the BaTiO3 structure before burning off residues at elevated temperatures.
3.2. X-ray Diffraction Results
Figure 2a depicts the X-ray diffraction results of pure multi-walled carbon nanotubes (MWCNT), pure barium titanate (BaTiO3), and BaTiO3/MWCNT composite with varying concentrations. The diffraction patterns (represented by the black line) display good agreement with standard data for MWCNT (JCPDS N° 41-1487) with peaks located at 26.14° and 43.04°, which correspond to the (002) and (100) reflection planes, respectively. These peaks are attributed to the hexagonal graphite structure of MWCNT [45,46]. In contrast, the results for pure BaTiO3 and BaTiO3/MWCNT composites do not exhibit peaks related to MWCNT. However, the effect of adding MWCNT in the synthesis process is reflected in the structural parameters and crystallite size, as seen in Table 1. The observed peaks correspond to BaTiO3 (JCPDS N° 01-075-2121), which has a tetragonal crystal structure and P4/mmm (number 123) space group.
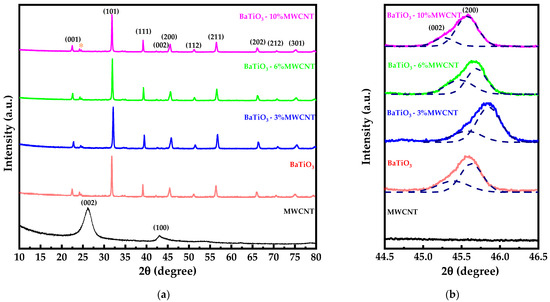
Figure 2.
(a) X-ray diffraction patterns of pure MWCNT, BaTiO3 and BaTiO3/MWCNT nanocomposite, and (b) detail observation of BaTiO3 (200) and (002) peaks.

Table 1.
Structural parameters obtained from Rietveld refinements of XRD analysis of pure BaTiO3 and BaTiO3/MWCNT nanocomposite.
The Rietveld refinement results indicate that the addition of 3% of MWCNT reduces the crystallite size of BaTiO3 from 83.27 to 57.71 nm, compared to pure BaTiO3. However, increasing the amount of MWCNT increases the crystallite size of BaTiO3 from 73.1 to 78.26 nm for 6 and 10% MWCNT, respectively. This effect can be appreciated in Figure 2b with the shift of the BaTiO3 peak from the center to the right side with the addition of 3% of MWCNT, and to the left side with increasing amounts of MWCNT. This result also shows that the peak located at 45.5° is composed of two peaks corresponding to the (002) and (200) reflection planes, which are indicative of the tetragonal crystal system [47].
Furthermore, the XRD patterns of pure BaTiO3 and BaTiO3/MWCNT nanocomposites showed a weak peak (labeled with “*”) at 24.16° and 24.61° that can be attributed to (111) and (102) reflection planes of BaCO3 (JCPDS N° 00-041-0373). This formation is associated with the result of a chemical reaction between barium from the starting reagent and citric acid during the combustion process [48].
3.3. Raman Spectroscopy
Figure 3 displays Raman spectra of pure BaTiO3 and BaTiO3/MWCNT composites. The obtained spectra from all the samples show similar quantities and positions of peaks. Figure 3 shows the commonly observed Raman peaks in the tetragonal BaTiO3 structure are the peaks located at 142 cm−1, which correspond to the symmetric stretching mode of the TiO6 octahedra, the peak at 191 cm−1, which corresponds to the antisymmetric stretching mode of the TiO6 octahedra, the peak at around 262 cm−1, which corresponds to the symmetric bending mode of the TiO6 octahedra, and the peak at around 717 cm−1, which corresponds to the antisymmetric stretching mode of the Ba atoms [49,50]. The results also display additional Raman peaks that were not shown in X-ray diffraction results, corresponding to the hexagonal structure of BaTiO3 located at 433 cm−1, which corresponds to the symmetric bending mode of the TiO6 octahedra, and 632 cm−1, which corresponds to the symmetric stretching mode of the Ba atom [51,52]. This effect can be attributed to a “core–shell” model, where submicron or nanosized crystals of BaTiO3 consist of a core with a hexagonal structure and a tetragonal phase shell [53]. This model has been similarly described by previous studies where a model with a cubic shell and tetragonal core was described [50,54,55]. The reduction in the intensity of the Raman spectra of the samples that contain MWCNTs is attributed to a reduction in the crystallinity and grain size of the samples [56].
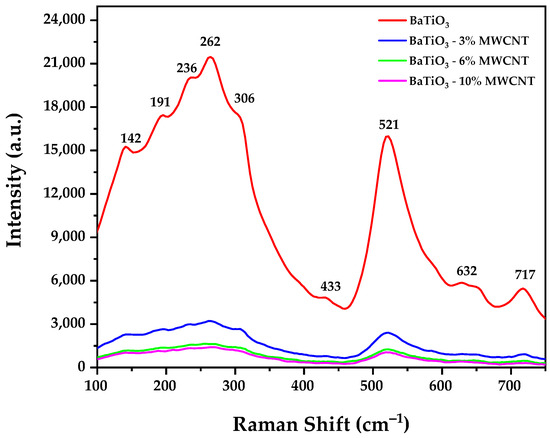
Figure 3.
Raman spectra of pure BaTiO3 and BaTiO3/MWCNT composite.
3.4. FTIR Spectroscopy
Figure 4 illustrates the FTIR spectra of pure BaTiO3 and BaTiO3/MWCNT nanocomposites. The observed spectral features include a characteristic band at around 492 cm−1, which is indicative of the Ti-O vibration mode. These spectral characteristics align with previous literature reports, thus affirming the crystal structure of BaTiO3 in the analyzed samples [57]. Additionally, a peak located at 865 cm−1 is observed, which corresponds to the in-plane and out-of-plane bending of from BaCO3 [58]. The peak located at 1440 cm−1 corresponds to COO− groups [59,60]. Furthermore, the peaks located at 2332 and 2360 cm−1 are related to the CO and CO2 groups [61], which may be attributed to adsorbed gasses on the sample surface during the synthesis process.
