Potential of Y2Sn2O7:Eu3+, Dy3+ Inorganic Nanophosphors in Latent Fingermark Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Phosphor Samples
2.2. Characterization
3. Results and Discussion
3.1. Powder XRD Analysis
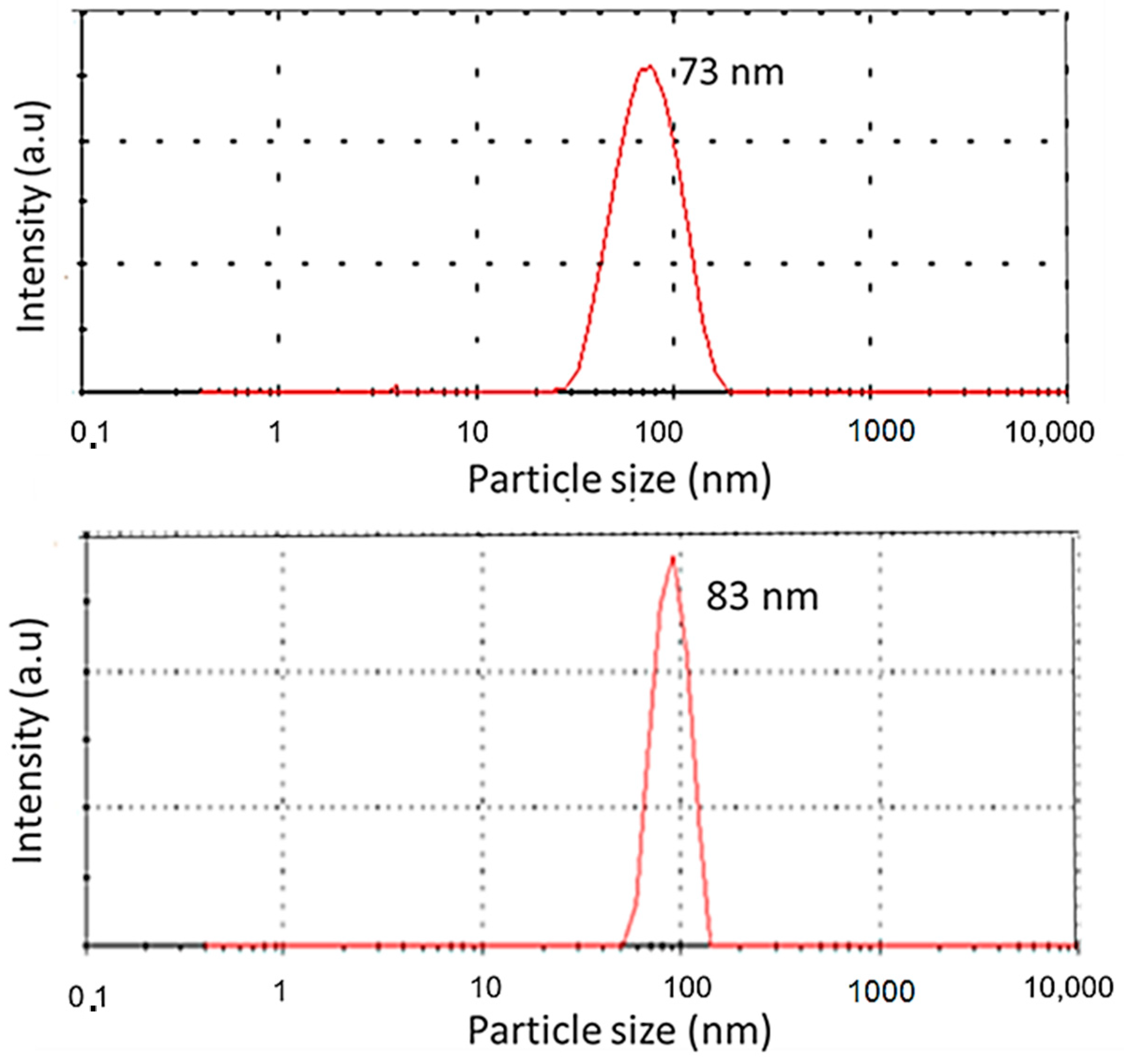
3.2. DLS Analysis
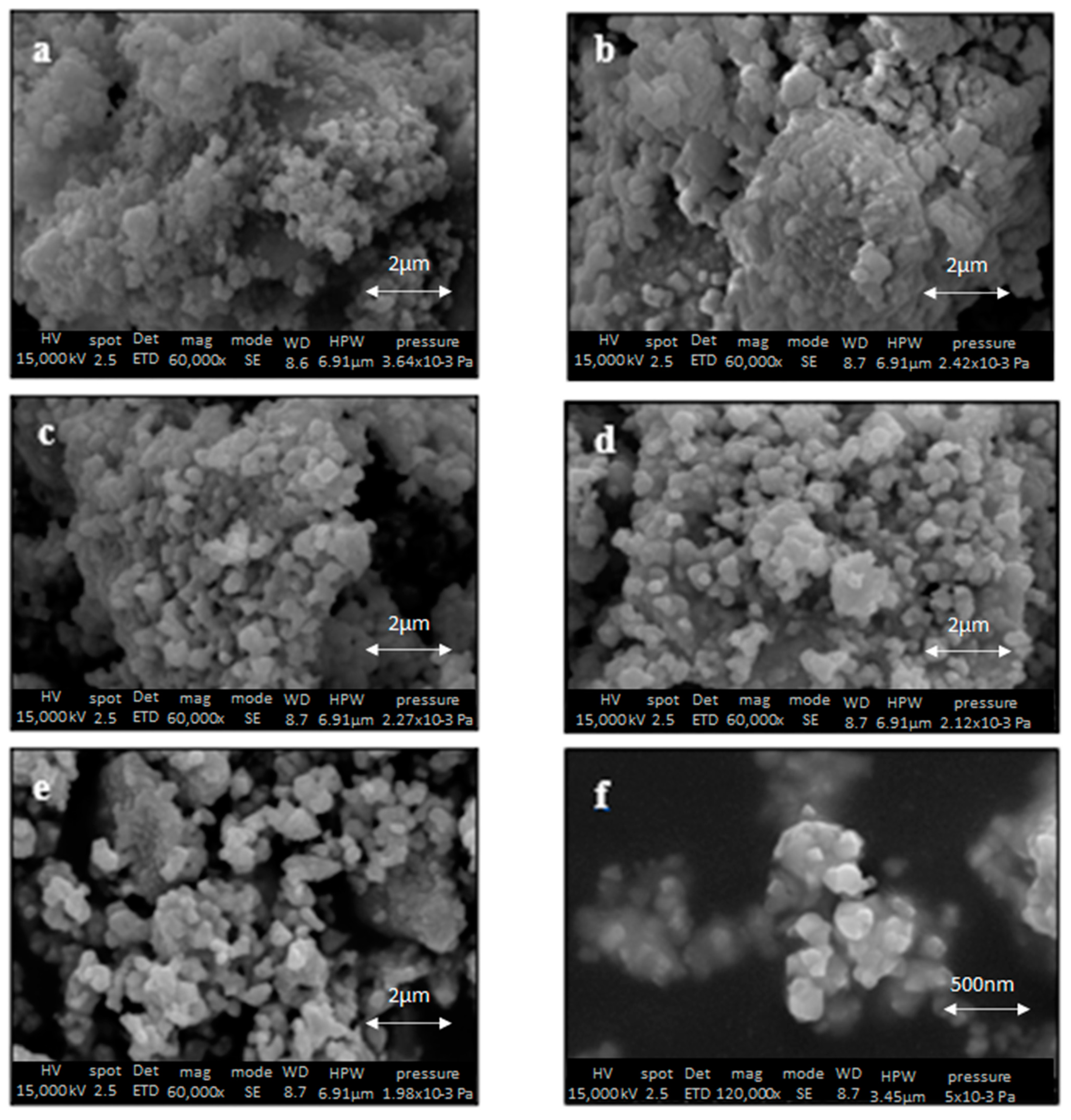
3.3. SEM Characterization
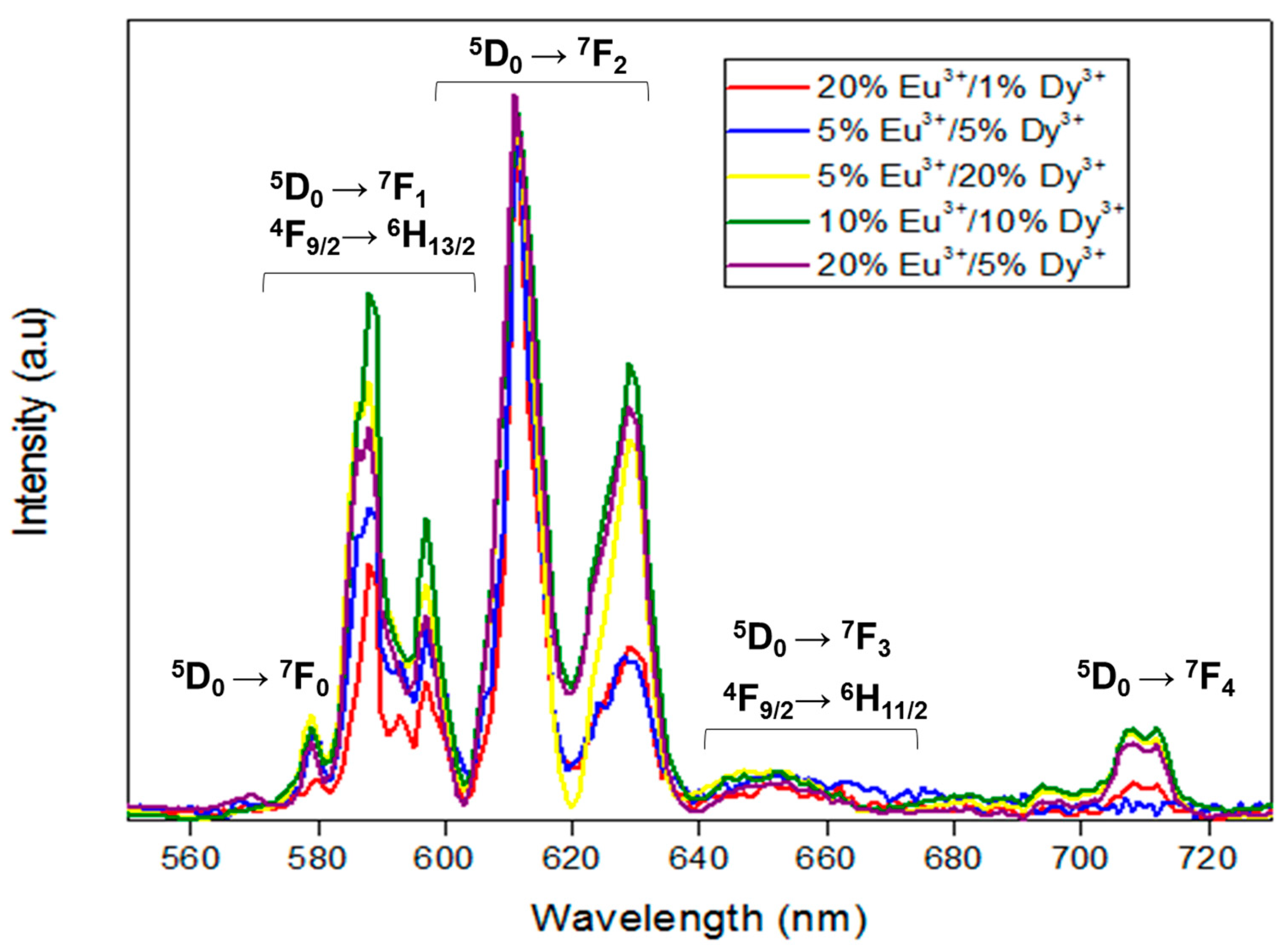
3.4. Photoluminescence
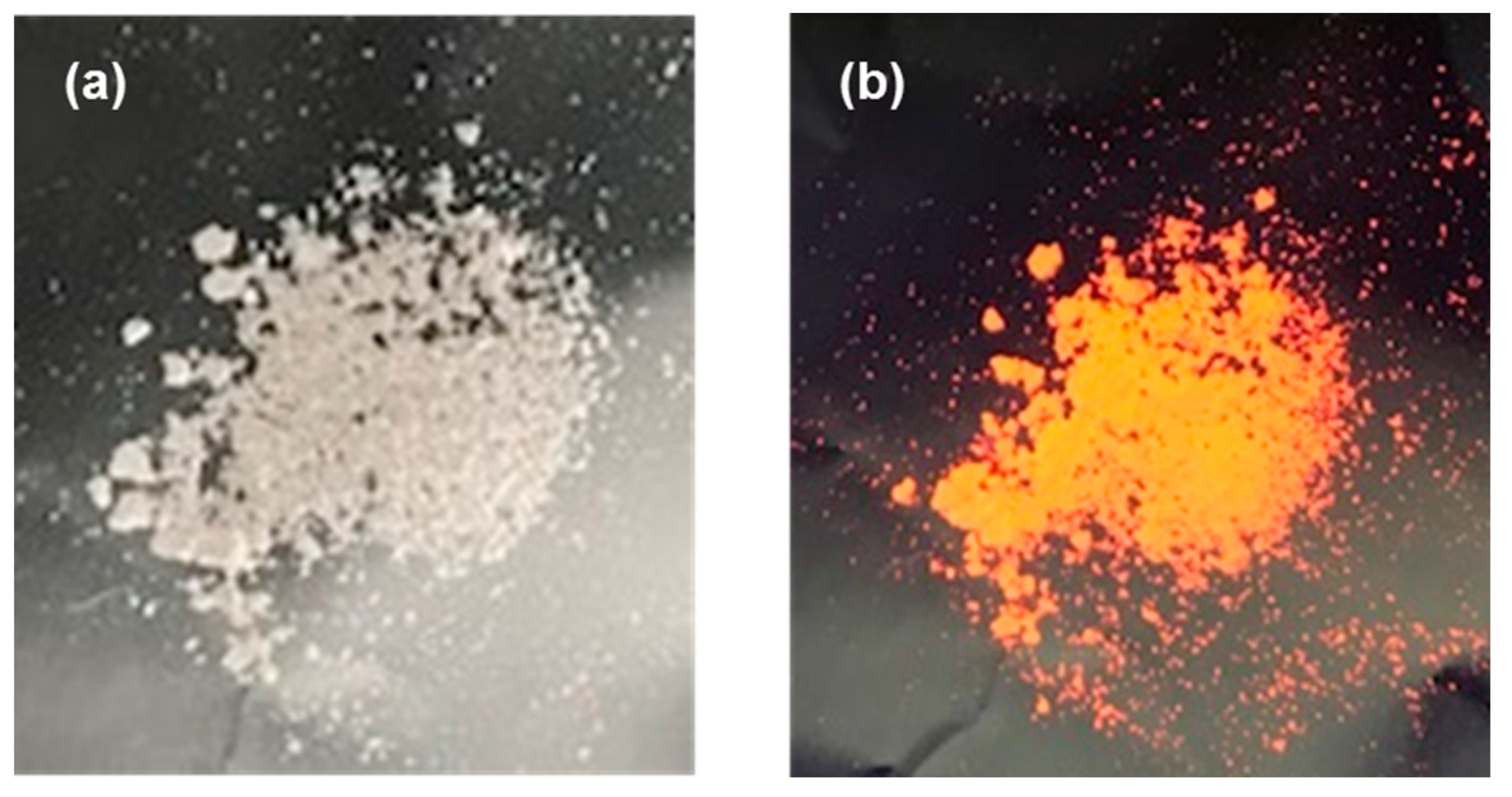
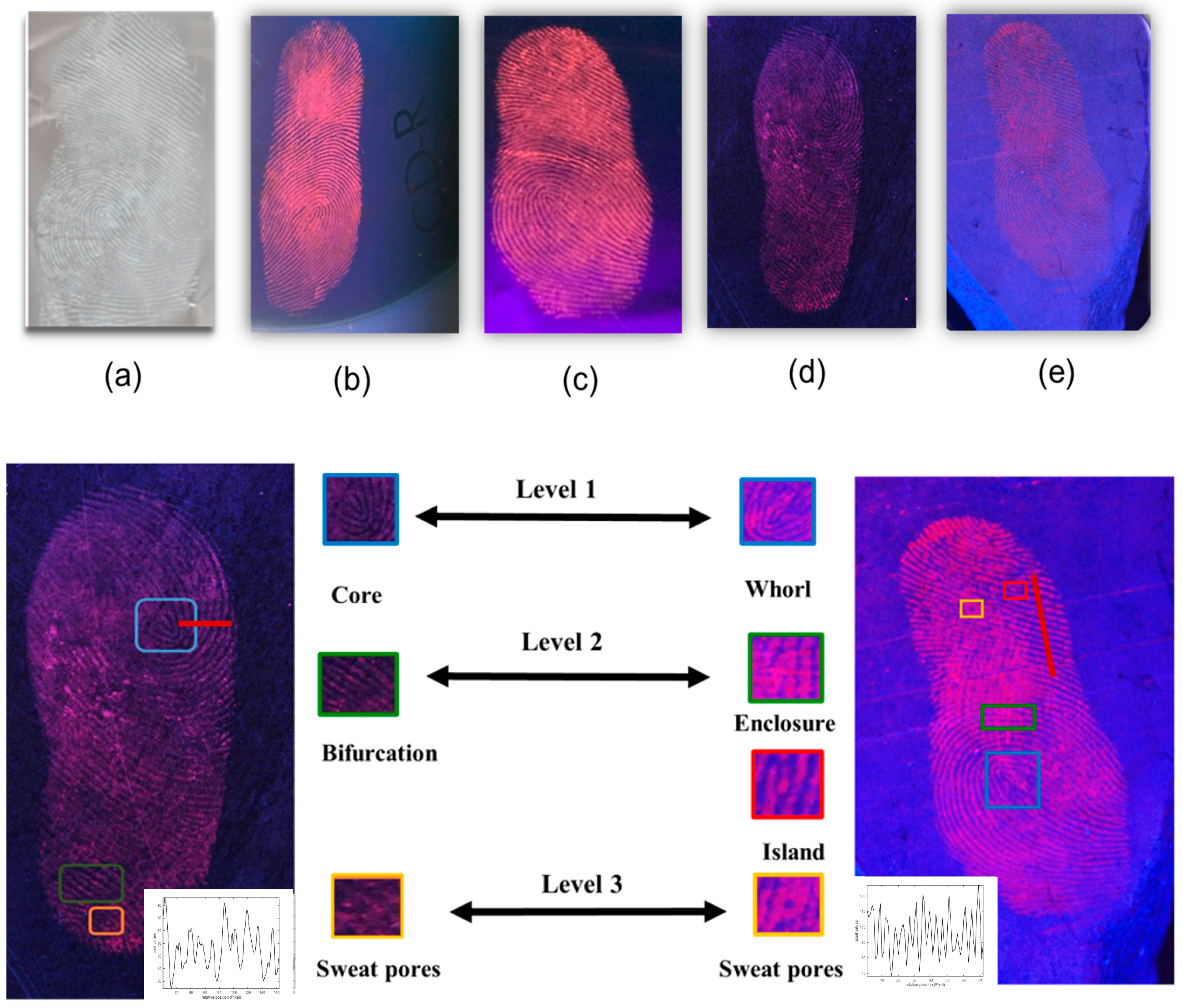
3.5. Detection of Latent Fingerprints
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sodhi, G.S.; Kaur, J. Powder method for detecting latent fingerprints: A review. J. Forensic Sci. Int. 2001, 120, 172–176. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Lulu, L.; Song, Q. Rapid Visualization of Latent Fingerprints with Color-Tunable Solid Fluorescent Carbon Dots. Part. Part. Syst. Charact. 2018, 35, 1700387. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Lu, Y.; Tang, L.; Zhang, J.; Ge, L.; Li, F. Visualization of latent fingerprints using a simple “silver imaging ink”. Anal. Methods 2016, 8, 6293. [Google Scholar] [CrossRef]
- Saferstein, R. Criminalistics: An Introduction to Forensic Science; Prentice-Hall: Englewood Cliffs, NJ, USA, 2001. [Google Scholar]
