Synthesis and Mechanism Study of Carbon Nanowires, Carbon Nanotubes, and Carbon Pompons on Single-Crystal Diamonds
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. SEM Observations
3.2. TEM Observations
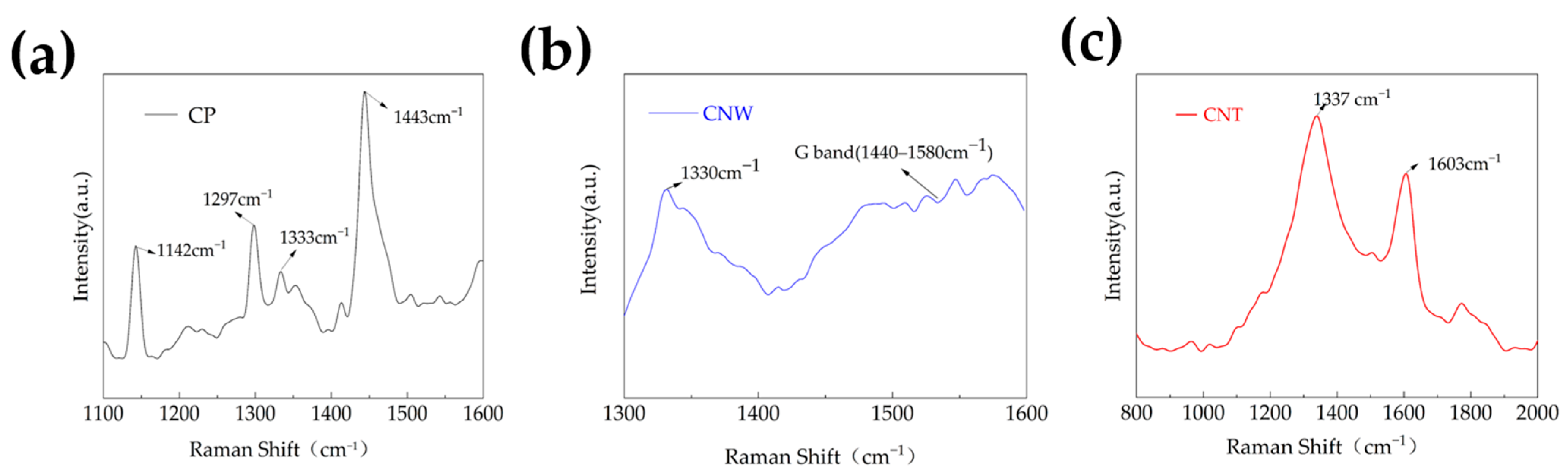
3.3. Raman Observations
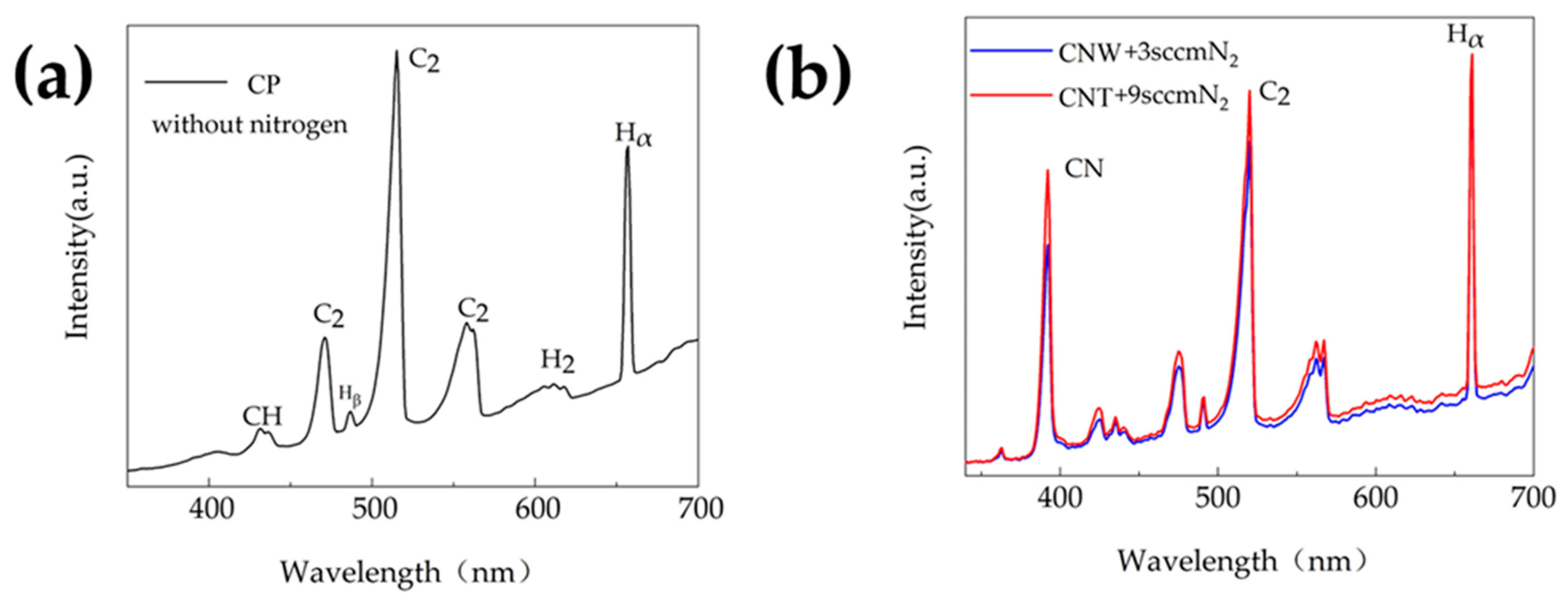
3.4. OES Observations
3.5. Mechanism Research
4. Conclusions
- (1)
- Carbon nanomaterials with different shapes were grown on single-crystal diamonds. CPs with an average diameter of 20 μm were observed inside the rectangular pits, and CNPs were grown with an average diameter of 10 μm outside the pits. The key factors affecting their growth were the temperature and position. The average diameter of the CNWs was 80 nm, and that of the CNT was 400 nm, with larger diameters formed owing to intense etching by hydrogen. The formation of CNWs was observed at a nitrogen flow rate of 3 sccm, whereas a flow rate of 9 sccm favored the deposition of CNTs.
- (2)
- According to the TEM image, many small carbon nanoparticles and lines were attached to the CP surface, which were formed via H plasma etching. In addition, black nodular defects were observed inside the CNWs.
- (3)
- The Raman spectrum of CP showed the presence of defects and stray peaks, which confirmed that the formation of CP is related to defects. In the spectrum of CNWs, a broadened graphite peak was observed. The two main peaks in the spectrum of CNT were blue-shifted due to the presence of stresses.
