Crystal and Electronic Structure of Ternary Bismuthides BaTM1.8Bi2 (TM = Au, Ag) with a New Variation of the BaAu2Sb2 Structure Type
Abstract
1. Introduction
2. Materials and Methods
2.1. Crystal Growth and Sample Characterization
2.2. Computational Details
3. Results and Discussion
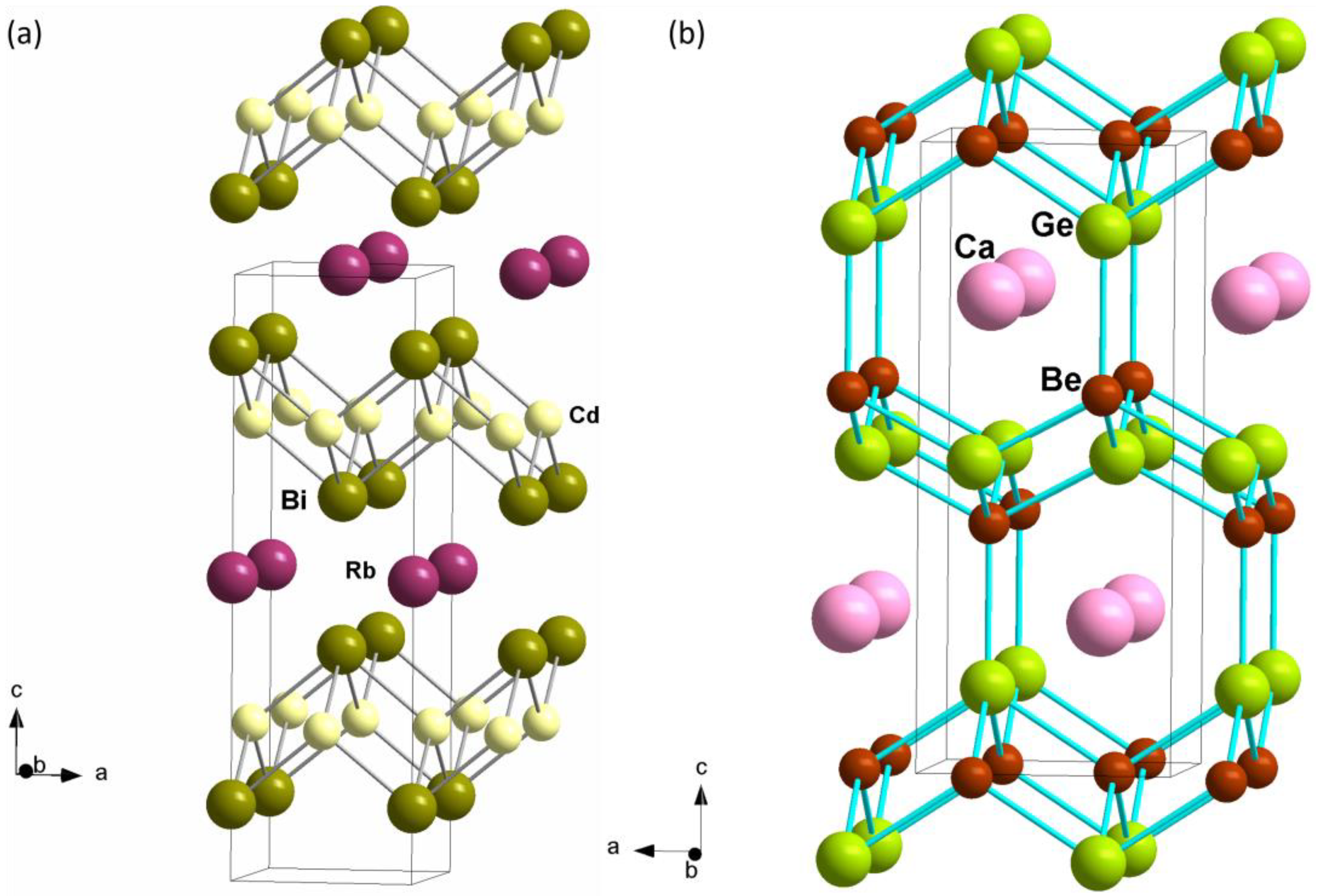
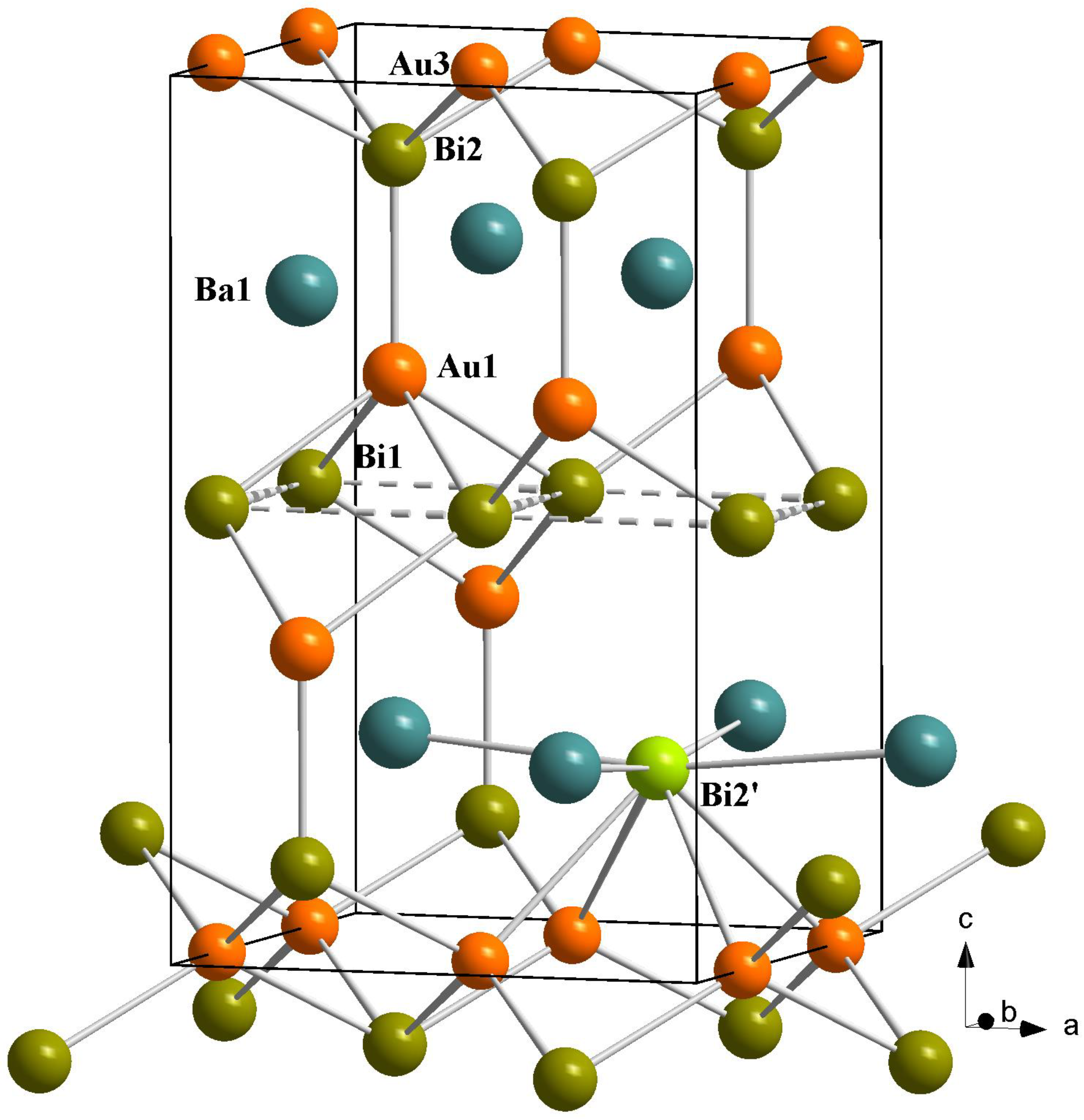
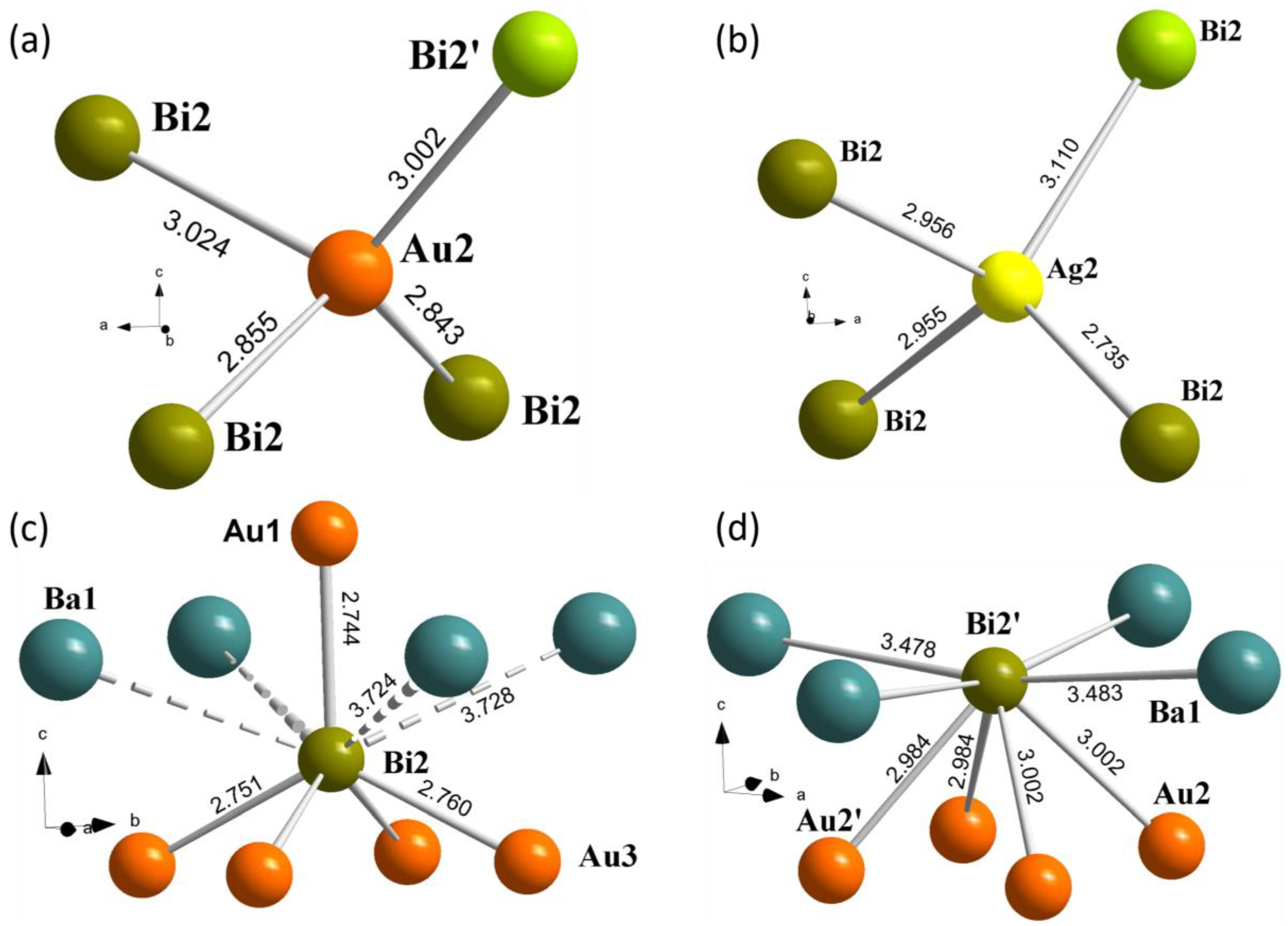
3.1. Synthesis and Crystal Structure
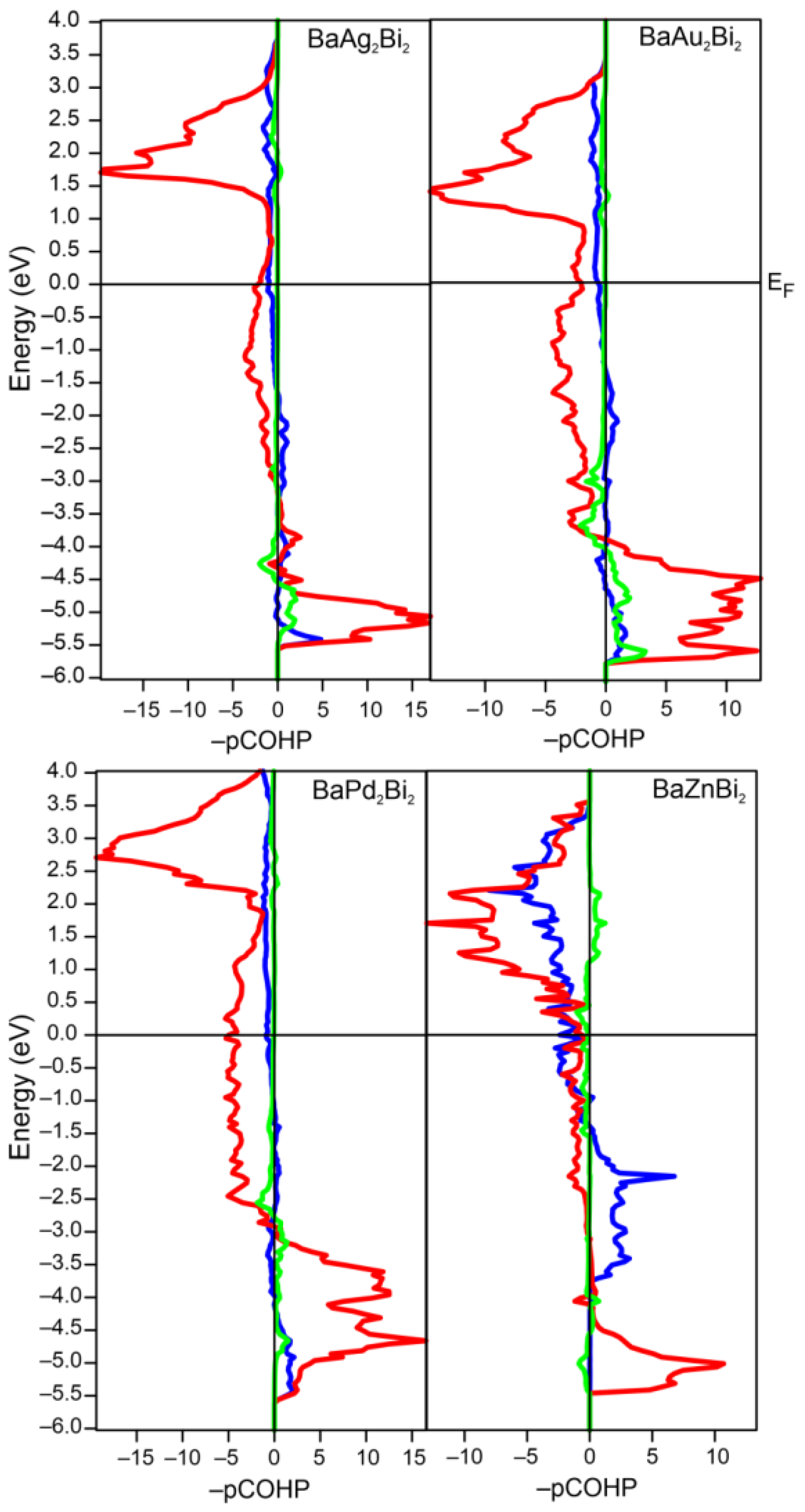
3.2. Electronic Structure and Bonding
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saparov, B.; Sefat, A.S. Crystals, Magnetic and Electronic Properties of a New ThCr2Si2-Type BaMn2Bi2 and K-Doped Compositions. J. Solid State Chem. 2013, 204, 32–39. [Google Scholar] [CrossRef]
