Effect of TiO2 on the Microstructure and Flexural Strength of Lunar Regolith Simulant
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Sample Preparation
2.3. Sintering Experiments
2.4. Characterization
3. Results and Discussion
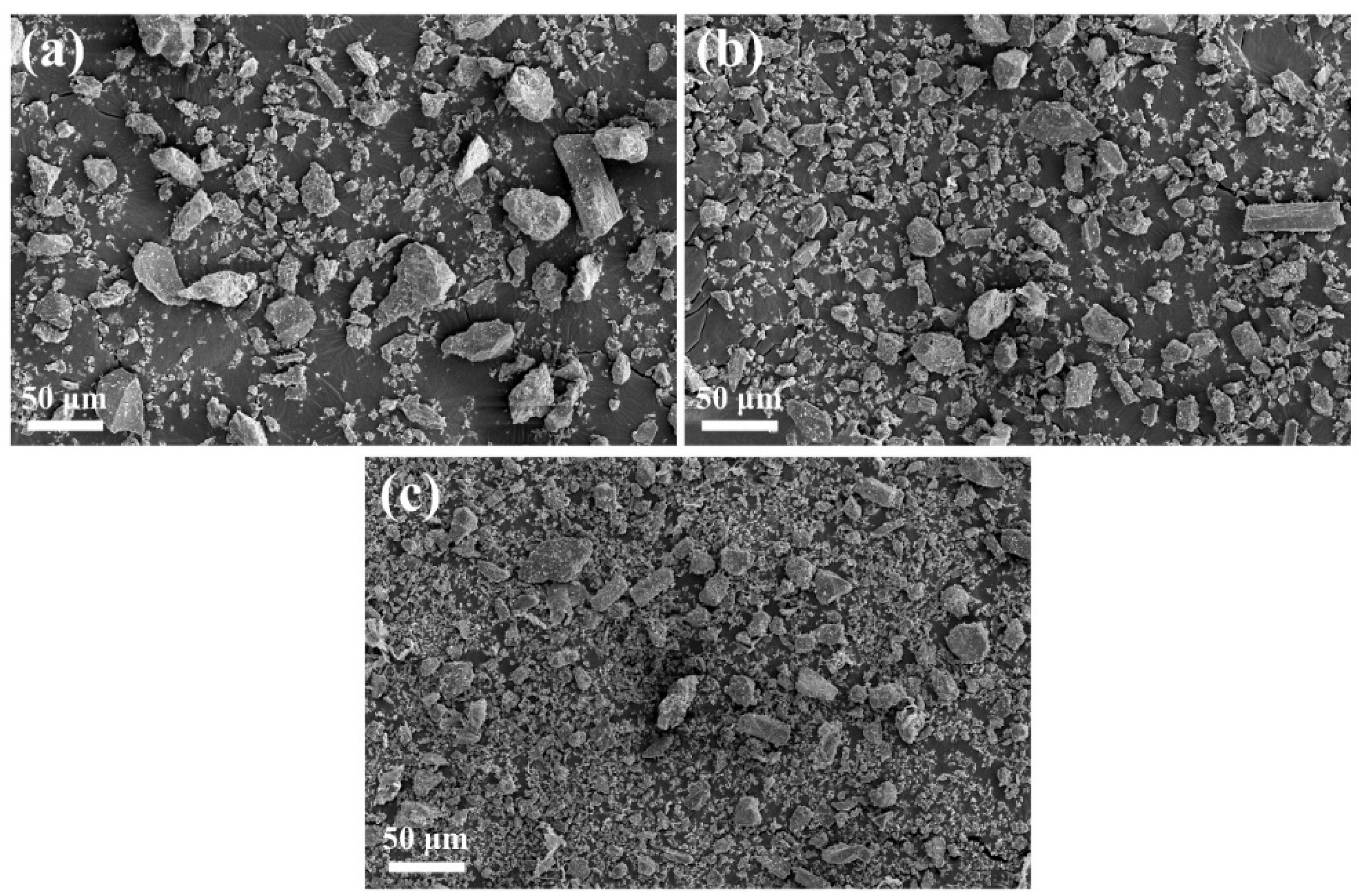
3.1. CUG-1A–TiO2 Ball-Milling Process
3.2. Phase Analysis
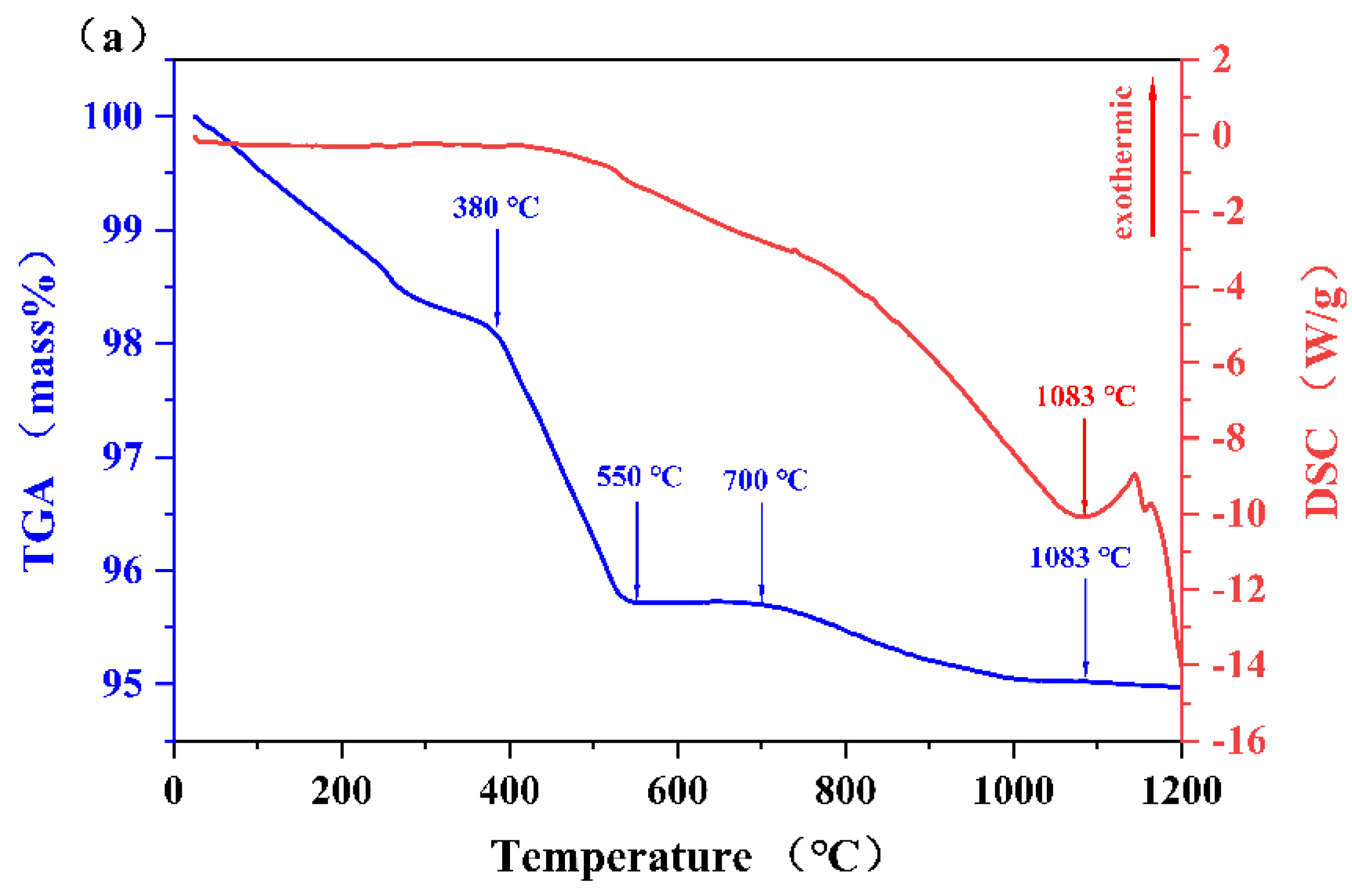
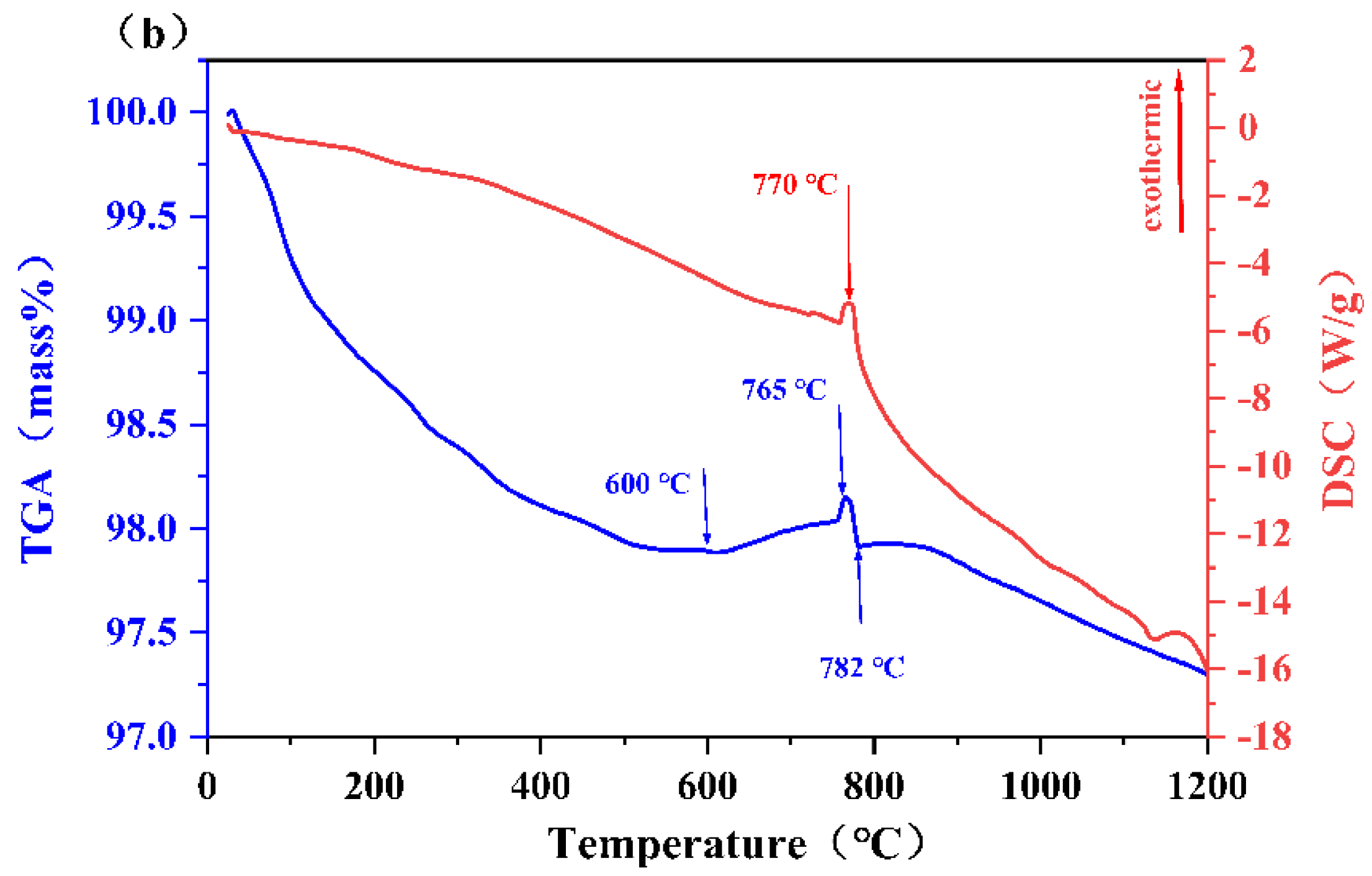
3.3. Thermal Analysis
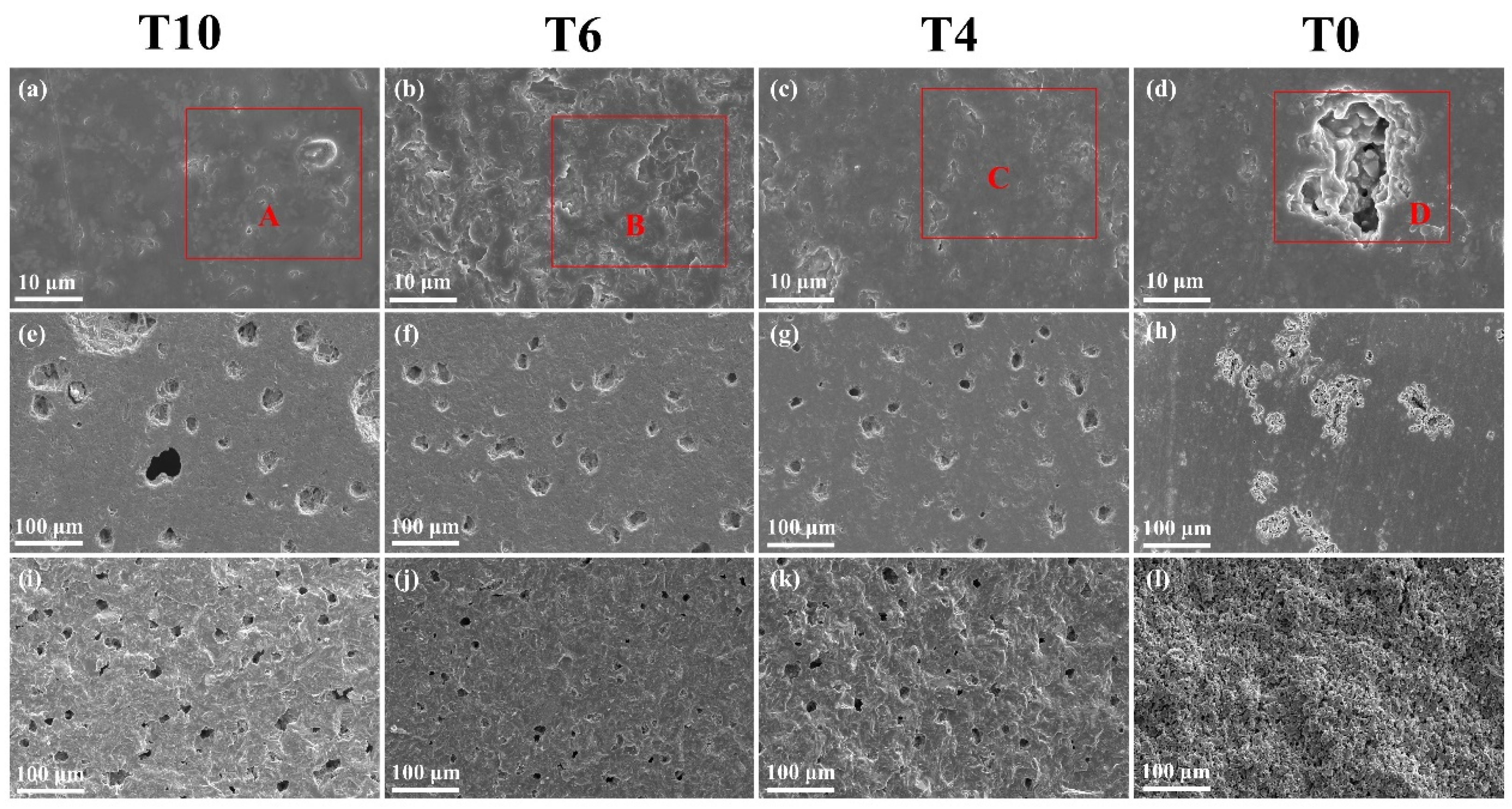
3.4. Microstructure and EDS Analysis
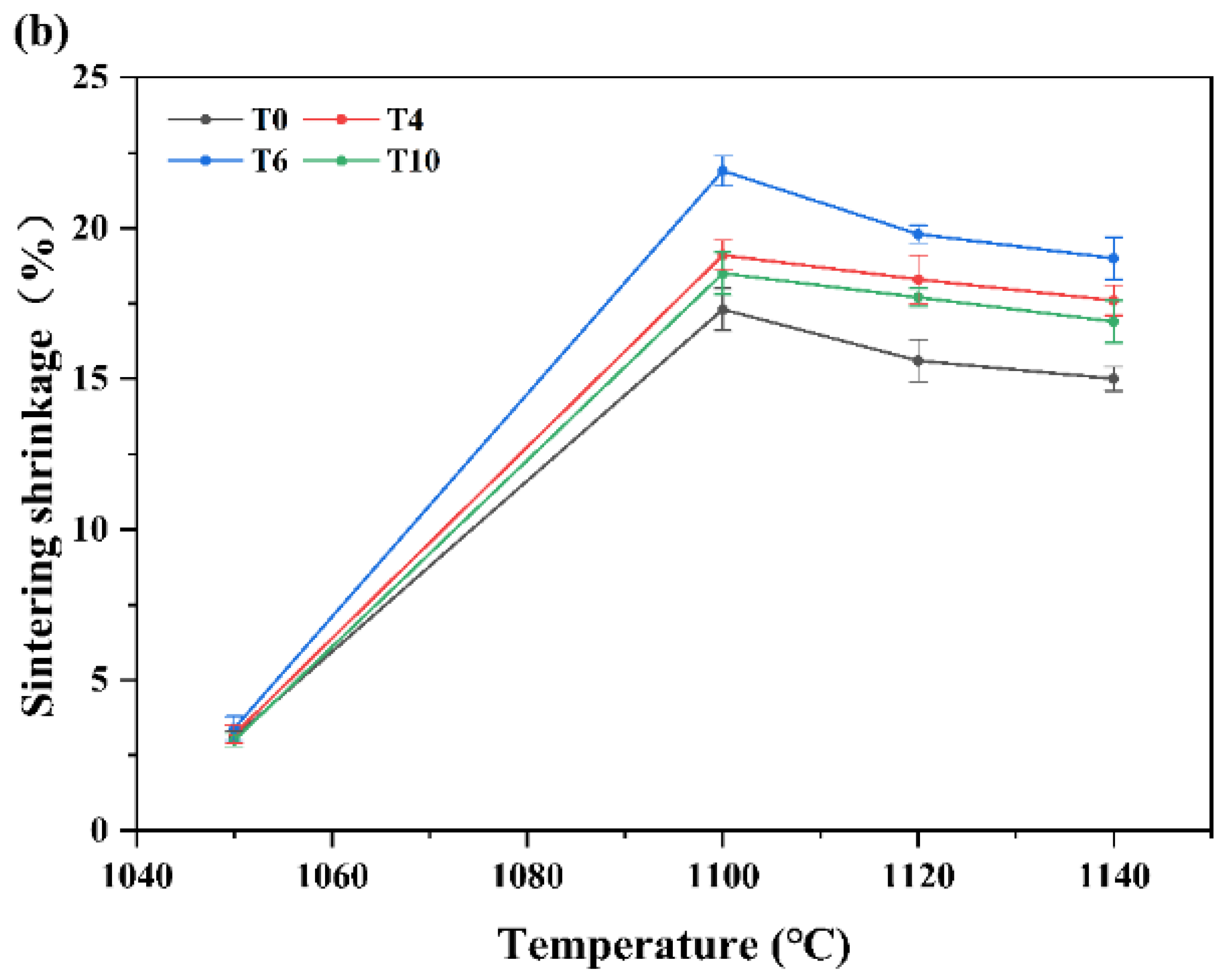
3.5. Sintering Shrinkage and Relative Density of CUG-1A–TiO2 Samples
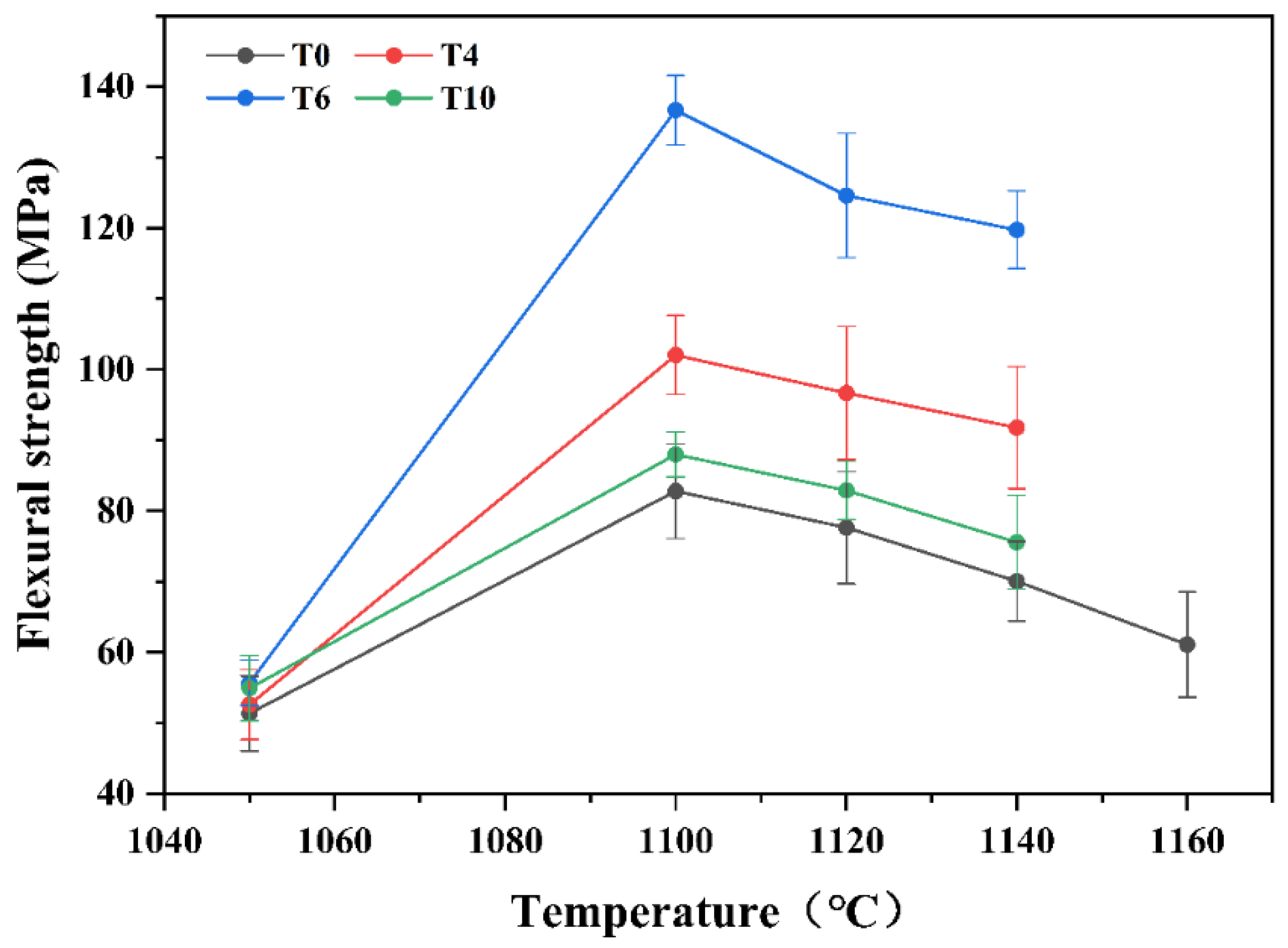
3.6. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruess, F.; Schaenzlin, J.; Benaroya, H. Structural design of a lunar habitat. J. Aerosp. Eng. 2006, 19, 135–157. [Google Scholar] [CrossRef]
- Detsis, E.; Doule, O.; Ebrahimi, A. Location selection and layout for LB10, a lunar base at the Lunar North Pole with a liquid mirror observatory. Acta Astronaut. 2013, 85, 61–72. [Google Scholar] [CrossRef]
- Wilhelm, S.; Curbach, M. Review of possible mineral materials and production techniques for a building material on the moon. Struct. Concr. 2014, 15, 419–428. [Google Scholar] [CrossRef]
- Labeaga-Martínez, N.; Sanjurjo-Rivo, M.; Díaz-Alvarez, J.; Martínez-Frías, J. Additive manufacturing for a Moon village. In Proceedings of the 7th Manufacturing-Engineering-Society International Conference (MESIC), Vigo, Spain, 28–30 June 2017; pp. 794–801. [Google Scholar]
- Lim, S.; Prabhu, V.L.; Anand, M.; Taylor, L.A. Extra-terrestrial construction processes—Advancements, opportunities and challenges. Adv. Space Res. 2017, 60, 1413–1429. [Google Scholar] [CrossRef]
- Anand, M.; Crawford, I.A.; Balat-Pichelin, M.; Abanades, S.; van Westrenen, W.; Péraudeau, G.; Jaumann, R.; Seboldt, W. A brief review of chemical and mineralogical resources on the Moon and likely initial in situ resource utilization (ISRU) applications. Planet Space Sci. 2012, 74, 42–48. [Google Scholar] [CrossRef]
