Two New Energetic Hexagonal Anti-Perovskites (N2H5)3X[B12H12] · H2O (X− = [NO3]− and [ClO4]−): Crystal Structure, Vibrational Spectra, and Thermal Decomposition
Abstract
1. Introduction
2. Materials and Methods
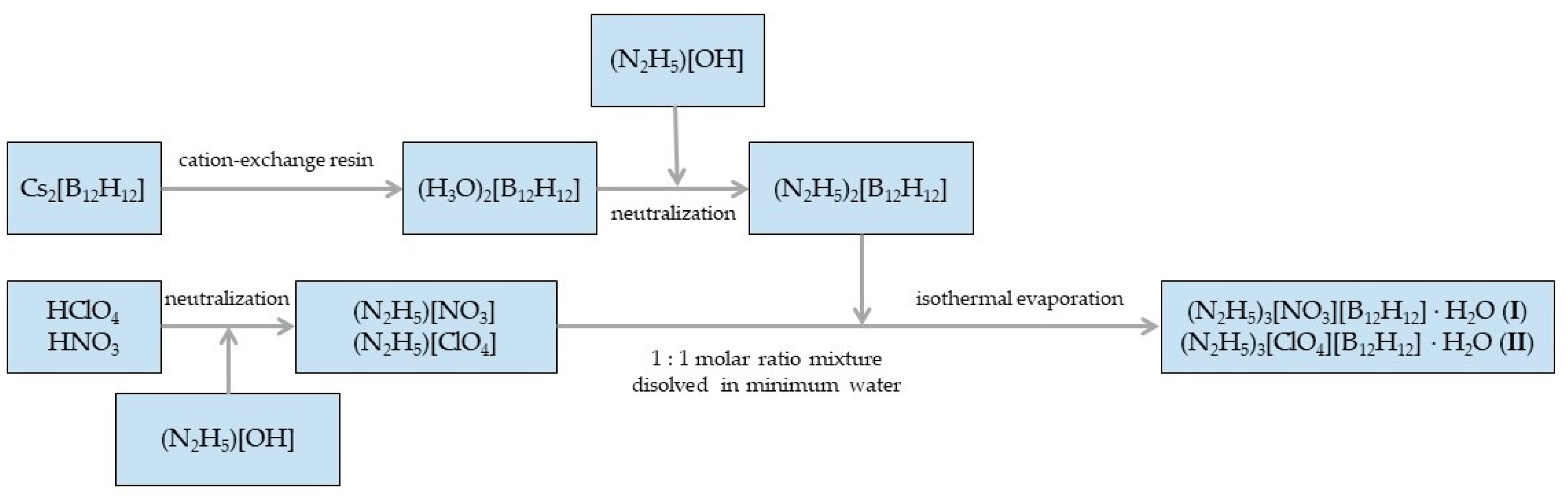
2.1. Synthesis
2.2. Characterization Techniques
2.2.1. X-ray Crystallography
2.2.2. Raman Spectroscopy
2.2.3. Thermal Analysis
2.2.4. Elemental Analysis
3. Results
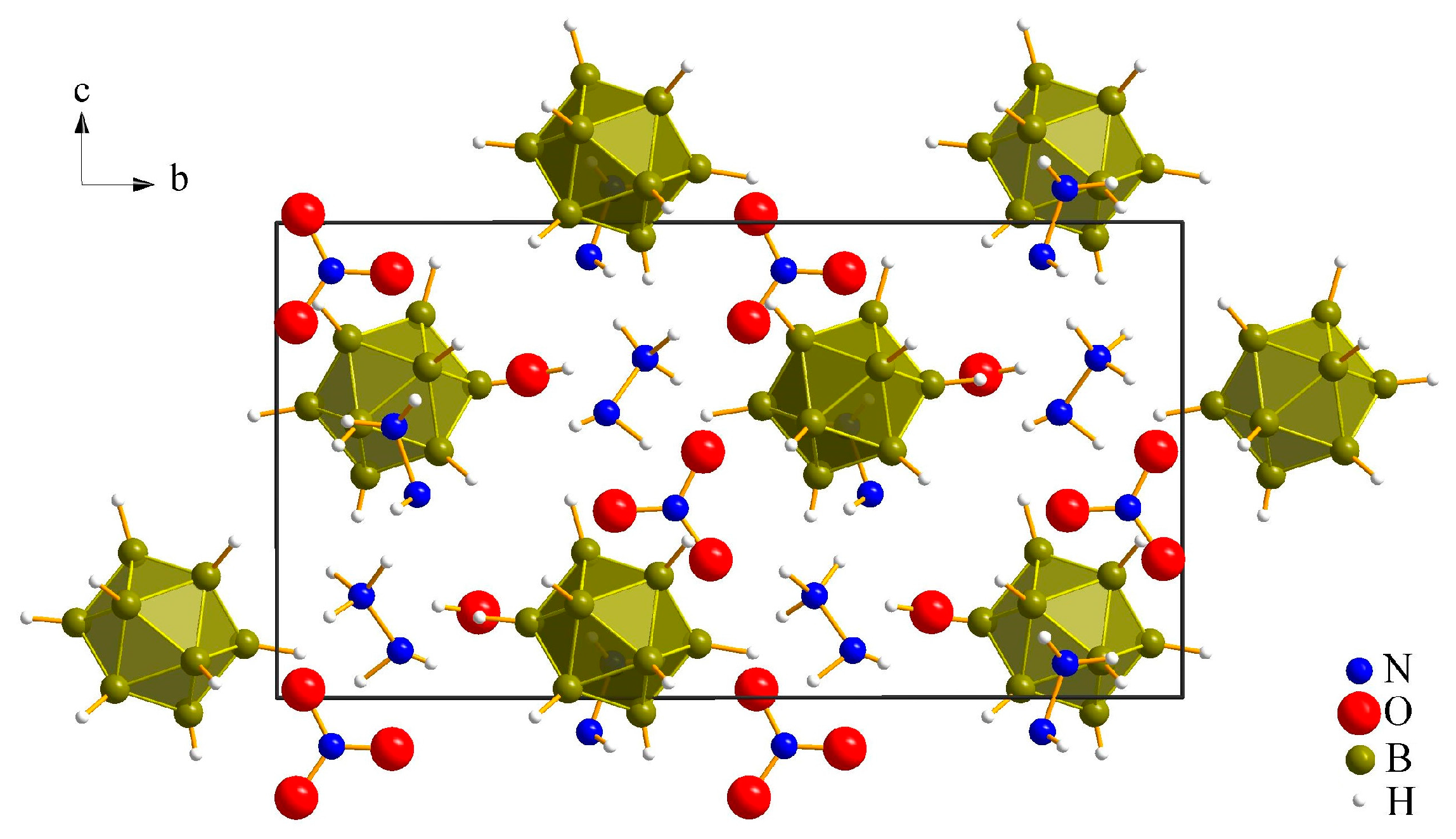
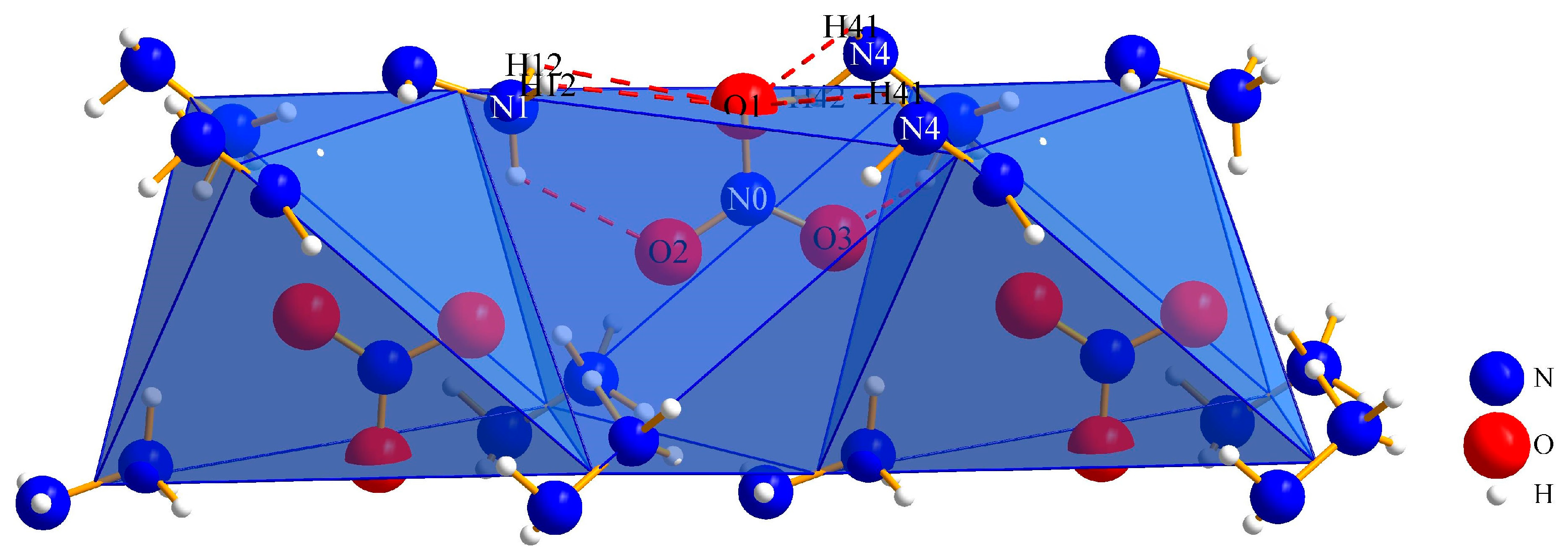
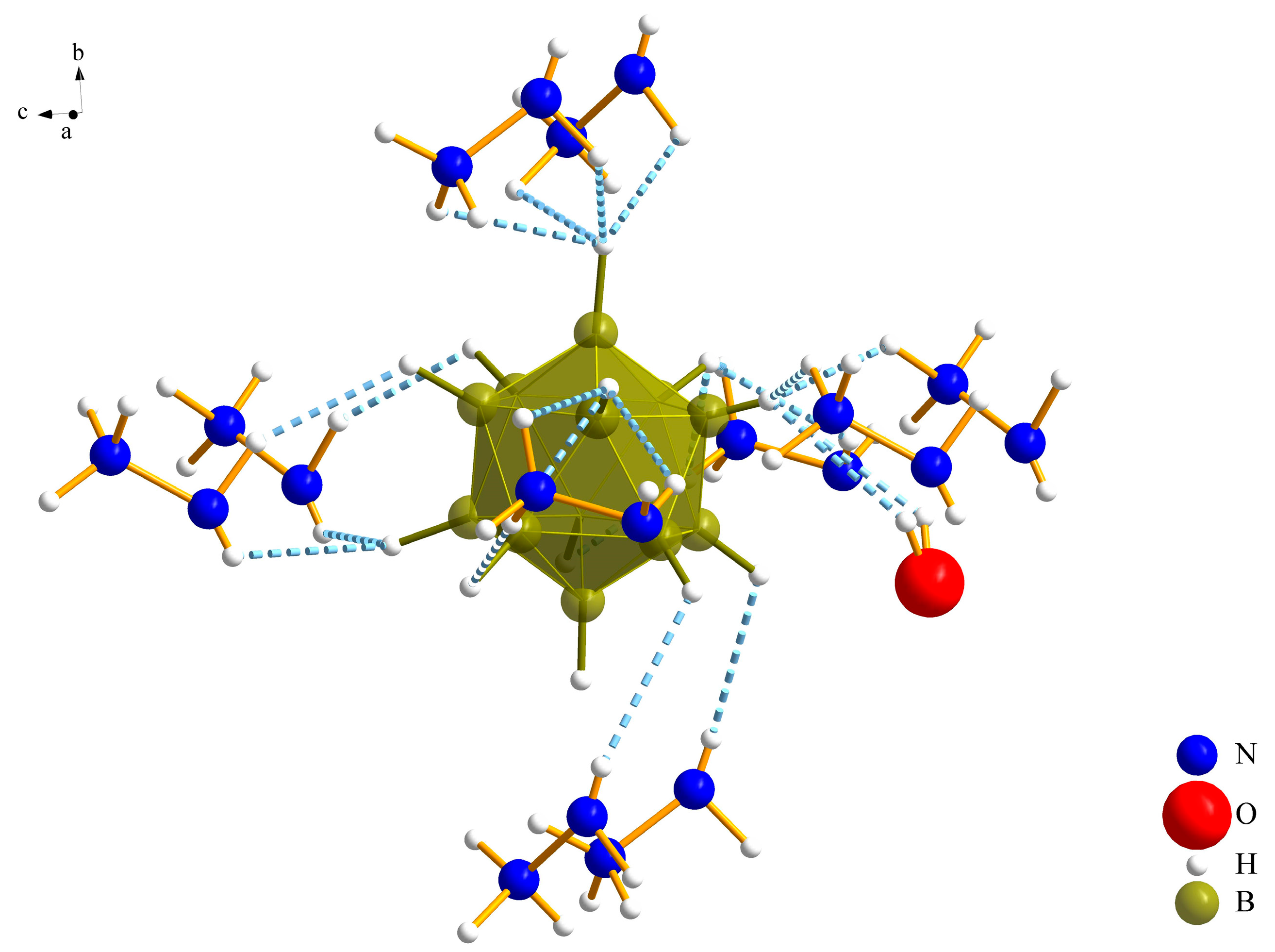
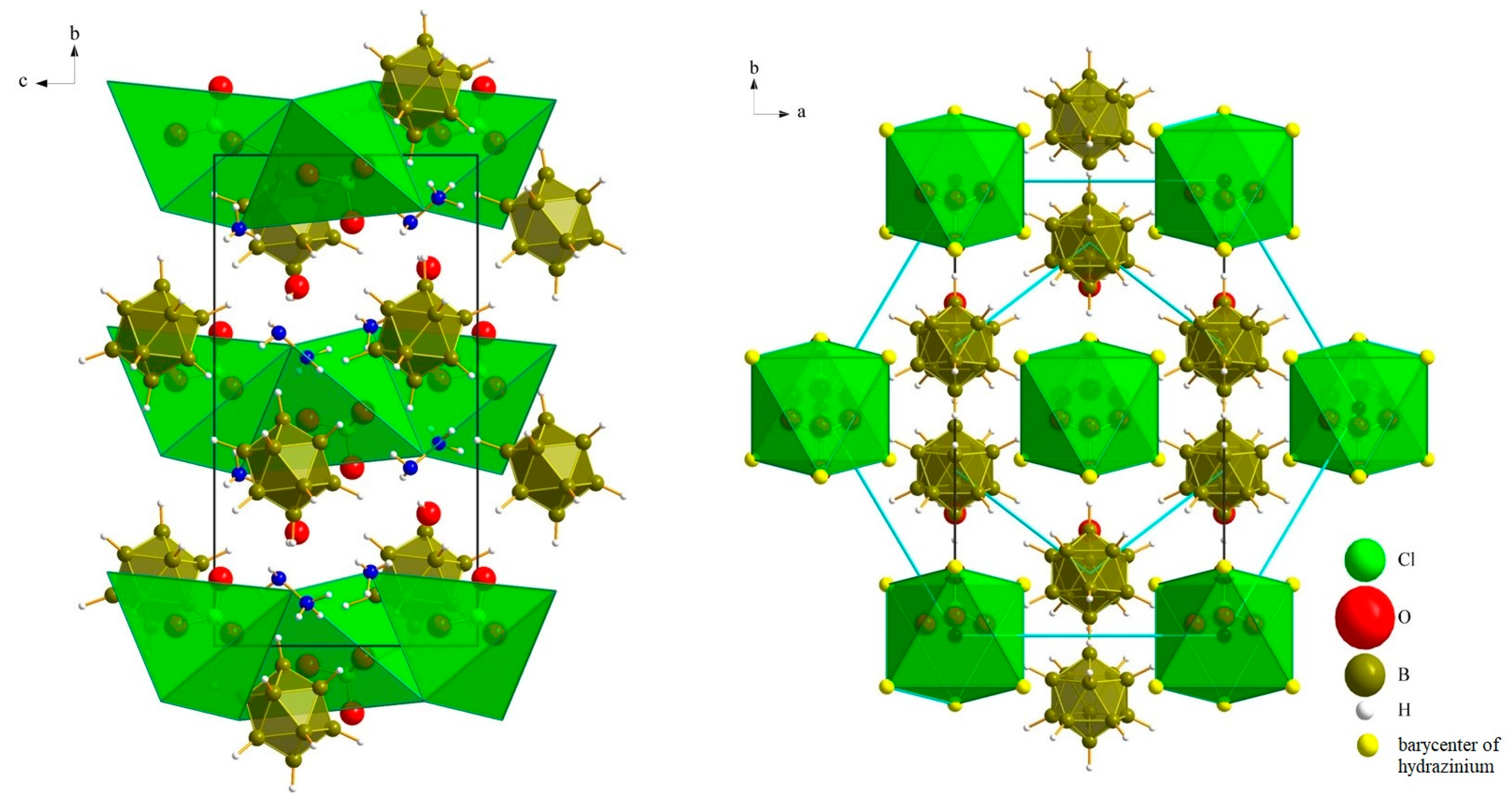
3.1. Crystal Structures
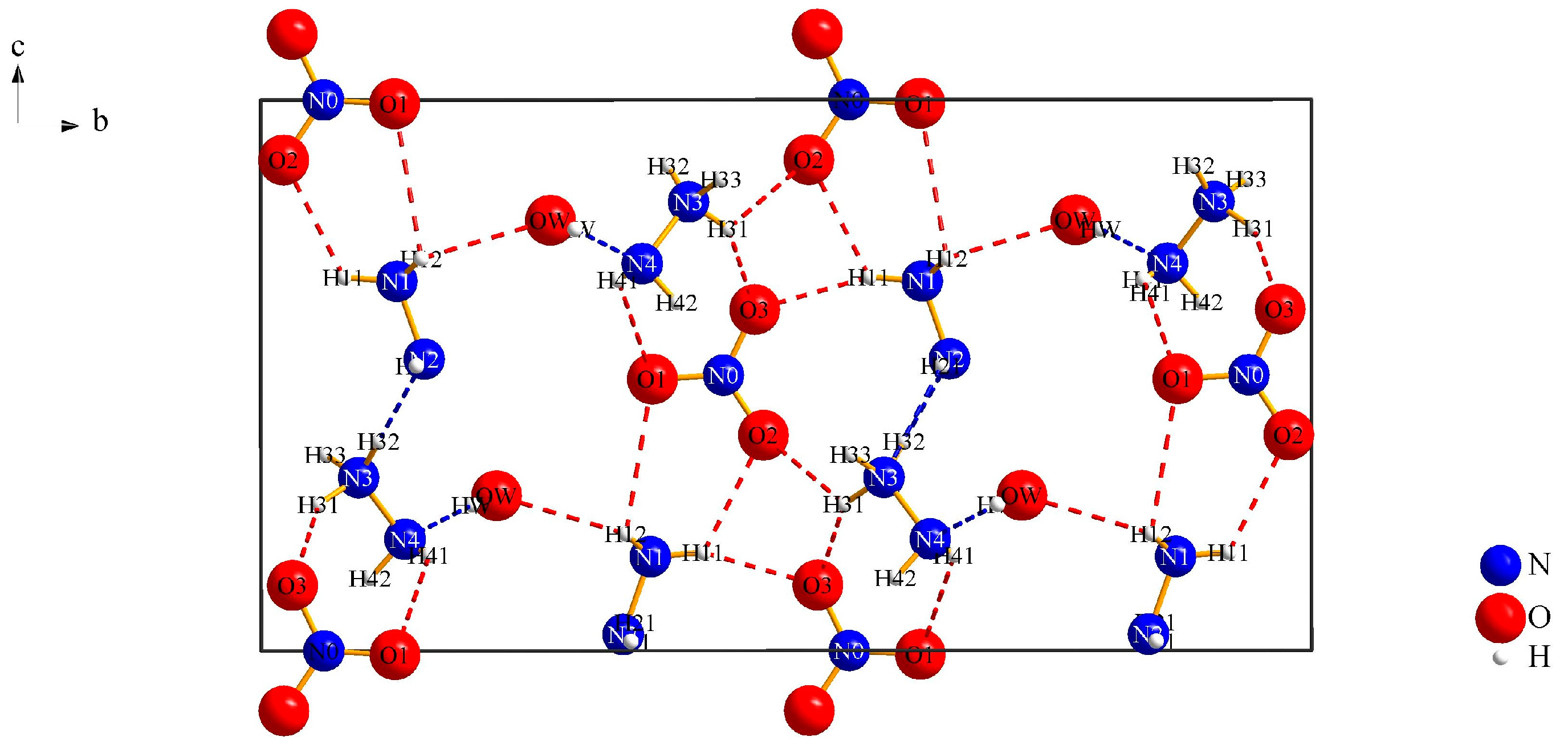
3.1.1. (N2H5)3[NO3][B12H12] · H2O (I)
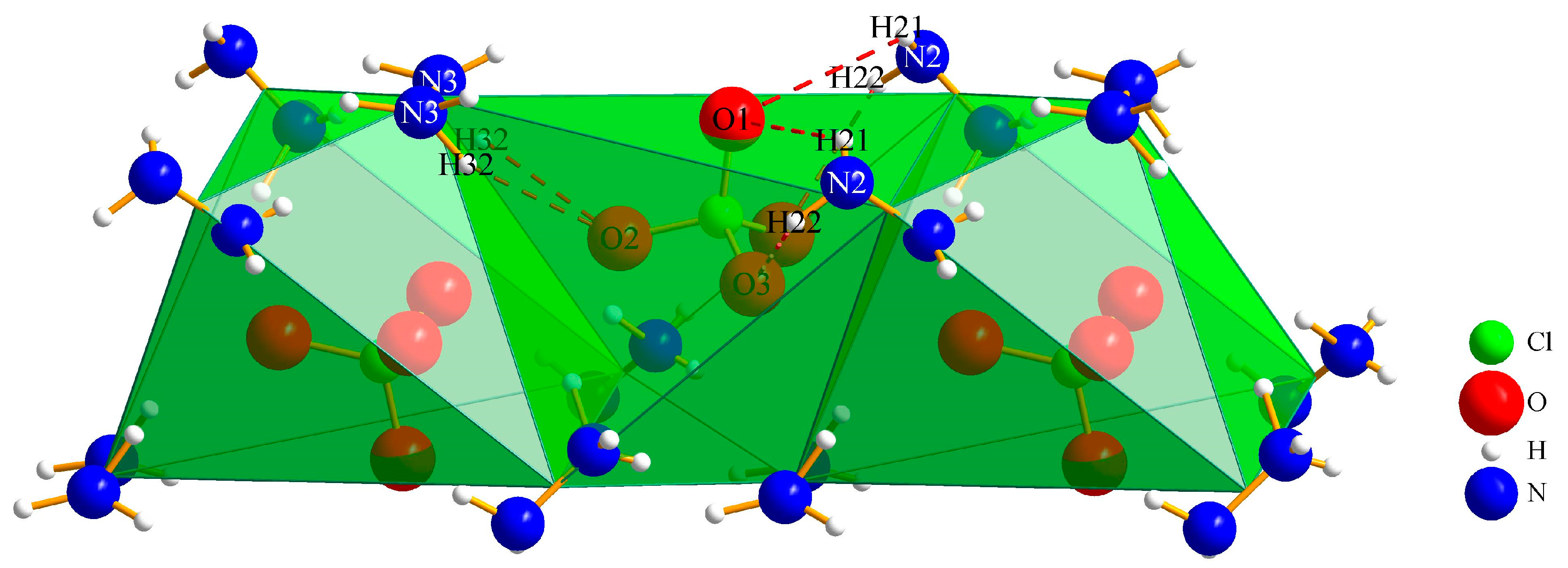
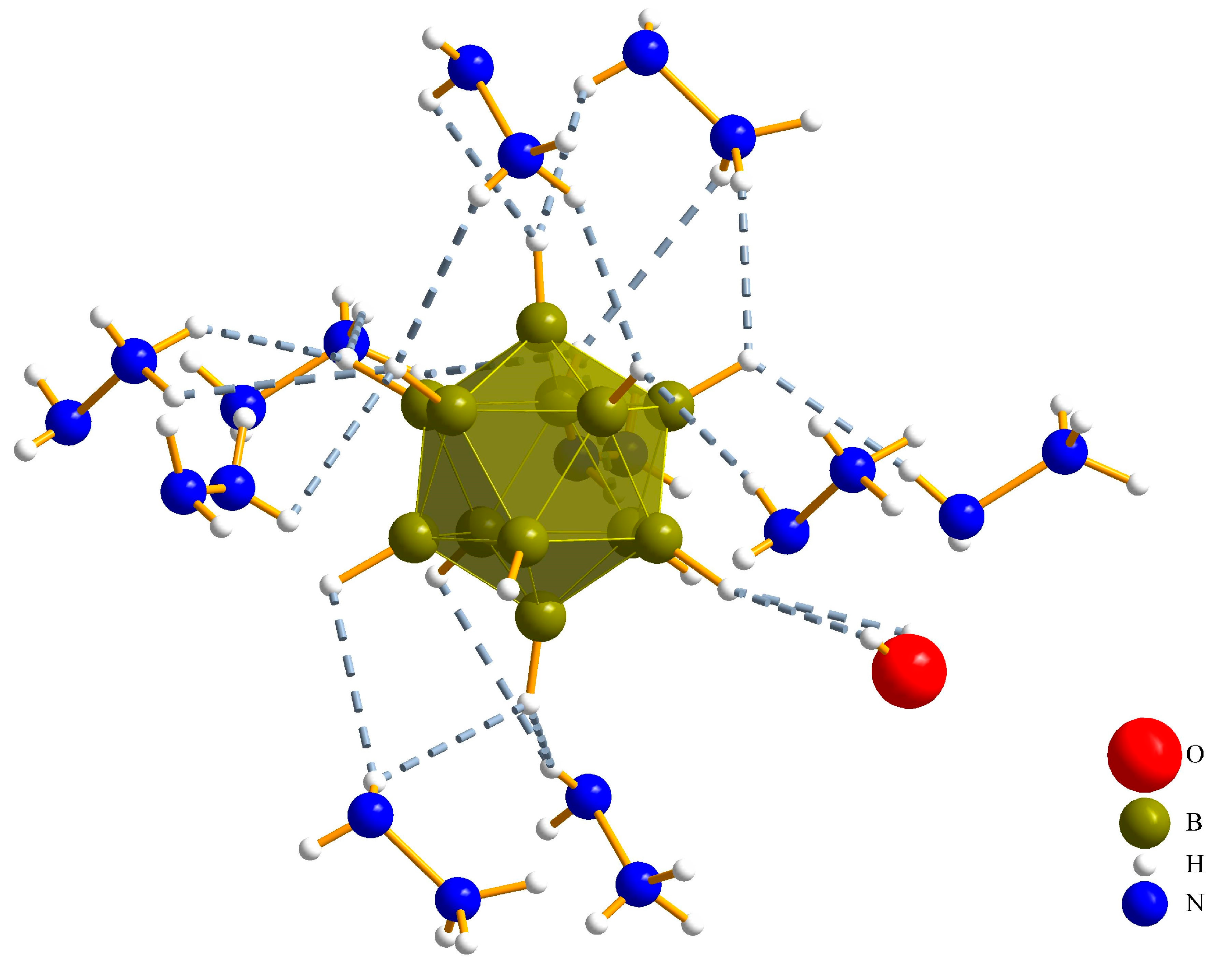
3.1.2. (N2H5)3[ClO4][B12H12] · H2O (II)
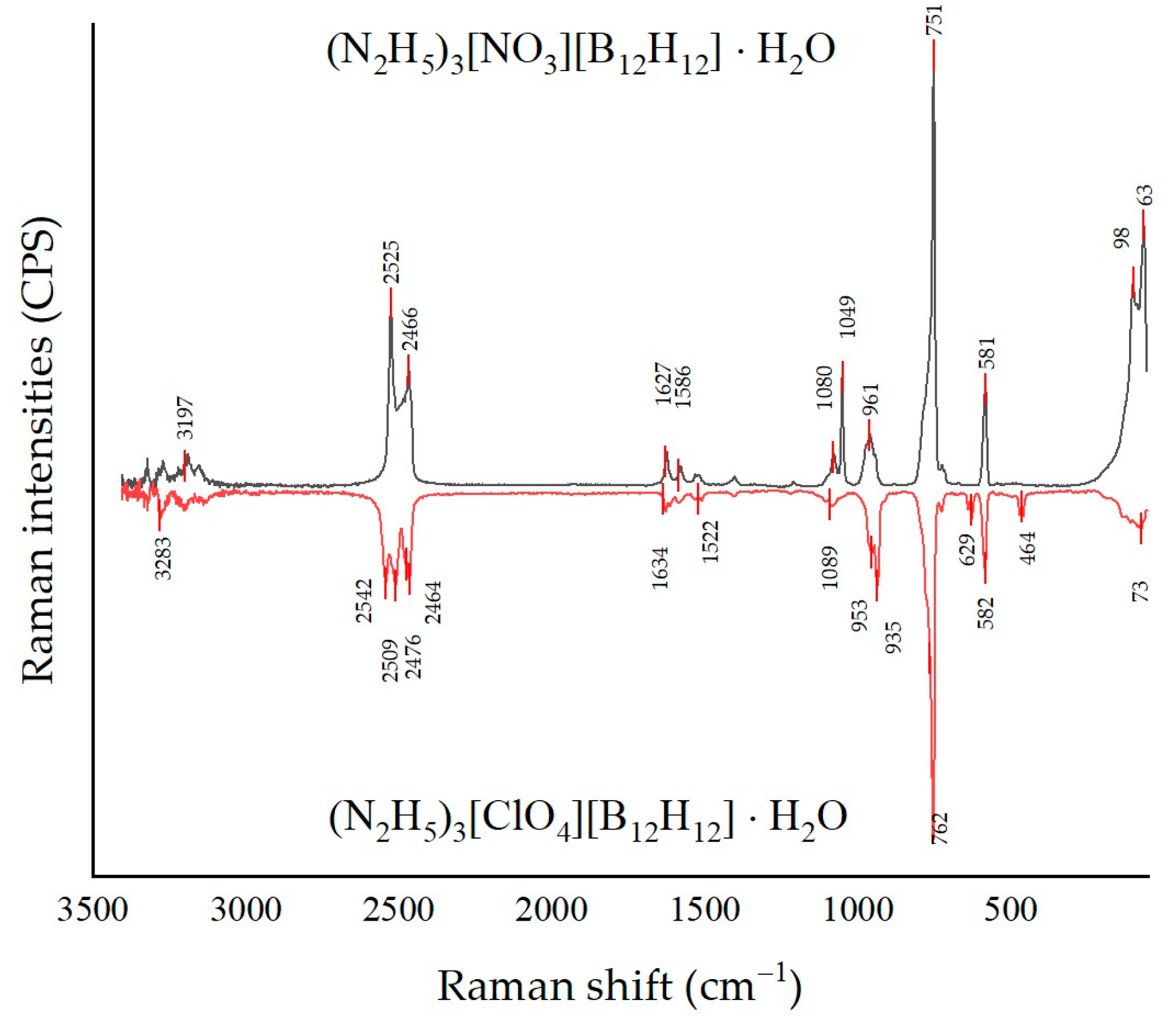
3.2. Raman Spectroscopy
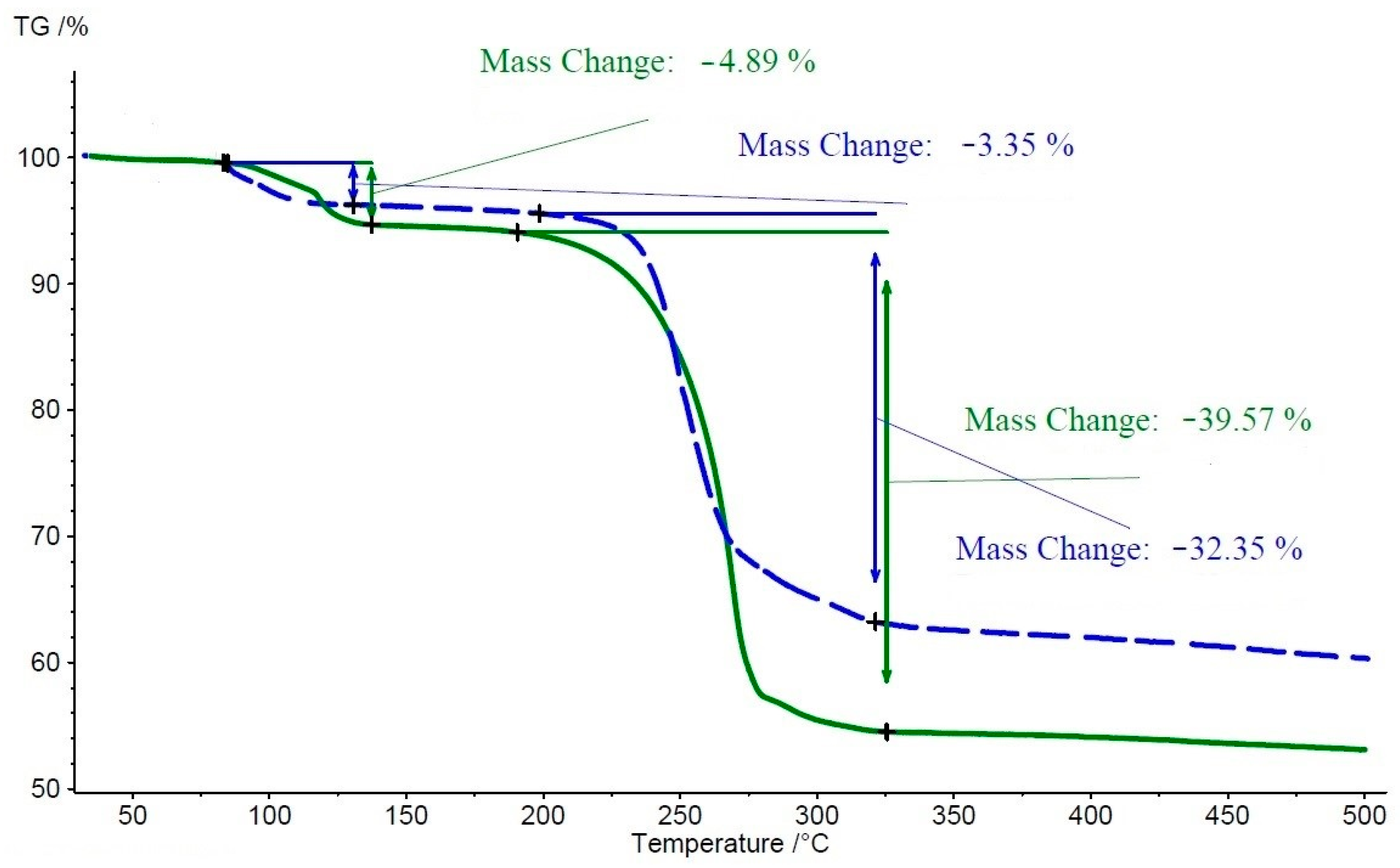
3.3. Thermal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klapötke, T.M. Chemistry of High-Energy Materials; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2022. [Google Scholar]
- Zawadzka-Małota, I. Testing of Mining Explosives with Regard to the Content of Carbon Oxides and Nitrogen Oxides in Their Detonation Products. J. Sustain. Min. 2015, 14, 173–178. [Google Scholar] [CrossRef]
- Isobe, D.; Jiang, R. Explosive Demolition Planning of Building Structures Using Key Element Index. J. Build. Eng. 2022, 59, 104935. [Google Scholar] [CrossRef]
