Excited State Calculations of Cu-Doped Anatase TiO2 (101) and (001) Nanofilms
Abstract
1. Introduction
2. Computation Details
3. Results and Discussion
3.1. Pristine Surfaces
3.2. Doped Surfaces
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Hazmi, F.; Alnowaiser, F.; Al-Ghamdi, A.; Al-Ghamdi, A.A.; Aly, M.; Al-Tuwirqi, R.M.; El-Tantawy, F. A new large—Scale synthesis of magnesium oxide nanowires: Structural and antibacterial properties. Superlattices Microstruct. 2012, 52, 200–209. [Google Scholar] [CrossRef]
- Lopez de Dicastillo, C.; Correa, M.; Martínez, F.; Streitt, C.; Galotto, M. Antimicrobial Effect of Titanium Dioxide Nanoparticles. In Antimicrobial Resistance—A One Health Perspective; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Maeda, K. Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol. C 2011, 12, 237–268. [Google Scholar] [CrossRef]
- Li, B.; Wu, S.; Gao, X. Theoretical calculation of a TiO2-based photocatalyst in the field of water splitting: A review. Nanotechnol. Rev. 2020, 9, 1080–1103. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.A.; Popov, A.I. Extraction–pyrolytic method for TiO2 polymorphs production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Liu, Z.; An, Y.; Zhong, Y.; Hu, Z.; Li, S.; Chen, Z.; Wang, S.; Sheng, X.; et al. Eu-doped zeolitic imidazolate framework-8 modified mixed-crystal TiO2 for efficient removal of basic fuchsin from effluent. Materials 2021, 14, 7265. [Google Scholar] [CrossRef] [PubMed]
- Ho, V.T.T.; Chau, D.H.; Bui, K.Q.; Nguyen, N.T.T.; Tran, T.K.N.; Bach, L.G.; Truong, S.N. A high-performing nanostructured Ir doped-TiO2 for efficient photocatalytic degradation of gaseous toluene. Inorganics 2022, 10, 29. [Google Scholar] [CrossRef]
- Permporn, D.; Khunphonoi, R.; Wilamat, J.; Khemthong, P.; Chirawatkul, P.; Butburee, T.; Sangkhun, W.; Wantala, K.; Grisdanurak, N.; Santatiwongchai, J.; et al. Insight into the roles of metal loading on CO2 photocatalytic reduction behaviors of TiO2. Nanomaterials 2022, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Tsebriienko, T.; Popov, A.I. Effect of poly(titanium oxide) on the viscoelastic and thermophysical properties of interpenetrating polymer networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Danish, M.S.S.; Estrella, L.L.; Alemaida, I.M.A.; Lisin, A.; Moiseev, N.; Ahmadi, M.; Nazari, M.; Wali, M.; Zaheb, H.; Senjyu, T. Photocatalytic applications of metal oxides for sustainable environmental eemediation. Metals 2021, 11, 80. [Google Scholar] [CrossRef]
