Fabrication and Properties for Thermal Neutron Detection of 6LiCl/Rb2CeCl5 Eutectic Scintillator
Abstract
1. Introduction
2. Materials and Methods
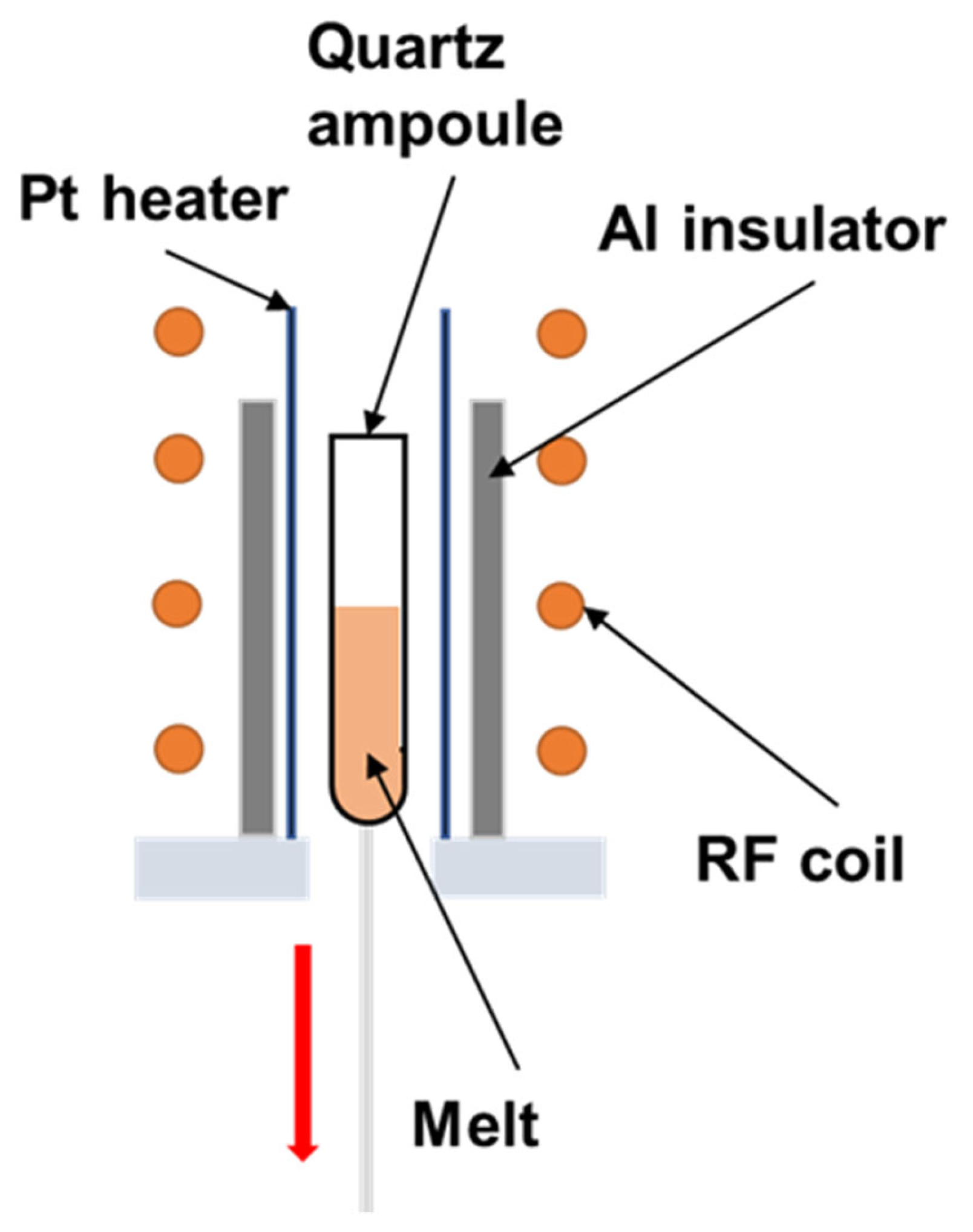
2.1. Crystal Growth
2.2. Evaluation of Microstructure and Scintillation Properties
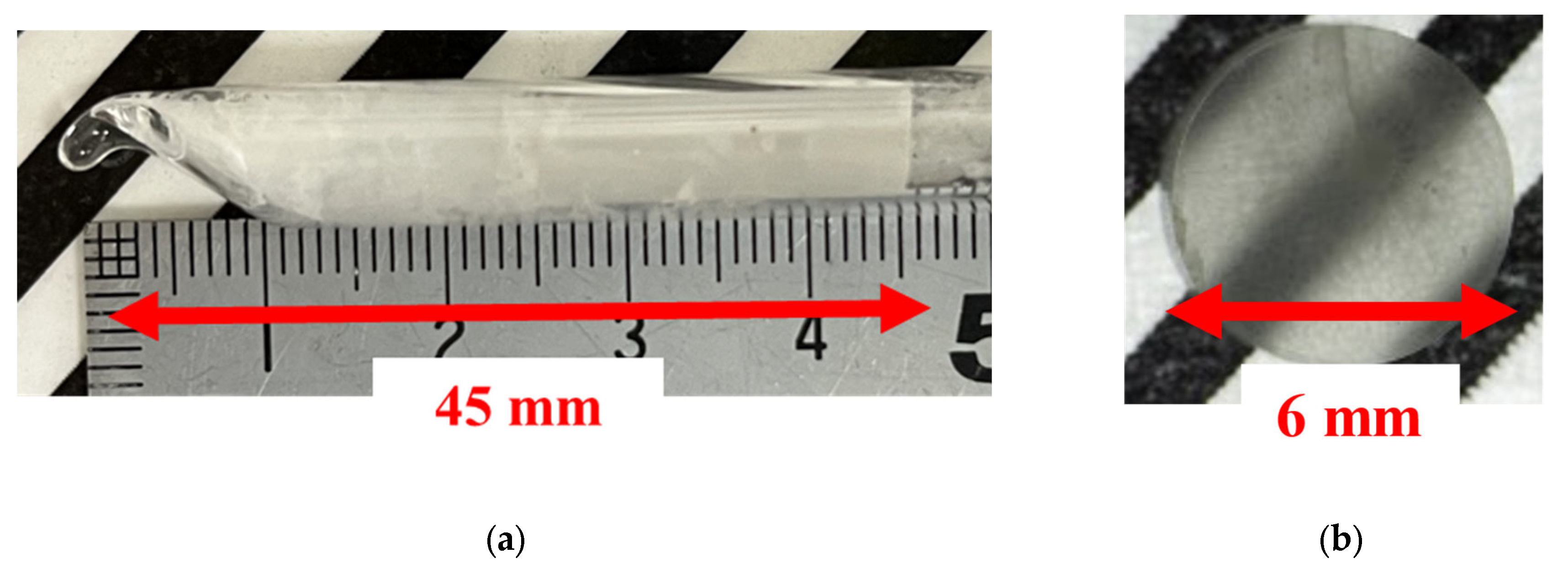
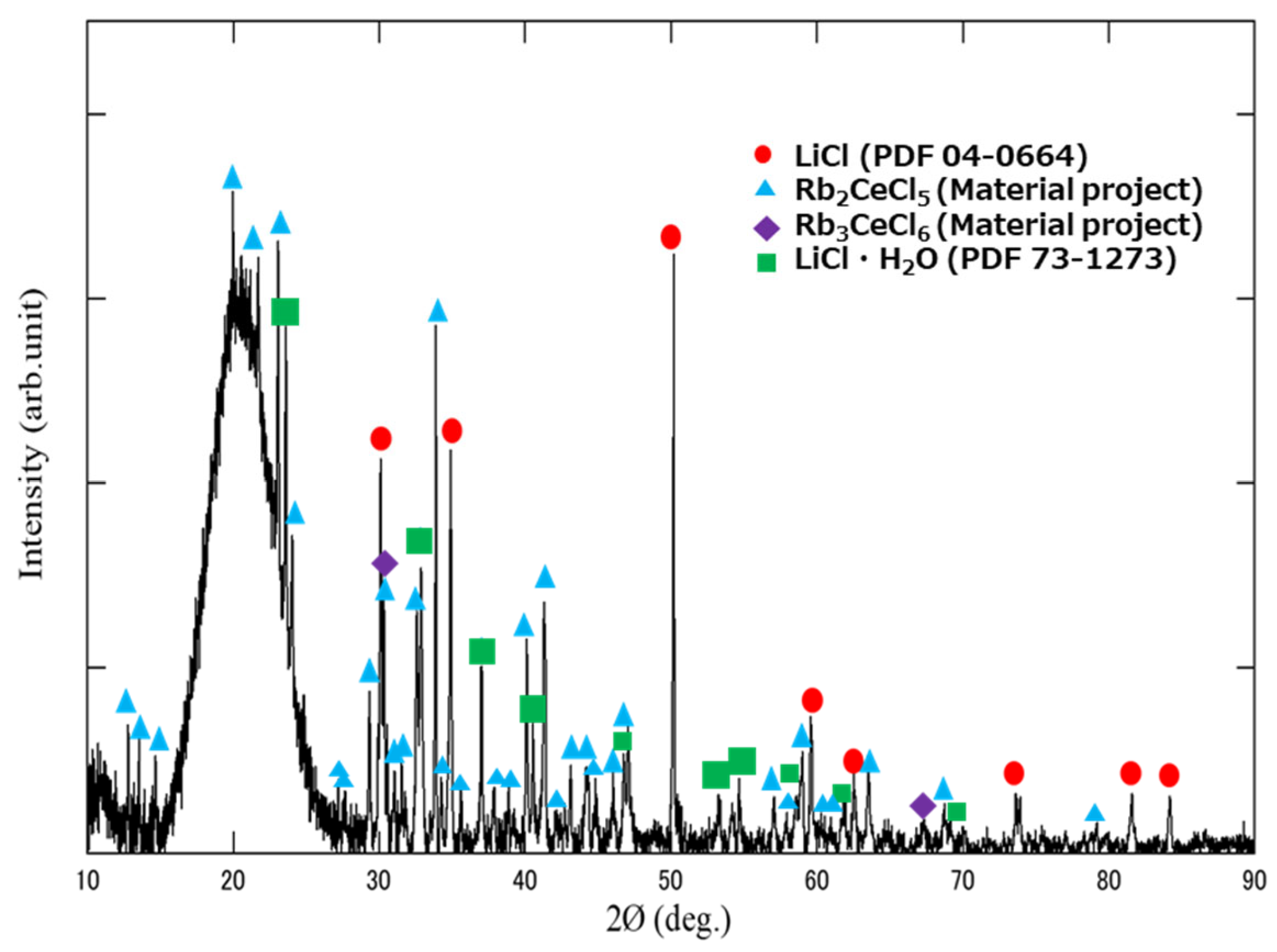
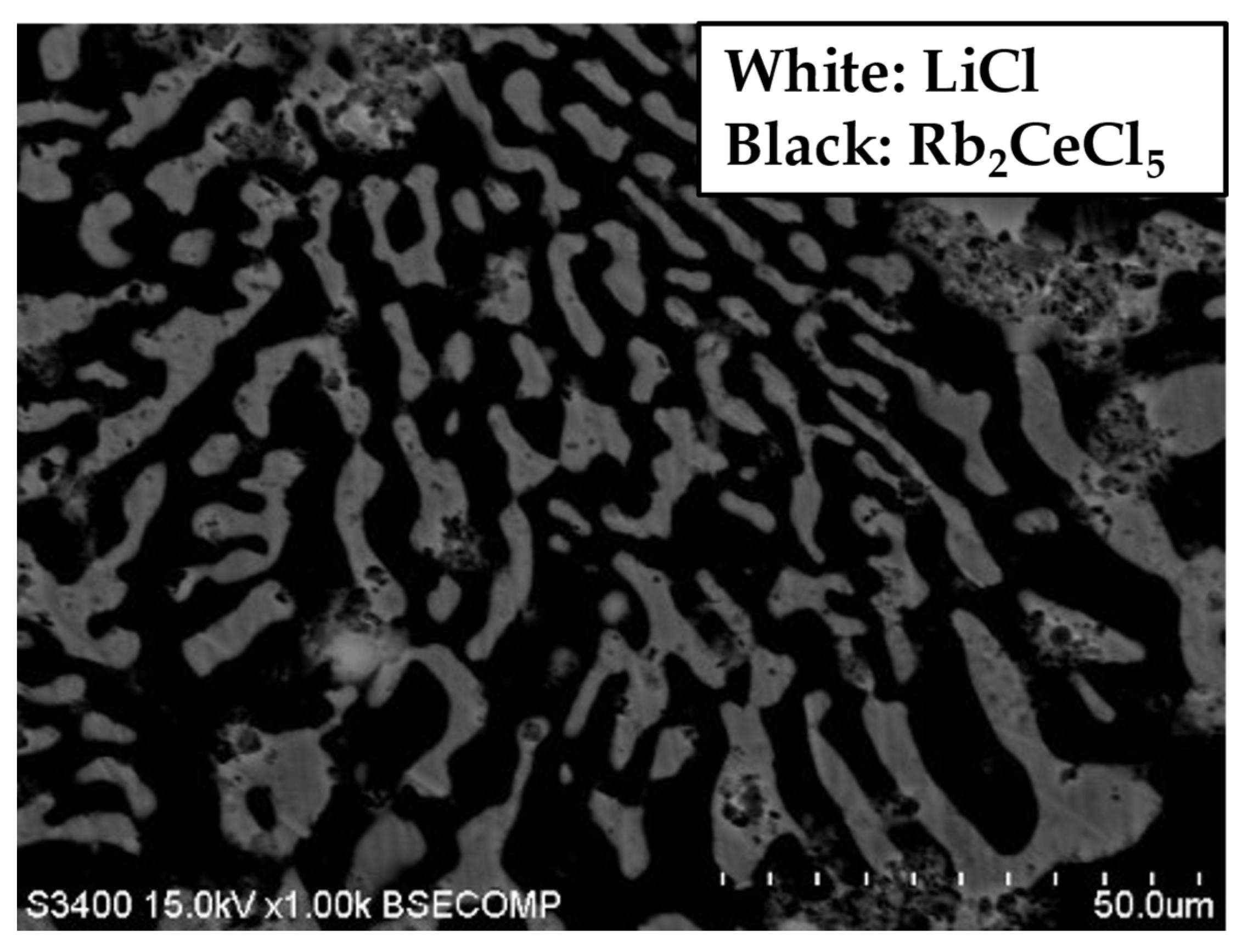
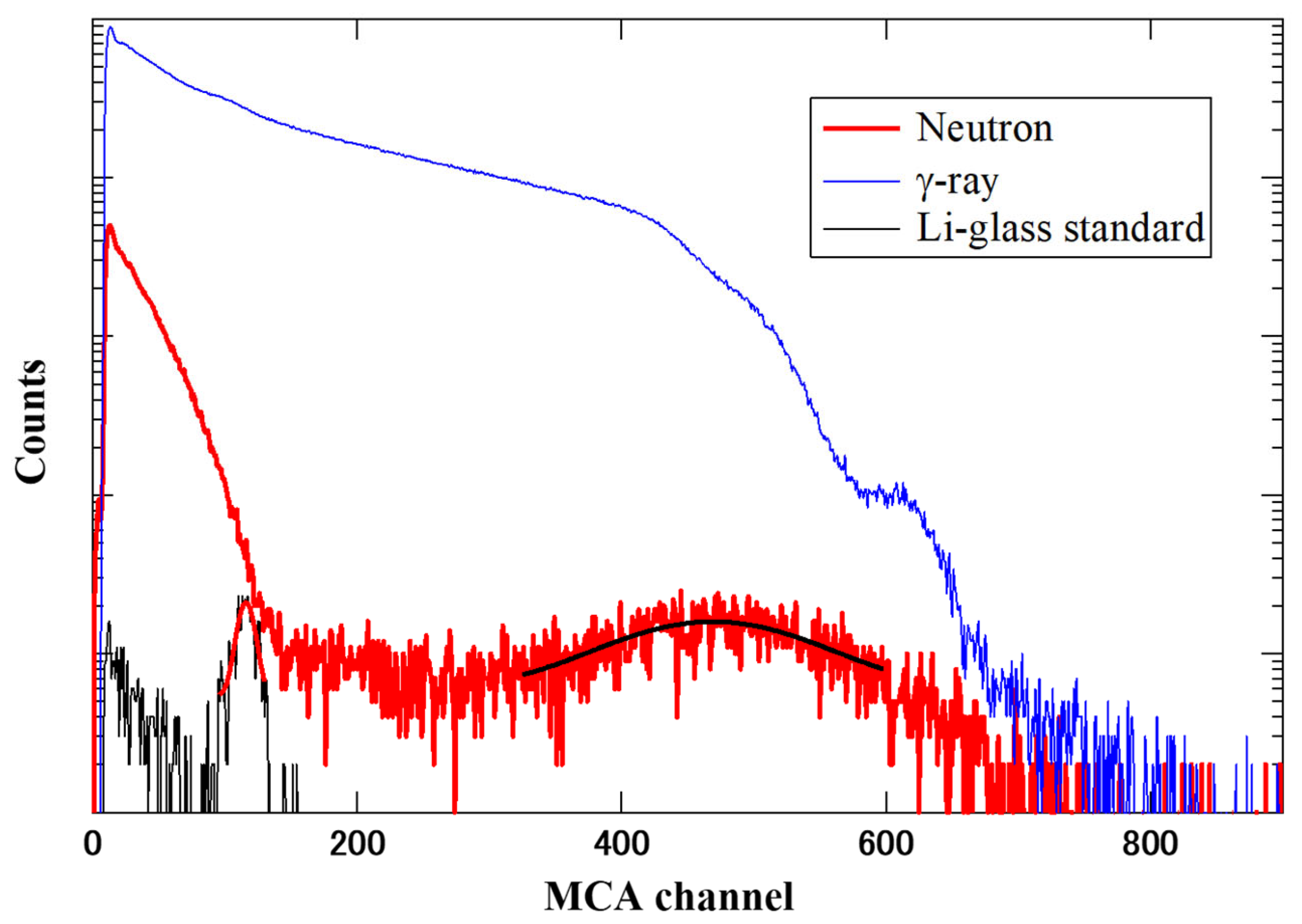
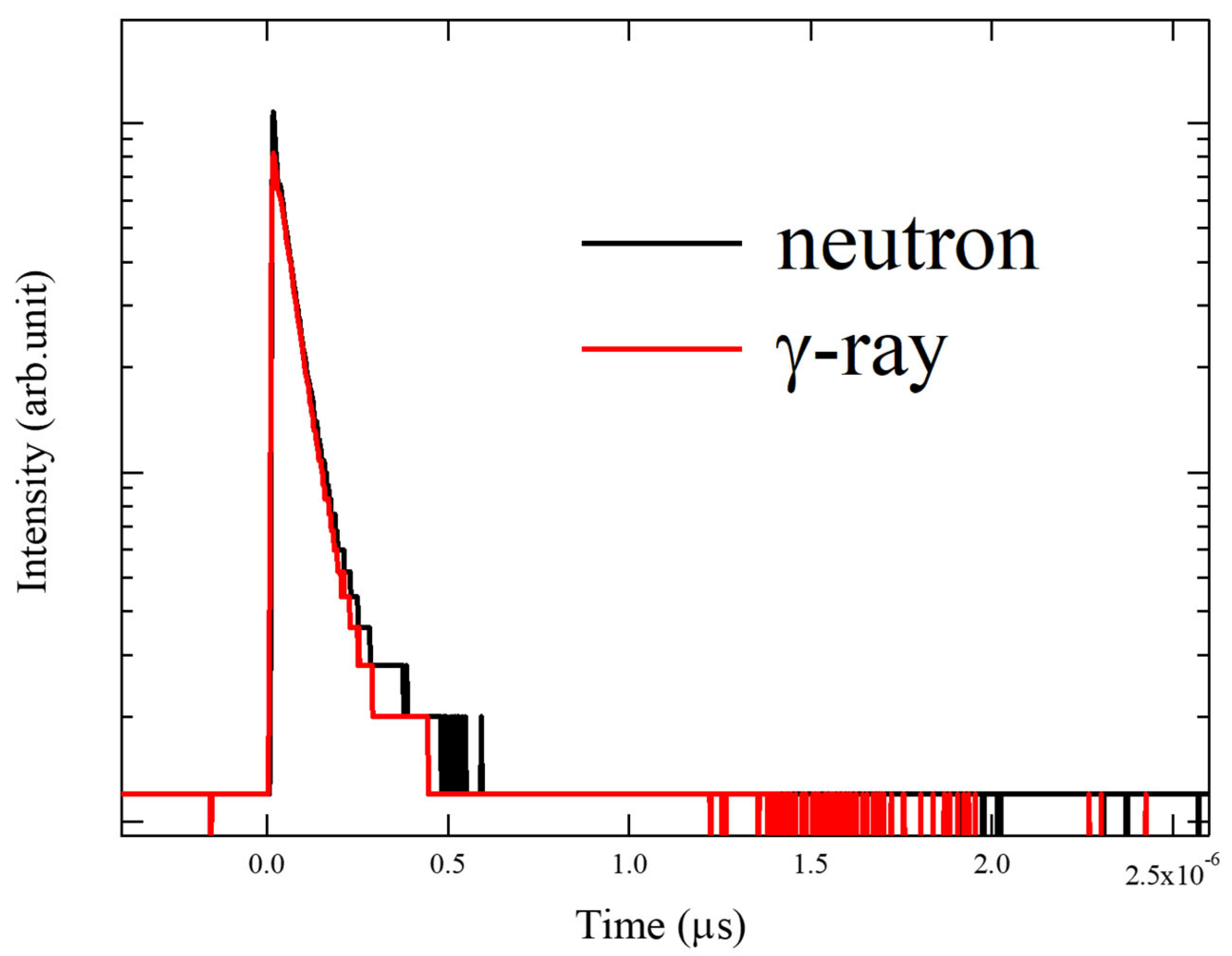
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kardjilov, N.; Manke, I.; Woracek, R.; Hilger, A.; Banhart, J. Advances in Neutron Imaging. Mater. Today 2018, 21, 652–672. [Google Scholar] [CrossRef]
- Butler, L.G.; Schillinger, B.; Ham, K.; Dobbins, T.A.; Liu, P.; Vajo, J.J. Neutron Imaging of a Commercial Li-Ion Battery during Discharge: Application of Monochromatic Imaging and Polychromatic Dynamic Tomography. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2011, 651, 320–328. [Google Scholar] [CrossRef]