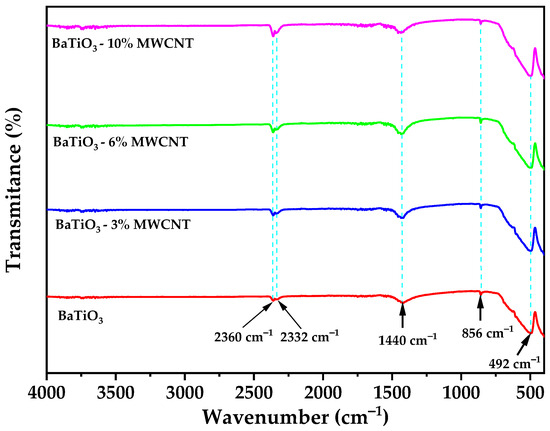
Figure 4.
FTIR Spectra of pure BaTiO3 and BaTiO3/MWCNT nanocomposite.
3.5. UV–Vis Spectroscopy
The optical absorption of the samples is displayed in Figure 5a. The results show that the absorption of light in the visible region increases proportionally with the amount of MWCNT, while there is no significant increase in absorption in the UV region. Furthermore, the collected data were used to analyze the optical bandgap energy (Eg) using the Tauc relation [62,63,64]:
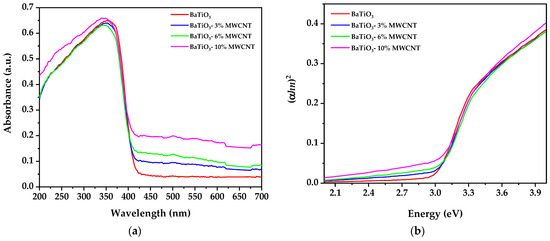
Figure 5.
(a) UV–visible absorption spectra of pure BaTiO3 and BaTiO3/MWCNT composites, (b) the Tauc plot to calculate the indirect bandgap energy.
The absorption coefficient (α) was calculated utilizing the well-established relation α = 4πk/λ [65,66], where k represents the extinction coefficient and λ denotes the wavelength of light, v is the transition frequency, A is a proportionality constant, hv is the photon energy, Eg refers to the optical bandgap, and n depends on the type of transition (n = 1/2 for direct allowed and n = 2 for indirect transitions). The optical bandgap of the samples was calculated by extrapolating the plot on the energy axis [hv versus (αhv)2] and obtaining the intercept of the energy axis, as shown in Figure 5b. The calculated optical bandgap of the samples is displayed in Table 1. The results indicate that the energy gap increases from 3.023 to 3.032 eV when 3% of MWCNT is added. However, this value decreases to 3.029 and 2.997 eV when the concentration of MWCNT is increased. This trend may be related to the corresponding decrease and increase in the crystallite size, as observed in the X-ray diffraction results. These changes in the crystallite size can alter the band structure of the material, leading to a corresponding change in the optical bandgap energy [18,67,68].
3.6. Photoluminescence Spectroscopy
The photoluminescence emission spectra spanning 300–700 nm for pure BaTiO3 nanoparticles and multi-walled carbon nanotube-reinforced BaTiO3 nanocomposites under 254 nm excitation are presented in Figure 6.
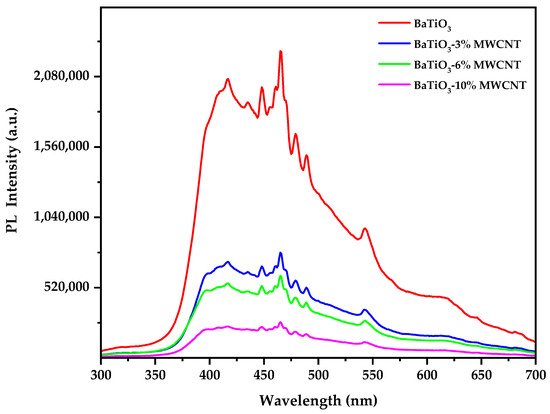
Figure 6.
Photoluminescence spectra of pure BaTiO3 and BaTiO3/MWCNT nanocomposites.
All the samples exhibit similar emission profiles, characterized by the presence of multiple peaks and shoulders, indicating that the incorporation of MWCNTs does not significantly alter the overall shape of the emission spectrum. The intrinsic visible luminescence in pure BaTiO3 originates from self-trapped excitons involving electron transfer between oxygen vacancies and adjacent Ti4+ sites [69]. The emission spectrum of pure BaTiO3 displays characteristic shoulders at 396 and 404 nm, which can be attributed to the presence of surface defect states [70]. The main emission peak at 416 nm is associated with the radiative recombination of self-trapped excitons [71]. Additional peaks are observed at 434, 447, 461, 465, 478, 489, and 542 nm, along with shoulders at 470 and 613 nm. These features may arise from the presence of different types of defects, such as oxygen vacancies and titanium interstitials, as well as the formation of a heterojunction between BaTiO3 and MWCNTs [72].
The most prominent effect of MWCNT incorporation on the photoluminescence behavior of BaTiO3 is the progressive quenching of the emission intensity with increasing MWCNT content. As the MWCNT loading increases from 0% to 10%, a significant decrease in the intensity of all the peaks and shoulders is observed, while their positions remain relatively unchanged. This quenching effect can be attributed to the efficient transfer of photogenerated electrons from BaTiO3 to MWCNTs, which act as electron acceptors and provide non-radiative relaxation channels [73,74]. The decrease in emission intensity becomes more pronounced with higher MWCNT content, suggesting a greater degree of charge transfer and a stronger interaction between the two components.
The preservation of the peak and shoulder positions in the emission spectra of the BaTiO3/MWCNT nanocomposites indicates that the incorporation of MWCNTs does not lead to a substantial modification of the BaTiO3 band structure. Instead, the MWCNTs primarily influence the charge carrier dynamics and recombination processes within the nanocomposites. The efficient transfer of photogenerated electrons from BaTiO3 to MWCNTs reduces the probability of radiative recombination, resulting in the observed quenching of the emission intensity. This non-radiative charge transfer pathway competes with the intrinsic radiative recombination processes in BaTiO3, leading to the suppression of luminescence.