- Meyers, R.A. Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentations, 1st ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Choi, M.J.; McDonagh, A.M.; Maynard, P.; Roux, C. Metal-containing nanoparticles and nano-structured particles in fingermark detection. Forensic Sci. Int. 2008, 179, 87–97. [Google Scholar] [CrossRef]
- Lee, H.C.; Gaensslen, R.E. Methods of Latent Fingerprint Development:Advances in Fingerprint Technology; CRC Press: Boca Raton, FL, USA, 2004; pp. 77–104. [Google Scholar]
- Sodhi, G.S.; Kaur, J. A novel cost-effective organic fingerprint powder based on fluorescent eosin yellow dye. Indian J. Criminol. 1999, 27, 73–74. [Google Scholar]
- Zhang, B.; Dewasurendra, S.; Zhang, F.X. Blue and red up-conversion light emission in TM-doped A2B2O7 oxides. Mater. Lett. 2016, 170, 53–57. [Google Scholar] [CrossRef]
- Belousov, A.S.; Suleimanov, E.V.; Fukina, D.G. Pyrochlore oxides as visible light-responsive photocatalysts. New J. Chem. 2021, 45, 22531–22558. [Google Scholar] [CrossRef]
- Anandkumar, M.; Bagul, P.M.; Deshpande, A.S. Structural and luminescent properties of Eu3+ doped multi-principal component Ce0.2Gd0.2Hf0.2La0.2Zr0.2O2 nanoparticles. J. Alloys Compd. 2020, 838, 155595. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Jin, J.; Qiao, J.; Wang, Y.; Molokeev, M.S.; Swart, H.C.; Xia, Z. Site Occupation Engineering toward Giant Red-Shifted Photoluminescence in (Ba,Sr)2LaGaO5:Eu2+. Phosphors Chem. Mater. 2023, 35, 8714–8721. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of Europium (III) Spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Ege, A.; Ayvacikli, M.; Dinçer, O.; Satılmış, S.U. Spectral emission of rare earth (Tb, Eu, Dy) doped Y2Sn2O7 phosphors. J. Lumin. 2013, 143, 653–656. [Google Scholar] [CrossRef]
- Chen, X.; Khamenok, A.V.; Khusainov, S.G.; Shestakov, M.V.; Moshchalkov, V.V. White Photoluminescence in Dy-Doped Oxyfluoride Glasses. Optics 2023, 4, 66–73. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, E.; Verma, J.; Dalal, J.; Kumar, A. Structural and photoluminescence properties of Dy-doped nanocrystalline ZrO2 for optoelectronics application. Ceram. Int. 2023, 49, 20185–20192. [Google Scholar] [CrossRef]
- Brini, L.; Bennour, I.; Toncelli, A.; Maalej, R.; Abdelhedi, M. Eu-Doped Pyrochlore Crystal Nano-Powders as Fluorescent Solid for Fingerprint Visualization and for Anti-Counterfeiting Applications. Materials 2022, 15, 2423. [Google Scholar] [CrossRef]