- Huang, H.; Huang, H.; Zhang, L.; Hu, P.; Ye, X.; Leung, D.Y. Enhanced degradation of gaseous benzene under vacuum ultraviolet (VUV) irradiation over TiO2 modified by transition metals. Chem. Eng. J. 2015, 259, 534–541. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Pankratova, V.; Popov, A.I.; Pankratov, V. Study of phase composition, photocatalytic activity, and photoluminescence of TiO2 with Eu additive produced by the extraction-pyrolytic method. J. Mater. Res. Technol. 2021, 13, 2350–2360. [Google Scholar] [CrossRef]
- Zhukovskii, Y.F.; Piskunov, S.; Lisovski, O.; Bocharov, D.; Evarestov, R.A. Doped 1D nanostructures of transition-metal oxides: First-principles evaluation of photocatalytic suitability. Isr. J. Chem. 2017, 57, 461–476. [Google Scholar] [CrossRef]
- Alfaifi, B.Y.; Ullah, H.; Alfaifi, S.Y.; Tahir, A.A.; Mallick, T.K. Photoelectrochemical solar water splitting: From basic principles to advanced devices. Veruscript Funct. Nanomater. 2018, 2, BDJOC3. [Google Scholar] [CrossRef]
- Pham, T.D.; Lee, B.-K. Effects of Ag doping on the photocatalytic disinfection of E. coli in bioaerosol by Ag–TiO2/GF under visible light. J. Colloid Interface Sci. 2014, 428, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.; Lee, B.-K. Cu doped TiO2/GF for photocatalytic disinfection of Escherichia coli in bioaerosols under visible light irradiation: Application and mechanism. Appl. Surf. Sci. 2014, 296, 9. [Google Scholar] [CrossRef]
- Foster, H.; Ditta, I.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, I.; Ozaki, Y. Far- and deep-ultraviolet spectroscopic investigations for titanium dioxide: Electronic absorption, Rayleigh scattering, and Raman spectroscopy. J. Mater. Chem. C 2016, 4, 7706–7717. [Google Scholar] [CrossRef]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.; Bartlett, J.; Nolan, M.; Pillai, S. Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Q.; Huang, J.; Zhu, C.; Deng, Z.; Shi, H.; Wu, L.; Liu, Z.; Cao, Y. Enhanced hydrogen production by water splitting using Cu-doped TiO2 film with preferred (0 0 1) orientation. Appl. Surf. Sci. 2014, 292, 161–164. [Google Scholar] [CrossRef]
- Lin, Y.P.; Bocharov, D.; Kotomin, E.A.; Brik, M.G.; Piskunov, S. Influence of Au, Ag, and Cu Adatoms on Optical Properties of TiO2 (110) Surface: Predictions from RT-TDDFT Calculations. Crystals 2022, 12, 452. [Google Scholar] [CrossRef]
- Kamalov, R.; Vokhmintsev, A.; Dorosheva, I.; Kravets, N.; Weinstein, I. Synthesis of Composite Based on Carbon Nanotubes and Anodic Titania. Adv. Sci. Lett. 2016, 22, 688–690. [Google Scholar] [CrossRef]