3.7. Scanning Electron Microscopy
The morphology and elemental composition of pure BaTiO3 and BaTiO3/MWCNT composites were analyzed through FE-SEM (Figure 7a–d) and energy-dispersive X-ray spectroscopy (EDS). The images of pure BaTiO3 show particles with an irregular and cubic shape, with an average size of 313.17 ± 97.18 nm. The addition of MWCNT and the post-thermal treatment had a significant impact on the morphology of the particles. The resulting images reveal irregular shapes, with the MWCNTs being fully covered, as reported previously [75]. It was also observed that an increase in the concentration of MWCNT led to its agglomeration [48].

Figure 7.
Field emission scanning electron microscopy images of (a) BaTiO3, (b) BaTiO3-3% MWCNT, (c) BaTiO3-6% MWCNT and (d) BaTiO3-10% MWCNT.
The EDS analysis was performed to further investigate the elemental composition of the synthesized powder. Table 2 displays the results of the EDS analysis, where the atomic ratio of Ba:Ti in all the samples is close to 1:1, confirming the formation of BaTiO3. The presence of carbon in the samples was also observed, which is consistent with the concentration of MWCNT.

Table 2.
Elemental composition of pure BaTiO3 and BaTiO3/MWCNT nanocomposites.
The energy-dispersive X-ray spectroscopy elemental mapping images (Figure S1) reveal the distribution of carbon in the BaTiO3/MWCNT nanocomposite samples with varying MWCNT contents. The pure BaTiO3 sample (Figure S1a) exhibits a low carbon content, which is consistent with the elemental composition data in Table 2. As the MWCNT content increases in the BaTiO3-3%MWCNT and BaTiO3-6%MWCNT samples (Figure S1b,c), the carbon content becomes well-distributed, with a higher frequency of carbon signals. However, the BaTiO3-10%MWCNT sample (Figure S1d) displays visible accumulations of carbon in certain regions, indicating MWCNT agglomeration. These carbon-rich areas suggest that, at higher MWCNT contents, the nanotubes tend to cluster together, potentially impacting the overall performance of the nanocomposite.
3.8. Solar Cell Evaluation
The incorporation of varying multi-walled carbon nanotube (MWCNT) weight fractions into BaTiO3-based dye-sensitized solar cells (DSSCs) under simulated AM1.5G illumination yields valuable photo-response signatures outlining the complex interplay between nanoscale interface engineering and bulk properties in modulating photoconversion. The introduction of MWCNTs is observed to considerably enhance power conversion efficiency (Figure 8a, Table 3), peaking at 4.044% for an optimal loading of 6 wt.%—nearly 6 times higher than pure BaTiO3 (0.693%). This stems from simultaneous improvements in the open-circuit voltage (VOC) and short-circuit current (JSC) enabled by favorable band structure alterations and accelerated charge kinetics, respectively [76,77]. However, further increasing MWCNT content to 10 wt.% reverses output metrics due to concentration-dependent limitations. Excess nanotubes impose a trapping effect via agglomerates, which agrees with the results obtained in scanning electron microscopy that hinder transport while also modifying interface recombination dynamics [78,79,80]. The recent reports also suggest that extensive carbon addition could downshift conduction bands in the nanocomposite matrix, marginally reducing VOC. Besides impeded light-harvesting due to competitive dye absorption, deteriorated JSC likely arises from additional recombination pathways stemming from non-optimal MWCNT dispersion [81].
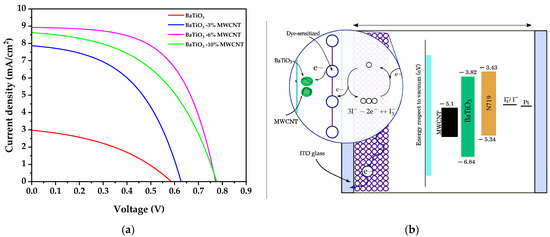
Figure 8.
(a) Current density–voltage curves of pure BaTiO3 and BaTiO3/MWCNT nanocomposites and (b) schematic energy level diagram.

Table 3.
Photovoltaic parameters of pure BaTiO3 and BaTiO3/MWCNT nanocomposites.
The fill factor (FF) is another critical parameter that influences the overall performance of DSSCs. As shown in Table 3, the FF values of the BaTiO3/MWCNT nanocomposite-based DSSCs are generally higher than that of the pure BaTiO3-based device. The BaTiO3-6%MWCNT nanocomposite exhibits the highest FF of 0.586, indicating an improved charge-collection efficiency and reduced recombination losses compared to the other samples. This enhancement in FF can be attributed to the optimal MWCNT loading, which facilitates efficient charge transport and suppresses recombination at the electrode–electrolyte interface. However, at higher MWCNT concentrations (10 wt.%), the FF decreases, likely due to the agglomeration effects and increased interfacial resistance, as discussed earlier.
To better understand the charge transfer processes in the BaTiO3/MWCNT nanocomposite-based DSSCs, it is essential to consider the energy levels of all the components, including the iodide/tri-iodide electrolyte and the Pt counter electrode. As shown in Figure 8b, the conduction band (CB) and valence band (VB) of BaTiO3 are located at −3.82 eV and −6.84 eV, respectively, while the work function of MWCNTs is approximately −5.1 eV. The N719 dye has a HOMO level of −5.34 eV and a LUMO level of −3.43 eV. Upon photoexcitation, electrons from the LUMO of the N719 dye are injected into the CB of BaTiO3, while the MWCNTs act as efficient electron transport pathways, facilitating the collection of electrons at the photoanode. The oxidized dye molecules are regenerated by the iodide/tri-iodide electrolyte, which has a redox potential of approximately −4.9 eV. The electrons collected at the Pt counter electrode, with a work function of −5.1 eV, reduce the tri-iodide ions back to iodide, completing the regenerative cycle. The well-aligned energy levels of BaTiO3 and MWCNTs with respect to the N719 dye, along with the properly positioned redox potential of the electrolyte and the work function of the Pt counter electrode, enable effective charge separation, transport, and regeneration, contributing to the enhanced photovoltaic performance of the nanocomposite-based DSSCs.