- Ryu, H.; Park, S.Y.; Li, L.; Ren, W.; Neaton, J.B.; Petrovic, C.; Hwang, C.; Mo, S.-K. Anisotropic Dirac Fermions in BaMnBi2 and BaZnBi2. Sci. Rep. 2018, 8, 15322. [Google Scholar] [CrossRef]
- Gvozdetskyi, V.; Wang, R.; Xia, W.; Zhang, F.; Lin, Z.; Ho, K.; Miller, G.; Zaikina, J.V. How to Look for Compounds: Predictive Screening and in Situ Studies in Na−Zn−Bi System. Chem. Eur. J. 2021, 27, 15954–15966. [Google Scholar] [CrossRef] [PubMed]
- Gvozdetskyi, V.; Owens-Baird, B.; Hong, S.; Zaikina, J. Thermal Stability and Thermoelectric Properties of NaZnSb. Materials 2018, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Shilov, A.I.; Pervakov, K.S.; Tafeenko, V.A.; Morozov, I.V. New Ternary Bismuthides NaZnBi and NaCdBi: Synthesis and Crystal Structures. Russ. J. Coord. Chem. 2020, 46, 622–630. [Google Scholar] [CrossRef]
- Hosono, H. Two Classes of Superconductors Discovered in Our Material Research: Iron-Based High Temperature Superconductor and Electride Superconductor. Physica C 2009, 469, 314–325. [Google Scholar] [CrossRef]