- Kukli, K.; Lu, J.; Link, J.; Kemell, M.; Puukilainen, E.; Heikkilä, M.; Hoxha, R.; Tamm, A.; Hultman, L.; Stern, R.; et al. Holmium and titanium oxide nanolaminates by atomic layer deposition. Thin Solid Films 2014, 565, 165–171. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, L.; Wu, Y.; Yu, Q. An experimental and theoretical study on the photocatalytic antibacterial activity of boron-doped TiO2 nanoparticles. Ceram. Int. 2021, 48, 604–614. [Google Scholar] [CrossRef]
- Alisiyonak, O.; Lavitskaya, A.; Khoroshko, L.; Kozlovskiy, A.L.; Zdorovets, M.; Korolkov, I.; Yauseichuk, M.; Kaniukov, E.; Shumskaya, A. Breathable Films with Self-Cleaning and Antibacterial Surfaces Based on TiO2-Functionalized PET Membranes. Membranes 2023, 13, 733. [Google Scholar] [CrossRef]
- Dukenbayev, K.; Kozlovskiy, A.; Kenzhina, I.; Berguzinov, A.; Zdorovets, M. Study of the effect of irradiation with Fe7+ ions on the structural properties of thin TiO2 foils. Mater. Res. Express 2019, 6, 046309. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Shlimas, I.; Dukenbayev, K.; Zdorovets, M. Structure and corrosion properties of thin TiO2 films obtained by magnetron sputtering. Vacuum 2019, 164, 224–232. [Google Scholar] [CrossRef]
- Zdorovets, M.; Kozlovskiy, A.; Tishkevich, D.; Zubar, T.; Trukhanov, A. The effect of doping of TiO2 thin films with low-energy O2+ ions on increasing the efficiency of hydrogen evolution in photocatalytic reactions of water splitting. J. Mater. Sci. Mater. Electron. 2020, 31, 21142–21153. [Google Scholar] [CrossRef]
- Wang, Z.; Labat, F. Modeling stoichiometric and oxygen defective TiO2 anatase bulk and (101) surface: Structural and electronic properties from hybrid DFT. J. Mol. Model. 2023, 29, 174. [Google Scholar] [CrossRef] [PubMed]
- Harb, M.; Sautet, P.; Raybaud, P. Anionic or Cationic S-Doping in Bulk Anatase TiO2: Insights on Optical Absorption from First Principles Calculations. J. Phys. Chem. C 2013, 117, 8892–8902. [Google Scholar] [CrossRef]
- Gustavsen, K.; Feng, T.; Huang, H.; Li, G.; Narkiewicz, U.; Wang, K. DFT Calculation of Carbon-Doped TiO2 Nanocomposites. Materials 2023, 16, 6117. [Google Scholar] [CrossRef]
- Fu, C.; Liu, L.; Li, Z.; Wei, Y.; Huang, W.; Zhang, X. Synergy of Bulk Defects and Surface Defects on TiO2 for Highly Efficient Photocatalytic Production of H2O2. J. Phys. Chem. Lett. 2023, 14, 7690–7696. [Google Scholar] [CrossRef] [PubMed]
- Zavatski, S.; Neilande, E.; Bandarenka, H.; Popov., A.I.; Piskunov, S.; Bocharov, D. Density functional theory for doped TiO2: Current research strategies and advancements. Nanotechnology 2024, 35, 192001. [Google Scholar] [CrossRef]