The obtained results show a significant improvement compared to the previous research displayed in Table 3.
4. Conclusions
Incorporating multi-walled carbon nanotubes (MWCNTs) into BaTiO3-based dye-sensitized solar cells is demonstrated to markedly boost power conversion efficiency by up to 6 times (4.044%) over the pure BaTiO3 sample (0.693%) under AM1.5G conditions. Fundamentally, it originated from the simultaneous enhancements in open-circuit voltage and short-circuit current density enabled via tailored band structure and accelerated charge kinetics, respectively, upon the optimal MWCNT addition. A loading of 6 wt.% of MWCNTs introduced donor states to favorably shift band edges for increasing voltage while also providing percolation pathways to improve the charge collection and photocurrent. However, the higher nanotube fractions prompt emerging limitations that reverse performance metrics. The concentration-dependent trade-offs that dominated at elevated MWCNT incorporation manifested as transport impediments from agglomeration along with worsened recombination through interfacial trap states. An extensive MWCNT filling could additionally downshift the conduction bands, marginally lowering photovoltage via the density of states modulation while also introducing competitive light absorption that lowers photocurrents. A precise balance between augmented interfacial area for transfer events and preserved percolation for efficient collection is imperative to maximize efficiency. The results outline design heuristics for organic–inorganic lead-free ferroelectric solar platforms that synergistically coalesce the intrinsic properties of the constituents towards addressing critical performance bottlenecks.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst14060489/s1, Figure S1: EDS Elemental mapping of (a) BaTiO3, (b) BaTiO3-3% MWCNT, (c) BaTiO3-6% MWCNT and (b) BaTiO3-10% MWCNT.
Author Contributions
Conceptualization, C.A.P.B., B.Y.C.O. and E.J.S.S.; methodology, C.A.P.B., B.Y.C.O. and E.J.S.S.; software, E.J.S.S.; validation, C.A.P.B. and E.J.S.S.; formal analysis, B.Y.C.O. and E.J.S.S.; investigation, C.A.P.B., B.Y.C.O. and E.J.S.S.; resources, J.A.C.G., A.B.Q.C., J.P.M.S. and F.G.G.; data curation, B.Y.C.O. and R.V.M.; writing—original draft preparation, E.J.S.S., B.Y.C.O., A.B.Q.C. and H.A.T.M.; writing—review and editing, E.J.S.S. and R.V.M.; visualization, B.Y.C.O. and E.J.S.S.; supervision, C.A.P.B. and E.J.S.S.; project administration, C.A.P.B. and J.A.C.G.; funding acquisition, C.A.P.B., and J.A.C.G.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universidad Nacional Jorge Basadre Grohmann through “Fondos del canon sobrecanon y regalias mineras”, approved by rectoral resolution N° 4516-2018-UN/JBG. With the project “Desarrollo de películas delgadas de nanotubos de carbono /TiO2 para mejorar la eficiencia de celdas solares sensibilizadas por colorantes (DSSC)” and the Project “Determinación de huellas digitales ópticas, de materiales sólidos, líquidos y orgánicos mediante espectroscopia visible e infrarroja” approved by rectoral resolution N° 5854-2019-UN/JBG.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We are grateful to Universidad Nacional Jorge Basadre Grohman and the projects: “Determinación de huellas digitales ópticas, de materiales sólidos, líquidos y orgánicos mediante espectroscopia visible e infrarroja”, “Estudio de la aplicación de la nanotecnología para la purificación del agua con arsénico en la región Tacna”, “Estudio de materiales ferroeléctricos (BiFeO3 y Bi2FeCrO6) y su aplicación en celdas solares” and “Generación fotocatalítica y foto-electrocatalítica de hidrógeno en la región Tacna empleando nanopartículas de NiTiO3 puras y dopadas” approved by rectoral resolutions N° 5854-2019-UN/JBG, N° 3780-2014-UN/JBG, N° 4516-2018-UN/JBG and N° 9155-2021-UN/JBG, respectively, for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- IEA. World Energy Outlook 2022; IEA: Paris, France, 2022; Available online: https://www.iea.org/reports/world-energy-outlook-2022 (accessed on 8 December 2023).