- Pang, W.; Klapötke, T.M.; DeLuca, L.T.; OuYang, D.; Qin, Z.; Hu, Y.; Fan, X.; Zhao, F. Effects of TKX-50 on the Performance of Solid Propellants and Explosives. Nano Micro-Scale Energ. Mater. Propellants Explos. 2023, 1, 149–190. [Google Scholar]
- Galante, E.; Haddad, A.; Marques, N. Application of Explosives in the Oil Industry. Int. J. Oil Gas Coal Eng. 2013, 1, 16–22. [Google Scholar] [CrossRef]
- Steinhauser, G.; Klapötke, T.M. “Green” Pyrotechnics: A Chemists’ Challenge. Angew. Chem. Int. Ed. 2008, 47, 3330–3347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wu, Y.; Zhang, W.; Mu, J. Evaluation of a New Primary Explosive: Nickel Hydrazine Nitrate (NHN) Complex. Propellants Explos. Pyrotech. 1997, 22, 317–320. [Google Scholar]
- Davis, S.M.; Yilmaz, N. Advances in Hypergolic Propellants: Ignition, Hydrazine, and Hydrogen Peroxide Research. Adv. Aerosp. Eng. 2014, 2014, 729313. [Google Scholar] [CrossRef]
- Karaghiosoff, K.; Klapötke, T.M.; Sabaté, C.M. Nitrogen-Rich Compounds in Pyrotechnics: Alkaline-Earth Metal Salts of 5,5′-Hydrazine-1, 2-diylbis(1H-tetrazole). Eur. J. Inorg. Chem. 2009, 2, 238–250. [Google Scholar] [CrossRef]
- Niemeier, J.K.; Kjell, D.P. Hydrazine and Aqueous Hydrazine Solutions: Evaluating Safety in Chemical Processes. Org. Proc. Res. Dev. 2013, 17, 1580–1590. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J.; Mao, Z.; Hong, Z.; Kang, B. Crystal Structure of Hydrazine Nitrate. Energ. Mater. 2008, 16, 73–77. [Google Scholar]
