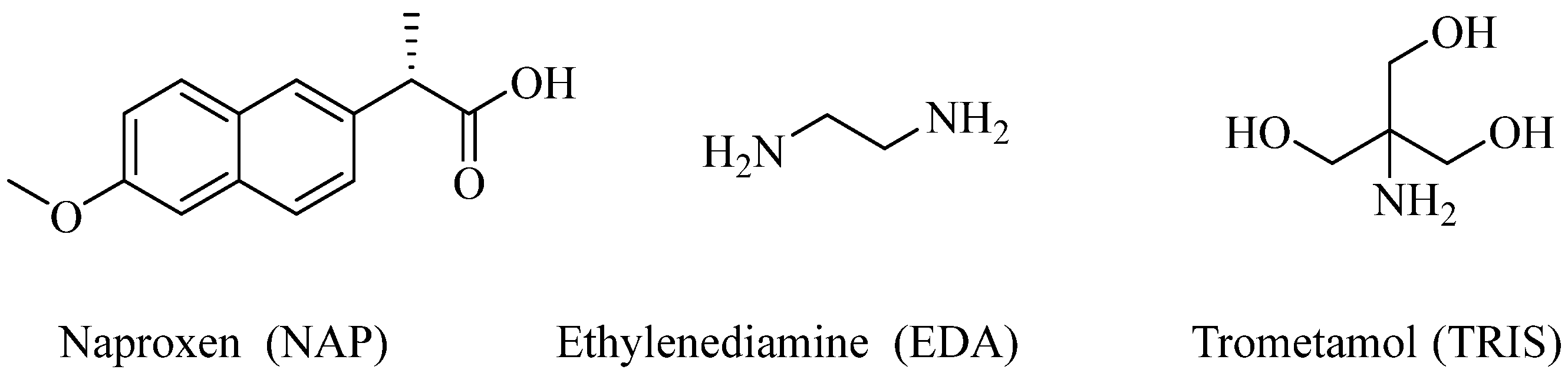
Simultaneous Improvement in Dissolution Behavior and Oral Bioavailability of Naproxen via Salt Formation
Abstract
1. Introduction
2. Results
2.1. Crystal Structure Analysis
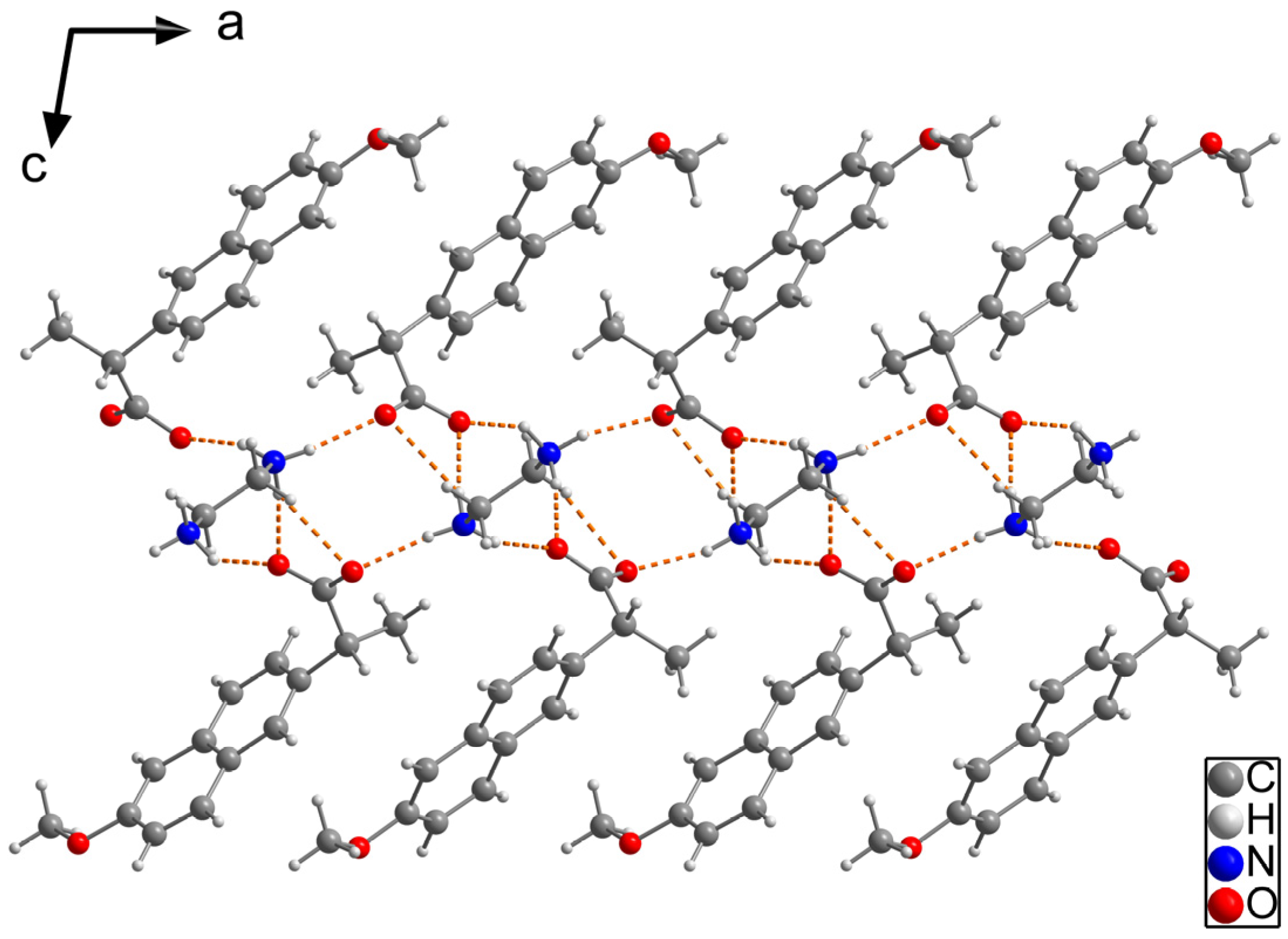
2.1.1. NAP-EDA Salt (2:1)
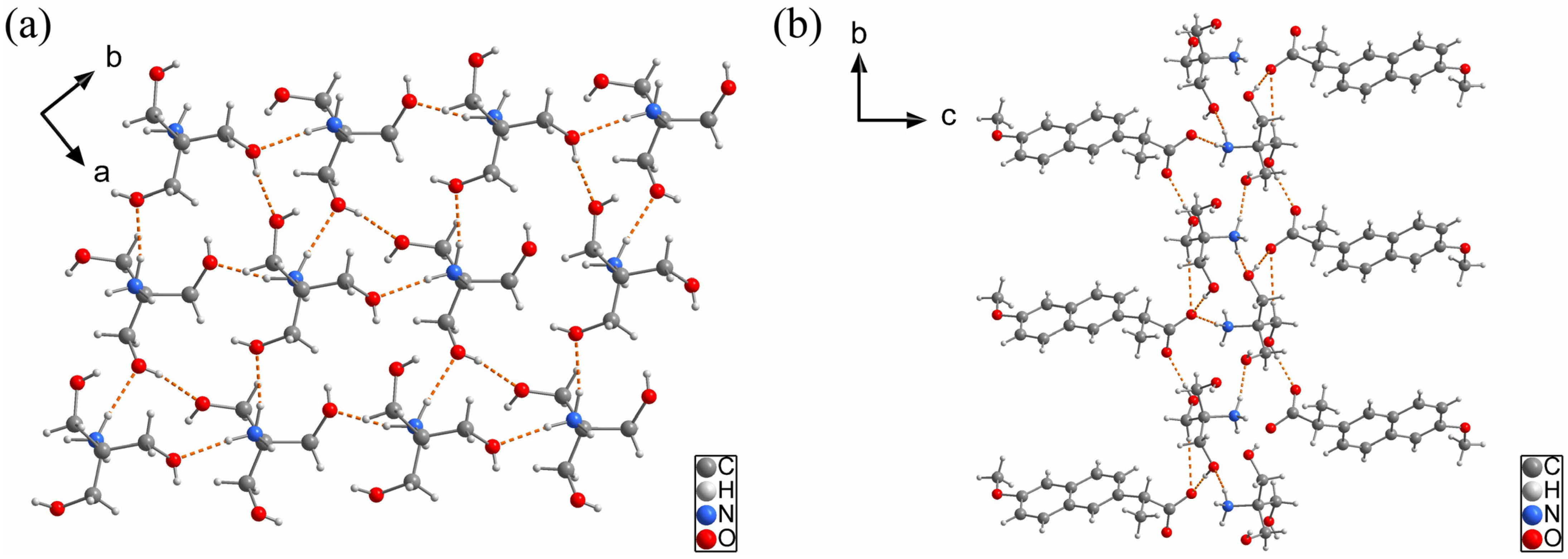
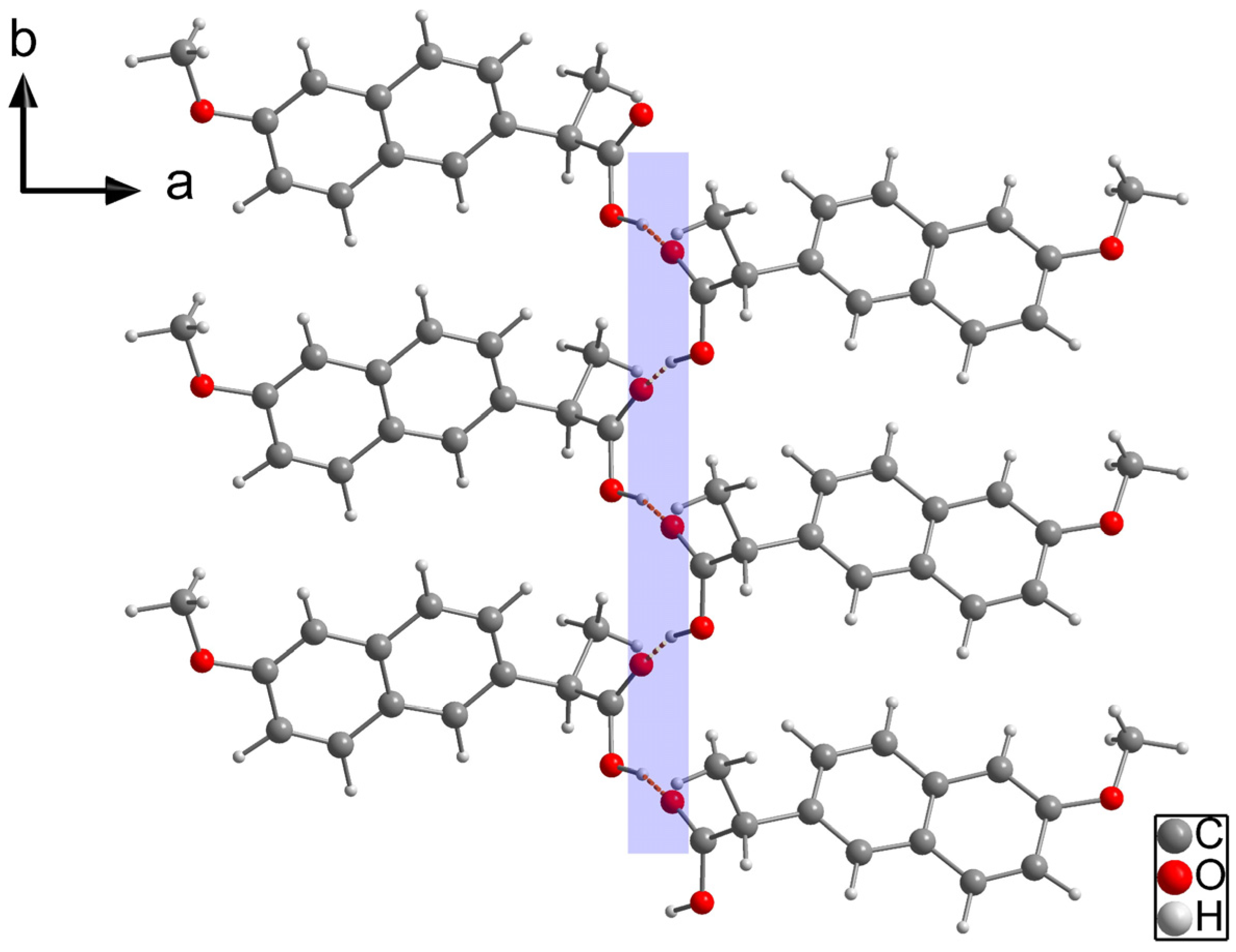
2.1.2. NAP-TRIS Salt (1:1)
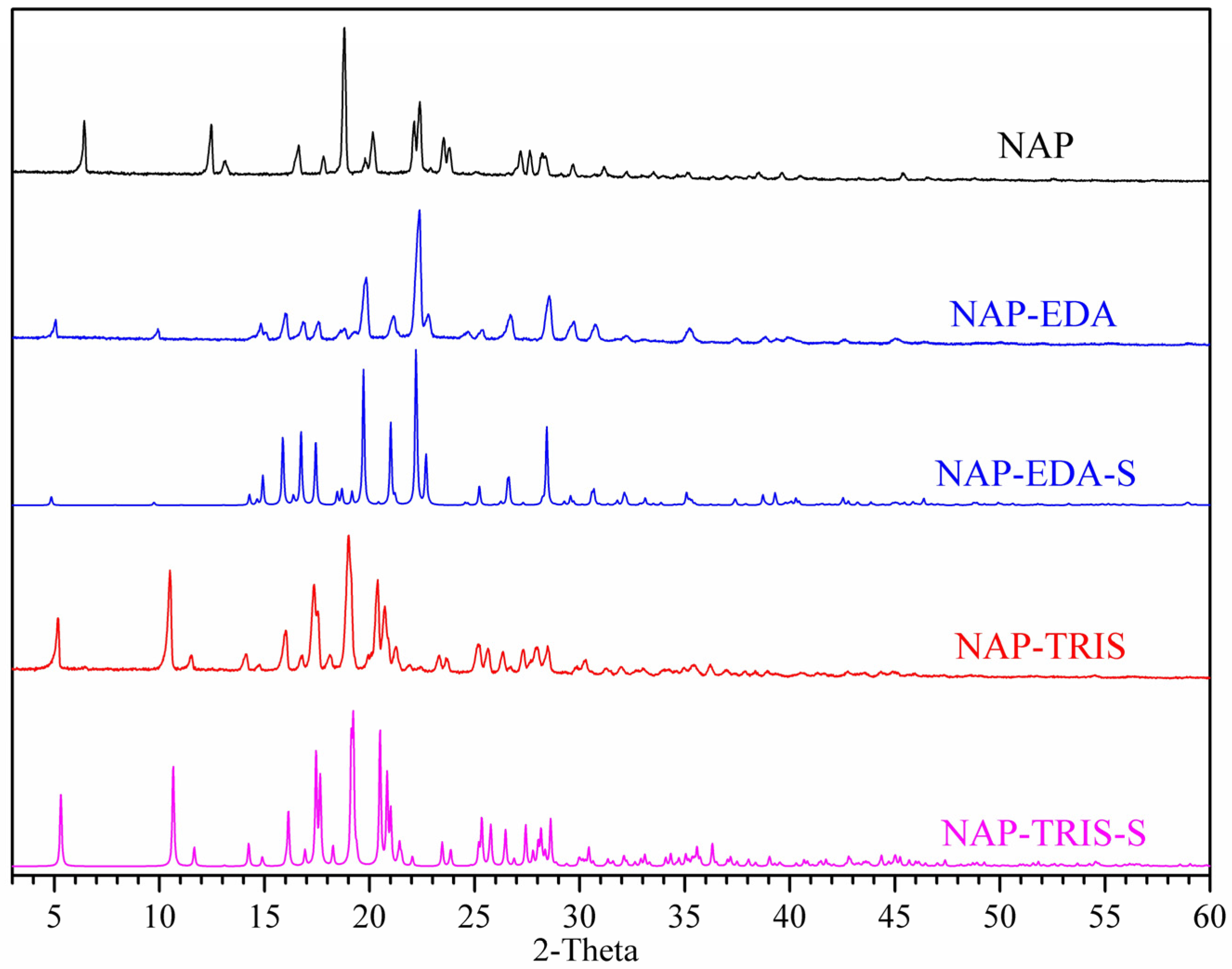
2.2. Powder X-Ray Diffraction (PXRD) Analysis
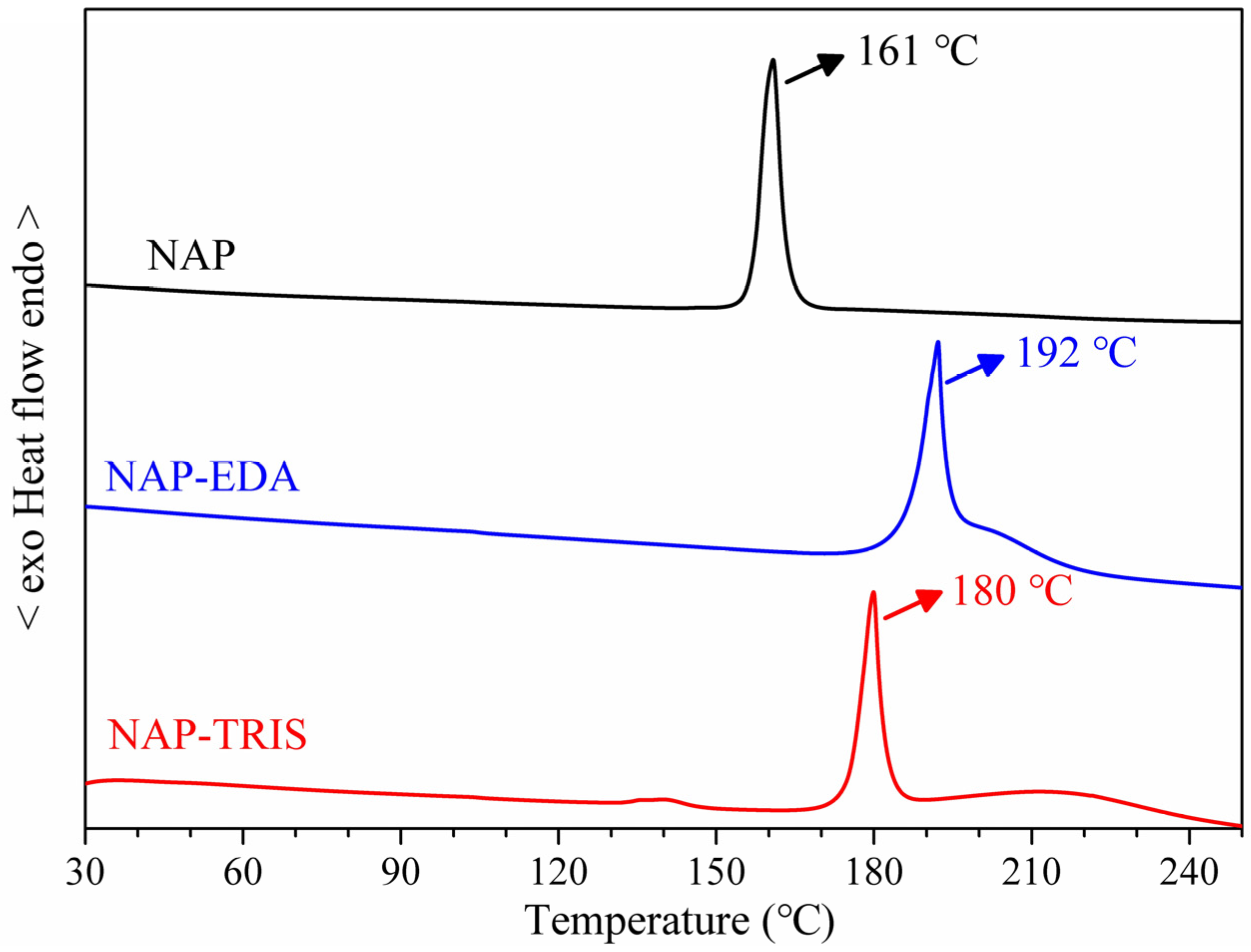
2.3. Differential Scanning Calorimetry (DSC) Analysis
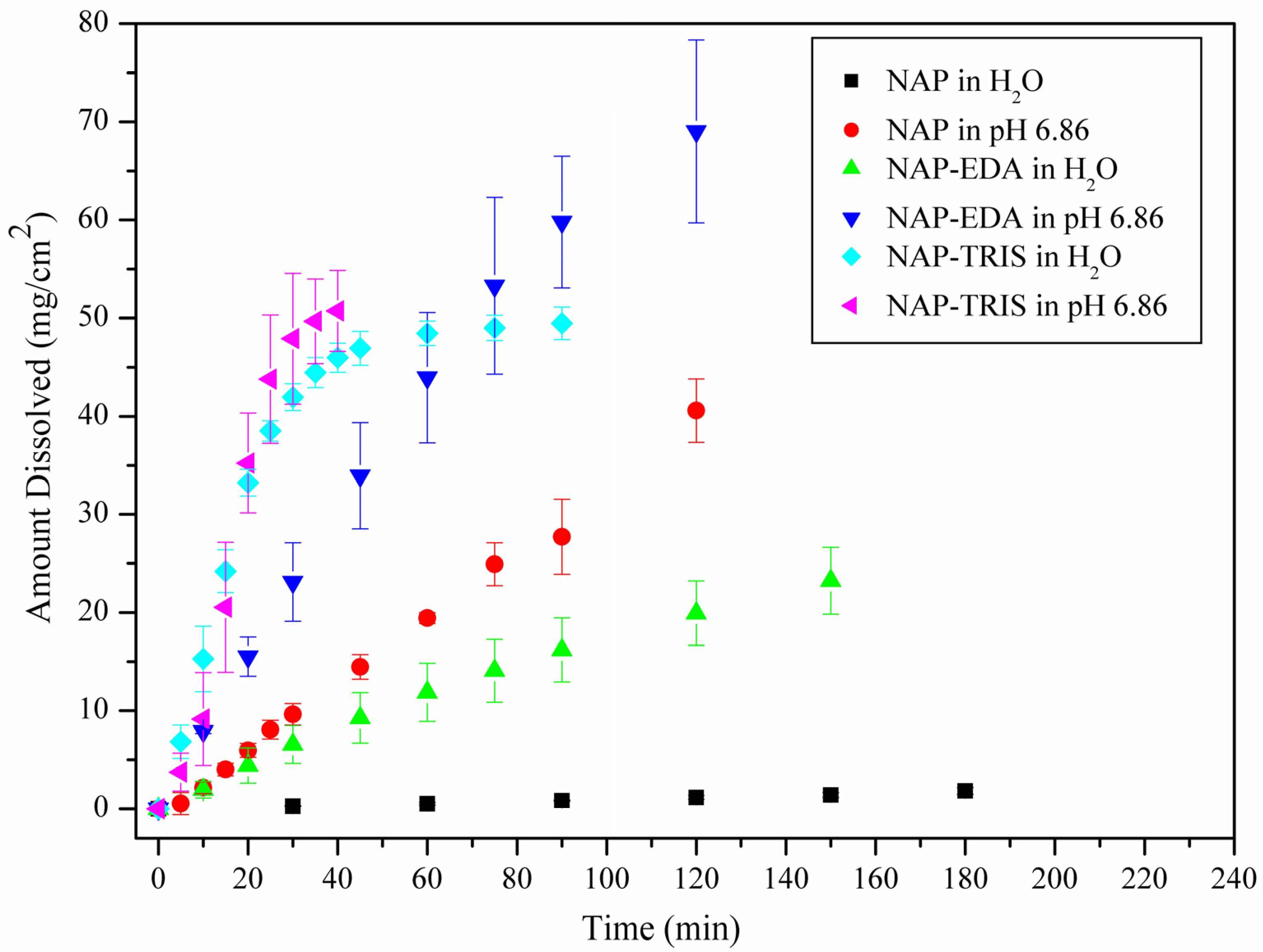
2.4. Solubility and Dissolution Rate Studies
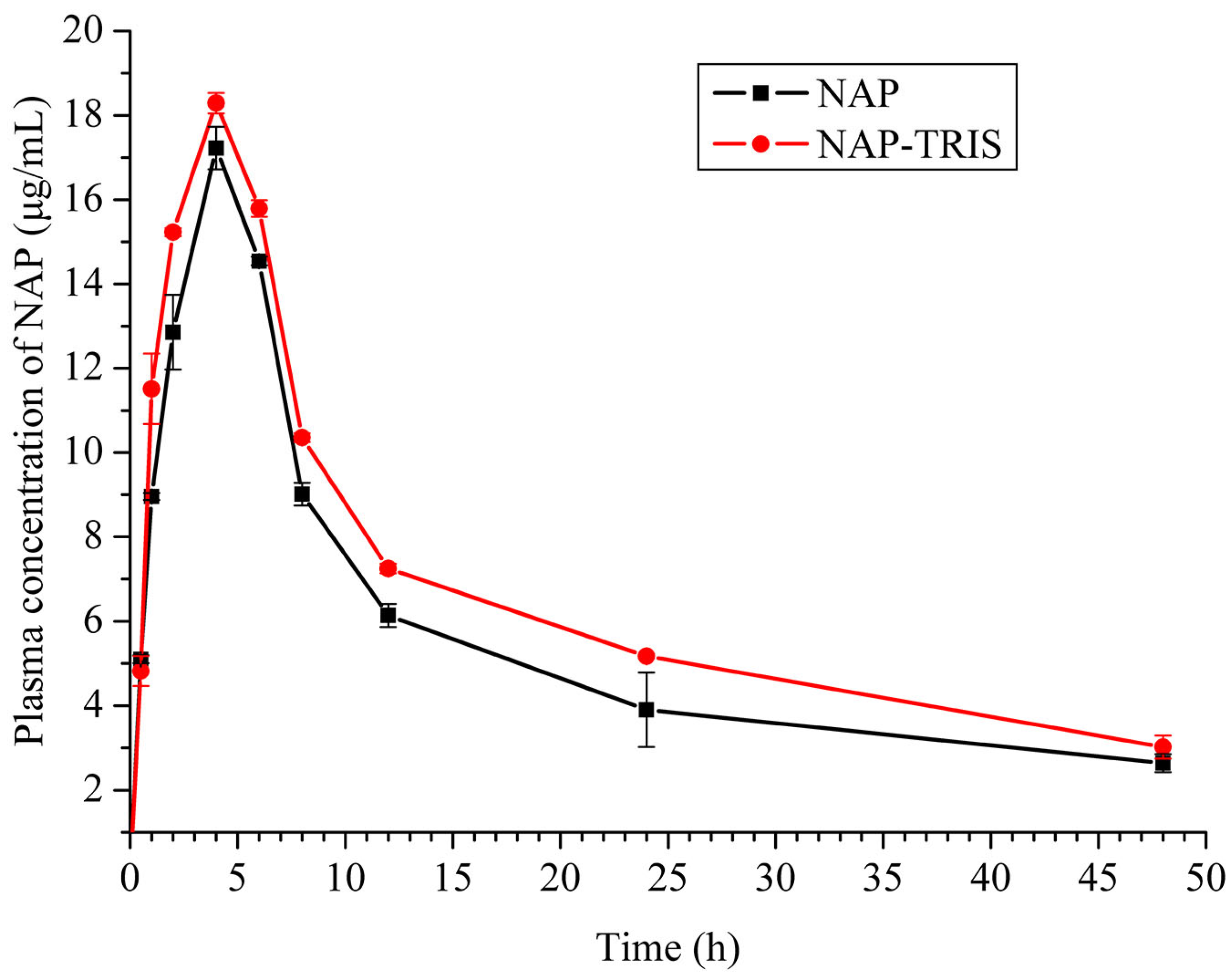
