Morphological Study of Tetra-n-Butylammonium Bromide Semi-Clathrate Hydrate in Confined Space
Abstract
1. Introduction
2. Materials and Methods
3. Results
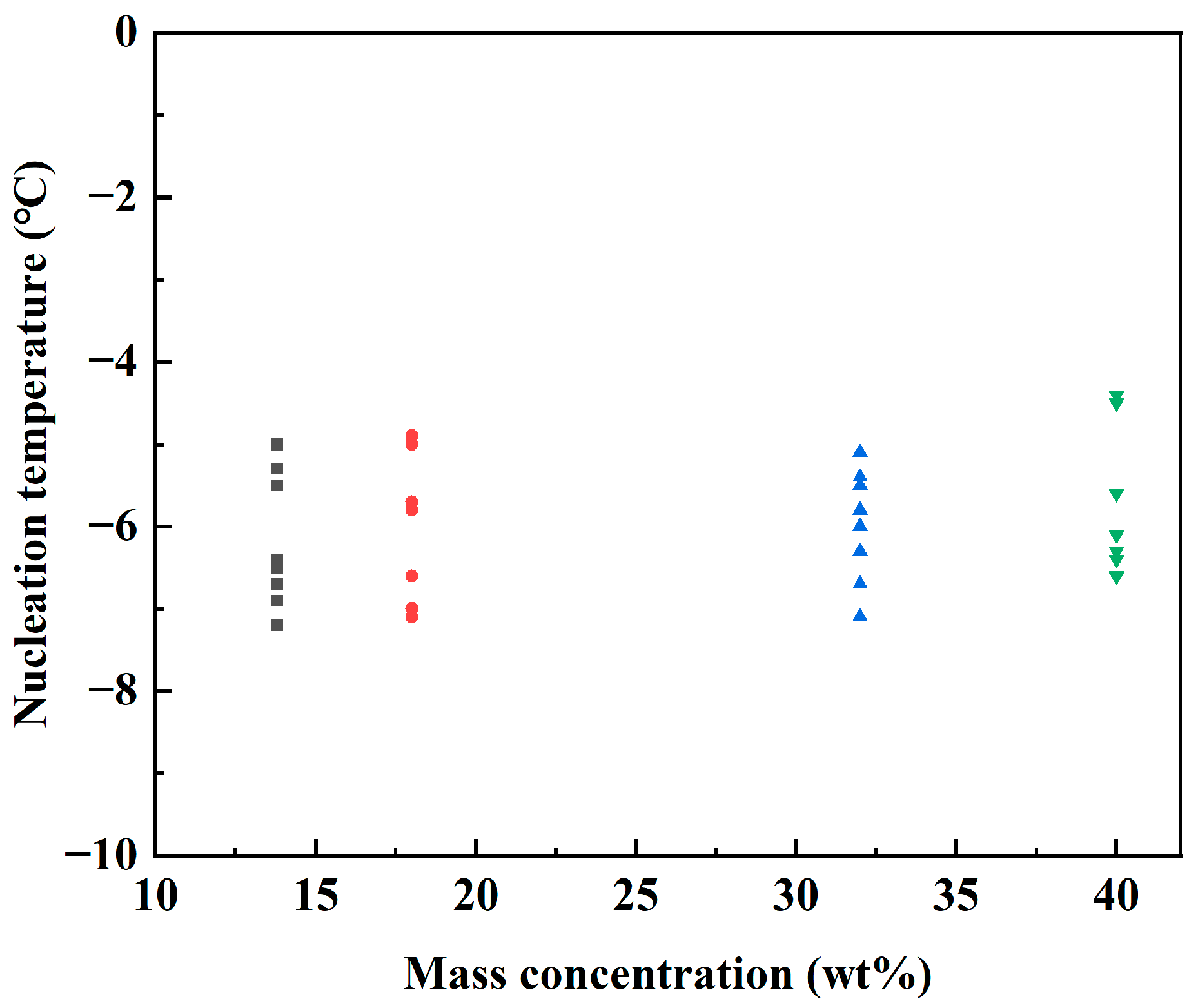
3.1. Hydrate Nucleation at Low Cooling Rate
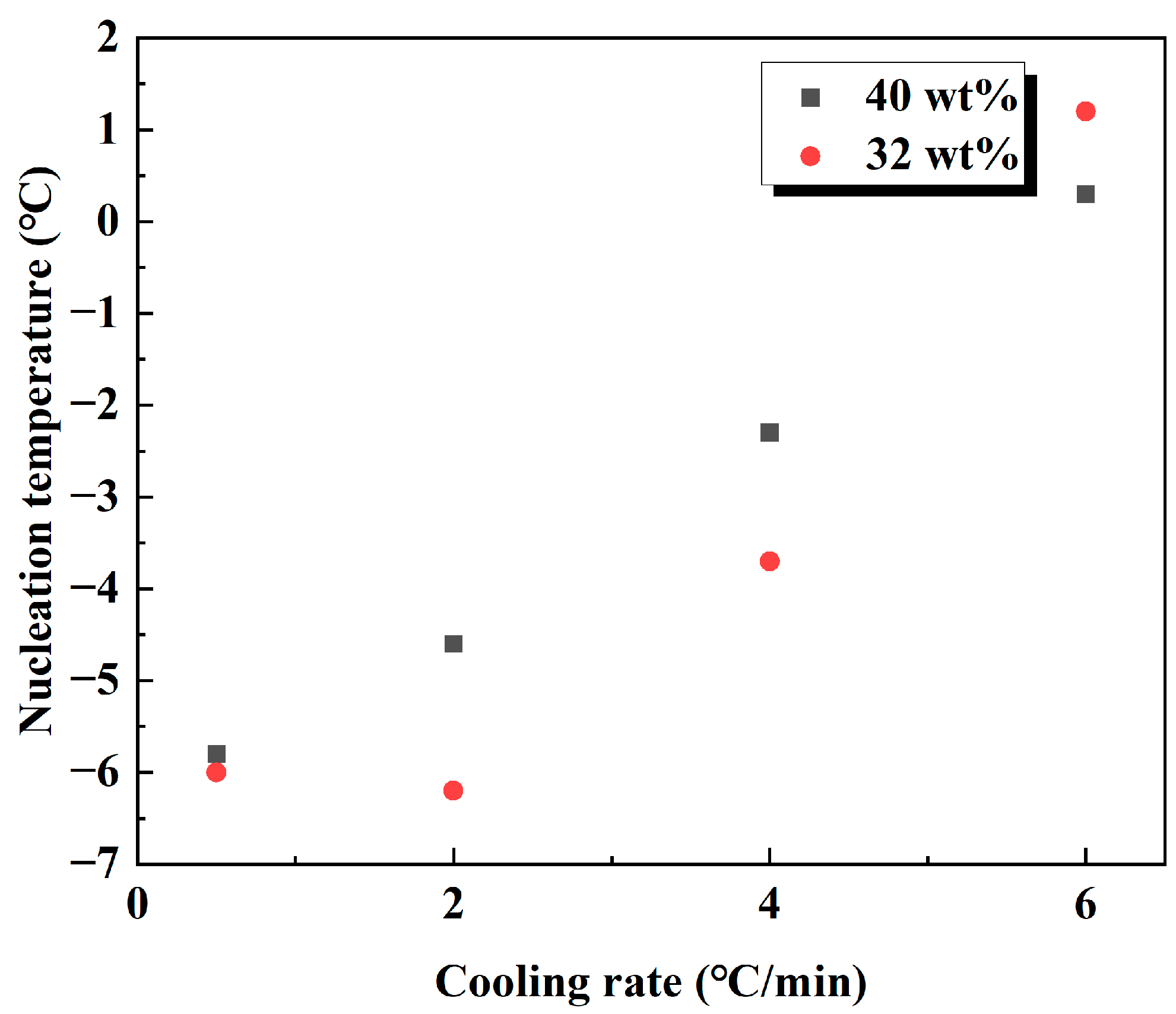
3.1.1. Supercooling Required for Hydrate Nucleation
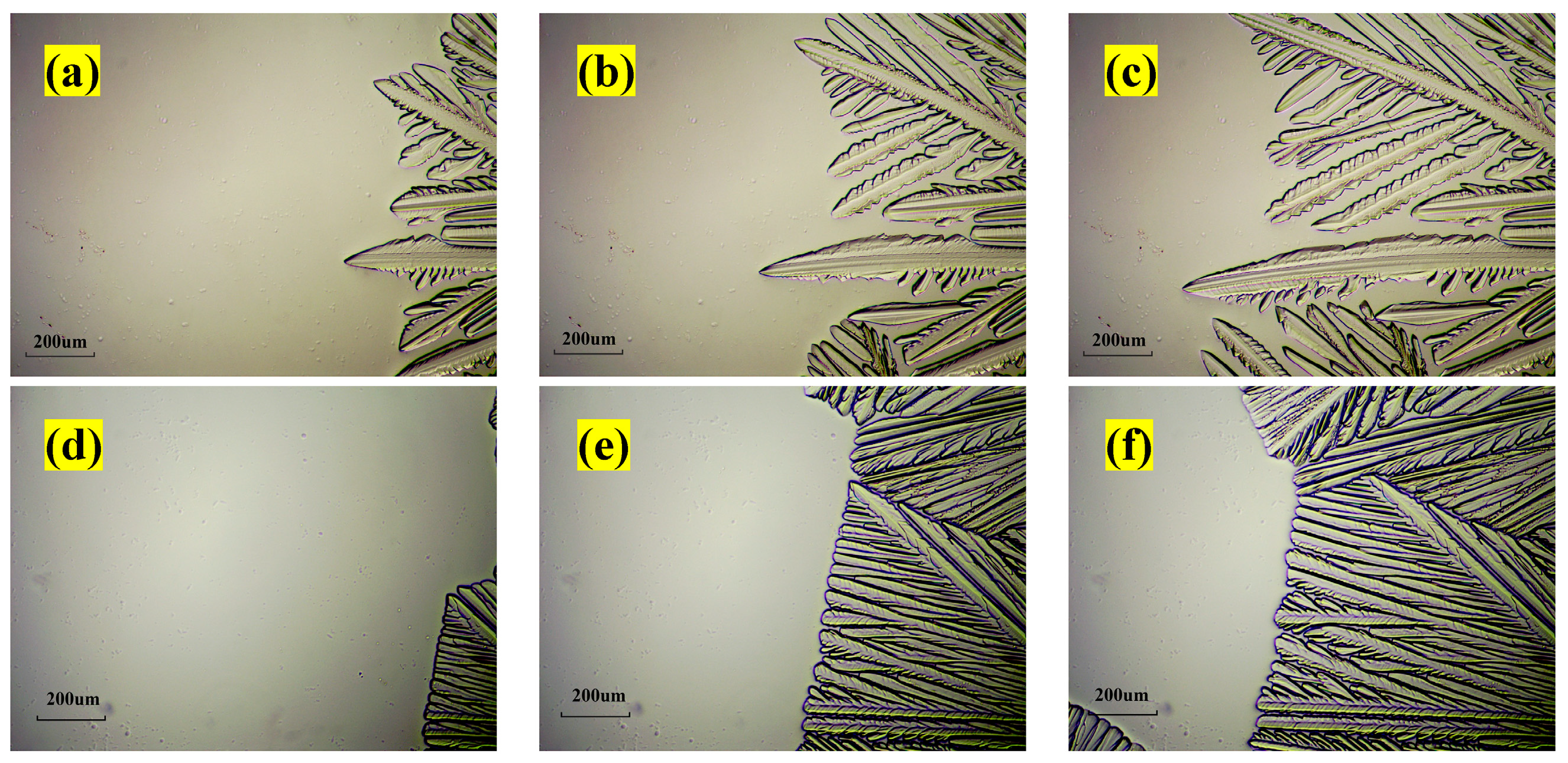
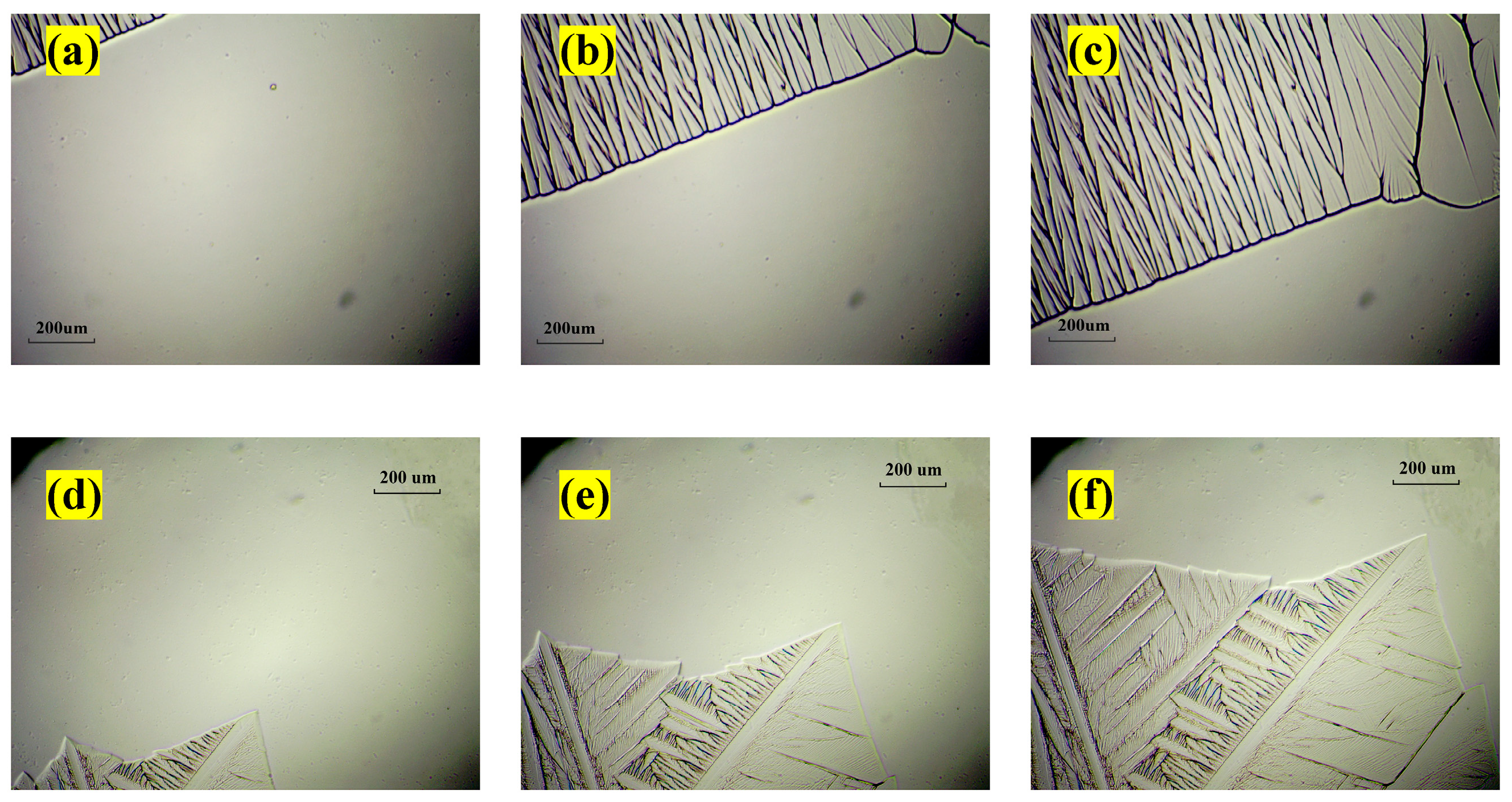
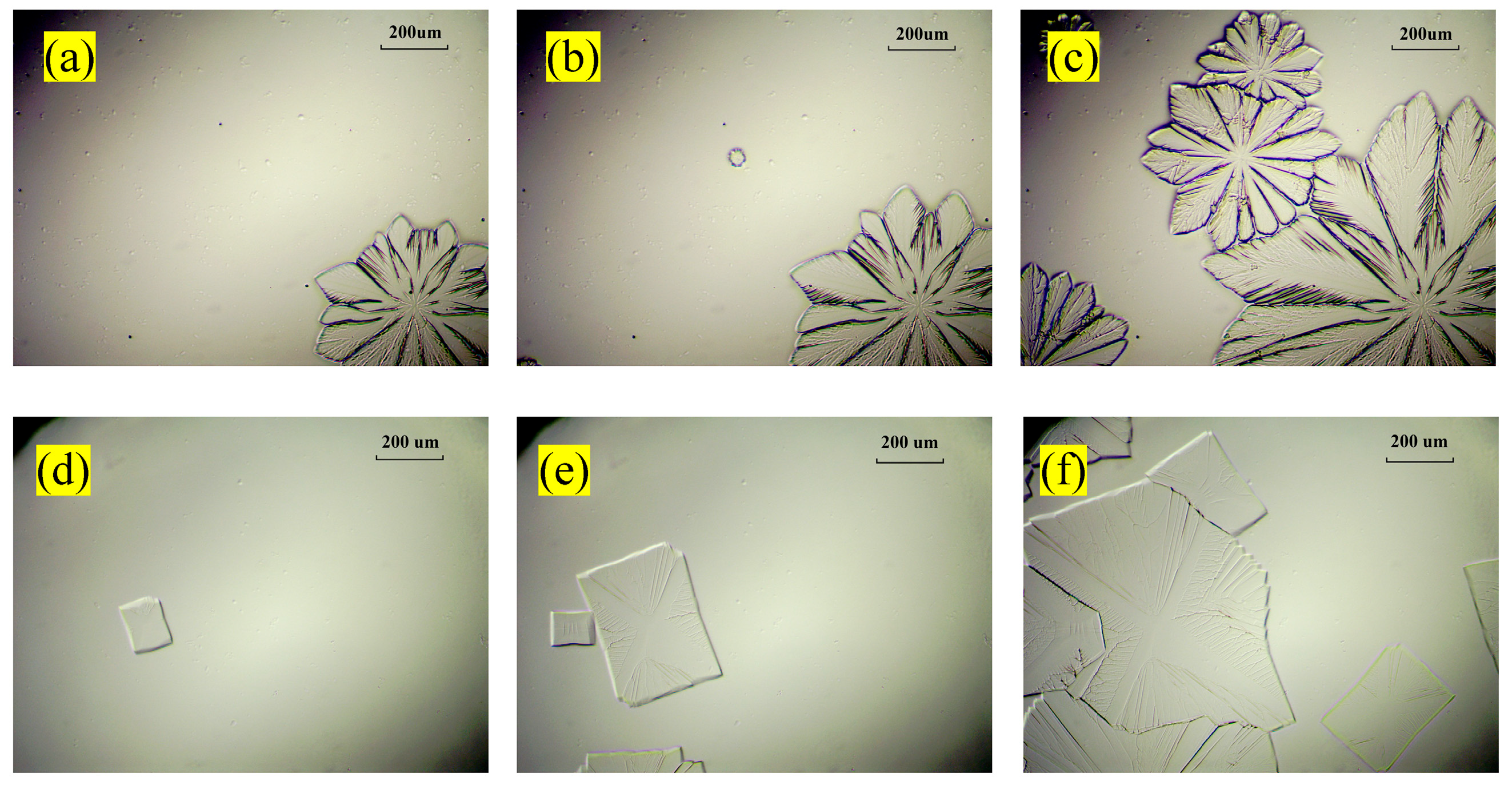
3.1.2. Optical Morphology
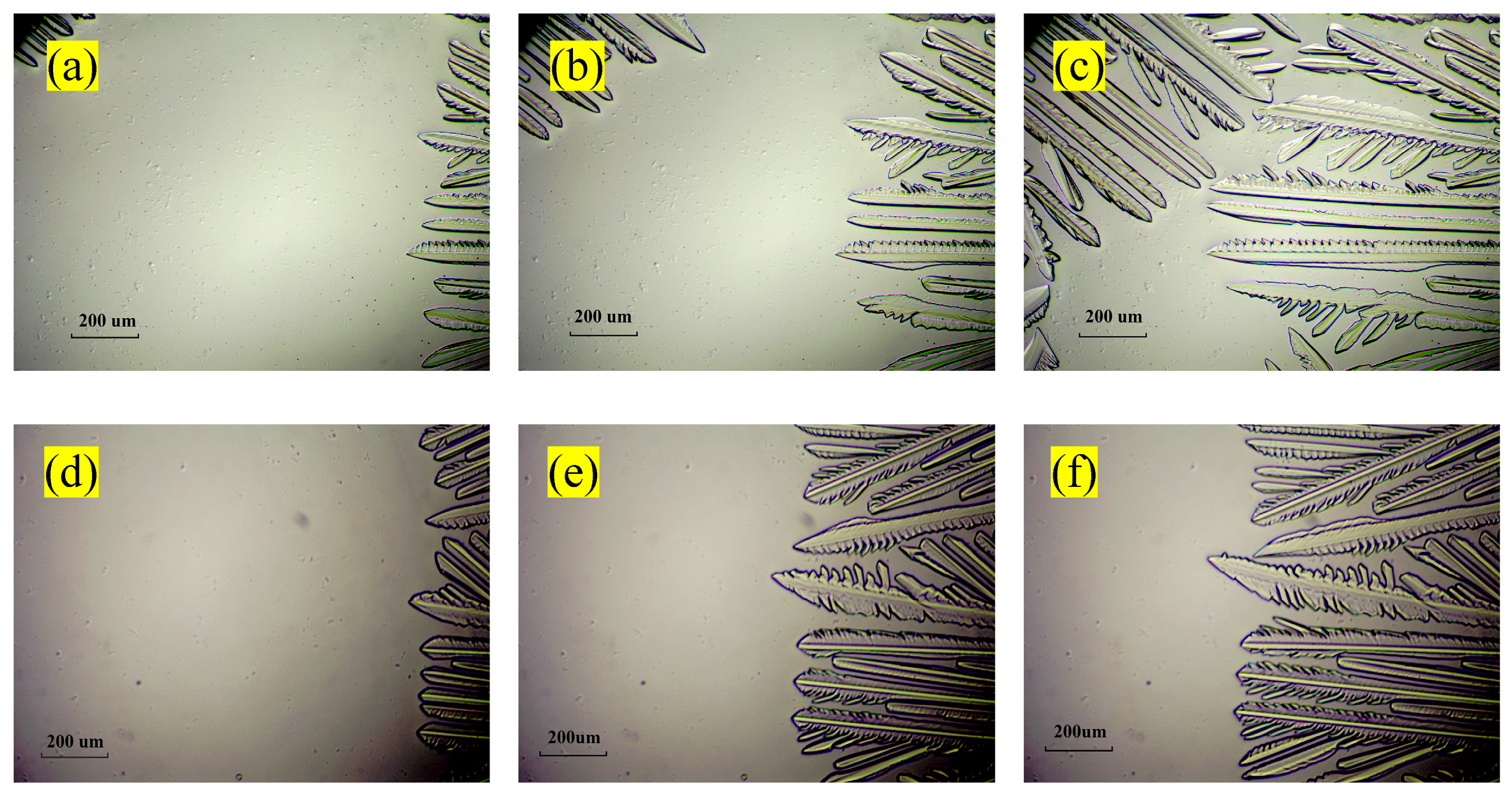
3.2. Hydrate Formation with Different Cooling Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, L.; Lu, H. Semi-clathrate hydrate based carbon dioxide capture and separation techniques. Front. Environ. Sci. Eng. 2023, 17, 144. [Google Scholar] [CrossRef]
- Fan, S.; Li, S.; Wang, J.; Lang, X.; Wang, Y. Efficient Capture of CO2 from Simulated Flue Gas by Formation of TBAB or TBAF Semiclathrate Hydrates. Energy Fuels 2009, 23, 4202–4208. [Google Scholar] [CrossRef]