- Sanders, G.B.; Larson, W.E. Integration of In-Situ Resource Utilization into lunar/Mars exploration through field analogs. Adv. Space Res. 2011, 47, 20–29. [Google Scholar] [CrossRef]
- Sanders, G.B.; Larson, W.E. Final review of analog field campaigns for In Situ Resource Utilization technology and capability maturation. Adv. Space Res. 2015, 55, 2381–2404. [Google Scholar] [CrossRef]
- Sanders, G.B.; Larson, W.E. Progress Made in Lunar In Situ Resource Utilization under NASA’s Exploration Technology and Development Program. J. Aerosp. Eng. 2013, 26, 5–17. [Google Scholar] [CrossRef]
- Isachenkov, M.; Chugunov, S.; Akhatov, I.; Shishkovsky, I. Regolith-based additive manufacturing for sustainable development of lunar infrastructure—An overview. Acta Astronaut. 2021, 180, 650–678. [Google Scholar] [CrossRef]
- Miller, J.; Taylor, L.; Zeitlin, C.; Heilbronn, L.; Guetersloh, S.; DiGiuseppe, M.; Iwata, Y.; Murakami, T. Lunar soil as shielding against space radiation. Radiat. Meas. 2009, 44, 163–167. [Google Scholar] [CrossRef]
- Zong, X.; Chen, J.H.; Chen, H.M.; Jiang, Z.S.; Wu, S.H. Progress in Preparation Technology of Lunar Regolith Simulant. Mech. Electr. Eng. Technol. 2023, 52, 23–29. [Google Scholar]
- Zhang, X.; Khedmati, M.; Kim, Y.R.; Shin, H.S.; Lee, J.G.; Kim, Y.J.; Cui, B. Microstructure evolution during spark plasma sintering of FJS-1 lunar soil simulant. J. Am. Ceram. Soc. 2020, 103, 899–911. [Google Scholar] [CrossRef]
- Matsushima, T.; Katagiri, J.; Uesugi, K.; Tsuchiyama, A.; Nakano, T. 3D Shape Characterization and Image-Based DEM Simulation of the Lunar Soil Simulant FJS-1. J. Aerosp. Eng. 2009, 22, 15–23. [Google Scholar] [CrossRef]
- Zheng, Y.C.; Wang, S.J.; Ouyang, Z.Y.; Zou, Y.L.; Liu, J.Z.; Li, C.L.; Li, X.Y.; Feng, J.M. CAS-1 lunar soil simulant. Adv. Space Res. 2009, 43, 448–454. [Google Scholar] [CrossRef]