- Conant, J.W.; Roof, R.B. The Crystal Structures of the Isostructural Compounds Hydrazinium Fluoroborate and Hydrazinium Perchlorate. Acta Crystallogr. 1970, 26, 1928–1932. [Google Scholar] [CrossRef]
- Wunderlich, J.A.; Lipscomb, W.N. Structure of B12H122– Ion. J. Am. Chem. Soc. 1960, 82, 4427–4428. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I.; Sjöberg, S. Chemistry of closo-Dodecaborate Anion [B12H12]2–: A Review. Collect. Czech. Chem. Commun. 2002, 67, 679–727. [Google Scholar] [CrossRef]
- Tiritiris, I. Untersuchungen zu Reaktivität, Aufbau und Struktureller Dynamik von Salzartigen closo-Dodekaboraten. Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 2004. [Google Scholar]
- Zimmermann, L.W.; Aghaei Hakkak, R.; Ranjbar, M.; Schleid, T. Crystal Structures and Thermal Analyses of Three New High-Energy Hydrazinium Hydro-closo-Borates. Int. J. Hydrogen Energy 2024, 49, 1469–1477. [Google Scholar] [CrossRef]
- Derdziuk, J.; Malinowski, P.J.; Jaroń, T. Synthesis, Structural Characterization and Thermal Decomposition Studies of (N2H5)2B12H12 and Its Solvates. Int. J. Hydrogen Energy 2019, 44, 27030–27038. [Google Scholar] [CrossRef]
- Wenk, H.-R.; Bulakh, A. Minerals: Their Constitution and Origin; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Zhang, L.; Mei, L.; Wang, K.; Lv, Y.; Zhang, S.; Lian, Y.; Liu, X.; Ma, Z.; Xiao, G.; Liu, Q. Advances in the Application of Perovskite Materials. Nano-Micro Lett. 2023, 15, 177. [Google Scholar] [CrossRef] [PubMed]