- Sharma, V.; Nigam, S.; Sudarsan, V.; Vatsa, R.K. High temperature stabilization of Y2Sn2O7: Eu luminescent nanoparticles—A facile synthesis. J. Lumin. 2016, 179, 248–253. [Google Scholar] [CrossRef]
- Vinothkumar, G.; Rengaraj, S.; Arunkumar, P.; Cha, S.W.; Suresh Babu, K. Ionic Radii and Concentration Dependency of RE3+ (Eu3+, Nd3+, Pr3+, and La3+)-Doped Cerium Oxide Nanoparticles for Enhanced Multienzyme-Mimetic and Hydroxyl Radical Scavenging Activity. J. Phys. Chem. C 2019, 123, 541–553. [Google Scholar] [CrossRef]
- Starecki, F.; Louvet, G.; Ari, J.; Braud, A.; Doualan, J.-L.; Chahal, R.; Hafienne, I.; Boussard-Pledel, C.; Nazabal, V.; Camy, P.; et al. Dy3+ doped GaGeSbSe fiber long-wave infrared emission Dy3+ doped GaGeSbSe fiber long-wave infrared emission. J. Lumin. 2020, 218, 116853. [Google Scholar] [CrossRef]
- Shrivastava, R.; Khaparde, S. Luminescence studies of diopside doped with various concentrations of Dysprosium (III). Res. Chem. Intermed. 2022, 48, 969–982. [Google Scholar] [CrossRef]