- Li, X.-S.; Xia, Z.-M.; Chen, Z.-Y.; Wu, H.-J. Precombustion Capture of Carbon Dioxide and Hydrogen with a One-Stage Hydrate/Membrane Process in the Presence of Tetra-n-butylammonium Bromide (TBAB). Energy Fuels 2011, 25, 1302–1309. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, D.-L.; Li, Z.; Li, J.-B. Application of tetra-n-butyl ammonium bromide semi-clathrate hydrate for CO2 capture from unconventional natural gases. Energy 2020, 197, 117209. [Google Scholar] [CrossRef]
- Chapoy, A.; Anderson, R.; Tohidi, B. Low-Pressure Molecular Hydrogen Storage in Semi-clathrate Hydrates of Quaternary Ammonium Compounds. J. Am. Chem. Soc. 2007, 129, 746–747. [Google Scholar] [CrossRef]
- Douzet, J.; Kwaterski, M.; Lallemand, A.; Chauvy, F.; Flick, D.; Herri, J.-M. Prototyping of a real size air-conditioning system using a tetra-n-butylammonium bromide semiclathrate hydrate slurry as secondary two-phase refrigerant—Experimental investigations and modelling. Int. J. Refrig. 2013, 36, 1616–1631. [Google Scholar] [CrossRef]
- Shimada, W.; Shiro, M.; Kondo, H.; Takeya, S.; Oyama, H.; Ebinuma, T.; Narita, H. Tetra-n-butylammonium bromide-water (1/38). Acta Crystallogr. C 2005, 61, o65–o66. [Google Scholar] [CrossRef]