- Kirmani, A.R.; Ostrowski, D.P.; VanSant, K.T.; Byers, T.A.; Bramante, R.C.; Heinselman, K.N.; Tong, J.; Stevens, B.; Nemeth, W.; Zhu, K. Metal Oxide Barrier Layers for Terrestrial and Space Perovskite Photovoltaics. Nat. Energy 2023, 8, 191–202. [Google Scholar] [CrossRef]
- Souri, M.; Amoli, H.S. Gas Sensing Mechanisms in ABO3 Perovskite Materials at Room Temperature: A Review. Mater. Sci. Semicond. Process. 2023, 156, 107271. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kang, W.; Yan, Z.; Shen, Y.; Huo, J. Anti-Perovskite Nitrides and Oxides: Properties and Preparation. Comput. Mater. Sci. 2023, 225, 112188. [Google Scholar] [CrossRef]
- Bareiß, K.U.; Bette, S.; Enseling, D.; Jüstel, T.; Schleid, T. Extraordinary Intense Blue Tl+ Lone-Pair Photoluminescence from Thallium(I) Chloride Hydroborate Tl3Cl[B12H12]. Dalton. Trans. 2022, 51, 13331–13341. [Google Scholar] [CrossRef]
- Tiritiris, I.; Weidlein, J.; Schleid, T. Dodekahydro-closo-Dodekaborat-Halogenide der schweren Alkalimetalle mit der Formel M3X[B12H12] (M = K–Cs, NH4; X = Cl und Br)/Dodecahydro-closo-Dodecaborate Halides of the Heavy Alkali Metals with the Formula M3X[B12H12] (M = K–Cs, NH4; X = Cl and Br). Z. Naturforschung B 2005, 60, 627–639. [Google Scholar] [CrossRef]