- Taylor, L.A.; Pieters, C.M.; Britt, D. Evaluations of lunar regolith simulants. Planet Space Sci. 2016, 126, 1–7. [Google Scholar] [CrossRef]
- Ray, C.S.; Reis, S.T.; Sen, S.; O’Dell, J.S. JSC-1A lunar soil simulant: Characterization, glass formation, and selected glass properties. J. Non-Cryst. Solids 2010, 356, 2369–2374. [Google Scholar] [CrossRef]
- Song, L.; Xu, J.; Fan, S.Q.; Tang, H.; Li, X.Y.; Liu, J.Z.; Duan, X.M. Vacuum sintered lunar regolith simulant: Pore-forming and thermal conductivity. Ceram. Int. 2019, 45, 3627–3633. [Google Scholar] [CrossRef]
- Fateri, M.; Meurisse, A.; Sperl, M.; Urbina, D.; Madakashira, H.K.; Govindaraj, S.; Gancet, J.; Imhof, B.; Hoheneder, W.; Waclavicek, R.; et al. Solar Sintering for Lunar Additive Manufacturing. J. Aerosp. Eng. 2019, 32, 04019101. [Google Scholar] [CrossRef]
- Lim, S.; Bowen, J.; Degli-Alessandrini, G.; Anand, M.; Cowley, A.; Levin Prabhu, V. Investigating the microwave heating behaviour of lunar soil simulant JSC-1A at different input powers. Sci. Rep. 2021, 11, 16. [Google Scholar] [CrossRef]
- Wilkerson, R.P.; Petkov, M.P.; Voecks, G.E.; Lynch, C.S.; Shulman, H.S.; Sundaramoorthy, S.; Choudhury, A.; Rickman, D.L.; Effinger, M.R. Outgassing behavior and heat treatment optimization of JSC-1A lunar regolith simulant. Icarus 2023, 400, 13. [Google Scholar] [CrossRef]
- Petkov, M.P.; Voecks, G.E. Characterization of volatiles evolved during vacuum sintering of lunar regolith simulants. Ceram. Int. 2023, 49, 33459–33468. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, G.; LÜ, X.C.; Yao, W. Experimental study of the parameters of laser melting molding process with regard to simulated lunar soil. Spacecr. Environ. Eng. 2021, 38, 575–580. [Google Scholar] [CrossRef]
- Toklu, Y.C.; Açikbas, N.; Açikbas, G.; Çerçevik, A.E.; Akpinar, P. Production of a set of lunar regolith simulants based on Apollo and Chinese samples. Adv. Space Res. 2023, 72, 565–576. [Google Scholar] [CrossRef]