- Gaponenko, L.A.; Solodovnikov, S.F.; Dyadin, Y.A.; Aladko, L.S.; Polyanskaya, T.M. Crystallographic study of tetra-n-butylammonium bromide polyhydrates. J. Struct. Chem. 1984, 25, 157–159. [Google Scholar] [CrossRef]
- Rodionova, T.V.; Komarov, V.Y.; Villevald, G.V.; Karpova, T.D.; Kuratieva, N.V.; Manakov, A.Y. Calorimetric and Structural Studies of Tetrabutylammonium Bromide Ionic Clathrate Hydrates. J. Phys. Chem. B 2013, 117, 10677–10685. [Google Scholar] [CrossRef]
- Lipkowski, J.; Komarov, V.Y.; Rodionova, T.V.; Dyadin, Y.A.; Aladko, L.S. The Structure of Tetrabutylammonium Bromide Hydrate (C4H9)4NBr·21/3H2O. J. Supramol. Chem. 2002, 2, 435–439. [Google Scholar] [CrossRef]
- Shimada, W.; Ebinuma, T.; Oyama, H.; Kamata, Y.; Takeya, S.; Uchida, T.; Nagao, J.; Narita, H. Separation of Gas Molecule Using Tetra-n-butyl Ammonium Bromide Semi-Clathrate Hydrate Crystals. Jpn. J. Appl. Phys. 2003, 42, L129–L131. [Google Scholar] [CrossRef]
- Servio, P.; Englezos, P. Morphology Study of Structure H Hydrate Formation from Water Droplets. Cryst. Growth Des. 2003, 3, 61–66. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Yang, T.; Linga, P. Crystal Growth of Hydrogen/Tetra-n-butylammonium Bromide Semiclathrates Based on Morphology Study. Cryst. Growth Des. 2014, 14, 1950–1960. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, N.; Liu, Q.; Cheng, Z.; Liu, Y.; Zhao, J. Review of Morphology Studies on Gas Hydrate Formation for Hydrate-Based Technology. Cryst. Growth Des. 2020, 20, 8148–8161. [Google Scholar] [CrossRef]
- Shimada, W.; Ebinuma, T.; Oyama, H.; Kamata, Y.; Narita, H. Free-growth forms and growth kinetics of tetra-n-butyl ammonium bromide semi-clathrate hydrate crystals. J. Cryst. Growth 2005, 274, 246–250. [Google Scholar] [CrossRef]
- Hashimoto, S.; Sugahara, T.; Moritoki, M.; Sato, H.; Ohgaki, K. Thermodynamic stability of hydrogen + tetra-n-butyl ammonium bromide mixed gas hydrate in nonstoichiometric aqueous solutions. Chem. Eng. Sci. 2008, 63, 1092–1097. [Google Scholar] [CrossRef]
- Akiba, H.; Ueno, H.; Ohmura, R. Crystal Growth of Ionic Semiclathrate Hydrate Formed at the Interface between CO2 Gas and Tetra-n-butylammonium Bromide Aqueous Solution. Cryst. Growth Des. 2015, 15, 3963–3968. [Google Scholar] [CrossRef]