- Pilar de Lara-Castells, M.; Hauser, A.W.; Ramallo-López, J.M.; Buceta, D.; Giovanetti, L.J.; López-Quintela, M.A.; Requejo, F.G. Increasing the optical response of TiO2 and extending it into the visible region through surface activation with highly stable Cu5 clusters. J. Mater. Chem. A 2019, 7, 7489–7500. [Google Scholar] [CrossRef]
- López-Caballero, P.; Ramallo-López, J.M.; Giovanetti, L.J.; Buceta, D.; Miret-Artés, S.; López-Quintela, M.A.; Requejo, F.G.; de Lara-Castells, M.P. Exploring the properties of Ag5–TiO2 interfaces: Stable surface polaron formation, UV-Vis optical response, and CO2 photoactivation. J. Mater. Chem. A 2020, 8, 6842–6853. [Google Scholar] [CrossRef]
- Yuan, W.; Zhu, B.; Li, X.Y.; Hansen, T.W.; Ou, Y.; Fang, K.; Yang, H.; Zhang, Z.; Wagner, J.B.; Gao, Y.; et al. Visualizing H2O molecules reacting at TiO2 active sites with transmission electron microscopy. Science 2020, 367, 428–430. [Google Scholar] [CrossRef]
- de Lara-Castells, M.P.; Cabrillo, C.; Micha, D.A.; Mitrushchenkov, A.O.; Vazhappilly, T. Ab initio design of light absorption through silver atomic cluster decoration of TiO2. Phys. Chem. Chem. Phys. 2018, 20, 19110–19119. [Google Scholar] [CrossRef]
- Kenmoe, S.; Lisovski, O.; Piskunov, S.; Zhukovskii, Y.F.; Spohr, E. Electronic and optical properties of pristine, N- and S-doped water-covered TiO2 nanotube surfaces. J. Chem. Phys. 2019, 150, 041714. [Google Scholar] [CrossRef] [PubMed]
- Rossi, T.P.; Kuisma, M.; Puska, M.J.; Nieminen, R.M.; Erhart, P. Kohn–Sham decomposition in real-time time-dependent density-functional theory: An efficient tool for analyzing plasmonic excitations. J. Chem. Theory Comput. 2017, 13, 4779–4790. [Google Scholar] [CrossRef]
- Conley, K.M.; Nayyar, N.; Rossi, T.P.; Kuisma, M.; Turkowski, V.; Puska, M.J.; Rahman, T.S. Plasmon excitations in mixed metallic nanoarrays. ACS Nano 2019, 13, 5344–5355. [Google Scholar] [CrossRef] [PubMed]
- Rossi, T.P.; Shegai, T.; Erhart, P.; Antosiewicz, T.J. Strong plasmon-molecule coupling at the nanoscale revealed by first-principles modeling. Nat. Commun. 2019, 10, 3336. [Google Scholar] [CrossRef] [PubMed]
- Mokkath, J.H. Strong collectivity of optical transitions in lead halide perovskite quantum dots. Plasmonics 2020, 15, 581–590. [Google Scholar] [CrossRef]
- Qu, Z.W.; Kroes, G.J. Theoretical study of adsorption of O(3P) and H2O on the rutile TiO2(110) surface. J. Phys. Chem. B 2006, 110, 23306–23314. [Google Scholar] [CrossRef] [PubMed]