- Moran, R.F.; Mckay, D.; Tornstrom, P.C.; Aziz, A.; Fernandes, A.; Grau-Crespo, R.; Ashbrook, S.E. Ensemble-Based Modeling of the NMR Spectra of Solid Solutions: Cation Disorder in Y2(Sn,Ti)2O7. J. Am. Chem. Soc. 2019, 141, 17838–17846. [Google Scholar] [CrossRef]
- Sellami, M.; Caignaert, V.; Hanni, F.Z.K.; Bettahar, N.; Benhadria, N.; Sari-Mohammed, E.; Imine, S.; Bahmani, A. Synthesis and characterization of new pyrochlore solid solution Bi1.5Sb1.5–xNbxCuO7. Comptes Rendus Chim. 2014, 17, 899–904. [Google Scholar] [CrossRef]
- Pokhrel, M.; Dimakis, N.; Dannangoda, C.; Gupta, S.K.; Martirosyan, K.S.; Mao, Y. Structural Evolution and Magnetic Properties of Gd2Hf2O7 Nanocrystals: Computational and Experimental Investigations. Molecules 2020, 25, 4847. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Umar, A. Glycols functionalized fluorescent Eu2O3 nanoparticles: Functionalization effect on the structural and optical properties. J. Alloys Compd. 2016, 682, 160–169. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Tang, Y.; Li, Y. Synthesis and Photoluminescence of Eu3+-Doped Y2Sn2O7 Nanocrystals. J. Solid State Chem. 2004, 177, 3075–3079. [Google Scholar] [CrossRef]
- Nigam, S.; Sudarsan, V.; Vatsa, R.K. Effect of Annealing Temperature on the Structural and Photoluminescence Properties of Y2Sn2O7: Eu Nanoparticles. Eur. J. Inorg. Chem. 2013, 2013, 357–363. [Google Scholar] [CrossRef]