- Kardjilov, N.; Manke, I.; Hilger, A.; Strobl, M.; Banhart, J. Neutron Imaging in Materials Science. Mater. Today 2011, 14, 248–256. [Google Scholar] [CrossRef]
- Beddingfield, D.H.; Johnson, N.H.; Menlove, H.O. 3He Neutron Proportional Counter Performance in High Gamma-Ray Dose Environments. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2000, 455, 670–682. [Google Scholar] [CrossRef]
- Kouzes, R.T. The 3He Supply Problem; Pacific Northwest National Lab.: Richland, WA, USA, 2009. [Google Scholar] [CrossRef]
- Kouzes, R.T.; Ely, J.H.; Erikson, L.E.; Kernan, W.J.; Lintereur, A.T.; Siciliano, E.R.; Stephens, D.L.; Stromswold, D.C.; Van Ginhoven, R.M.; Woodring, M.L. Neutron Detection Alternatives to 3He for National Security Applications. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2010, 623, 1035–1045. [Google Scholar] [CrossRef]
- Rezaei-Ochbelagh, D. Comparison of 3He and BF3 Neutron Detectors Used to Detect Hydrogenous Material Buried in Soil. Radiat. Phys. Chem. 2012, 81, 379–382. [Google Scholar] [CrossRef]