- (4)
- By OES observation of the plasma in the experiments, the intensity of the C2 group was lower than that of the Hα group during the deposition of CNWs and CNTs. However, it was higher than that of the Hα group in the CP deposition, suggesting that the C2 group could indicate the morphology of the products.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Xie, J.; Ma, H.; Zhang, X.; Pan, Y.; Lv, J.; Ge, H.; Ren, N.; Su, H.; Xie, X.; et al. From graphite to graphene oxide and graphene oxide quantum dots. Small 2017, 13, 1601001. [Google Scholar] [CrossRef] [PubMed]
- Ghavamian, A.; Banerjee, S.; Rahmandoust, M.; Öchsner, A. On the vibrational behavior of the conventional and hetero-junction carbon nanotubes. Mater. Today Commun. 2024, 39, 108656. [Google Scholar] [CrossRef]
- Huang, H.-D.; Fan, J.-W.; Liu, H.-Y.; Su, B.; Ha, X.-Y.; Guo, Z.-Y.; Ren, Y.-F. High-performance carbon nanofiber conductive films induced by titanium carbide. J. Mater. Chem. C 2024, 12, 5122–5137. [Google Scholar] [CrossRef]
- Liu, Z.-G.; He, X.-X.; Zhao, J.-H.; Xu, C.-M.; Qiao, Y.; Li, L.; Chou, S.-L. Carbon nanosphere synthesis and applications for rechargeable batteries. Chem. Commun. 2023, 59, 4257–4273. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Kitta, M.; Hong, J.; Suenaga, K.; Tsumori, N.; Liu, Z.; Xu, Q. Fabrication of a spherical superstructure of carbon nanorods. Adv. Mater. 2019, 31, 1900440. [Google Scholar] [CrossRef] [PubMed]
- Qasemnazhand, M.; Khoeini, F.; Marsusi, F. Fullerene, fullerane and the fulleryne: A comparative thermodynamic study for a new member of the carbon cage family. Results Phys. 2022, 43, 106066. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, W.-K.; Yang, Y.-Q.; Gao, Z.-Y.; Wang, J.-W.; Wei, X.-X. Preparation and microwave absorption properties of magnetic carbon nano-onion matrix composites. Carbon 2019, 150, 551. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Carbon-Based Nanomaterials. Int. J. Mol. Sci. 2021, 22, 7726. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, Z.; Yang, J.; Wang, C.; Sun, B.; Wang, G. Nanoengineering of Advanced Carbon Materials for Sodium-Ion Batteries. Small 2021, 17, 2007431. [Google Scholar] [CrossRef]
- Sibera, D.; Narkiewicz, U.; Kapica, J.; Serafin, J.; Michalkiewicz, B.; Wróbel, R.J.; Morawski, A.W. Preparation and characterisation of carbon spheres for carbon dioxide capture. J. Porous Mater. 2019, 26, 19–27. [Google Scholar] [CrossRef]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of Carbon Nanotubes for Biomedical Applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Long, Z.; Jiang, L.; Liu, Z.; Liu, C.; Chen, Z. 3D printed crack-free SiOC(Fe) structures with pyrolysis-induced carbon nanowires for enhanced wave absorption performance. Ceram. Int. 2023, 49, 33205–33213. [Google Scholar] [CrossRef]