- Mason, L.R.; Melton, C.C.; Gray, D.; Swallow, A.L. Climate Change, Social Work, and the Transition Away from Fossil Fuels: A Scoping Review. Sustainability 2022, 14, 7086. [Google Scholar] [CrossRef]
- Stepanov, I.A.; Makarov, I.A. Greenhouse gas emissions regulation in fossil fuels exporting countries: Opportunities and challenges for Russia. Post-Communist Econ. 2022, 34, 916–943. [Google Scholar] [CrossRef]
- Perera, F.; Nadeau, K. Climate Change, Fossil-Fuel Pollution, and Children’s Health. N. Engl. J. Med. 2022, 386, 2303–2314. [Google Scholar] [CrossRef] [PubMed]
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res. Int. 2022, 29, 42539–42559. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.H.; Ghazvini, M.; Alhuyi Nazari, M.; Ahmadi, M.A.; Pourfayaz, F.; Lorenzini, G.; Ming, T. Renewable energy harvesting with the application of nanotechnology: A review. Int. J. Energy Res. 2019, 43, 1387–1410. [Google Scholar] [CrossRef]
- Handayani, K.; Krozer, Y.; Filatova, T. From fossil fuels to renewables: An analysis of long-term scenarios considering technological learning. Energy Policy 2019, 127, 134–146. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A. Renewable energy and climate change. Renew. Sustain. Energy Rev. 2022, 158, 112111. [Google Scholar] [CrossRef]
- Aljibory, M.W.; Hashim, H.T.; Abbas, W.N. A Review of Solar Energy Harvesting Utilising a Photovoltaic–Thermoelectric Integrated Hybrid System. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1067, 12115. [Google Scholar] [CrossRef]
- Hao, D.; Qi, L.; Tairab, A.M.; Ahmed, A.; Azam, A.; Luo, D.; Pan, Y.; Zhang, Z.; Yan, J. Solar energy harvesting technologies for PV self-powered applications: A comprehensive review. Renew. Energy 2022, 188, 678–697. [Google Scholar] [CrossRef]
- Lau, D.; Song, N.; Hall, C.; Jiang, Y.; Lim, S.; Perez-Wurfl, I.; Ouyang, Z.; Lennon, A. Hybrid solar energy harvesting and storage devices: The promises and challenges. Mater. Today Energy 2019, 13, 22–44. [Google Scholar] [CrossRef]
- Nayak, P.K.; Mahesh, S.; Snaith, H.J.; Cahen, D. Photovoltaic solar cell technologies: Analysing the state of the art. Nat. Rev. Mater. 2019, 4, 269–285. [Google Scholar] [CrossRef]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Luo, X.; Wu, T.; Wang, Y.; Lin, X.; Su, H.; Han, Q.; Han, L. Progress of all-perovskite tandem solar cells: The role of narrow-bandgap absorbers. Sci. China Chem. 2021, 64, 218–227. [Google Scholar] [CrossRef]
- Zatirostami, A. SnO2− based DSSC with SnSe counter electrode prepared by sputtering and selenization of Sn: Effect of selenization temperature. Mater. Sci. Semicond. Process. 2021, 135, 106044. [Google Scholar] [CrossRef]
- Abdulah, H.I.; Rheima, A.M.; Hussain, D.H.; Abed, H.J. of Fe2O3Nanoparticles by Photolysis Method for Novel Dye-sensitized Solar Cell. J. Adv. Sci. Nanotechnol. 2022, 1, 1–8. [Google Scholar] [CrossRef]
- Ursu, D.; Vajda, M.; Miclau, M. Investigation of the p-type dye-sensitized solar cell based on full Cu2O electrodes. J. Alloys Compd. 2019, 802, 86–92. [Google Scholar] [CrossRef]
- Tiburcio, J.; Sacari, E.; Chacaltana, J.; Medina, J.; Gamarra, F.; Polo, C.; Mamani, E.; Quispe, A. Influence of Cr Doping on Structural, Optical, and Photovoltaic Properties of BiFeO3 Synthesized by Sol-Gel Method. Energies 2023, 16, 786. [Google Scholar] [CrossRef]
- Lilge, T.S.; Ramires das Neves Stigger, A.; Fernandes, C.D.; Gularte, L.T.; Raubach, C.W.; Da Silva Cava, S.; Gomes Jardim, P.L.; Giroldo Valerio, M.E.; Moreira, M.L. Increase of Voc using heterojunctions of BaTiO3 without sensitization. Ceram. Int. 2020, 46, 4907–4913. [Google Scholar] [CrossRef]
- Bhojanaa, K.B.; Soundarya Mary, A.; Shalini Devi, K.S.; Pavithra, N.; Pandikumar, A. Account of Structural, Theoretical, and Photovoltaic Properties of ABO3 Oxide Perovskites Photoanode-Based Dye-Sensitized Solar Cells. Sol. RRL 2022, 6, 2100792. [Google Scholar] [CrossRef]
- Cui, Y.; Briscoe, J.; Dunn, S. Effect of Ferroelectricity on Solar-Light-Driven Photocatalytic Activity of BaTiO3—Influence on the Carrier Separation and Stern Layer Formation. Chem. Mater. 2013, 25, 4215–4223. [Google Scholar] [CrossRef]
- Zhong, M.; Shi, J.; Zhang, W.; Han, H.; Li, C. Charge recombination reduction in dye-sensitized solar cells by depositing ultrapure TiO2 nanoparticles on “inert” BaTiO3 films. Mater. Sci. Eng. B 2011, 176, 1115–1122. [Google Scholar] [CrossRef]
- Clabel, H.J.L.; Rivera, V.; Nogueira, I.C.; Leite, E.R.; Pereira-da-Silva, M.A.; Li, M.S.; Marega, E. Effects of defects, grain size, and thickness on the optical properties of BaTiO3 thin films. J. Lumin. 2017, 192, 969–974. [Google Scholar] [CrossRef]
- Thomas, R.; Dube, D.C.; Kamalasanan, M.N.; Chandra, S. Optical and electrical properties of BaTiO3 thin films prepared by chemical solution deposition. Thin Solid. Film. 1999, 346, 212–225. [Google Scholar] [CrossRef]