- Enkovaara, J.; Rostgaard, C.; Mortensen, J.; Chen, J.; Dulak, M.; Ferrighi, L.; Gavnholt, J.; Glinsvad, C.; Haikola, V.; Hansen, H.; et al. Electronic structure calculations with GPAW: A real-space implementation of the projector augmented-wave method. J. Condens. Matter Phys. 2010, 22, 253202. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Häkkinen, H.; Lehtovaara, L.; Puska, M.; Enkovaara, J.; Rostgaard, C.; Mortensen, J. Time-dependent density-functional theory in the projector augmented-wave method. J. Chem. Phys. 2008, 128, 244101. [Google Scholar] [CrossRef]
- Larsen, A.; Mortensen, J.; Blomqvist, J.; Castelli, I.; Christensen, R.; Dulak, M.; Friis, J.; Groves, M.; Hammer, B.; Hargus, C.; et al. The Atomic Simulation Environment — A Python library for working with atoms. J. Condens. Matter Phys. 2017, 29, 273002. [Google Scholar] [CrossRef] [PubMed]
- Kuisma, M.; Sakko, A.; Rossi, T.; Larsen, A.; Enkovaara, J.; Lehtovaara, L.; Rantala, T. Localized surface plasmon resonance in silver nanoparticles: Atomistic first-principles time-dependent density-functional theory calculations. Phys. Rev. B 2015, 91, 115431. [Google Scholar] [CrossRef]
- Makkonen, E.; Rossi, T.; Larsen, A.; Acevedo, O.L.; Rinke, P.; Kuisma, M.; Chen, x. Real-time time-dependent density functional theory implementation of electronic circular dichroism applied to nanoscale metal–organic clusters. J. Chem. Phys. 2021, 154, 114102. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Jónsson, E.Ö.; Vegge, T.; Jónsson, H. Direct energy minimization based on exponential transformation in density functional calculations of finite and extended systems. Comput. Phys. Commun. 2021, 267, 108047. [Google Scholar] [CrossRef]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.H.; Vanin, M.; Mortensen, J.J.; Thygesen, K.S.; Jacobsen, K.W. Localized atomic basis set in the projector augmented wave method. Phys. Rev. B 2009, 80, 195112. [Google Scholar] [CrossRef]
- Kuisma, M.; Ojanen, J.; Enkovaara, J.; Rantala, T.T. Kohn-Sham potential with discontinuity for band gap materials. Phys. Rev. B 2010, 82, 115106. [Google Scholar] [CrossRef]
- Castelli, I.E.; Hüser, F.; Pandey, M.; Li, H.; Thygesen, K.S.; Seger, B.; Jain, A.; Persson, K.A.; Ceder, G.; Jacobsen, K.W. New Light-Harvesting Materials Using Accurate and Efficient Bandgap Calculations. Adv. Energy Mater. 2015, 5, 1400915. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- van der Walt, S.; Colbert, S.; Varoquaux, G. The NumPy Array: A Structure for Efficient Numerical Computation. Comput. Sci. Eng. 2011, 13, 22–30. [Google Scholar] [CrossRef]