- Schouwink, P.; Sadikin, Y.; van Beek, W.; Černý, R. Experimental Observation of Polymerization from BH4− to B12H122− in Mixed-Anion A3BH4B12H12 (A = Rb+, Cs+). Int. J. Hydrogen Energy 2015, 40, 10902–10907. [Google Scholar] [CrossRef]
- Aghaei Hakkak, R.; Tiritiris, I.; Schleid, T. Synthesis and Characterization of High-Energy Anti-Perovskite Compounds Cs3X[B12H12] Based on Cesium Dodecahydro-closo-Borate with Molecular Oxoanions (X− = [NO3]−, [ClO3]− and [ClO4]−). Molecules 2024, 29, 382. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shang, Y.; Jiang, J.; Huang, M.; Ren, J.; Guo, T.; Yu, C.; Zhang, W.; Chen, X. A New Nitrate-Based Energetic Molecular Perovskite as a Modern Edition of Black Powder. Energ. Mater. Front. 2022, 3, 122–127. [Google Scholar] [CrossRef]
- Zhou, J.; Ding, L.; Zhao, F.; Wang, B.; Zhang, J. Thermal Studies of Novel Molecular Perovskite Energetic Material (C6H14N2)[NH4(ClO4)3]. Chin. Chem. Lett. 2020, 31, 554–558. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Chen, S.; Zhao, F.; Qiu, L.; Meng, Z.; Ding, L.; Wang, B.; Pan, Q. Comparative Thermal Research on Energetic Molecular Perovskite Structures. Molecules 2022, 27, 805. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXS-97 and SHELXL-97, Program for Crystal Structure Solution and Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sass, R.L.; Vidale, R.; Donohue, J. Interatomic Distances and Thermal Anisotropy in Sodium Nitrate and Calcite. Acta Crystallogr. 1957, 10, 567–570. [Google Scholar] [CrossRef]