- Shatruk, M. ThCr2Si2 Structure Type: The “Perovskite” of Intermetallics. J. Solid State Chem. 2019, 272, 198–209. [Google Scholar] [CrossRef]
- Wang, K.; Petrovic, C. Large Thermopower in the Antiferromagnetic Semiconductor BaMn2Bi2. Appl. Phys. Lett. 2013, 103, 192104. [Google Scholar] [CrossRef]
- Calder, S.; Saparov, B.; Cao, H.B.; Niedziela, J.L.; Lumsden, M.D.; Sefat, A.S.; Christianson, A.D. Magnetic Structure and Spin Excitations in BaMn2Bi2. Phys. Rev. B 2014, 89, 064417. [Google Scholar] [CrossRef]
- Shilov, A.I.; Pervakov, K.S.; Lyssenko, K.A.; Vlasenko, V.A.; Efremov, D.V.; Aswartham, S.; Simonov, S.V.; Morozov, I.V.; Shevelkov, A.V. Synthesis and Crystal Growth of Novel Layered Bismuthides ATM2Bi2 (A = K, Rb, Cs; TM = Zn, Cd), Electron-deficient Compounds with the ThCr2Si2 Structure. Z. Anorg. Allg. Chem. 2023, 649, e202200298. [Google Scholar] [CrossRef]
