Interplay of Isomorphs and Polymorphs of Amidino-Copper(II) Complexes with Different Halides
Abstract
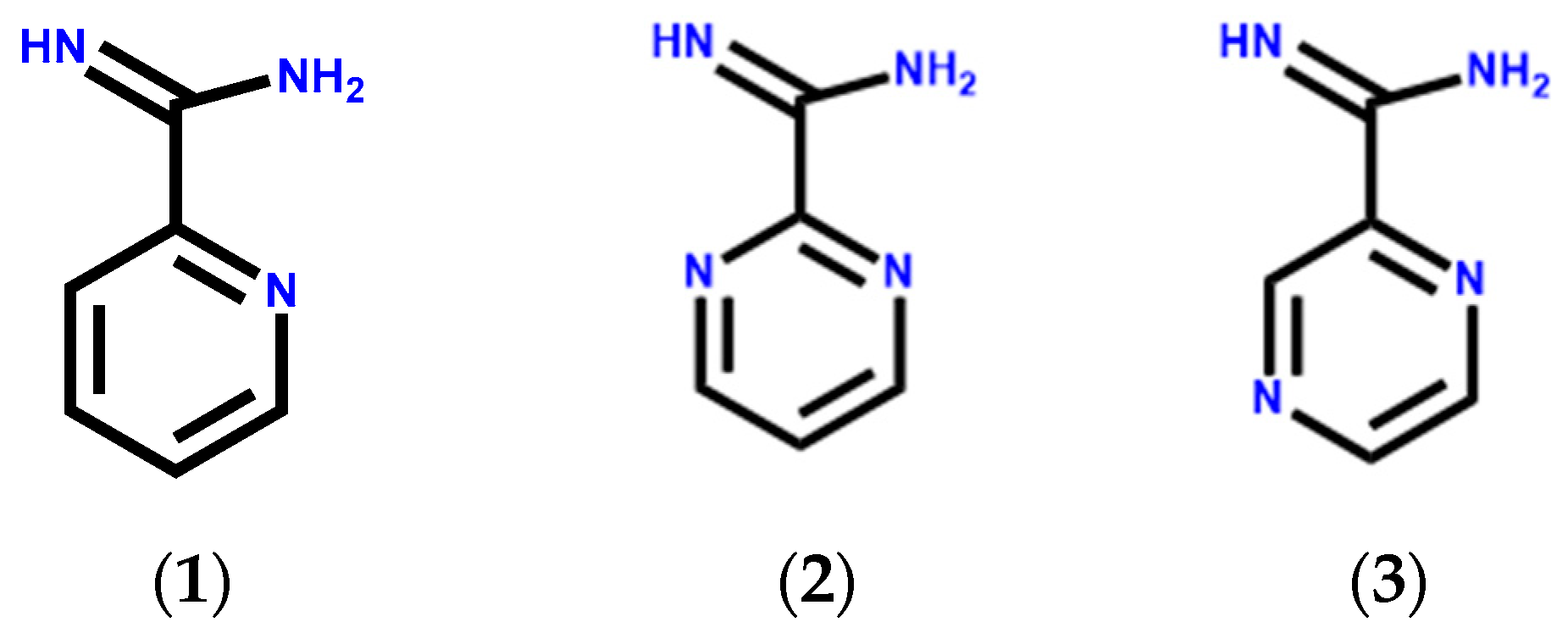
1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis of Pyrimidine-2-Carboximidamide Salt [2]+[O2C2F3]−
2.3. Synthesis of Pyrazine-2-Carboximidamide Salt [3]+[O2C2F3]−
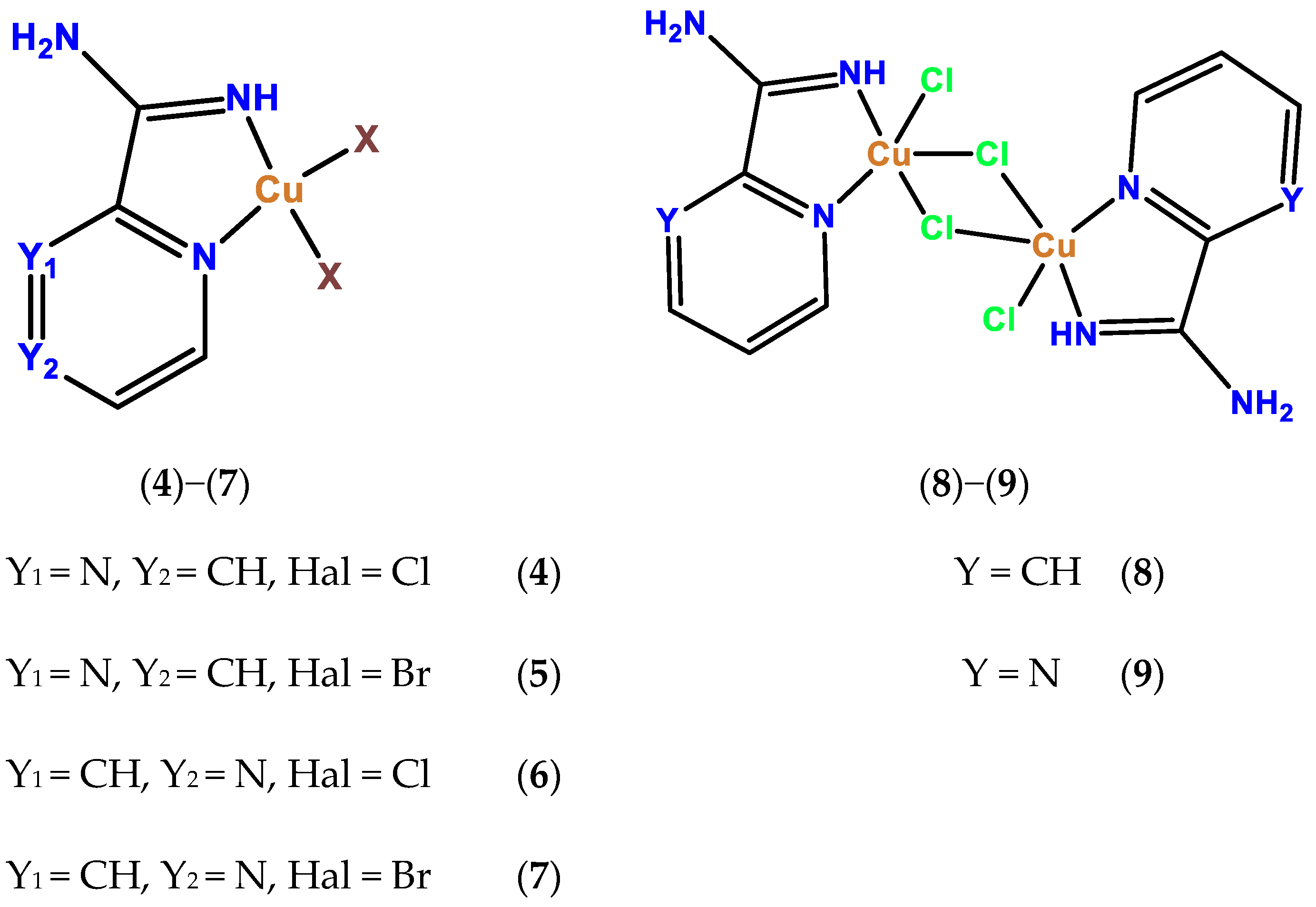
2.4. Synthesis of Complex 4
2.5. Synthesis of Complex 5
2.6. Synthesis of Complex 6
2.7. Synthesis of Complex 7
2.8. Synthesis of Complex 8
2.9. Synthesis of 9
2.10. X-ray Structure Analysis
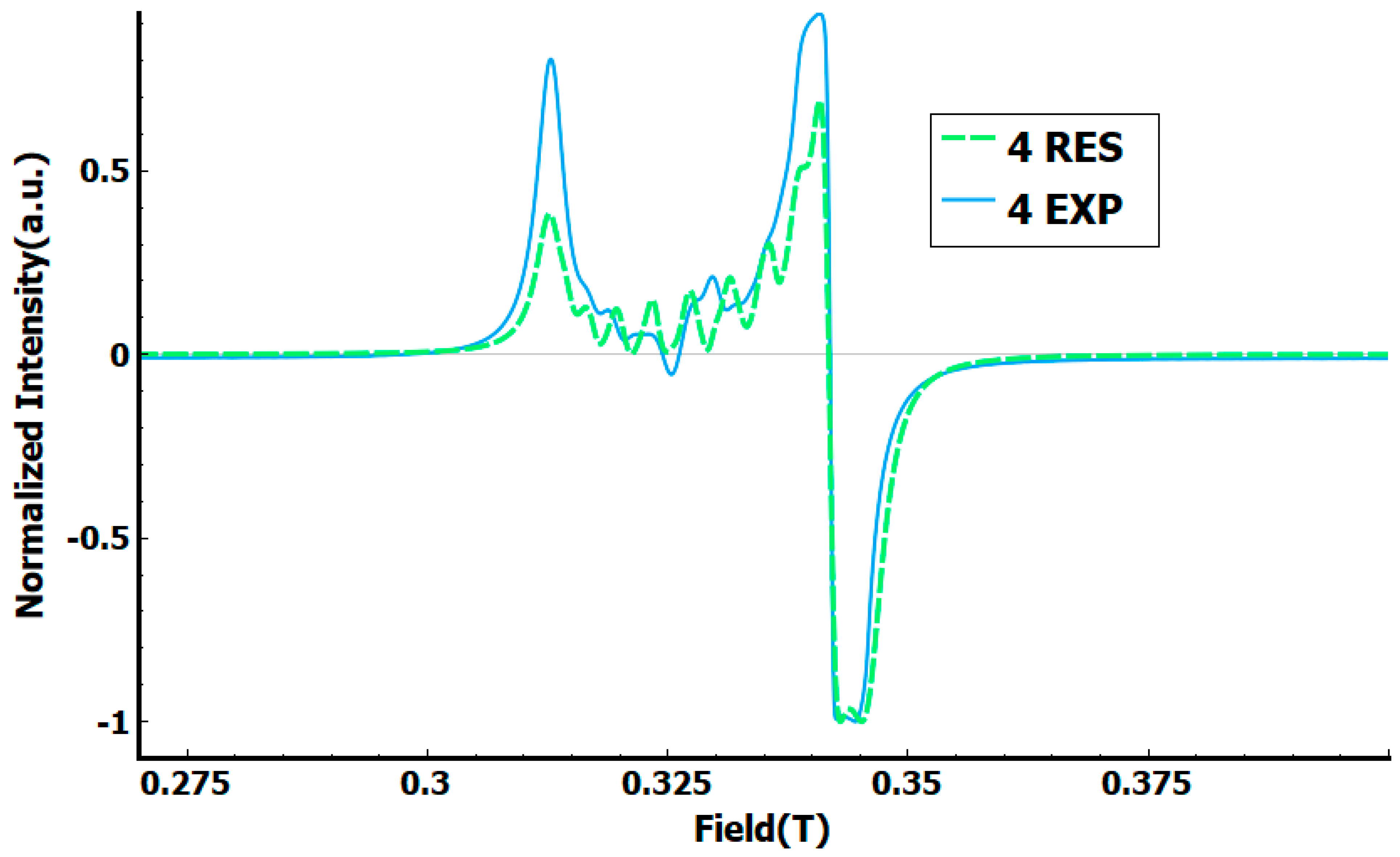
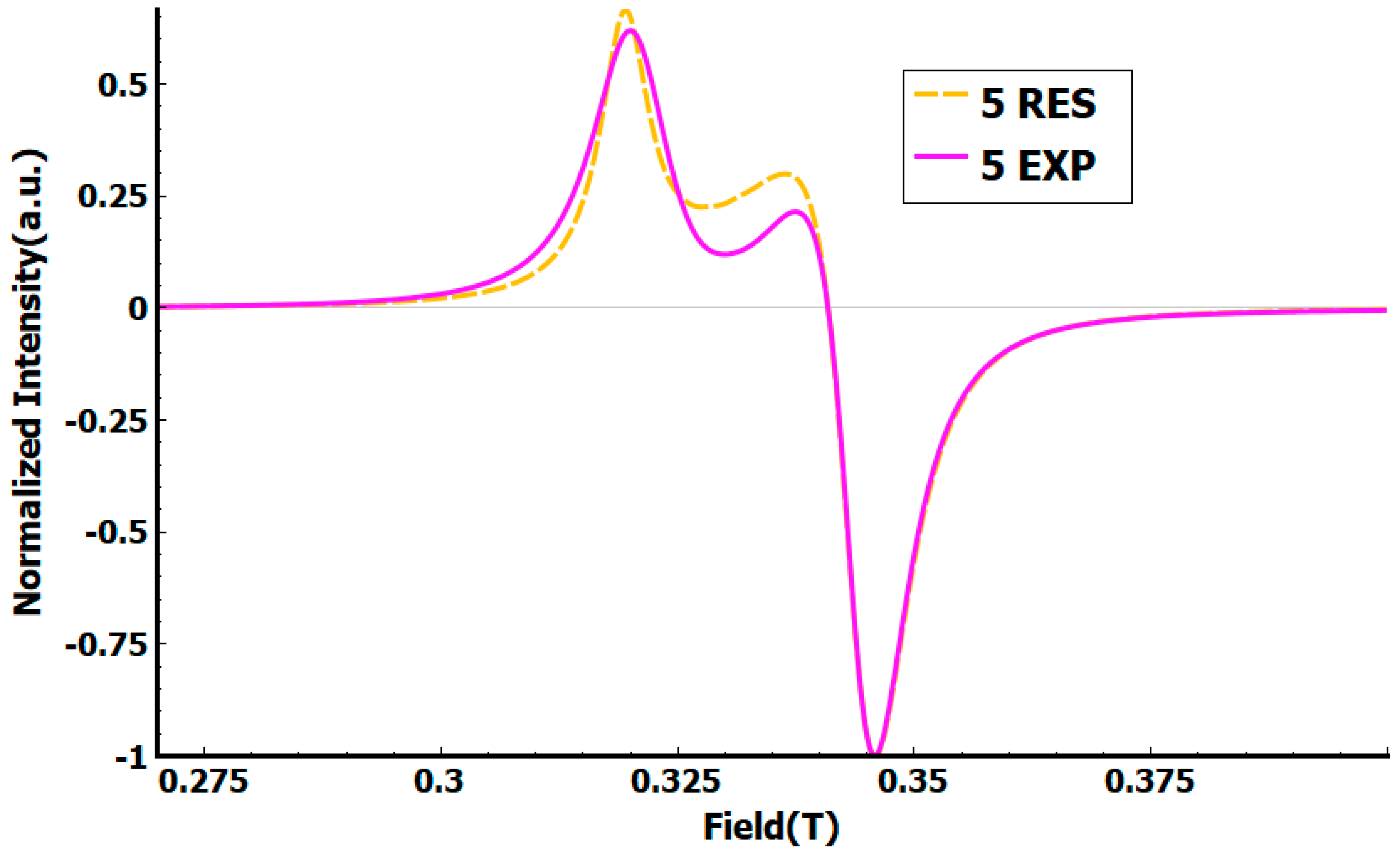
3. Results and Discussion
3.1. Crystal Structure of Ligand
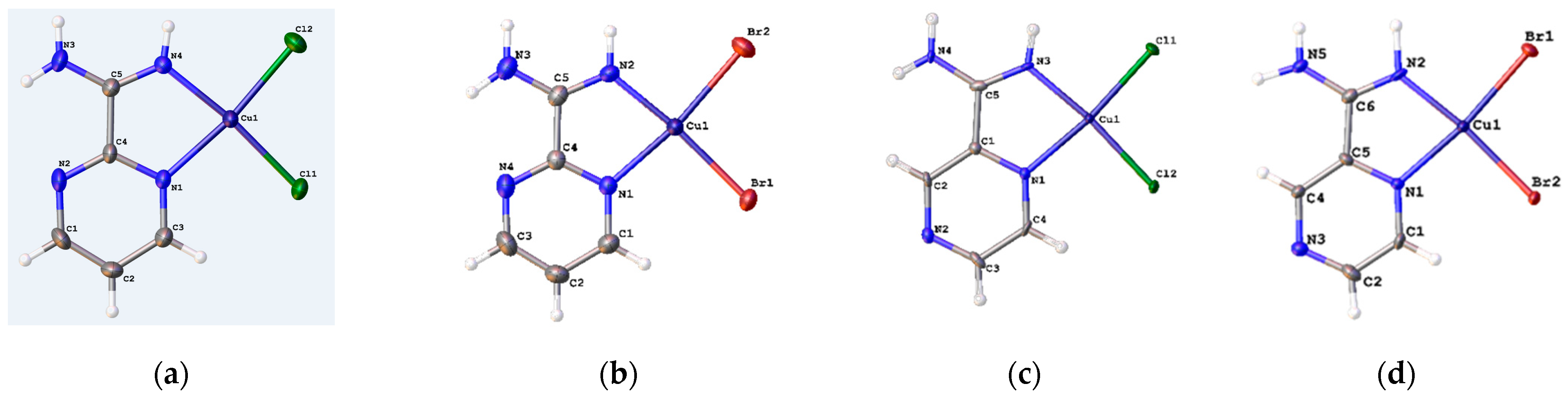
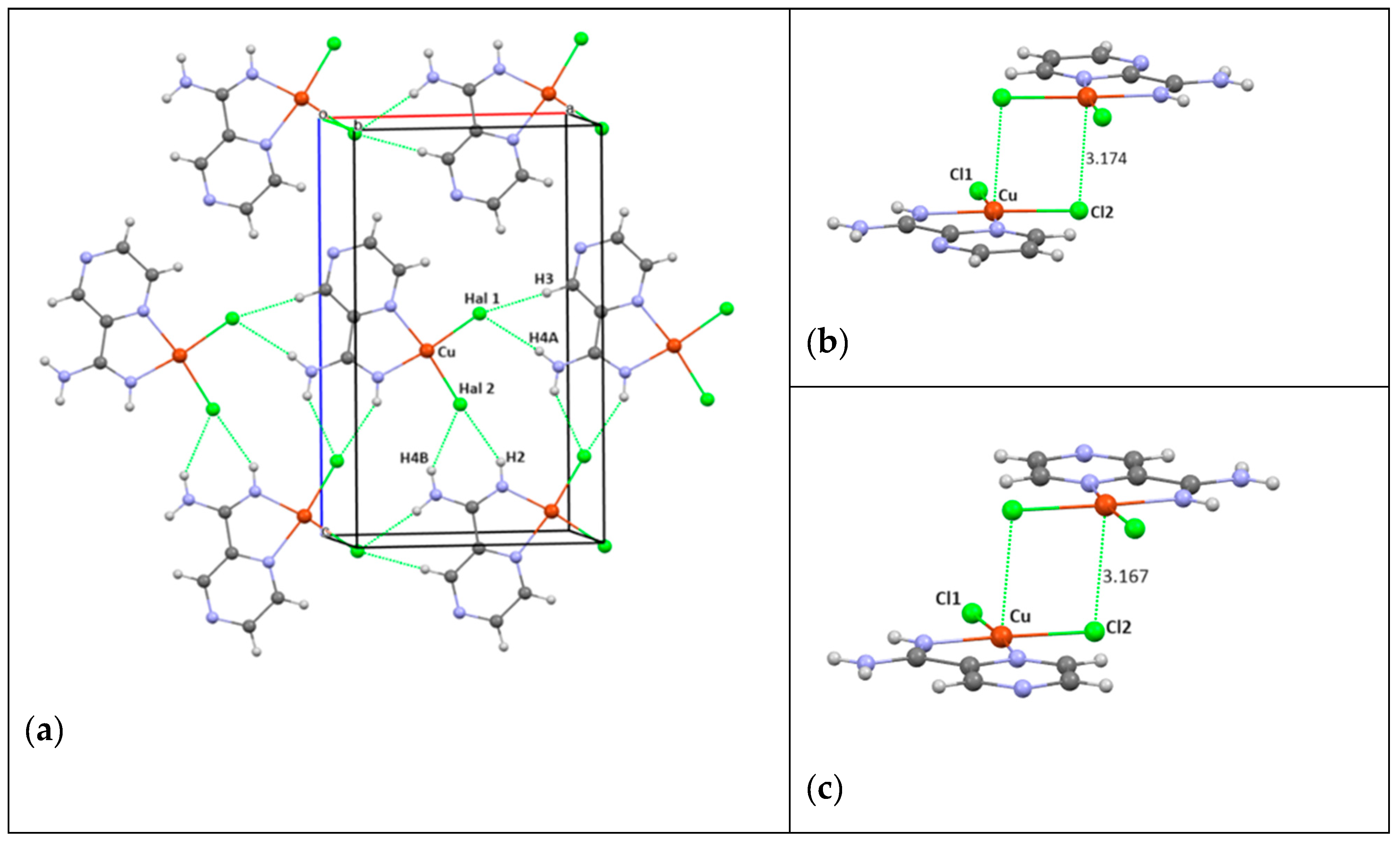
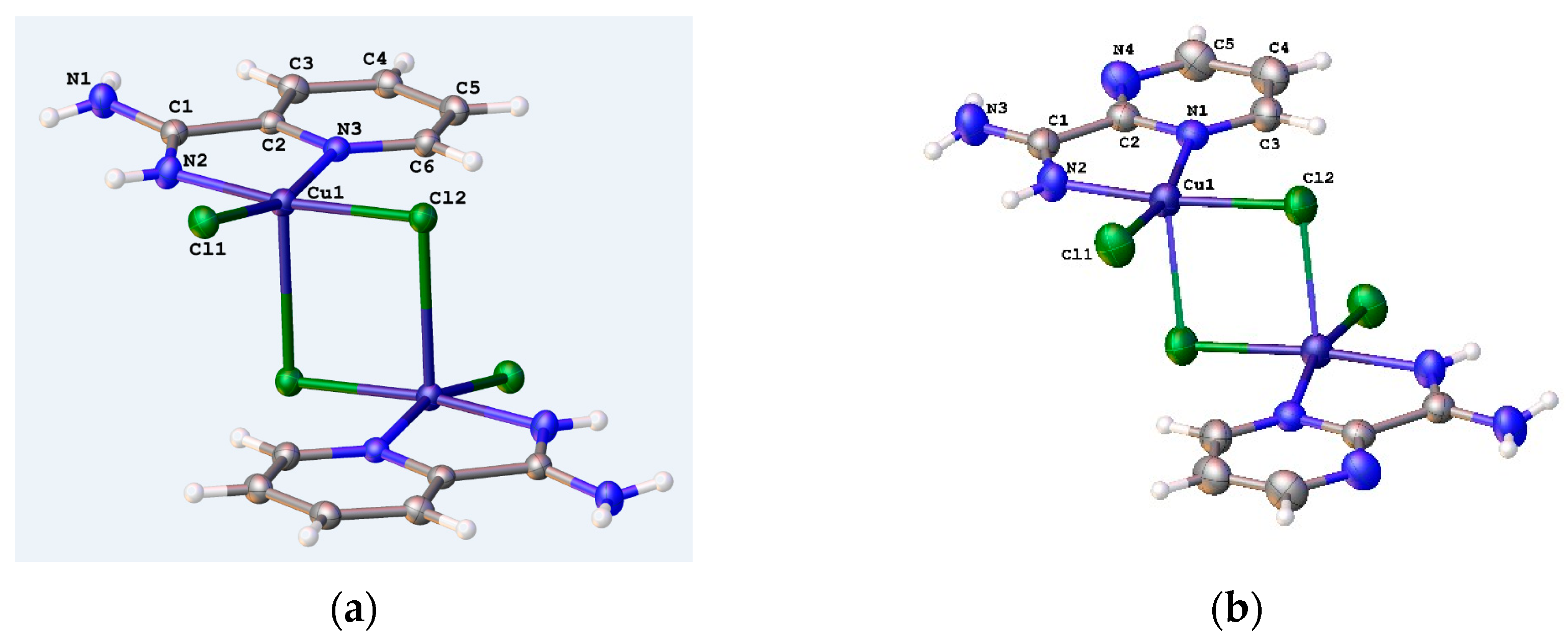
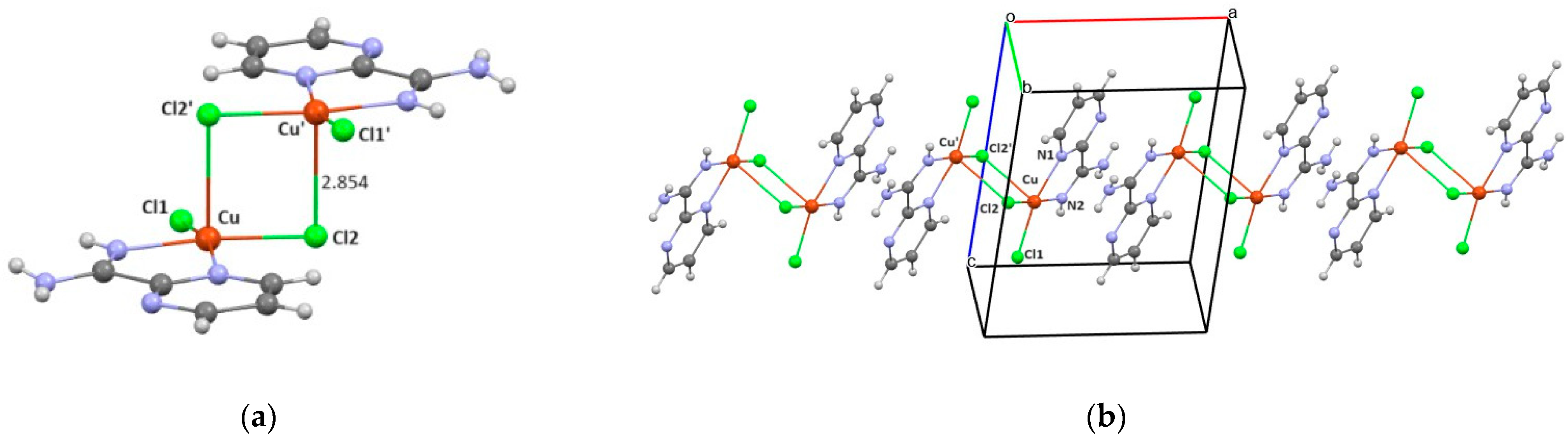
3.2. Crystal Structure of Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Grobner, T. Gadolinium—A specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transplant. 2006, 21, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Yu-Dong, X.; Ramchandra, P.; Jun, L.; Cong, M.; Zi-Shu, Z.; Shun-Ke, Z. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar]
- Valerie, C.P.; Matthew, J.A.; Peter, C. Contrast agents for MRI: 30+ years and where are we going? J. Biol. Inorg. Chem. 2014, 19, 127–131. [Google Scholar]