- Bolewski, A.; Ciechanowski, M.; Dydejczyk, A.; Kreft, A. On the Optimization of the Isotopic Neutron Source Method for Measuring the Thermal Neutron Absorption Cross Section: Advantages and Disadvantages of BF3 and 3He Counters. Appl. Radiat. Isot. 2008, 66, 457–462. [Google Scholar] [CrossRef]
- Glodo, J.; Hawrami, R.; Shah, K.S. Development of Cs2LiYCl6 Scintillator. J. Cryst. Growth 2013, 379, 73–78. [Google Scholar] [CrossRef]
- Lee, D.W.; Stonehill, L.C.; Klimenko, A.; Terry, J.R.; Tornga, S.R. Pulse-Shape Analysis of Cs2LiYCl6:Ce Scintillator for Neutron and Gamma-Ray Discrimination. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2012, 664, 1–5. [Google Scholar] [CrossRef]
- Nagarkar, V.V.; Ovechkina, E.; Bhandari, H.; Miller, S.R.; Marton, Z.; Glodo, J.; Soundara-Pandian, L.; Mengesha, W.; Gerling, M.; Brubaker, E. Lithium Alkali Halides-New Thermal Neutron Detectors with n-γ Discrimination. In Proceedings of the 2013 IEEE Nuclear Science Symposium and Medical Imaging Conference (2013 NSS/MIC), Seoul, Republic of Korea, 27 October–2 November 2013; pp. 1–4. [Google Scholar] [CrossRef]