- Hoffmann, R.; Zheng, C. Making and Breaking Bonds in the Solid State: The ThCr2Si2 Structure. J. Phys. Chem. 1985, 89, 4175–4181. [Google Scholar] [CrossRef]
- Sun, Z.-M.; Xie, J.-Y.; Pan, D.-C.; Mao, J.-G. Synthesis and Crystal Structure of CaAgBi and BaAg1.837Bi2. J. Alloys Compd. 2007, 430, 71–76. [Google Scholar] [CrossRef]
- Gui, X.; Xing, L.; Wang, X.; Bian, G.; Jin, R.; Xie, W. Pt–Bi Antibonding Interaction: The Key Factor for Superconductivity in Monoclinic BaPt2Bi2. Inorg. Chem. 2018, 57, 1698–1701. [Google Scholar] [CrossRef] [PubMed]
- Frik, L.; Johrendt, D.; Mewis, A. Eine neue Verzerrungsvariante des CaBe2Ge2-Typs—Die Kristallstrukturen von SrPd2Bi2, BaPd2Bi2 und BaAu2Sb2. Z. Anorg. Allg. Chem. 2006, 632, 1514–1517. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.; Stuart, D. An Empirical Method for Correcting Diffractometer Data for Absorption Effects. Acta Crystallogr. A 1983, 39, 158–166. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Vienna Ab Initio Simulation Package (VASP), v.5.4.4. Available online: https://journals.aps.org/prb/abstract/10.1103/PhysRevB.54.11169 (accessed on 19 January 2024).
- Furness, J.W.; Kaplan, A.D.; Ning, J.; Perdew, J.P.; Sun, J. Correction to “Accurate and Numerically Efficient r2SCAN Meta-Generalized Gradient Approximation”. J. Phys. Chem. Lett. 2020, 11, 9248. [Google Scholar] [CrossRef]
- Kaplan, A.D.; Furness, J.W. r2SCAN Subroutines: Repository for Subroutines/Patches Needed to Implement r2SCAN in Popular Electronic Structure Codes. Available online: https://gitlab.com/dhamil/r2scan-subroutines/-/tree/master (accessed on 25 January 2024).