- Kuo, P.H. Gadolinium-Containing MRI Contrast Agents: Important Variations on a Theme for NSF. J. Am. Coll. Radiol. 2008, 5, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Abu-Alfa, A. The Impact of NSF on the Care of Patients With Kidney Disease. J. Am. Coll. Radiol. 2008, 5, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gallez, B.; Baudelet, C.; Geurts, M. Regional distribution of manganese found in the brain after injection of a single dose of manganese-based contrast agents. Magn. Reson. Imaging 1998, 16, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, W.; Zhou, S.; Kong, D.; Yang, H.; Wu, L. Mn12 single-molecule magnet aggregates as magnetic resonance imaging contrast agents. Chem. Commun. 2011, 47, 3541–3543. [Google Scholar] [CrossRef]
- Viswanathan, S.; Kovacs, Z.; Green, K.N.; Ratnakar, S.J.; Sherry, A.D. Alternatives to gadolinium-based metal chelates for magnetic resonance imaging. Chem. Rev. 2010, 110, 2960–3018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gorden, J.D.; Beyers, R.J.; Goldsmith, C.R. Manganese(II)-containing MRI contrast agent employing a neutral and non-macrocyclic ligand. Inorg. Chem. 2011, 50, 9365–9373. [Google Scholar] [CrossRef] [PubMed]
- Dasa, S.; Pargaa, K.; Chakrabortya, I.; Tinocoa, A.D.; Delgadoa, Y.; Lópeza, P.M.; Vegaa, L.F.; Sanakisb, Y.; Ghoshc, S.; Banksond, J.; et al. Magnetic resonance imaging contrast enhancement in vitro and in vivo by octanuclear iron-oxo cluster-based agents. J. Inorg. Biochem. 