- Fugel, M.; Malaspina, L.A.; Pal, R.; Thomas, S.P.; Shi, M.W.; Spackman, M.A.; Sugimoto, K.; Grabowsky, S. Revisiting a Historical Concept by Using Quantum Crystallography: Are Phosphate, Sulfate and Perchlorate Anions Hypervalent? Chem. Eur. J. 2019, 25, 6523–6532. [Google Scholar] [CrossRef]
- Muller, P.; Herbst-Irmer, R.; Spek, A.; Schneider, T.; Sawaya, M. Crystal Structure Refinement: A Crystallographer’s Guide to SHELXL; OUP Oxford: Oxford, UK, 2006; Volume 8. [Google Scholar]
- Fontana, M.D.; Mabrouk, K.B.; Kauffmann, T.H. Raman Probe of Pollutants in Water: Measurement Process. In Proceedings of the 4th Imeko TC19 Symposium on Environmental Instrumentation and Measurements Protecting Environment, Climate Changes and Pollution Control, IMEKO, Budapest, Hungary, 3–4 June 2013; pp. 27–29. [Google Scholar]
- Zapata, F.; García-Ruiz, C. The Discrimination of 72 Nitrate, Chlorate and Perchlorate Salts Using IR and Raman Spectroscopy. Spectrochim. Acta 2018, 189, 535–542. [Google Scholar] [CrossRef]
- Aghaei Hakkak, R.; Schleid, T. Crystal Structure and Thermal Behavior of Three Potential High-Energy Compounds of Hydro-closo-Borates with Guanidinium. J. Solid State Chem. 2024, 329, 124416. [Google Scholar] [CrossRef]
- Simoes, M.C.; Hughes, K.J.; Ingham, D.B.; Ma, L.; Pourkashanian, M. Estimation of the Thermochemical Radii and Ionic Volumes of Complex Ions. Inorg. Chem. 2017, 56, 7566–7573. [Google Scholar] [CrossRef] [PubMed]