- Tang, W.; Sanville, E.; Henkelman, G. A Grid-Based Bader Analysis Algorithm without Lattice Bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef]
- Sanville, E.; Kenny, S.D.; Smith, R.; Henkelman, G. Improved Grid-based Algorithm for Bader Charge Allocation. J. Comput. Chem. 2007, 28, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A Fast and Robust Algorithm for Bader Decomposition of Charge Density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Yu, M.; Trinkle, D.R. Accurate and Efficient Algorithm for Bader Charge Integration. J. Chem. Phys. 2011, 134, 064111. [Google Scholar] [CrossRef] [PubMed]
- M Ganose, A.; J Jackson, A.; O Scanlon, D. Sumo: Command-Line Tools for Plotting and Analysis of Periodic Ab Initio Calculations. JOSS 2018, 3, 717. [Google Scholar] [CrossRef]
- Dronskowski, R.; Bloechl, P.E. Crystal Orbital Hamilton Populations (COHP): Energy-Resolved Visualization of Chemical Bonding in Solids Based on Density-Functional Calculations. J. Phys. Chem. 1993, 97, 8617–8624. [Google Scholar] [CrossRef]
- Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. Crystal Orbital Hamilton Population (COHP) Analysis As Projected from Plane-Wave Basis Sets. J. Phys. Chem. A 2011, 115, 5461–5466. [Google Scholar] [CrossRef] [PubMed]
- Maintz, S.; Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. Analytic Projection from Plane-Wave and PAW Wavefunctions and Application to Chemical-Bonding Analysis in Solids. J. Comput. Chem. 2013, 34, 2557–2567. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.; Ertural, C.; George, J.; Deringer, V.L.; Hautier, G.; Dronskowski, R. LOBSTER: Local Orbital Projections, Atomic Charges, and Chemical-bonding Analysis from projector-augmented-wave-based Density-functional Theory. J. Comput. Chem. 2020, 41, 1931–1940. [Google Scholar] [CrossRef]
- Maintz, S.; Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. LOBSTER: A Tool to Extract Chemical Bonding from Plane-wave Based DFT. J. Comput. Chem. 2016, 37, 1030–1035. [Google Scholar] [CrossRef]
- wxDragon, ver. 2.2.2. Available online: https://github.com/pritampanda15/wxDragon (accessed on 25 January 2024).
- Selter, S.; Scaravaggi, F.; Kappenberger, R.; Naumann, M.; Romaka, V.V.; Knupfer, M.; Aswartham, S.; Wolter, A.U.B.; Wurmehl, S.; Büchner, B. Evolution of Structure and Electronic Correlations in a Series of BaT2As2 (T = Cr–Cu) Single Crystals. Inorg. Chem. 2020, 59, 16913–16923. [Google Scholar] [CrossRef]
- Xu, S.; Wang, H.; Wang, Y.-Y.; Su, Y.; Wang, X.-Y.; Xia, T.-L. Crystal Growth of BaAgAs Family Topological Materials via Flux Method. J. Cryst. Growth 2020, 531, 125304. [Google Scholar] [CrossRef]