- Fujihara, S.; Tokumo, K. Multiband Orange-Red Luminescence of Eu3+ Ions Based on the Pyrochlore-Structured Host Crystal. Chem. Mater. 2005, 17, 5587–5593. [Google Scholar] [CrossRef]
- Hansel, R.A.; Desai, S.K.; Allison, S.W.; Heyes, A.L.; Walker, D.G. Emission lifetimes of europium-doped pyrochlores for phosphor thermometry. J. Appl. Phys. 2010, 107, 016101. [Google Scholar] [CrossRef]
- Xia, J.; Lei, L.; Dai, X.; Ling, J.; Li, Y.; Xu, S. Excitation-dependent multi-color emissions in Yb/Er/Eu: Gd2Ti2O7 pyrochlore for anti-counterfeiting. Mater. Res. Bull. 2018, 107, 213–217. [Google Scholar] [CrossRef]
- Sebai, S.; Zambon, D.; Watras, A.; Dereń, P.J.; Megriche, A.; Mahiou, R. Synthesis and photoluminescence of Eu3+ activated alkali mixed (Li, Na)Y(PO3)4 under VUV-UV excitation. Opt. Mater. 2019, 92, 217–222. [Google Scholar] [CrossRef]
- Yang, M.; Sui, Y.; Wang, S.; Wang, X.; Wang, Y.; Lü, S.; Lü, T.; Liu, W. Correlation between the surface state and optical properties of S6 site and C2 site in nanocrystalline Eu3+:Y2O3. J. Alloys Compd. 2011, 509, 266–270. [Google Scholar] [CrossRef]