- Marshall, M.S.J.; More, M.J.; Bhandari, H.B.; Riedel, R.A.; Waterman, S.; Crespi, J.; Nickerson, P.; Miller, S.; Nagarkar, V.V. Novel Neutron Detector Material: Microcolumnar LixNa1-XI:Eu. IEEE Trans. Nucl. Sci. 2017, 64, 2878–2882. [Google Scholar] [CrossRef]
- Kaburagi, M.; Shimazoe, K.; Terasaka, Y.; Tomita, H.; Yoshihashi, S.; Yamazaki, A.; Uritani, A.; Takahashi, H. Neutron/γ-Ray Discrimination Based on the Property and Thickness Controls of Scintillators Using Li Glass and LiCAF (Ce) in a γ-Ray Field. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2023, 1046, 167636. [Google Scholar] [CrossRef]
- Yokota, Y.; Yamaji, A.; Kurosawa, S.; Ohashi, Y.; Kamada, K.; Yoshikawa, A. Engineering of Eu Dopant Segregation in Colquiriite-Type Fluoride Single Crystal Scintillators. AIP Adv. 2017, 7, 125312. [Google Scholar] [CrossRef]
- Seeger, P.A. Neutron Detection Systems for Small-Angle Scattering. J. Appl. Crystallogr. 1988, 21, 613–617. [Google Scholar] [CrossRef]
- Chuirazzi, W.; Craft, A.; Schillinger, B.; Cool, S.; Tengattini, A. Boron-Based Neutron Scintillator Screens for Neutron Imaging. J. Imaging 2020, 6, 124. [Google Scholar] [CrossRef]
- Yajima, R.; Kamada, K.; Yoshino, M.; Sasaki, R.; Horiai, T.; Murakami, R.; Kim, K.J.; Kochurikhin, V.V.; Ohashi, Y.; Yamaji, A.; et al. Tl Doped CsI/6LiBr Scintillator for Thermal Neutron Detection with Ultra-High Light Yield. IEEE Trans. Nucl. Sci. 2023, 70, 1331–1336. [Google Scholar] [CrossRef]
- Takizawa, Y.; Kamada, K.; Yoshino, M.; Kim, K.J.; Yamaji, A.; Kurosawa, S.; Yokota, Y.; Sato, H.; Toyoda, S.; Ohashi, Y.; et al. Growth and Scintillation Properties of Ce Doped 6LiBr/LaBr3 Eutectic Scintillator for Neutron Detection. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2022, 1028, 166384. [Google Scholar] [CrossRef]