- Shkuratov, Y.; Kaydash, V.; Sysolyatina, X.; Razim, A.; Videen, G. Lunar surface traces of engine jets of Soviet sample return probes: The enigma of the Luna-23 and Luna-24 landing sites. Planet Space Sci. 2013, 75, 28–36. [Google Scholar] [CrossRef]
- Arslan, H.; Sture, S.; Batiste, S. Experimental simulation of tensile behavior of lunar soil simulant JSC-1. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2008, 478, 201–207. [Google Scholar] [CrossRef]
- Kanamori, H. Properties of lunar soil simulant inanufactured in Japan. In Proceedings of the Proceeding of the International Conference & Exposition on Engineering, Albuquerque, NM, USA, 26–30 April 1998. [Google Scholar]
- Yoshida, H.; Watanabe, T.; Kanamori, H.; Yoshida, T.; Ogiwara, S.; Eguchi, K. Experimental Study on Water Production by Hydrogen Reduction of Lunar Soil Simulant in a Fixed Bed Reactor. Pain 2000, 148, 36–42. [Google Scholar]
- Weiblen, P.W.; Murawa, M.J.; Reid, K.J. Preparation of Simulants for Lunar Surface Materials. Eng. Constr. Oper. Space II 1990, 98–106. [Google Scholar]
- He, X.X.; Xiao, L.; Huang, J.; He, Q.; Gao, R.; Yang, G. Lunar Soil Simulant Development and Lunar Soil Simulant CUG-1A. Geol. Sci. Technol. Inf. 2011, 30, 6. [Google Scholar]
- James, O.B.; Jackson, E.D. Petrology of the Apollo 11 ilmenite basalts. J. Geophys. Res. 1970, 75, 5793–5824. [Google Scholar] [CrossRef]
- Cameron, E.N. Apollo 11 ilmenite revisited. In Proceedings of the 3rd International Conference, Denver, CO, USA, 31 May–4 June 1992. [Google Scholar]
- Zhou, C.J.; Tang, H.; Li, X.Y.; Zeng, X.J.; Yu, W.; Mo, B.; Li, R.; Liu, J.Z.; Zou, Y.L. Effects of Ilmenite on the Properties of Microwave-Sintered Lunar Regolith Simulant. J. Aerosp. Eng. 2021, 34, 8. [Google Scholar] [CrossRef]
- Taylor, L.A.; Mckay, D.S. An Ilmenite Feedstock on the Moon: Beneficiation of Rocks Versus Soil. Abstr. Lunar Planet. Sci. Conf. 1992, 23, 1411. [Google Scholar]