- Baig, N.; Dhoble, N.S.; Park, K.; Kokode, N.S.; Dhoble, S.J. Enhanced luminescence and white light emission from Eu3+-co-doped K3Ca2(SO4)3Cl:Dy3+ phosphor with near visible ultraviolet excitation for white LEDs. Luminescence 2015, 30, 479–484. [Google Scholar] [CrossRef]


| Atom | Site | Symmetry | x | y | z |
|---|---|---|---|---|---|
| Y/Eu/Dy | 16d | D3d | 0.5 | 0.5 | 0.5 |
| Sn | 16c | D3d | 0 | 0 | 0 |
| O | 48f | C2v | 0.337 | 0.125 | 0.125 |
| O′ | 8b | Td | 0.375 | 0.375 | 0.375 |
| 2x/2y | Lattice Constant (Å) | Observed Phase | Rp | Rwp | χ2 |
|---|---|---|---|---|---|
| 0.2/0.01 | 10.3954 | Y1.79Eu0.2Dy0.01Sn2O7 | 23.1 | 24.6 | 5.93 |
| 0.05/0.05 | 10.3857 | Y1.9Eu0.05Dy0.05Sn2O7 | 22.6 | 23.56 | 4.33 |
| 0.05/0.2 | 10.3861 | Y1.75Eu0.05Dy0.2Sn2O7 | 26.3 | 26.9 | 6.17 |
| 0.2/0.05 | 10.3930 | Y1.75Eu0.2Dy0.05Sn2O7 | 17.3 | 17.8 | 2.29 |
| 0.1/0.1 | 10.3884 | Y1.8Eu0.1Dy0.1Sn2O7 | 20.5 | 21.5 | 4.52 |
| Sample | Size (nm) |
|---|---|
| Y2Sn2O7:20%Eu/1%Dy | 102 |
| Y2Sn2O7:5%Eu/5%Dy | 96 |
| Y2Sn2O7:5%Eu/20%Dy | 119 |
| Y2Sn2O7:20%Eu/5%Dy | 73 |
| Y2Sn2O7:10%Eu/10%Dy | 86 |
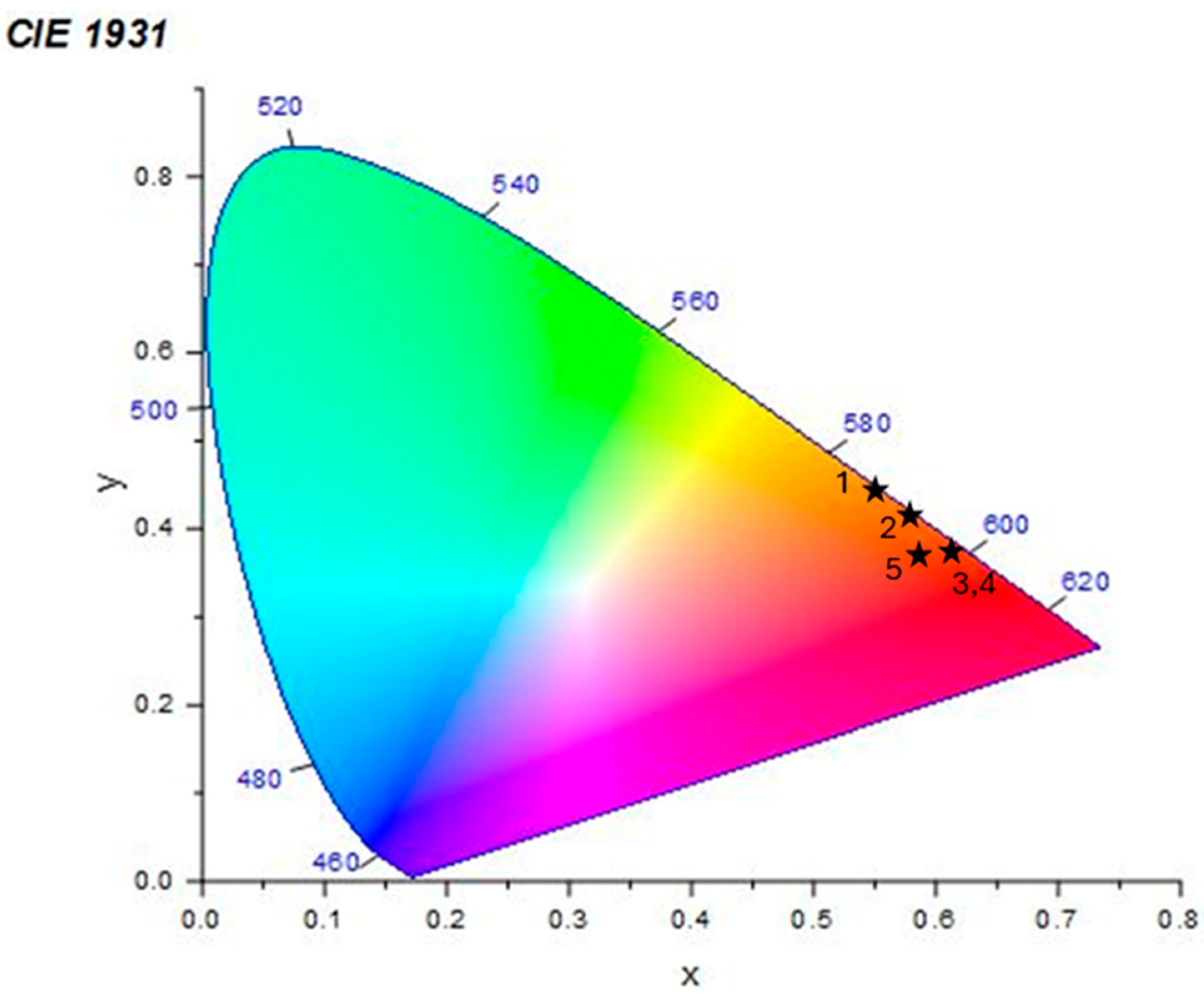
| Sample Number | Eu3+/Dy3+ Concentration | CIE x | y |
|---|---|---|---|
| 1 | 5/20 mol % | 0.563 | 0.436 |