- Yajima, R.; Kamada, K. Fabrication and Characterization of K2CeCl5/6LiCl and CeCl3/SrCl2/6LiCl Eutectics for Thermal Neutron Detection. Crystals 2022, 12, 1795. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Saeki, K.; Nakauchi, D.; Yanagida, T.; Koshimizu, M.; Asai, K. Photoluminescence and Scintillation Properties of Rb2CeCl5 Crystal. Sensors Mater. 2019, 31, 1241–1248. [Google Scholar] [CrossRef]
- He, M.; Lu, G.; Kang, Z.; Zhang, Y. Thermodynamic Assessment of the LiCl-KCl-CeCl3 System. Calphad Comput. Coupling Phase Diagr. Thermochem. 2015, 49, 1–7. [Google Scholar] [CrossRef]
- Holl, I.; Lorenz, E.; Mageras, G. A Measurement of the Light Yield of Common Inorganic Scintillators. IEEE Trans. Nucl. Sci. 1988, 35, 105–109. [Google Scholar] [CrossRef]
- Yang, K.; Menge, P.R.; Ouspenski, V. Enhanced α-γ Discrimination in Co-Doped LaBr3:Ce. In Proceedings of the 2014 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Seattle, WA, USA, 8–15 November 2014; pp. 1–5. [Google Scholar] [CrossRef]