- Dou, R.; Tang, W.Z.; Wang, L.; Li, S.; Duan, W.Y.; Liu, M.; Zhang, Y.B.; Wang, G. Sintering of lunar regolith structures fabricated via digital light processing. Ceram. Int. 2019, 45, 17210–17215. [Google Scholar] [CrossRef]
- Schlüter, L.; Cowley, A.; Pennec, Y.; Roux, M. Gas purification for oxygen extraction from lunar regolith. Acta Astronaut. 2021, 179, 371–381. [Google Scholar] [CrossRef]
- Reiss, P.; Kerscher, F.; Grill, L. Thermogravimetric analysis of chemical reduction processes to produce oxygen from lunar regolith. Planet Space Sci. 2020, 181, 8. [Google Scholar] [CrossRef]
- Chen, H.M.; Nie, G.L.; Li, Y.H.; Zong, X.; Wu, S.H. Improving relative density and mechanical strength of lunar regolith structures via DLP-stereolithography integrated with powder surface modification process. Ceram. Int. 2022, 48, 26874–26883. [Google Scholar] [CrossRef]
- Wang, C.Y.; Gong, H.Q.; Wei, W.; Wu, H.; Luo, X.; Li, N.; Liang, J.H.; Khan, S.B.; Xiao, C.; Lu, B.H.; et al. Vat photopolymerization of low-titanium lunar regolith simulant for optimal mechanical performance. Ceram. Int. 2022, 48, 29752–29762. [Google Scholar] [CrossRef]
- Xiao, C.; Zheng, K.; Chen, S.G.; Li, N.; Shang, X.; Wang, F.H.; Liang, J.H.; Khan, S.B.; Shen, Y.F.; Lu, B.H.; et al. Additive manufacturing of high solid content lunar regolith simulant paste based on vat photopolymerization and the effect of water addition on paste retention properties. Addit. Manuf. 2023, 71, 14. [Google Scholar] [CrossRef]
- Song, L.; Xu, J.; Tang, H.; Fan, S.Q.; Liu, J.Z.; Li, X.Y.; Liu, J.Q. Research Progress. of the Simulated Lunar Soil Molding. Acta Mineral. Sin. 2020, 40, 47–57. [Google Scholar]
- Street, K.W.; Ray, C.; Rickman, D.; Scheiman, D.A. Thermal Properties of Lunar Regolith Simulants. In Proceedings of the Earth and Space 2010 Conference Sponsored by the American Society of Civil Engineers, Honolulu, HI, USA, 14–17 March 2010. [Google Scholar]