2018, 186, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Henoumont, C.; Devreux, M.; Laurent, S. Mn-Based MRI Contrast Agents: An Overview. Molecules 2023, 28, 7275. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M.; Popa, C.-V. Pyridine Compounds with Antimicrobial and Antiviral Activities. Int. J. Mol. Sci. 2022, 23, 5659. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Ma, S. Recent Development of Pyrimidine-Containing Antimicrobial Agents. ChemMedChem 2020, 15, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Dai, W.; Huang, H.; Liu, S.-L.; Liu, J.; Huang, L.-J.; Huang, X.-H.; Zeng, J.-L.; Gan, Z.-W.; Zhang, Z.-Y.; et al. Pharmacological activity and mechanism of pyrazines. Eur. J. Med. Chem. 2023, 258, 115544. [Google Scholar] [CrossRef] [PubMed]
- Perontsis, S.; Geromichalos, G.D.; Pekou, A.; Hatzidimitriou, A.G.; Pantazaki, A.; Fylaktakidou, K.C.; Psomas, G. Structure and biological evaluation of pyridine-2-carboxamidine copper(II) complex resulting from N′-(4-nitrophenylsulfonyloxy)2-pyridine-carboxamidoxime. J. Inorg. Biochem. 2020, 208, 111085. [Google Scholar] [CrossRef] [PubMed]
- Grineva, O.V.; Zorkii, P.M. Isostructural and Nonisostructural Compounds in Series of Halogenated Organic Crystal Substances. Structure of Hal-Aggregates. J. Struct. Chem. 2001, 42, 16–23. [Google Scholar] [CrossRef]