2.5. Pharmacokinetic Study
3. Materials and Methods
3.1. Instrumentations and Materials
3.2. Preparation of NAP-EDA and NAP-TRIS Salts
3.2.1. Preparation of NAP-EDA and NAP-TRIS Powder Samples
3.2.2. Preparation of NAP-EDA and NAP-TRIS Crystals
3.3. Solubility and Dissolution Rate Studies
3.4. Pharmacokinetic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karmi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating cocrystals: A review of pharmaceutical cocrystal preparation routes and applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Yang, F.; Zhang, X.; Hu, W. Cocrystal engineering: A collaborative strategy toward functional materials. Adv. Mater. 2019, 31, 1902328. [Google Scholar] [CrossRef] [PubMed]
- Bolla, G.; Sarma, B.; Nangia, A.K. Crystal engineering of pharmaceutical cocrystals in the discovery and development of improved drugs. Chem. Rev. 2022, 122, 11514–11603. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.S. Recent developments in pharmaceutical salts: FDA approvals from 2015–2019. Drug Discov. Today 2021, 26, 384–398. [Google Scholar] [CrossRef]
- Nugrahani, I.; Parwati, R.D. Challeges and progress in nonsteroidal anti-inflammatory drugs co-crystal development. Molecules 2021, 26, 4185. [Google Scholar] [CrossRef]