- Ye, N.; Zhang, P. Equilibrium Data and Morphology of Tetra-n-butyl Ammonium Bromide Semiclathrate Hydrate with Carbon Dioxide. J. Chem. Eng. Data 2012, 57, 1557–1562. [Google Scholar] [CrossRef]
- Shimada, W.; Oshima, M.; Masaki, T.; Tsutsui, H.; Momma, K.; Takai, K. Nucleation probability of Type-A tetra-n-butyl ammonium bromide semi-clathrate hydrate via the memory effect. J. Cryst. Growth 2022, 583, 126560. [Google Scholar] [CrossRef]
- Cai, W.; Zhan, L.; Huang, X.; Lu, H. Raman Micro-Imaging of the Coexistence of sI and sII Hydrates Formed from a Mixed Methane-Propane Gas in a Confined Space. Acta Geol. Sin. Engl. Ed. 2022, 96, 674–679. [Google Scholar] [CrossRef]
- Sugahara, T.; Machida, H. Dissociation and Nucleation of Tetra-n-butyl Ammonium Bromide Semi-Clathrate Hydrates at High Pressures. J. Chem. Eng. Data 2017, 62, 2721–2725. [Google Scholar] [CrossRef]
- Chazallon, B.; Ziskind, M.; Carpentier, Y.; Focsa, C. CO2 Capture Using Semi-Clathrates of Quaternary Ammonium Salt: Structure Change Induced by CO2 and N2 Enclathration. J. Phys. Chem. B 2014, 118, 13440–13452. [Google Scholar] [CrossRef]