| 2 | 10/10 mol % | 0.580 | 0.418 |
| 3 | 20/5 mol % | 0.614 | 0.381 |
| 4 | 20/1 mol% | 0.610 | 0.375 |
| 5 | 5/5 mol% | 0.586 | 0.379 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brini, L.; Douiri, H.; Abid, M.; Toncelli, A.; Qasymeh, M.; Maalej, R.; Abdelhedi, M. Potential of Y2Sn2O7:Eu3+, Dy3+ Inorganic Nanophosphors in Latent Fingermark Detection. Crystals 2024, 14, 300. https://doi.org/10.3390/cryst14040300
Brini L, Douiri H, Abid M, Toncelli A, Qasymeh M, Maalej R, Abdelhedi M. Potential of Y2Sn2O7:Eu3+, Dy3+ Inorganic Nanophosphors in Latent Fingermark Detection. Crystals. 2024; 14(4):300. https://doi.org/10.3390/cryst14040300
Chicago/Turabian StyleBrini, Layla, Hanen Douiri, Marwa Abid, Alessandra Toncelli, Montasir Qasymeh, Ramzi Maalej, and Mohamed Abdelhedi. 2024. "Potential of Y2Sn2O7:Eu3+, Dy3+ Inorganic Nanophosphors in Latent Fingermark Detection" Crystals 14, no. 4: 300. https://doi.org/10.3390/cryst14040300
APA StyleBrini, L., Douiri, H., Abid, M., Toncelli, A., Qasymeh, M., Maalej, R., & Abdelhedi, M. (2024). Potential of Y2Sn2O7:Eu3+, Dy3+ Inorganic Nanophosphors in Latent Fingermark Detection. Crystals, 14(4), 300. https://doi.org/10.3390/cryst14040300