- Ramakanth, S.; James Raju, K.C. Band gap narrowing in BaTiO3 nanoparticles facilitated by multiple mechanisms. J. Appl. Phys. 2014, 115, 173507. [Google Scholar] [CrossRef]
- Yang, F.; Yang, L.; Ai, C.; Xie, P.; Lin, S.; Wang, C.-Z.; Lu, X. Tailoring Bandgap of Perovskite BaTiO3 by Transition Metals Co-Doping for Visible-Light Photoelectrical Applications: A First-Principles Study. Nanomaterial 2018, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-T.; Lu, F.-H. Corrosion resistance of BaTiO3 films prepared by plasma electrolytic oxidation. Surf. Coat. Technol. 2003, 166, 31–36. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Cao, X.; Wu, S.; Liu, C.; Li, G.; Jiang, W.; Wang, H.; Wang, N.; Ding, W. Preparation and characterization of TiO2 nanoparticles by two different precipitation methods. Ceram. Int. 2020, 46, 15333–15341. [Google Scholar] [CrossRef]
- Gupta, T.; Samriti, C.J.; Prakash, J. Hydrothermal synthesis of TiO2 nanorods: Formation chemistry, growth mechanism, and tailoring of surface properties for photocatalytic activities. Mater. Today Chem. 2021, 20, 100428. [Google Scholar] [CrossRef]
- Gamarra, F.; Medina, J.; Lanchipa, W.; Tamayo, R.; Sacari, E. Structural, Optical, and Arsenic Removal Properties of Sol-Gel Synthesized Fe-Doped TiO2 Nanoparticles. Nanomaterial 2022, 12, 3402. [Google Scholar] [CrossRef]
- Shakeel Ahmad, M.; Pandey, A.K.; Abd Rahim, N. Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications. A review. Renew. Sustain. Energy Rev. 2017, 77, 89–108. [Google Scholar] [CrossRef]
- Mujtahid, F.; Gareso, P.L.; Armynah, B.; Tahir, D. Review effect of various types of dyes and structures in supporting performance of dye-sensitized solar cell TiO2-based nanocomposites. Int. J. Energy Res. 2022, 46, 726–742. [Google Scholar] [CrossRef]
- Jun, H.K.; Careem, M.A.; Arof, A.K. Quantum dot-sensitized solar cells—Perspective and recent developments: A review of Cd chalcogenide quantum dots as sensitizers. Renew. Sustain. Energy Rev. 2013, 22, 148–167. [Google Scholar] [CrossRef]
- Kouhnavard, M.; Ikeda, S.; Ludin, N.A.; Ahmad Khairudin, N.B.; Ghaffari, B.V.; Mat-Teridi, M.A.; Ibrahim, M.A.; Sepeai, S.; Sopian, K. A review of semiconductor materials as sensitizers for quantum dot-sensitized solar cells. Renew. Sustain. Energy Rev. 2014, 37, 397–407. [Google Scholar] [CrossRef]
- Brennan, L.J.; Byrne, M.T.; Bari, M.; Gun’ko, Y.K. Carbon Nanomaterials for Dye-Sensitized Solar Cell Applications: A Bright Future. Adv. Energy Mater. 2011, 1, 472–485. [Google Scholar] [CrossRef]
- Batmunkh, M.; Biggs, M.J.; Shapter, J.G. Carbonaceous Dye-Sensitized Solar Cell Photoelectrodes. Adv. Sci. 2015, 2, 1400025. [Google Scholar] [CrossRef] [PubMed]
- Batmunkh, M.; Biggs, M.J.; Shapter, J.G. Carbon Nanotubes for Dye-Sensitized Solar Cells. Small 2015, 11, 2963–2989. [Google Scholar] [CrossRef] [PubMed]
- Sugathan, V.; John, E.; Sudhakar, K. Recent improvements in dye sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2015, 52, 54–64. [Google Scholar] [CrossRef]
- Najm, A.S.; Alwash, S.A.; Sulaiman, N.H.; Chowdhury, M.S.; Techato, K. N719 dye as a sensitizer for dye-sensitized solar cells (DSSCs): A review of its functions and certain rudimentary principles. Env. Prog. Sustain. Energy 2023, 42, e13955. [Google Scholar] [CrossRef]
- Uk Lee, S.; Seok Choi, W.; Hong, B. A comparative study of dye-sensitized solar cells added carbon nanotubes to electrolyte and counter electrodes. Sol. Energy Mater. Sol. Cells 2010, 94, 680–685. [Google Scholar] [CrossRef]
- Mehmood, U.; Hussein, I.A.; Harrabi, K.; Mekki, M.B.; Ahmed, S.; Tabet, N. Hybrid TiO2–multiwall carbon nanotube (MWCNTs) photoanodes for efficient dye sensitized solar cells (DSSCs). Sol. Energy Mater. Sol. Cells 2015, 140, 174–179. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Yao, D.S.; Song, C.B.; Zhu, L.; Song, J.; Gu, X.Q.; Qiang, Y.H. CNT–G–TiO2 layer as a bridge linking TiO2 nanotube arrays and substrates for efficient dye-sensitized solar cells. RSC Adv. 2015, 5, 43805–43809. [Google Scholar] [CrossRef]
- Anjidani, M.; Milani Moghaddam, H.; Ojani, R. Binder-free MWCNT/TiO2 multilayer nanocomposite as an efficient thin interfacial layer for photoanode of dye sensitized solar cell. Mater. Sci. Semicond. Process. 2017, 71, 20–28. [Google Scholar] [CrossRef]
- Thanki, A.A.; Goyal, R.K. Study on effect of cubic- and tetragonal phased BaTiO3 on the electrical and thermal properties of polymeric nanocomposites. Mater. Chem. Phys. 2016, 183, 447–456. [Google Scholar] [CrossRef]
- Saravanan, A.; Prasad, K.; Gokulakrishnan, N.; Kalaivani, R.; Somanathan, T. Efficiency of Transition Metals in Combustion Catalyst for High Yield Helical Multi-Walled Carbon Nanotubes. Adv. Sci. Eng. Med. 2014, 6, 809–813. [Google Scholar] [CrossRef]
- Nie, P.; Min, C.; Song, H.-J.; Chen, X.; Zhang, Z.; Zhao, K. Preparation and Tribological Properties of Polyimide/Carboxyl-Functionalized Multi-walled Carbon Nanotube Nanocomposite Films Under Seawater Lubrication. Tribol. Lett. 2015, 58, 7. [Google Scholar] [CrossRef]