- Kim, H.; Zheng, J.; Yin, Z.; Kumar, S.; Tee, J.; Seo, Y.; Linga, P. An electrical resistivity-based method for measuring semi-clathrate hydrate formation kinetics: Application for cold storage and transport. Appl. Energy 2022, 308, 118397. [Google Scholar] [CrossRef]
- Oyama, H.; Shimada, W.; Ebinuma, T.; Kamata, Y.; Takeya, S.; Uchida, T.; Nagao, J.; Narita, H. Phase diagram, latent heat, and specific heat of TBAB semiclathrate hydrate crystals. Fluid Phase Equilibria 2005, 234, 131–135. [Google Scholar] [CrossRef]
- Meldrum, F.C.; O’Shaughnessy, C. Crystallization in Confinement. Adv. Mater. 2020, 32, e2001068. [Google Scholar] [CrossRef]
- Meldrum, F.C.; Cölfen, H. Controlling Mineral Morphologies and Structures in Biological and Synthetic Systems. Chem. Rev. 2008, 108, 4332–4432. [Google Scholar] [CrossRef]
- Kuang, Y.; Feng, Y.; Yang, L.; Song, Y.; Zhao, J. Effects of micro-bubbles on the nucleation and morphology of gas hydrate crystals. Phys. Chem. Chem. Phys. 2019, 21, 23401–23407. [Google Scholar] [CrossRef]