- Inyushkin, A.V.; Taldenkov, A.N.; Ralchenko, V.G.; Bolshakov, A.P.; Koliadin, A.V.; Katrusha, A.N. Thermal conductivity of high purity synthetic single crystal diamonds. Phys. Rev. B 2018, 97, 144305. [Google Scholar] [CrossRef]
- Volpe, P.-N.; Muret, P.; Pernot, J.; Omnès, F.; Teraji, T.; Jomard, F.; Planson, D.; Brosselard, P.; Dheilly, N.; Vergne, B.; et al. High breakdown voltage Schottky diodes synthesized on p-type CVD diamond layer. Phys. Status Solidi (A) 2010, 207, 2088–2092. [Google Scholar] [CrossRef]
- Tordjman, M. Diamond electronics with high carrier mobilities. Nat. Electron. 2022, 5, 21–22. [Google Scholar] [CrossRef]
- Wildi, T.; Kiss, M.; Quack, N. Diffractive optical elements in single crystal diamond. Opt. Lett. 2020, 45, 3458–3461. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Ali, F.; Yang, B.; Abbas, A. A comprehensive account of biomedical applications of CVD diamond coatings. J. Phys. D Appl. Phys. 2021, 54, 443001. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y. Preparation of polymer-derived graphene-like carbon-silicon carbide nanocomposites as electromagnetic interference shielding material for high temperature applications. J. Alloys Compd. 2017, 709, 313–321. [Google Scholar] [CrossRef]
- Patil, A.; Walunj, G.; Ozdemir, F.; Gupta, R.K.; Borkar, T. Tribological Behavior of Carbon-Based Nanomaterial-Reinforced Nickel Metal Matrix Composites. Materials 2021, 14, 3536. [Google Scholar] [CrossRef]
- Saleem, A.; Iqbal, R.; Hussain, A.; Javed, M.S.; Ashfaq, M.Z.; Imran, M.; Hussain, M.M.; Akbar, A.R.; Jun, S.; Majeed, M.K. Recent advances and perspectives in carbon-based fillers reinforced Si3N4 composite for high power electronic devices. Ceram. Int. 2022, 48, 13401–13419. [Google Scholar] [CrossRef]
- Gu, C.; Li, W.; Xu, J.; Xu, S.; Lu, C.; Xu, L.; Li, J.; Zhang, S. Graphene grown out of diamond. Appl. Phys. Lett. 2016, 109, 162105. [Google Scholar] [CrossRef]
- Ismail, R.A.; Mohsin, M.H.; Ali, A.K.; Hassoon, K.I.; Erten-Ela, S. Preparation and characterization of carbon nanotubes by pulsed laser ablation in water for optoelectronic application. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 119, 113997. [Google Scholar] [CrossRef]
- Oulanti, H.; Laurent, F.; Le-Huu, T.; Durand, B.; Donnet, J.B. Growth of carbon nanotubes on carbon fibers using the combustion flame oxy-acetylene method. Carbon 2015, 95, 261–267. [Google Scholar] [CrossRef]
- Panickar, R.; Sobhan, C.B.; Chakravorti, S. Chemical vapor deposition synthesis of carbon spheres: Effects of temperature and hydrogen. Vacuum 2020, 172, 109108. [Google Scholar] [CrossRef]