- Vuong, P.Q.; Kim, H.; Luan, N.T.; Kim, S. Neutron Spectroscopy Using Pure LaCl3 Crystal and the Dependence of Pulse Shape Discrimination on Ce-Doped Concentrations. Nucl. Eng. Technol. 2021, 53, 3784–3789. [Google Scholar] [CrossRef]
- Pino, F.; Polo, M.; Delgado, J.C.; Mantovani, G.; Carturan, S.M.; Fabris, D.; Brunelli, D.; Pancheri, L.; Quaranta, A.; Moretto, S. Evidence of Fast Neutron Detection Capability of the CLLB Scintillation Detector. Radiat. Phys. Chem. 2023, 202, 110494. [Google Scholar] [CrossRef]
- Ishikawa, A.; Yamazaki, A.; Watanabe, K.; Yoshihashi, S.; Uritani, A.; Fukuda, K.; Koike, A.; Ogawara, R.; Suda, M.; Hamano, T. Sensitivity and Linearity of Optical Fiber-Based Neutron Detectors Using Small 6Li-Based Scintillators. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2020, 954, 161661. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, R.; Kamada, K.; Yoshino, M.; Kim, K.J.; Murakami, R.; Horiai, T.; Yamaji, A.; Kurosawa, S.; Yokota, Y.; Sato, H.; et al. Fabrication and Properties for Thermal Neutron Detection of 6LiCl/Rb2CeCl5 Eutectic Scintillator. Crystals 2024, 14, 154. https://doi.org/10.3390/cryst14020154