- Sunagawa, I. Crystals: Growth, Morphology, and Perfection; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Faure, F.; Trolliard, G.; Nicollet, C.; Montel, J.-M. A developmental model of olivine morphology as a function of the cooling rate and the degree of undercooling. Contrib. Mineral. Petrol. 2003, 145, 251–263. [Google Scholar] [CrossRef]
- Li, M.-y.; Gao, M.; Zuo, Q.-r.; Zhao, Y.-g.; Zhang, L.-x. Experimental and theoretical investigation on hydrate nucleation in TBAB droplets. Fuel 2022, 308, 121994. [Google Scholar] [CrossRef]
- Shi, X.J.; Zhang, P. Crystallization of tetra-n-butyl ammonium bromide clathrate hydrate slurry and the related heat transfer characteristics. Energy Convers. Manag. 2014, 77, 89–97. [Google Scholar] [CrossRef]
- Kim, H.; Zheng, J.; Babu, P.; Kumar, S.; Tee, J.; Linga, P. Key factors influencing the kinetics of tetra-n-butylammonium bromide hydrate formation as a cold storage and transport material. Chem. Eng. J. 2022, 446, 136843. [Google Scholar] [CrossRef]
- Vysniauskas, A.; Bishnoi, P.R. A kinetic study of methane hydrate formation. Chem. Eng. Sci. 1983, 38, 1061–1072. [Google Scholar] [CrossRef]
- Yin, Z.; Khurana, M.; Tan, H.K.; Linga, P. A review of gas hydrate growth kinetic models. Chem. Eng. J. 2018, 342, 9–29. [Google Scholar] [CrossRef]