- Grineva, O.V.; Zorkii, P.M. Aggregation of Halogen Atoms in Crystalline Isomers. J. Struct. Chem. 2002, 43, 995–1005. [Google Scholar] [CrossRef]
- Reddy, C.M.; Kirchner, M.T.; Gundakaram, R.C.; Padmanabhan, K.A.; Desiraju, G.R. Isostructurality, Polymorphism and Mechanical Properties of Some Hexahalogenated Benzenes: The Nature of Halogen···Halogen Interactions. Chem. Eur. J. 2006, 12, 2222–2234. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Desiraju, G.R. Crystal Engineering of Hand-Twisted Helical Crystals. J. Am. Chem. Soc. 2017, 139, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Fotović, L.; Bedeković, N.; Stilinović, V. Isostructural Halogen Exchange and Halogen Bonds: The Case of N-(4-Halogenobenzyl)-3-halogenopyridinium Halogenides. Cryst. Growth Des. 2022, 22, 1333–1344. [Google Scholar] [CrossRef]
- Kariuki, B.M.; Abdel-Wahab, B.F.; El-Hiti, G.A. Synthesis and Structural Characterization of Isostructural 4-(4-Aryl)-2-(5-(4-fluorophenyl)-3-(1-(4-fluorophenyl)-5-methyl-1H-1,2,3-triazol-4-yl)-4,5-dihydro-1H-pyrazol-1-yl)thiazoles. Crystals 2021, 11, 795. [Google Scholar] [CrossRef]
- Kashina, M.V.; Ivanov, D.M.; Kinzhalov, M.A. The Isocyanide Complexes cis-[MCl2(CNC6H4-4-X)2] (M = Pd, Pt; X = Cl, Br) as Tectons in Crystal Engineering Involving Halogen Bonds. Crystals 2021, 11, 799. [Google Scholar] [CrossRef]