- Zhang, G.Q.; Ostrovski, O. Effect of preoxidation and sintering on properties of ilmenite concentrates. Int. J. Miner. Process. 2002, 64, 201–218. [Google Scholar] [CrossRef]
- Chen, X.; Deng, J.X.; Yu, R.B.; Chen, J.; Hu, P.H.; Xing, X.R. A Simple Oxidation Route to Prepare Pseudobrookite from Panzhihua Raw Ilmenite. J. Am. Ceram. Soc. 2010, 93, 2968–2971. [Google Scholar] [CrossRef]
- Song, L.; Xu, J.; Tang, H.; Liu, J.Q.; Liu, J.Z.; Li, X.Y.; Fan, S.Q. Vacuum sintering behavior and magnetic transformation for high-Ti type basalt simulated lunar regolith. Icarus 2020, 347, 8. [Google Scholar] [CrossRef]
- Taylor, S.L.; Jakus, A.E.; Koube, K.D.; Ibeh, A.J.; Geisendorfer, N.R.; Shah, R.N.; Dunand, D.C. Sintering of micro-trusses created by extrusion-3D-printing of lunar regolith inks. Acta Astronaut. 2018, 143, 1–8. [Google Scholar] [CrossRef]
- Alekseeva, I.P.; Dymshits, O.S.; Zhilin, A.A.; Mikhailov, M.D.; Khubetsov, A.A. Phase transformations in glass of the MgO-Al2O3-SiO2-TiO2 system doped with yttrium oxide. Glass Phys. Chem. 2015, 41, 597–606. [Google Scholar] [CrossRef]
- Alekseeva, I.P.; Dymshits, O.S.; Zhilin, A.A.; Mikha?Lov, M.D.; Khubetsov, A.A. Effect of yttrium oxide on the crystallization of glasses of the MgO–Al2O3–SiO2 system, nucleated by a mix of titanium and zirconium dioxides, and the transparency of glass--crystalline materials in the superhigh-frequency spectral region. J. Opt. Technol. Opt. Zhurnal 2015, 82, 262. [Google Scholar] [CrossRef]
- Lim, S.; Nakamoto, M.; Fuji-ta, K.; Tanaka, T. Effect of Titanium Dioxide on Aggregation of Reduced Metallic Iron in Molten Slag. Mater. Trans. 2023, 64, 672–680. [Google Scholar] [CrossRef]
- He, D.F.; Ma, H.; Zhong, H. Effect of different nucleating agent ratios on the crystallization and properties of MAS glass ceramics. J. Eur. Ceram. Soc. 2021, 41, 342–350. [Google Scholar] [CrossRef]