| Compound | N (Theoretical/Found) % | H (Theoretical/Found) % |
|---|---|---|
| (N2H5)3[NO3][B12H12] · H2O | 30.54/30.48 | 9.11/9.09 |
| (N2H5)3[ClO4][B12H12] · H2O | 23.45/23.34 | 8.15/8.10 |
| Compound | (N2H5)3[NO3][B12H12] · H2O (I) | (N2H5)3[ClO4][B12H12] · H2O (II) |
|---|---|---|
| temperature, K | 293 | 293 |
| crystal system | orthorhombic | orthorhombic |
| space group | Cmc21 (no. 36) | Cmc21 (no. 36) |
| formula units, Z | 4 | 4 |
| a, pm | 915.94(5) | 1040.51(6) |
| b, pm | 1817.45(9) | 1757.68(9) |
| c, pm | 952.67(5) | 942.34(5) |
| Dcal, g cm−3 | 1.34 | 1.22 |
| Vm, cm3 mol−1 | 238.8(1) | 259.5(1) |
| μ(MoKα), mm−1 | 0.09 | 0.24 |
| F(000), e− | 680 | 752 |
| hkl range | ±(12, 23, 12) | ±(13, 22, 12) |
| θmax,° | 28.55 | 27.48 |
| reflections measured | 11,324 | 14,845 |
| reflections unique | 1102 | 1144 |
| Rint, Rσ | 0.087, 0.042 | 0.096, 0.051 |
| R1, wR2 | 0.055, 0.144 | 0.045, 0.107 |
| GooF | 1.068 | 1.047 |
| CSD number | 2331546 | 2331555 |
| Interaction | Hydrogen-Bond Length (d/pm) | Angle (∡/°) |
|---|---|---|
| Ow∙∙∙H12–N1 | 242(4) | 111(3) |
| O1∙∙∙H41–N4 | 284(5) | 120(53) |
| O1∙∙∙H12–N1 | 280(4) | 99(3) |
| O2∙∙∙H11–N1 | 228(6) | 121(4) |
| O2∙∙∙H31–N3 | 235(4) | 120(3) |
| O3∙∙∙H11–N1 | 205(5) | 160(5) |
| N2∙∙∙H32–N3 | 215(4) | 177(3) |
| N4∙∙∙Ow–Hw | 218(4) | 166(5) |
| Interaction | Hydrogen-Bond Length (d/pm) | Angle (∡/°) |
|---|---|---|
| O1∙∙∙Hw–Ow | 265(3) | 128(4) |