- Hunter, J. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Himmetoglu, B.; Floris, A.; De Gironcoli, S.; Cococcioni, M. Hubbard-corrected DFT energy functionals: The LDA+U description of correlated systems. Int. J. Quantum Chem. 2014, 114, 14–49. [Google Scholar] [CrossRef]
- Tritsaris, G.; Vinichenko, D.; Kolesov, G.; Friend, C.; Kaxiras, E. Dynamics of the Photogenerated Hole at the Rutile TiO2(110)/Water Interface: A Nonadiabatic Simulation Study. J. Phys. Chem. C 2014, 118, 27393–27401. [Google Scholar] [CrossRef]
- Deng, Q.; Zhang, W.; Lan, T.; Xie, J.; Xie, W.; Liu, Z.; Huang, Y.; Wei, M. Anatase TiO2 quantum dots with a narrow bandgap energy of 2.85 eV exhibiting significant photodegradation property. Eur. J. Inorg. Chem. 2018, 2018, 1506–1510. [Google Scholar] [CrossRef]
- Lin, Y.P.; Bocharov, D.; Isakoviča, I.; Pankratov, V.; Popov, A.A.; Popov, A.I.; Piskunov, S. Chlorine Adsorption on TiO2 (110)/Water Interface: Nonadiabatic Molecular Dynamics Simulations for Photocatalytic Water Splitting. Electron. Mater. 2023, 4, 33–48. [Google Scholar] [CrossRef]
- Lin, Y.P.; Isakoviča, I.; Gopejenko, A.; Ivanova, A.; Začinskis, A.; Eglitis, R.I.; D’yachkov, P.N.; Piskunov, S. Time-dependent density functional theory calculations of N- and S-doped TiO2 nanotube for water-splitting applications. Nanomaterials 2021, 11, 2900. [Google Scholar] [CrossRef] [PubMed]
- Estévez Ruiz, E.P.; Lago, J.L.; Thirumuruganandham, S.P. Experimental Studies on TiO2 NT with Metal Dopants through Co-Precipitation, Sol–Gel, Hydrothermal Scheme and Corresponding Computational Molecular Evaluations. Materials 2023, 16, 3076. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.J.; McKenna, K.P. Screening doping strategies to mitigate electron trapping at anatase TiO2 surfaces. J. Phys. Chem. C 2019, 123, 22358–22367. [Google Scholar] [CrossRef] [PubMed]
- Di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Paganini, M.C.; Giamello, E. N-doped TiO2: Theory and experiment. Chem. Phys. 2007, 339, 44–56. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef]
- Sultana, M.; Mondol, A.; Islam, S.; Khatun, M.A.; Rahman, M.H.; Chakraborty, A.K.; Rahman, M.S.; Rahman, M.M.; Nur, A.S. Strategic development of metal doped TiO2 photocatalysts for enhanced dye degradation activity under UV–Vis irradiation: A review. Curr. Res. Green Sustain. Chem. 2023, 7, 100383. [Google Scholar] [CrossRef]