- Hayashi, H.; Nakamura, T.; Ebina, T. In-situ Raman spectroscopy of BaTiO3 particles for tetragonal–cubic transformation. J. Phys. Chem. Solids 2013, 74, 957–962. [Google Scholar] [CrossRef]
- Pitiphattharabun, S.; Sato, N.; Panomsuwan, G.; Jongprateep, O. Electrocatalytic Properties of a BaTiO3/MWCNT Composite for Citric Acid Detection. Catalysts 2022, 12, 49. [Google Scholar] [CrossRef]
- Dang, N.V.; Thanh, T.D.; Hong, L.V.; Lam, V.D.; Phan, T.-L. Structural, optical and magnetic properties of polycrystalline BaTi1−xFexO3 ceramics. J. Appl. Phys. 2011, 110, 43914. [Google Scholar] [CrossRef]
- Kholodkova, A.A.; Danchevskaya, M.N.; Ivakin, Y.D.; Muravieva, G.P.; Tyablikov, A.S. Crystalline barium titanate synthesized in sub- and supercritical water. J. Supercrit. Fluids 2016, 117, 194–202. [Google Scholar] [CrossRef]
- Colson, T.A.; Spencer, M.J.; Yarovsky, I. A DFT study of the perovskite and hexagonal phases of BaTiO3. Comput. Mater. Sci. 2005, 34, 157–165. [Google Scholar] [CrossRef]
- Buixaderas, E.; Kamba, S.; Petzelt, J.; Wada, M.; Yamanaka, A.; Inoue, K. Study of Phase Transitions in Hexagonal BaTiO3 by means of Far-infrared Spectroscopy. J.-Korean Phys. Soc. 1998, 32, S578–S580. [Google Scholar]
- Gajović, A.; Pleština, J.V.; Žagar, K.; Plodinec, M.; Šturm, S.; Čeh, M. Temperature-dependent Raman spectroscopy of BaTiO3 nanorods synthesized by using a template-assisted sol-gel procedure. J. Raman Spectrosc. 2013, 44, 412–420. [Google Scholar] [CrossRef]
- Aoyagi, S.; Kuroiwa, Y.; Sawada, A.; Kawaji, H.; Atake, T. Size effect on crystal structure and chemical bonding nature in BaTiO3 nanopowder. J. Therm. Anal. Calorim. 2005, 81, 627–630. [Google Scholar] [CrossRef]
- Hoshina, T. Size effect of barium titanate: Fine particles and ceramics. J. Ceram. Soc. Jpn. 2013, 121, 156–161. [Google Scholar] [CrossRef]
- Frey, M.H.; Payne, D.A. Grain-size effect on structure and phase transformations for barium titanate. Phys. Rev. B Condens. Matter 1996, 54, 3158–3168. [Google Scholar] [CrossRef]
- Kappadan, S.; Gebreab, T.W.; Thomas, S.; Kalarikkal, N. Tetragonal BaTiO3 nanoparticles: An efficient photocatalyst for the degradation of organic pollutants. Mater. Sci. Semicond. Process. 2016, 51, 42–47. [Google Scholar] [CrossRef]
- Sreedhar, B.; Satya Vani, C.; Keerthi Devi, D.; VBasaveswara Rao, M.; Rambabu, C. Shape Controlled Synthesis of Barium Carbonate Microclusters and Nanocrystallites using Natural Polysachharide—Gum Acacia. Materials 2012, 2, 5–13. [Google Scholar] [CrossRef]
- Singh, M.; Yadav, B.C.; Ranjan, A.; Kaur, M.; Gupta, S.K. Synthesis and characterization of perovskite barium titanate thin film and its application as LPG sensor. Sens. Actuators B Chem. 2017, 241, 1170–1178. [Google Scholar] [CrossRef]
- Khan, T.M.; Zakria, M.; Shakoor, R.I.; Hussain, S. Composite-hydroxide-mediated approach an effective synthesis route for BaTiO3 functional nanomaterials. Appl. Phys. A 2016, 122, 274. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Khazani, Y.; Gozali Balkanloo, P.; Enayati, M. Poly(diallyldimethylammonium chloride)-grafted carboxylated-MWCNT as an additive in the polyethersulfone membrane. Polym. Bull. 2021, 78, 4313–4332. [Google Scholar] [CrossRef]
- Changshi, L.; Feng, L. Natural path for more precise determination of band gap by optical spectra. Opt. Commun. 2012, 285, 2868–2873. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Sangiorgi, N.; Aversa, L.; Tatti, R.; Verucchi, R.; Sanson, A. Spectrophotometric method for optical band gap and electronic transitions determination of semiconductor materials. Opt. Mater. 2017, 64, 18–25. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Azarhoosh, P.; Alonso, M.I.; Campoy-Quiles, M.; Weber, O.J.; Yao, J.; Bryant, D.; Weller, M.T.; Nelson, J.; Walsh, A.; et al. Experimental and theoretical optical properties of methylammonium lead halide perovskites. Nanoscale 2016, 8, 6317–6327. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-J.; Zhang, R.-J.; Zheng, H.; Li, D.-H.; Wei, W.; Chen, X.; Sun, Y.; Wei, Y.-F.; Lu, H.-L.; Dai, N.; et al. Optical Constants and Band Gap Evolution with Phase Transition in Sub-20-nm-Thick TiO2 Films Prepared by ALD. Nanoscale Res. Lett. 2017, 12, 243. [Google Scholar] [CrossRef] [PubMed]
- El-Hagary, M.; Shaaban, E.R.; Moustafa, S.H.; Gad, G. The particle size-dependent optical band gap and magnetic properties of Fe-doped CeO2 nanoparticles. Solid State Sci. 2019, 91, 15–22. [Google Scholar] [CrossRef]
- Madhan, K.; Murugaraj, R. Structural and electron paramagnetic resonance of Fe3+ Sm3+ co-doped BaTiO3. In Proceedings of the DAE Solid State Physics Symposium 2019, Jodhpur, India, 18–22 December 2019; AIP Publishing: Melville, NY, USA, 2020; p. 30495. [Google Scholar]
- Rastogi, M.; Kushwaha, H.S.; Vaish, R. Highly efficient visible light mediated azo dye degradation through barium titanate decorated reduced graphene oxide sheets. Electron. Mater. Lett. 2016, 12, 281–289. [Google Scholar] [CrossRef]