- Cordero, B.; Gómez, V.; Platero-Prats, A.E.; Revés, M.; Echeverría, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent Radii Revisited. Dalton Trans. 2008, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Li, G.N.; Huang, Q.; Chen, G.F.; He, J.B.; Green, M.A.; Qiu, Y.; Wang, D.M.; Luo, J.L. Superconductivity Tuned by the Iron Vacancy Order in KxFe2−ySe2. Physica C 2012, 474, 1–4. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Revised Values of Effective Ionic Radii. Acta Crystallogr. B 1970, 26, 1046–1048. [Google Scholar] [CrossRef]
- Shevelkov, A.V.; Kovnir, K. Zintl Clathrates. In Zintl Phases: Principles and Recent Developments; Springer: Berlin/Heidelberg, Germany, 2011; pp. 97–142. [Google Scholar]
- Plokhikh, I.V.; Kuznetsov, A.N.; Charkin, D.O.; Shevelkov, A.V.; Pfitzner, A. Layered Compounds BaFMgPn (Pn = P, As, Sb, and Bi), Transition-Metal-Free Representatives of the 1111 Structure Type. Inorg. Chem. 2019, 58, 3435–3443. [Google Scholar] [CrossRef] [PubMed]
- Brechtel, E.; Cordier, G.; Schäfer, H. Neue ternäre Erdalkali-Übergangselement-Pnictide. J. Less-Comm. Metals 1981, 78, 131–138. [Google Scholar] [CrossRef]
- Sun, Z.-M.; Mao, J.-G. Synthesis and Crystal Structure of EuBi2. J. Solid. State Chem. 2004, 177, 3752–3756. [Google Scholar] [CrossRef]
- Papoian, A.G.; Hoffmann, R. Hypervalent Bonding in One, Two, and Three Dimensions: Extending the Zintl-Klemm Concept to Nonclassical Electron-Rich Networks. Angew. Chem. Int. Ed. 2000, 39, 2408–2448. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Guo, P.-J.; Yu, Q.-H.; Xu, S.; Liu, K.; Xia, T.-L. Magneto-Transport and Electronic Structures of BaZnBi2. New J. Phys. 2017, 19, 123044. [Google Scholar] [CrossRef]



| EDX Composition | Ba1.06(3)Ag1.81(2)Bi2.13(4) | Ba0.97(5)Au1.80(6)Bi2.23(5) |
|---|---|---|
| Chemical formula | BaAg1.79Bi2 | BaAu1.79Bi2 |
| Mr | 749.93 | 894.57 |
| Crystal system, space group | Monoclinic, C2/m | |
| a, Å | 6.9058(7) | 6.9298(5) |
| b, Å | 6.9047(7) | 6.9340(5) |
| c, Å | 11.5384(12) | 11.1159(8) |
| β, deg. | 90.010(5) | 90.020(4) |
| V, Å3 | 550.18(10) | 534.13(7) |
| Z | 4 | 4 |
| Radiation type | Mo Kα1 | |
| Temperature | 116(2) | 116(2) |
| µ (mm−1) | 76.916 | 119,911 |
| Diffractometer | Bruker Quest D8 | |
| Absorption correction | SADABS | |
| Tmin, Tmax | 0.034, 0.199 | 0.022, 0.119 |
| No. of measured, independent, and observed [I > 2σ(I)] reflections | 3988, 792, 713 | 2486, 756, 649 |
| Rint | 0.1248 | 0.0631 |