- Safin, D.; Tumanov, N.; Leitch, A.A.; Brusso, J.L.; Filinchuk, Y.; Murugesu, M. Elucidating the elusive crystal structure of 2,4,6-tris(2-pyrimidyl)-1,3,5-triazine (TPymT) Through X-ray Powder Diffraction. CrystEngComm 2015, 17, 2190–2195. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Michalczyk, M.; Piec, K.; Zierkiewicz, W.; Ejfler, J.; Łukasz, J. Possible coordination modes of copper(II) atom in model silsesquioxanes complexes at various pH conditions: DFT study. Chem. Phys. Lett. 2021, 778, 138739. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals Radii of Elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S.J. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Reinen, D. Cu2+, a Chameleon in Coordination Chemistry. Comments Inorg. Chem. 2006, 2, 227–246. [Google Scholar] [CrossRef]
- Wertz, J.E.; Bolton, J.R. Basic Principles of Electron Spin Resonance. In Electron Spin Resonance; Springer: Dordrecht, The Netherlands, 1986; 497p. [Google Scholar]



| 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|
| Formula | C5H6Cl2CuN4 | C5H6Br2CuN4 | C5H6Cl2CuN4 | C5H6Br2CuN4 | C12H14Cl4Cu2N6 | C10H12Cl4Cu2N8 |
| Formula weight | 256.58 | 345.484 | 256.58 | 345.50 | 255.59 | 256.581 |
| Temperature/K | 100 | 100 | 100 | 100 | 100 | 100 |
| Crystal system | Orthorhombic | Orthorhombic | Orthorhombic | Orthorhombic | Monoclinic | Monoclinic |
| Space group, Z | Pnma, 4 | Pnma, 4 | Pnma, 4 | Pnma, 4 | P21/n, 4 | P21/n, 4 |