- Khater, G.A. Influence of Cr2O, LiF, CaF2 and TiO2 nucleants on the crystallization behavior and microstructure of glass-ceramics based on blast-furnace slag. Ceram. Int. 2011, 37, 2193–2199. [Google Scholar] [CrossRef]
- Allen, C.C.; Morris, R.V.; Mckay, D.S. Experimental reduction of lunar mare soil and volcanic glass. J. Geophys. Res. Planets 1994, 99, 23173–23185. [Google Scholar] [CrossRef]
- Suzuki, Y.; Shinoda, Y. Magnesium dititanate (MgTi2O5) with pseudobrookite structure: A review. Sci. Technol. Adv. Mater. 2011, 12, 6. [Google Scholar] [CrossRef]
- Son, H.W.; Maki, R.S.S.; Kim, B.N.; Suzuki, Y. High-strength pseudobrookite-type MgTi2O5 by spark plasma sintering. J. Ceram. Soc. Jpn. 2016, 124, 838–840. [Google Scholar] [CrossRef][Green Version]



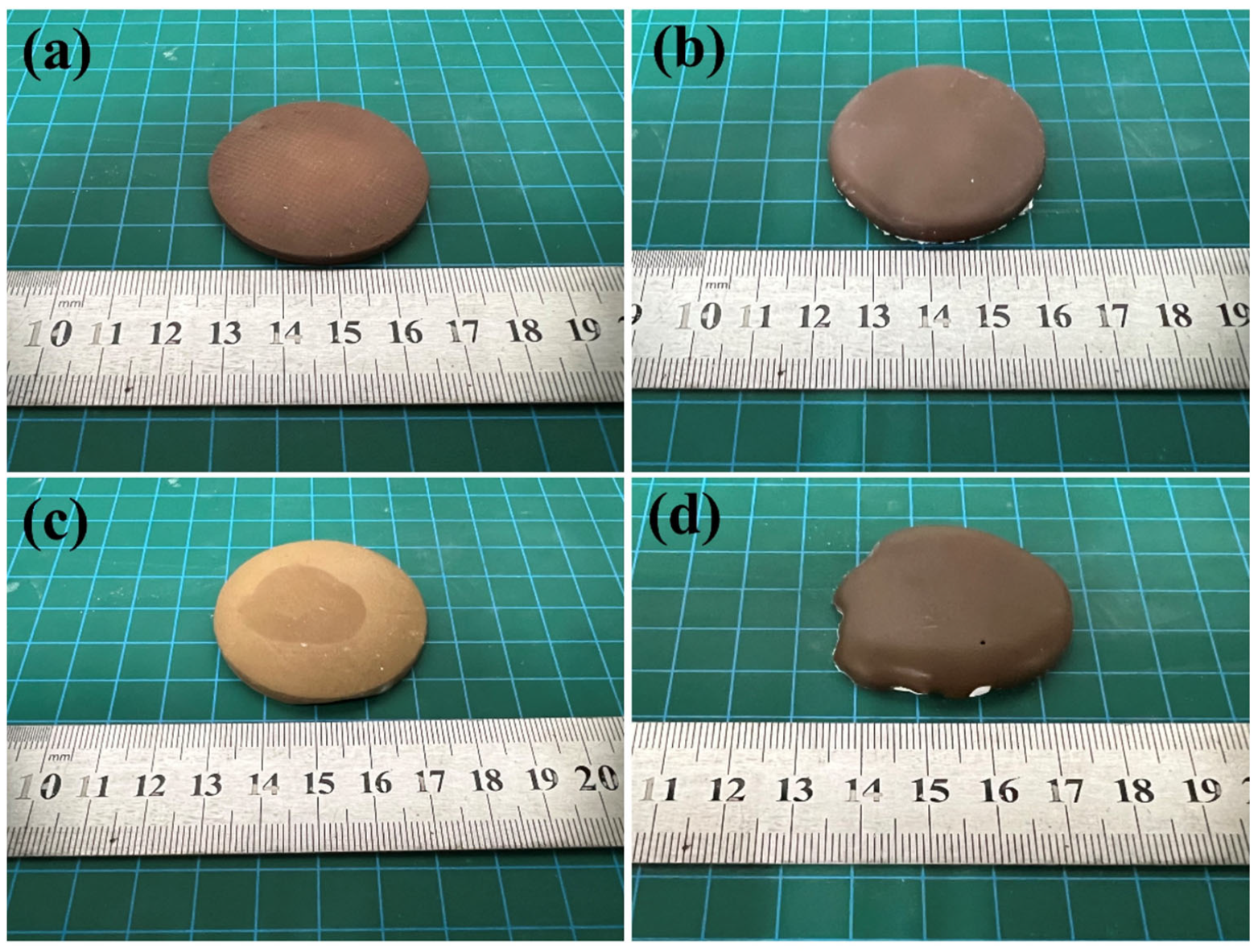


| Element | SiO2 | Al2O3 | FeO | MgO | CaO | Na2O | TiO2 | K2O | P2O5 | MnO | SrO | Cr2O3 | NiO | SO3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 45.9 | 14.3 | 11.5 | 9.86 | 6.92 | 4.58 | 1.9 | 2.33 | 0.63 | 0.14 | 0.1 | 0.06 | 0.06 | 0.05 |
| Sample Number | CUG-1A | TiO2 |
|---|---|---|
| T0 | 100 | 0 |
| T4 | 96 | 4 |
| T6 | 94 | 6 |
| T10 | 90 | 10 |
| Time (h) | D10 (μm) | D50 (μm) | D90 (μm) | |||
|---|---|---|---|---|---|---|
| T0 | T6 | T0 | T6 | T0 | T6 | |
| 0 | 2.91 ± 0.03 | 2.89 ± 0.02 | 19.10 ± 0.06 | 19.49 ± 0.04 | 46.30 ± 0.03 | 46.09 ± 0.04 |
| 2 | 1.81 ± 0.05 | 1.79 ± 0.06 | 10.94 ± 0.02 | 10.52 ± 0.05 | 26.37 ± 0.08 | 25.59 ± 0.07 |
| 4 | 1.44 ± 0.04 | 1.42 ± 0.03 | 8.25 ± 0.03 | 8.44 ± 0.01 | 20.83 ± 0.02 | 19.94 ± 0.05 |
| 6 | 1.26 ± 0.03 | 1.25 ± 0.05 | 5.74 ± 0.07 | 5.63 ± 0.08 | 17.09 ± 0.02 | 16.90 ± 0.03 |
| 8 | 1.27 ± 0.02 | 1.28 ± 0.01 | 5.77 ± 0.01 | 5.60 ± 0.03 | 16.94 ± 0.05 | 16.86 ± 0.03 |
| 10 | 1.23 ± 0.01 | 1.21 ± 0.02 | 5.72 ± 0.02 | 5.58 ± 0.05 | 16.97 ± 0.03 | 16.89 ± 0.04 |
| Element | Atomic % | |||
|---|---|---|---|---|
| A | B | C | D | |