- Fernandes, A.J.S.; Pinto, M.; Neto, M.A.; Oliveira, F.J.; Silva, R.F.; Costa, F.M. Nano carbon hybrids from the simultaneous synthesis of CNT/NCD by MPCVD. Diam. Relat. Mater. 2009, 18, 160–163. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. A Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11, 822. [Google Scholar] [CrossRef]
- Li, Y.F.; Su, J.J.; Liu, Y.Q.; Ding, M.H.; Wang, G.; Tang, W.Z. A circumferential antenna ellipsoidal cavity type MPCVD reactor developed for diamond film deposition. Diam. Relat. Mater. 2015, 51, 24–29. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, S.; Ji, Z.; Shen, B. Covalent graphene-diamond heterostructure coating on tool surface with enhanced lubricity and anti-wear performance. Carbon 2024, 217, 118648. [Google Scholar] [CrossRef]
- Kumaran, C.R.; Chandran, M.; Krishna Surendra, M.; Bhattacharya, S.S.; Ramachandra Rao, M.S. Growth and characterization of diamond particles, diamond films, and CNT-diamond composite films deposited simultaneously by hot filament CVD. J. Mater. Sci. 2015, 50, 144–156. [Google Scholar] [CrossRef]
- Yan, B.; Chen, N.; Xu, C.; Wu, Y.; Li, L.; Lee, C.; He, N. Covalently Bonded Heterostructures with Mixed-Dimensional Carbons for Suppressing Mechanochemical Wear of Diamond under Heavy Loads. ACS Appl. Mater. Interfaces 2024, 16, 19751–19763. [Google Scholar] [CrossRef]
- Kleshch, V.I.; Orekhov, A.S.; Pishchulina, A.E.; Sapkov, I.V.; Khmelenin, D.N.; Loginov, A.B.; Ismagilov, R.R.; Obraztsov, A.N. All-carbon heterostructures self-assembly during field electron emission from diamond nanotip. Carbon 2024, 221, 118936. [Google Scholar] [CrossRef]
- Lee, S.-K.; Song, M.-J.; Kim, J.-H.; Lim, Y.-K.; Chun, Y.-S.; Lim, D.-S. Selective growth of carbon nanotubes on boron-doped diamond for electrochemical biosensor application. RSC Adv. 2015, 5, 23395–23400. [Google Scholar] [CrossRef]
- Tumilty, N.; Kasharina, L.; Prokhoda, T.; Sinelnikov, B.; Jackman, R.B. Synthesis of carbon nanotubes on single crystal diamond. Carbon 2010, 48, 3027–3032. [Google Scholar] [CrossRef]
- Bokhonov, B.B.; Ukhina, A.V.; Dudina, D.V.; Katsui, H.; Goto, T.; Kato, H. Multiwalled carbon nanotube forests grown on the surface of synthetic diamond crystals. Ceram. Int. 2017, 43, 10606–10609. [Google Scholar] [CrossRef]
- Li, Y.; Cui, R.; Ding, L.; Liu, Y.; Zhou, W.; Zhang, Y.; Jin, Z.; Peng, F.; Liu, J. How Catalysts Affect the Growth of Single-Walled Carbon Nanotubes on Substrates. Adv. Mater. 2010, 22, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Z.; Tseng, S.-C.; Chen, Y.-H.; Tsai, H.-Y. The mechanisms of carbon nano-flake balls growth by laser ablation and microwave plasma chemical vapor deposition. Surf. Coat. Technol. 2021, 425, 127668. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, J.; Huang, Y.; Zhang, T. Synthesis and applications of carbon nanospheres: A review. Particuology 2024, 87, 325–338. [Google Scholar] [CrossRef]