| a/Å | 8.710 (6) | 8.924 (2) | 8.6664 (2) | 8.98 (5) | 8.3731 (4) | 8.4622 (12) |
| b/Å | 6.292 (5) | 6.3937 (16) | 6.2964 (2) | 6.43 (3) | 10.9315 (6) | 10.6392 (14) |
| c/Å | 15.260 (11) | 15.626 (4) | 14.8940 (4) | 15.22 (14) | 9.5637 (3) | 9.6678 (13) |
| α/° | 90 | 90 | 90 | 90 | 90 | 90 |
| β/° | 90 | 90 | 90 | 90 | 103.022 (4) | 99.036 (5) |
| γ/° | 90 | 90 | 90 | 90 | 90 | 90 |
| Volume/Å3 | 836.4 (11) | 891.5 (4) | 812.72 (4) | 879 (11) | 852.86 (7) | 859.6 (2) |
| ρcalcg/cm3 | 2.038 | 2.574 | 2.097 | 2.610 | 1.991 | 1.983 |
| μ/mm−1 | 3.193 | 11.359 | 9.398 | 13.745 | 3.127 | 3.106 |
| F(000) | 508.0 | 652.1 | 508.0 | 652.0 | 508.0 | 508.0 |
| Crystal size/mm3 | 0.3 × 0.09 × 0.04 | 0.4 × 0.15 × 0.05 | 0.06 × 0.03 × 0.01 | 0.05 × 0.02 × 0.01 | 0.5 × 0.25 × 0.15 | 0.4 × 0.15 × 0.15 |
| Radiation | Mo Kα (λ = 0.71073) | Mo Kα (λ = 0.71073) | Cu Kα (λ = 1.54184) | Cu Kα (λ = 1.54184) | Mo Kα (λ = 0.71073) | Mo Kα (λ = 0.71073) |
| Reflections Collected | 17,318 | 11,741 | 7265 | 4587 | 7902 | 16,659 |
| Independent Reflections | 1376 | 1167 | 817 | 954 | 2956 | 2610 |
| Rint | 0.0488 | 0.0522 | 0.0455 | 0.0296 | 0.0463 | 0.0327 |
| Data/parameters | 1376/73 | 1167/82 | 817/75 | 954/76 | 2956/121 | 2610/121 |
| Goodness-of-fit on F2 | 1.344 | 1.073 | 1.103 | 1.049 | 1.050 | 1.017 |
| R1 [I ≥ 2σ (I)] | 0.0697 | 0.0328 | 0.0604 | 0.0574 | 0.0420 | 0.0269 |