- Nawanil, C.; Panprom, P.; Khaosa Ard, K.; Makcharoen, W.; Vittayakorn, N. Effect of surface treatment on electrical properties of barium titanate/carbon nanotube/polydimethylsiloxane nanocomposites. AIP Conf. Proc. 2018, 2010, 20029. [Google Scholar]
- Clabel, H.J.L.; Nicolodelli, G.; Lozano, C.G.; Rivera, V.A.G.; Ferreira, S.O.; Pinto, A.H.; Li, M.S.; Marega, E. The extrinsic nature of double broadband photoluminescence from the BaTiO3 perovskite: Generation of white light emitters. Phys. Chem. Chem. Phys. 2021, 23, 18694–18706. [Google Scholar] [CrossRef]
- Woong Lee, K.; Siva Kumar, K.; Heo, G.; Seong, M.-J.; Yoon, J.-W. Characterization of hollow BaTiO3 nanofibers and intense visible photoluminescence. J. Appl. Phys. 2013, 114, 134303. [Google Scholar] [CrossRef]
- Jiang, L.-C.; Zhang, W.-D. Charge transfer properties and photoelectrocatalytic activity of TiO2/MWCNT hybrid. Electrochim. Acta 2010, 56, 406–411. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Lee, J.Y.; Bajaj, H.C.; Jo, W.-K.; Tayade, R.J. Synthesis of multiwall carbon nanotubes/TiO2 nanotube composites with enhanced photocatalytic decomposition efficiency. Catal. Today 2017, 282, 13–23. [Google Scholar] [CrossRef]
- Ni, Q.-Q.; Zhu, Y.-F.; Yu, L.-J.; Fu, Y.-Q. One-dimensional carbon nanotube@ barium titanate@ polyaniline multiheterostructures for microwave absorbing application. Nanoscale Res. Lett. 2015, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, U.; Ishfaq, A.; Sufyan, M. Nanocomposites of Multi-walled Carbon Nanotubes and Titanium dioxide (MWCNTs/TiO2) as affective counter electrode materials for Platinum-free Dye-Sensitized Solar Cells (DSSCs). Sol. Energy 2021, 220, 949–952. [Google Scholar] [CrossRef]
- Song, C.B.; Qiang, Y.H.; Zhao, Y.L.; Gu, X.Q.; Song, D.M.; Zhu, L. Adhesion of TiO2 nanotube arrays on transparent conducting substrates using CNT–TiO2 composite pastes. Appl. Surf. Sci. 2014, 305, 792–796. [Google Scholar] [CrossRef]
- Hu, J.; Xie, Y.; Bai, T.; Zhang, C.; Wang, J. A novel triple-layer zinc oxide/carbon nanotube architecture for dye-sensitized solar cells with excellent power conversion efficiency. J. Power Sources 2015, 286, 175–181. [Google Scholar] [CrossRef]
- Wei, Y.; Du, H.; Kong, J.; Lu, X.; Ke, L.; Sun, X.W. Multi-walled Carbon Nanotubes Modified ZnO Nanorods: A Photoanode for Photoelectrochemical Cell. Electrochim. Acta 2014, 143, 188–195. [Google Scholar] [CrossRef]
- Yue, G.; Wu, W.; Liu, X.; Zheng, H. Enhanced photovoltaic performance of dye-sensitized solar cells based on a promising hybrid counter electrode of CoSe2/MWCNTs. Sol. Energy 2018, 167, 137–146. [Google Scholar] [CrossRef]
- Kiran, S.; Naveen Kumar, S.K.; Yogananda, K.C.; Rangappa, D. Optimization of TiO2/MWCNT composites for efficient dye sensitized solar cells. J. Mater. Sci. Mater. Electron. 2018, 29, 12681–12689. [Google Scholar] [CrossRef]
- Sadikin, S.N.; Rahman, M.Y.; Umar, A.A. TiO2-BaTiO3 Composite Films as Photoanode for Dye Sensitized Solar Cell: Effect of BaTiO3 Content//TiO2-BaTiO3 Composite Films as Photoanode for Dye Sensitized Solar Cell: Effect of BaTiO3 Content. J. New Mater. Electrochem. Syst. 2017, 20, 109–113. [Google Scholar] [CrossRef]
- Rajamanickam, N.; Jayakumar, K.; Ramachandran, K. Influence of Mn ion on flower shaped perovskite BaTiO3 nanostructures based dye-sensitized solar cell. Nano-Struct. Nano-Objects 2017, 9, 19–25. [Google Scholar] [CrossRef]
- Sharma, S.; Tomar, M.; Puri, N.K.; Gupta, V. BiFeO3 /BaTiO3 Multilayer Structures for Solar Energy Harvesting Application. Energy Harvest. Syst. 2016, 3, 237–243. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Zhang, W.; Yang, S. Effects of solvents on the band energetics of nanostructured BaTiO3 electrodes for dye sensitised solar cells. Int. J. Nanomanuf. 2016, 12, 216. [Google Scholar] [CrossRef]
- Van-Pham, D.-T.; Phat, V.V.; Hoa, N.T.Q.; Ngoc, N.H.; Thao Ngan, D.T.; Don, T.N.; Mai, N.T.N.; Quyen, T.T.B.; van Thien, D.H. Fabrication of electrospun BaTiO3/chitosan/PVA nanofibers and application for dye-sensitized solar cells. IOP Conf. Ser. Earth Environ. Sci. 2021, 947, 12017. [Google Scholar] [CrossRef]
- Peter, I.J.; Vignesh, G.; Vijaya, S.; Anandan, S.; Ramachandran, K.; Nithiananthi, P. Enhancing the power conversion efficiency of SrTiO3/CdS/Bi2S3quantum dot based solar cell using phosphor. Appl. Surf. Sci. 2019, 494, 551–560. [Google Scholar] [CrossRef]
- Aravinthkumar, K.; Praveen, E.; Jacquline Regina Mary, A.; Raja Mohan, C. Investigation on SrTiO3 nanoparticles as a photocatalyst for enhanced photocatalytic activity and photovoltaic applications. Inorg. Chem. Commun. 2022, 140, 109451. [Google Scholar] [CrossRef]
- Okamoto, Y.; Suzuki, Y. Perovskite-type SrTiO3, CaTiO3 and BaTiO3 porous film electrodes for dye-sensitized solar cells. J. Ceram. Soc. Jpn. 2014, 122, 728–731. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).