- Rusevich, L.L.; Kotomin, E.A.; Zvejnieks, G.; Kržmanc, M.M.; Gupta, S.; Daneu, N.; Wu, J.C.; Lee, Y.G.; Yu, W.Y. Effects of Al Doping on Hydrogen Production Efficiency upon Photostimulated Water Splitting on SrTiO3 Nanoparticles. J. Phys. Chem. C 2022, 126, 21223–21233. [Google Scholar] [CrossRef]
- Tai, Y.Y.; Wu, J.C.; Yu, W.Y.; Kržmanc, M.M.; Kotomin, E. Photocatalytic water splitting of improved strontium titanate for simultaneous separation of H2 in a twin photoreactor. Appl. Catal. B 2023, 324, 122183. [Google Scholar] [CrossRef]
- LeCoultre, S.; Rydlo, A.; Félix, C.; Buttet, J.; Gilb, S.; Harbich, W. Optical absorption of small copper clusters in neon: Cun, (n = 1–9). J. Chem. Phys. 2011, 134, 074303. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, X.; Chen, M.; Hou, B. Effect of the existing form of Cu element on the mechanical properties, bio-corrosion and antibacterial properties of Ti-Cu alloys for biomedical application. Mater. Sci. Eng. C 2016, 69, 1210–1221. [Google Scholar] [CrossRef] [PubMed]



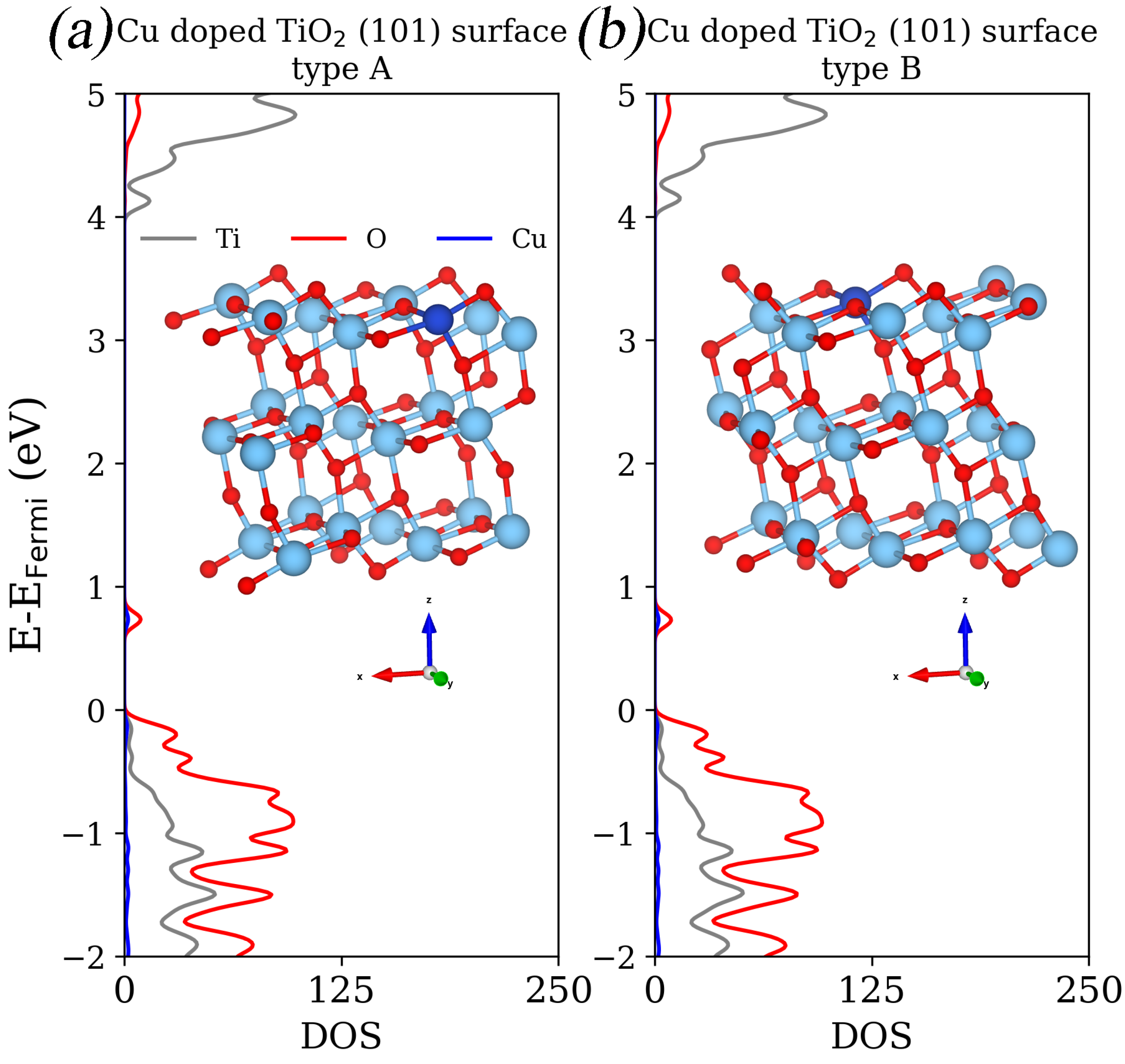

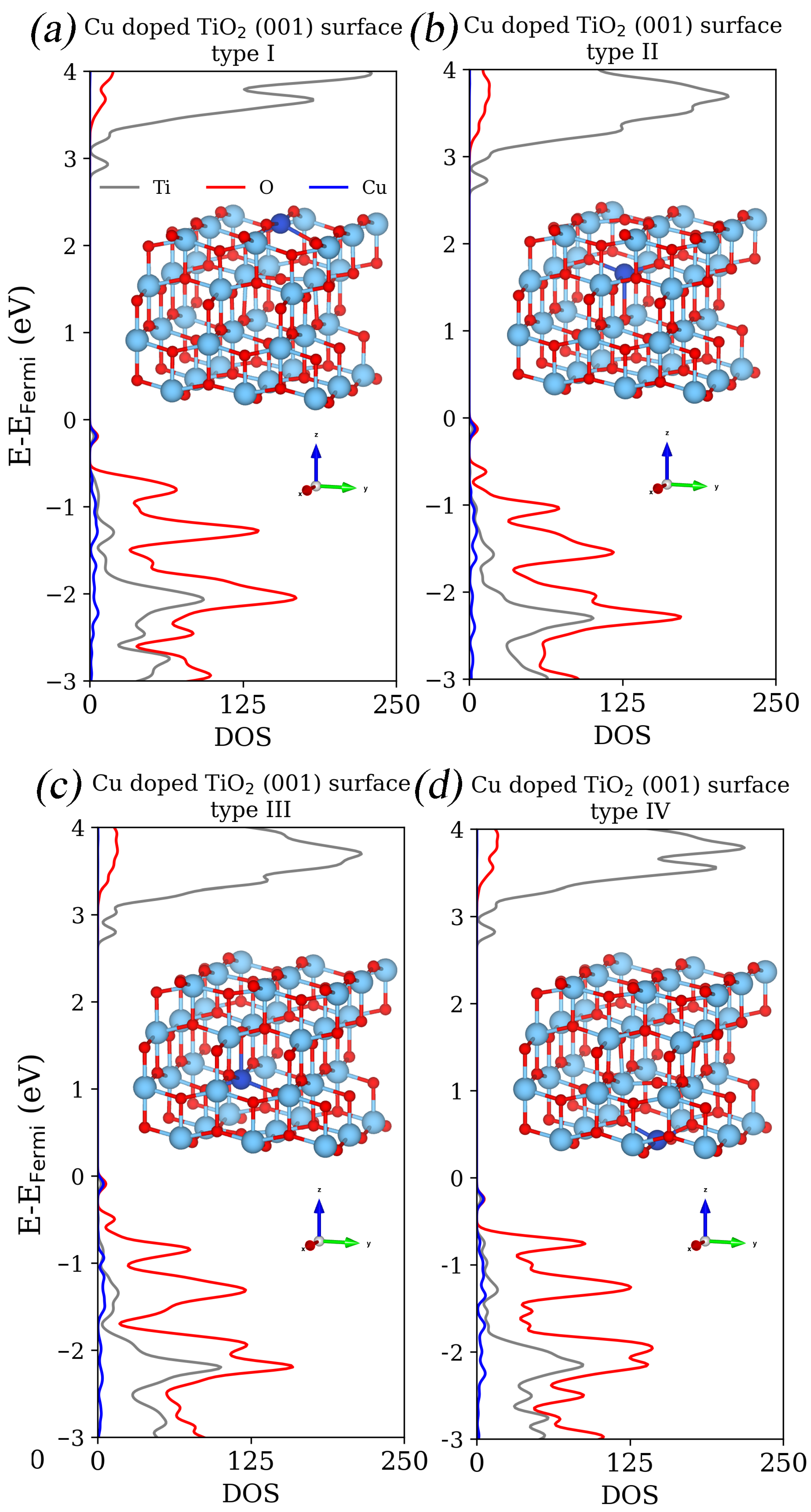

| eV | eV | |
|---|---|---|
| TiO2 (101) surface type A | 3.32 | 4.18 |
| TiO2 (101) surface type B | 3.31 | 4.17 |
| TiO2 (001) surface | 2.55 | 3.39 |
| TiO2 (101) surface type A | 3.97 | 0.75 |
| TiO2 (101) surface type B | 4.00 | 0.77 |
| TiO2 (001) surface type I | 3.32 | 0.33 |
| TiO2 (001) surface type II | 3.15 | 0.41 |
| TiO2 (001) surface type III | 3.17 | 0.27 |
| TiO2 (001) surface type IV | 3.25 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-P.; Neilande, E.; Bandarenka, H.; Zavatski, S.; Isakoviča, I.; Piskunov, S.; Bocharov, D.; Kotomin, E.A. Excited State Calculations of Cu-Doped Anatase TiO2 (101) and (001) Nanofilms. Crystals 2024, 14, 247. https://doi.org/10.3390/cryst14030247
Lin Y-P, Neilande E, Bandarenka H, Zavatski S, Isakoviča I, Piskunov S, Bocharov D, Kotomin EA. Excited State Calculations of Cu-Doped Anatase TiO2 (101) and (001) Nanofilms. Crystals. 2024; 14(3):247. https://doi.org/10.3390/cryst14030247
Chicago/Turabian StyleLin, Yin-Pai, Elina Neilande, Hanna Bandarenka, Siarhei Zavatski, Inta Isakoviča, Sergei Piskunov, Dmitry Bocharov, and Eugene A. Kotomin. 2024. "Excited State Calculations of Cu-Doped Anatase TiO2 (101) and (001) Nanofilms" Crystals 14, no. 3: 247. https://doi.org/10.3390/cryst14030247
APA StyleLin, Y.-P., Neilande, E., Bandarenka, H., Zavatski, S., Isakoviča, I., Piskunov, S., Bocharov, D., & Kotomin, E. A. (2024). Excited State Calculations of Cu-Doped Anatase TiO2 (101) and (001) Nanofilms. Crystals, 14(3), 247. https://doi.org/10.3390/cryst14030247