| wR2 [I ≥ 2σ (I)] | 0.1601 | 0.0811 | 0.1548 | 0.1527 | 0.0889 | 0.0674 |
| R1 [all data] | 0.0767 | 0.0440 | 0.0658 | 0.0602 | 0.0560 | 0.0343 |
| Complexes | ||||||
|---|---|---|---|---|---|---|
| Bond, Å | 4 | 5 | 6 | 7 | 8 | 9 |
| Cu1-N1 | 2.058 (7) | 2.062 (4) | 1.950 (5) | 2.071 (15) | 2.045 (2) | 2.0607 (15) |
| Cu1-N2 | 1.973 (7) | 1.951 (5) | 2.050 (6) | 1.958 (12) | 1.949 (2) | 1.9504 (15) |
| Cu1-Hal2 | 2.283 (3) | 2.4307 (10) | 2.2570 (2) | 2.403 (17) | 2.2745 (7) | 2.2520 (6) |
| Cu1-Hal1 | 2.249 (3) | 2.3874 (9) | 2.2578 (18) | 2.396 (12) | 2.2747 (7) | 2.2540 (5) |
| Cu1-Hal3 | 2.7360 (7) | 2.854 (6) | ||||
| Angle, degr. | ||||||
| N1-Cu1-N4 | 80.62 (3) | 80.44 (19) | 79.9 (2) | 79.2 (6) | 80.14 (9) | 79.99 (6) |
| N1-Cu1-Hal1 | 92.84 (2) | 93.99 (13) | 93.28 (16) | 94.4 (6) | 94.83 (6) | 93.85 (4) |
| N4-Cu1-Hal2 | 91.70 (2) | 91.47 (14) | 92.87 (17) | 93.2 (6) | 90.90 (7) | 91.10 (5) |
| Hal1-Cu1-Hal2 | 94.84 (11) | 94.10 (4) | 93.94 (7) | 93.2 (6) | 92.61 (3) | 94.23 (19) |
| Hal1-Cu1-Hal3 | 91.86 (2) | 92.987 (18) | ||||
| Hal2-Cu1-Hal3 | 101.08 (3) | 106.225 (18) | ||||
| N1-Cu1-Hal3 | 92.85 (6) | 84.73 (4) | ||||
| N2-Cu1-Hal3 | 94.15 (7) | 90.09 (5) | ||||
| Contact, Å | Structure | |||
|---|---|---|---|---|
| In Layer | 4/Cl | 5/Br | 6/Cl | 7/Br |
| Hal1…H3 (B) | 2.612 (3) | 2.629 (5) | 2.459 (13) | 2.589 (8) |
| Hal1…H4A | - | - | 2.501 (16) | Disordered H |