- Ozden, S.; Tiwary, C.S.; Yao, J.; Brunetto, G.; Bhowmick, S.; Asif, S.; Vajtai, R.; Ajayan, P.M. Highly ordered carbon-based nanospheres with high stiffness. Carbon 2016, 105, 144–150. [Google Scholar] [CrossRef]
- Zhang, M.-C.; Guo, G.-C.; Wang, R.-Z.; Cui, Y.-L.; Feng, X.-Y.; Wang, B.-R. Coupling enhanced growth by nitrogen and hydrogen plasma of carbon nanotubes. CrystEngComm 2019, 21, 4653–4660. [Google Scholar] [CrossRef]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Ni, Z.H.; Fan, H.M.; Feng, Y.P.; Shen, Z.X.; Yang, B.J.; Wu, Y.H. Raman spectroscopic investigation of carbon nanowalls. J. Chem. Phys. 2006, 124, 204703. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, J.; Zhang, N.; Zhang, W.; Zhou, Y.; Huang, K. Advances of microwave plasma-enhanced chemical vapor deposition in fabrication of carbon nanotubes: A review. J. Mater. Sci. 2021, 56, 12559–12583. [Google Scholar] [CrossRef]
- Hsu, C.M.; Lin, C.H.; Lai, H.J.; Kuo, C.T. Root growth of multi-wall carbon nanotubes by MPCVD. Thin Solid Film. 2005, 471, 140–144. [Google Scholar] [CrossRef]
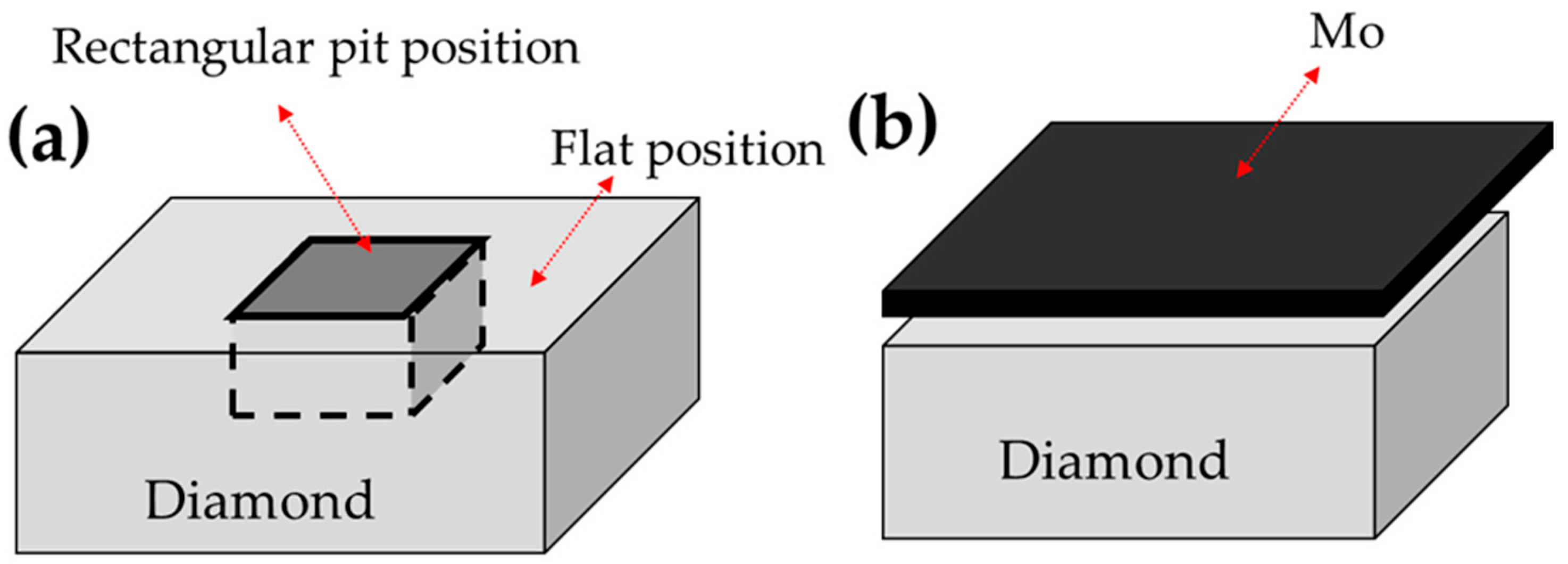



| Expt. No. | Gas Mixture | Microwave Power (kW) | Operating Pressure (Torr) | Substrate Details | Substrate Temperature (K) | Deposition Time (min) | Outcome |
|---|---|---|---|---|---|---|---|
| 1. | 300 sccmH2 45 sccmCH4 | 5 | 100 | Rectangular pit position | 1258 | 60 | CP |
| 2. | 300 sccmH2 45 sccmCH4 | 5 | 100 | Flat position | 1203 | 60 | CNP |
| 3. | 200 sccmH2 45 sccmCH4 3 sccmN2 | 4 | 80 | Mo | 973 | 40 | CNW |
| 4. | 200 sccmH2 45 sccmCH4 9 sccmN2 | 4.5 | 80 | Mo | 993 | 40 | CNT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Wang, Q.; Guo, K.; Liu, L.; Bai, J.; Yang, Z.; Li, X.; Liu, H. Synthesis and Mechanism Study of Carbon Nanowires, Carbon Nanotubes, and Carbon Pompons on Single-Crystal Diamonds. Crystals 2024, 14, 481. https://doi.org/10.3390/cryst14060481
Wu S, Wang Q, Guo K, Liu L, Bai J, Yang Z, Li X, Liu H. Synthesis and Mechanism Study of Carbon Nanowires, Carbon Nanotubes, and Carbon Pompons on Single-Crystal Diamonds. Crystals. 2024; 14(6):481. https://doi.org/10.3390/cryst14060481
Chicago/Turabian StyleWu, Shuai, Qiang Wang, Kesheng Guo, Lei Liu, Jie Bai, Zhenhuai Yang, Xin Li, and Hong Liu. 2024. "Synthesis and Mechanism Study of Carbon Nanowires, Carbon Nanotubes, and Carbon Pompons on Single-Crystal Diamonds" Crystals 14, no. 6: 481. https://doi.org/10.3390/cryst14060481
APA StyleWu, S., Wang, Q., Guo, K., Liu, L., Bai, J., Yang, Z., Li, X., & Liu, H. (2024). Synthesis and Mechanism Study of Carbon Nanowires, Carbon Nanotubes, and Carbon Pompons on Single-Crystal Diamonds. Crystals, 14(6), 481. https://doi.org/10.3390/cryst14060481