| R, wR, GooF | 0.0463, 0.1088, 1.133 | 0.0392, 0.0891, 1.104 |
| No. of parameters | 41 | 41 |
| Largest difference in peak/hole (e/Å3) | −2.638/3.397 | −3.470/3.621 |
| CCDC | 2312786 | 2312785 |
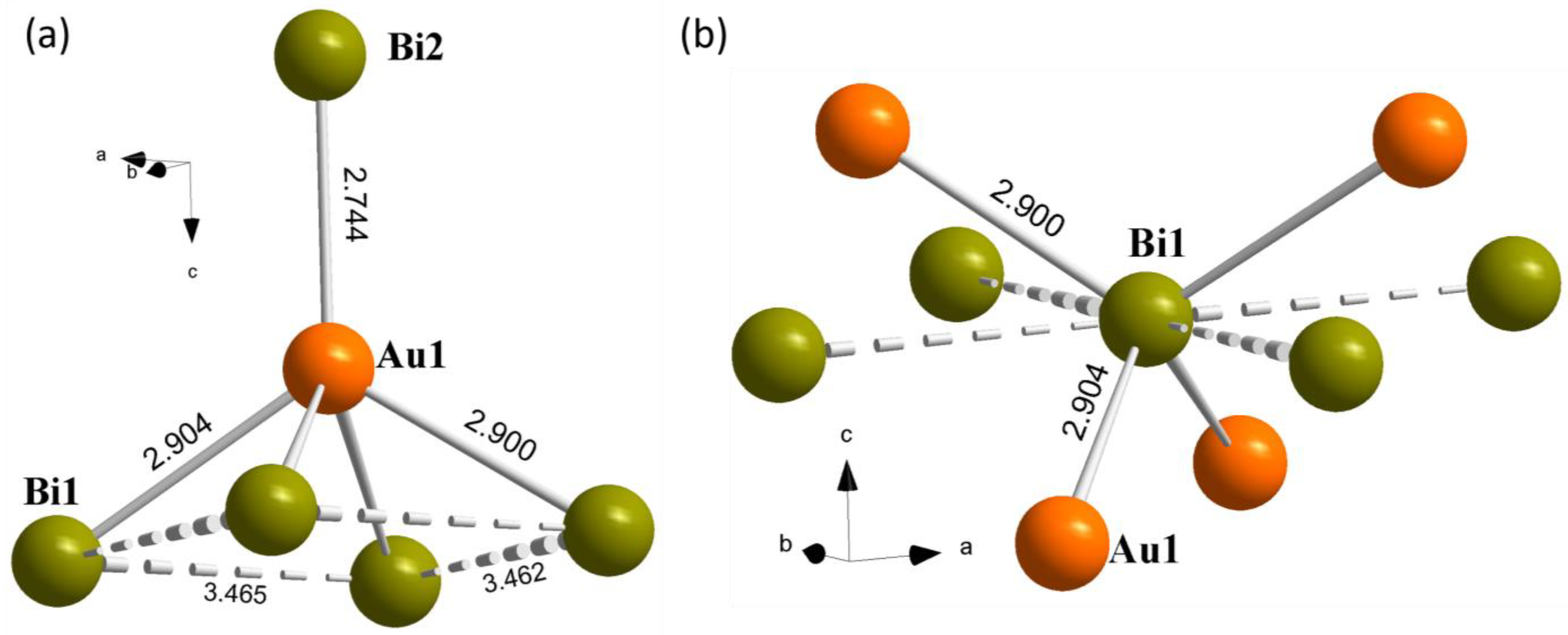
| Polyhedron | Bond | 1 (TM = Ag) | 2 (TM = Au) |
|---|---|---|---|
| Bi square net | Bi1−Bi1 | 3.4529(4) × 2 | 3.4649(3) × 2 |
| 3.4460(16) | 3.462(3) | ||
| 3.4587(16) | 3.472(2) | ||
| [Bi1(TM1)4] | Bi1−TM1 | 2.8986(19) × 2 | 2.9001(13) × 2 |
| 2.9012(14) × 2 | 2.9042(11) × 2 | ||
| TM1−Bi2 | 2.758(2) | 2.7438(13) | |
| [TM2(Bi2)3(Bi2′)] | TM2−Bi2 | 2.955(7) | 2.843(12) |
| 2.956(7) | 2.855(12) | ||
| 2.735(8) | 3.024(13) | ||
| TM2−Bi2′ | 3.110(8) | 3.002(6) | |
| [TM2′(Bi2)3(Bi2′)] | TM2′−Bi2 | 2.955(7) | 2.853(12) |
| 2.957(7) | 2.855(12) | ||
| 2.720(7) | 3.035(13) | ||
| TM2′−Bi2′ | 3.120(9) | 2.984(5) |
| Compound | Bond | ||
|---|---|---|---|
| Bi−TM | Bi−Bi | TM−TM | |
| BaAg2Bi2 | −1.27 | −0.82 | −0.20 |
| BaAu2Bi2 | −1.36 | −0.67 | −0.28 |
| BaPd2Bi2 | −1.51 | −0.90 | −0.18 |
| BaZnBi2 | −1.04 | −1.24 | −0.03 |
| Compound | Atom | ||
|---|---|---|---|
| Ba | TM | Bi | |
| BaAg2Bi2 | +1.24 | −0.29 | −0.33 |
| BaAu2Bi2 | +1.37 | −0.72 | +0.03 |
| BaPd2Bi2 | +1.28 | −0.57 | −0.07 |
| BaZnBi2 | +1.25 | +0.15 | −0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shilov, A.I.; Rakhmanov, E.O.; Lyssenko, K.A.; Kuznetsov, A.N.; Morozov, I.V.; Shevelkov, A.V. Crystal and Electronic Structure of Ternary Bismuthides BaTM1.8Bi2 (TM = Au, Ag) with a New Variation of the BaAu2Sb2 Structure Type. Crystals 2024, 14, 155. https://doi.org/10.3390/cryst14020155
Shilov AI, Rakhmanov EO, Lyssenko KA, Kuznetsov AN, Morozov IV, Shevelkov AV. Crystal and Electronic Structure of Ternary Bismuthides BaTM1.8Bi2 (TM = Au, Ag) with a New Variation of the BaAu2Sb2 Structure Type. Crystals. 2024; 14(2):155. https://doi.org/10.3390/cryst14020155
Chicago/Turabian StyleShilov, Andrey I., Evgeny O. Rakhmanov, Konstantin A. Lyssenko, Alexey N. Kuznetsov, Igor V. Morozov, and Andrei V. Shevelkov. 2024. "Crystal and Electronic Structure of Ternary Bismuthides BaTM1.8Bi2 (TM = Au, Ag) with a New Variation of the BaAu2Sb2 Structure Type" Crystals 14, no. 2: 155. https://doi.org/10.3390/cryst14020155
APA StyleShilov, A. I., Rakhmanov, E. O., Lyssenko, K. A., Kuznetsov, A. N., Morozov, I. V., & Shevelkov, A. V. (2024). Crystal and Electronic Structure of Ternary Bismuthides BaTM1.8Bi2 (TM = Au, Ag) with a New Variation of the BaAu2Sb2 Structure Type. Crystals, 14(2), 155. https://doi.org/10.3390/cryst14020155