| Hal2…H2 | 2.537 (3) | 2.561 (6) | 2.517 (15) | 2.580 (5) |
| Hal2…H4B(A) | 2.615 (3) | 2.615 (6) | 2.560 (15) | Disordered H |
| Between Layers | ||||
| Cu…Hal * | 3.174 (3) | 3.210 (8) | 3.167 (3) | 3.223 (14) |
| Complex | Ligands | Temperature, °C |
|---|---|---|
| 4 | 2, Cl | 266 |
| 5 | 2, Br | 263 |
| 6 | 3, Cl | 199.5 |
| 7 | 3, Br | 238 |
| 8 | 1, Cl | 362 |
| 9 | 2, Cl | 226 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamba, Z.; Peoble, A.; Novikov, E.M.; Castañeda, R.; Timofeeva, T.V. Interplay of Isomorphs and Polymorphs of Amidino-Copper(II) Complexes with Different Halides. Crystals 2024, 14, 319. https://doi.org/10.3390/cryst14040319
Yamba Z, Peoble A, Novikov EM, Castañeda R, Timofeeva TV. Interplay of Isomorphs and Polymorphs of Amidino-Copper(II) Complexes with Different Halides. Crystals. 2024; 14(4):319. https://doi.org/10.3390/cryst14040319
Chicago/Turabian StyleYamba, Zaina, Anna Peoble, Egor M. Novikov, Raúl Castañeda, and Tatiana V. Timofeeva. 2024. "Interplay of Isomorphs and Polymorphs of Amidino-Copper(II) Complexes with Different Halides" Crystals 14, no. 4: 319. https://doi.org/10.3390/cryst14040319
APA StyleYamba, Z., Peoble, A., Novikov, E. M., Castañeda, R., & Timofeeva, T. V. (2024). Interplay of Isomorphs and Polymorphs of Amidino-Copper(II) Complexes with Different Halides. Crystals, 14(4), 319. https://doi.org/10.3390/cryst14040319