- Nangia, A.K.; Desiraju, G.R. Heterosynthons, solid form design and enhanced drug bioavailability. Angew. Chem. Int. Ed. 2022, 134, e202207484. [Google Scholar] [CrossRef]
- Drozd, K.V.; Manin, A.N.; Boycov, D.E.; Perlovich, G.L. Simultaneous improvement of dissolution behavior and oral bioavailability of antifungal miconazole via cocrystal and salt formation. Pharmaceutics 2022, 14, 1107. [Google Scholar] [CrossRef]
- Florindo, C.; Costa, A.; Matos, C.; Nunes, S.L.; Matias, A.N.; Duarte, C.M.M.; Rebelo, L.P.N.; Branco, L.C.; Marrucho, I.M. Novel organic salts based on fluoroquinolone drugs: Synthesis, bioavailability and toxicological profiles. Int. J. Pharm. 2014, 469, 179–189. [Google Scholar] [CrossRef]
- Guo, C.; Wang, Y.; Xie, J.; Zhu, B.; Qi, M.H.; Hong, M.; Ren, G.B. Novel salts of the atypical antipsychotic drug lurasidone with improved solubility and bioavailability. Cryst. Growth Des. 2022, 23, 326–332. [Google Scholar] [CrossRef]
- Gwak, H.S.; Choi, J.S.; Choi, H.K. Enhanced bioavailability of piroxicam via salt formation with ethanolamines. Int. J. Pharm. 2005, 297, 156–161. [Google Scholar] [CrossRef]
- Liu, L.; Zou, Y.; Zhang, Y.; Zhang, D.; Zhang, Y.; Zhang, Q.; Wang, J.; Zeng, S.; Wang, C. Assembly of threepharmaceutical salts/cocrystals of tetrahydroberine with sulfophenyl acids: Improving the properties by formation of charge-assisted hydrogen bonds. New J. Chem. 2019, 43, 4886–4894. [Google Scholar] [CrossRef]