- Aysal, N.; Kurt, Y.; Öztürk, H.; Ildiz, G.O.; Yesiltas, M.; Laçin, D.; Öngen, S.; Nikitin, T.; Fausto, R. Crystallization Kinetics: Relationship between Crystal Morphology and the Cooling Rate—Applications for Different Geological Materials. Crystals 2023, 13, 1130. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, L.; Lu, H. Morphological Study of Tetra-n-Butylammonium Bromide Semi-Clathrate Hydrate in Confined Space. Crystals 2024, 14, 408. https://doi.org/10.3390/cryst14050408
Gu L, Lu H. Morphological Study of Tetra-n-Butylammonium Bromide Semi-Clathrate Hydrate in Confined Space. Crystals. 2024; 14(5):408. https://doi.org/10.3390/cryst14050408
Chicago/Turabian StyleGu, Lijuan, and Hailong Lu. 2024. "Morphological Study of Tetra-n-Butylammonium Bromide Semi-Clathrate Hydrate in Confined Space" Crystals 14, no. 5: 408. https://doi.org/10.3390/cryst14050408
APA StyleGu, L., & Lu, H. (2024). Morphological Study of Tetra-n-Butylammonium Bromide Semi-Clathrate Hydrate in Confined Space. Crystals, 14(5), 408. https://doi.org/10.3390/cryst14050408