Sasaki R, Kamada K, Yoshino M, Kim KJ, Murakami R, Horiai T, Yamaji A, Kurosawa S, Yokota Y, Sato H, et al. Fabrication and Properties for Thermal Neutron Detection of 6LiCl/Rb2CeCl5 Eutectic Scintillator. Crystals. 2024; 14(2):154. https://doi.org/10.3390/cryst14020154
Chicago/Turabian StyleSasaki, Rei, Kei Kamada, Masao Yoshino, Kyoung Jin Kim, Rikito Murakami, Takahiko Horiai, Akihiro Yamaji, Shunsuke Kurosawa, Yuui Yokota, Hiroki Sato, and et al. 2024. "Fabrication and Properties for Thermal Neutron Detection of 6LiCl/Rb2CeCl5 Eutectic Scintillator" Crystals 14, no. 2: 154. https://doi.org/10.3390/cryst14020154
APA StyleSasaki, R., Kamada, K., Yoshino, M., Kim, K. J., Murakami, R., Horiai, T., Yamaji, A., Kurosawa, S., Yokota, Y., Sato, H., Ohashi, Y., Hanada, T., & Yoshikawa, A. (2024). Fabrication and Properties for Thermal Neutron Detection of 6LiCl/Rb2CeCl5 Eutectic Scintillator. Crystals, 14(2), 154. https://doi.org/10.3390/cryst14020154