| O | 39.87 | 39.76 | 43.62 | 41.62 |
| Na | 2.97 | 2.71 | 2.94 | 4.89 |
| Mg | 4.80 | 5.54 | 4.34 | 3.56 |
| Al | 7.45 | 7.53 | 7.03 | 9.64 |
| Si | 29.35 | 28.26 | 28.49 | 27.98 |
| K | 1.70 | 1.61 | 1.86 | 2.09 |
| Ca | 3.81 | 3.60 | 3.92 | 4.86 |
| Ti | 4.02 | 3.35 | 2.06 | 1.05 |
| Fe | 6.03 | 7.64 | 5.74 | 4.31 |
| Total: | 100.00 | 100.00 | 100.00 | 100.00 |
| Samples | Temperature (°C) | |||
|---|---|---|---|---|
| 1050 | 1100 | 1120 | 1140 | |
| T0 | 2.236 ± 0.31 | 2.338 ± 0.69 | 2.303 ± 0.94 | 2.281 ± 0.60 |
| T4 | 2.362 ± 0.93 | 2.497 ± 0.58 | 2.460 ± 0.40 | 2.391 ± 0.43 |
| T6 | 2.403 ± 0.25 | 2.558 ± 0.94 | 2.509 ± 0.79 | 2.431 ± 0.50 |
| T10 | 2.472 ± 0.61 | 2.537 ± 0.18 | 2.521 ± 0.13 | 2.497 ± 0.62 |
| Samples | Temperature (°C) | |||
|---|---|---|---|---|
| 1050 | 1100 | 1120 | 1140 | |
| T0 | 3.478 ± 0.51 | 2.884 ± 0.28 | 2.968 ± 0.72 | 3.217 ± 0.74 |
| T4 | 3.325 ± 0.25 | 2.840 ± 0.2 | 2.995 ± 0.69 | 3.002 ± 0.81 |
| T6 | 3.387 ± 0.68 | 2.809 ± 0.16 | 2.836 ± 0.41 | 2.904 ± 0.49 |
| T10 | 3.542 ± 0.49 | 2.964 ± 0.79 | 3.105 ± 0.29 | 3.164 ± 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Chen, H.; Zhao, Z.; Zong, X. Effect of TiO2 on the Microstructure and Flexural Strength of Lunar Regolith Simulant. Crystals 2024, 14, 110. https://doi.org/10.3390/cryst14020110
Chen J, Chen H, Zhao Z, Zong X. Effect of TiO2 on the Microstructure and Flexural Strength of Lunar Regolith Simulant. Crystals. 2024; 14(2):110. https://doi.org/10.3390/cryst14020110
Chicago/Turabian StyleChen, Junhao, Haoming Chen, Zhe Zhao, and Xiao Zong. 2024. "Effect of TiO2 on the Microstructure and Flexural Strength of Lunar Regolith Simulant" Crystals 14, no. 2: 110. https://doi.org/10.3390/cryst14020110
APA StyleChen, J., Chen, H., Zhao, Z., & Zong, X. (2024). Effect of TiO2 on the Microstructure and Flexural Strength of Lunar Regolith Simulant. Crystals, 14(2), 110. https://doi.org/10.3390/cryst14020110