| O1∙∙∙H21–N2 | 310(4) | 126(4) |
| O2∙∙∙H13–N1 | 225(4) | 147(3) |
| O2∙∙∙H32–N3 | 234(5) | 158(4) |
| O3∙∙∙H13–N1 | 234(4) | 126(3) |
| O3∙∙∙H22–N2 | 271(4) | 111(3) |
| Ow∙∙∙H31–N3 | 246(6) | 126(5) |
| N3∙∙∙H11–N1 | 237(5) | 166(6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghaei Hakkak, R.; Klapötke, T.M.; Schleid, T. Two New Energetic Hexagonal Anti-Perovskites (N2H5)3X[B12H12] · H2O (X− = [NO3]− and [ClO4]−): Crystal Structure, Vibrational Spectra, and Thermal Decomposition. Crystals 2024, 14, 310. https://doi.org/10.3390/cryst14040310
Aghaei Hakkak R, Klapötke TM, Schleid T. Two New Energetic Hexagonal Anti-Perovskites (N2H5)3X[B12H12] · H2O (X− = [NO3]− and [ClO4]−): Crystal Structure, Vibrational Spectra, and Thermal Decomposition. Crystals. 2024; 14(4):310. https://doi.org/10.3390/cryst14040310
Chicago/Turabian StyleAghaei Hakkak, Rouzbeh, Thomas M. Klapötke, and Thomas Schleid. 2024. "Two New Energetic Hexagonal Anti-Perovskites (N2H5)3X[B12H12] · H2O (X− = [NO3]− and [ClO4]−): Crystal Structure, Vibrational Spectra, and Thermal Decomposition" Crystals 14, no. 4: 310. https://doi.org/10.3390/cryst14040310
APA StyleAghaei Hakkak, R., Klapötke, T. M., & Schleid, T. (2024). Two New Energetic Hexagonal Anti-Perovskites (N2H5)3X[B12H12] · H2O (X− = [NO3]− and [ClO4]−): Crystal Structure, Vibrational Spectra, and Thermal Decomposition. Crystals, 14(4), 310. https://doi.org/10.3390/cryst14040310