- Berge, S.M.; Bighley, L.D.; Monkhouse, D.C. Pharmaceutical salts. J. Pharm. Sci. 1977, 66, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Serajuddin, A.T. Salt forming to improve drug solubility. Adv. Drug Deliv. Rev. 2007, 59, 603–616. [Google Scholar] [CrossRef]
- Nechipadappu, S.K.; Truvedi, D.R. Pharmaceutical salts of ethionamide with GRAS counter ion donors to enhance the solubility. Eur. J. Pharm. Sci. 2017, 96, 578–589. [Google Scholar] [CrossRef]
- Nechipadappu, S.K.; Reddy, I.R.; Tarafder, K.; Trivedi, D.R. Salt/cocrystal of anti-fibrinolytic hemostatic drug tranexamic acid: Structural, DFT, and stability study of salt/cocrystal with GRAS molecules. Cryst. Growth Des. 2018, 19, 347–361. [Google Scholar] [CrossRef]
- He, S.F.; Zhang, X.R.; Zhang, S.; Guan, S.; Li, J.; Li, S. An investigation into vortioxetine salts: Crystal structure, thermal stability, and solubilization. J. Pharm. Sci. 2016, 105, 2123–2128. [Google Scholar] [CrossRef]
- Li, J.; Li, L.Y.; He, S.F.; Li, S.; Dong, C.Z.; Zhang, L. Crystal structures, X-ray photoelectron spectroscopy, thermodynamic stabilities, and improved solubilities of 2-hydrochloride salts of vortioxetine. J. Pharm. Sci. 2017, 106, 1069–1074. [Google Scholar] [CrossRef]
- Carvalho, P.S.; Diniz, L.F.; Tenorio, J.C.; Souza, M.S.; Franco, C.H.J.; Rial, R.C.; de Oliveira, K.R.W.; Nazario, C.E.D.; Ellena, J. Pharmaceutical paroxetine-based organic salts of carboxylic acids with optimized properties: The identification and characterization of potential novel API solid forms. CrystEngComm 2019, 21, 3668–3678. [Google Scholar] [CrossRef]
- Owoyemi, B.C.D.; da Silva, C.C.; Diniz, L.F.; Souza, M.S.; Ellena, J.; Carneiro, R.L. Fluconazolium oxalate: Synthesis and structural characterization of a highly soluble crystalline form. CrystEngComm 2019, 21, 1114–1121. [Google Scholar] [CrossRef]
- Sanphui, P.; Tothadi, S.; Ganguly, S.; Desiraju, G.R. Salt and cocrystals of sildenafil with dicarboxylic acids: Solubility and pharmacokinetic advantage of the glutarate salt. Mol. Pharm. 2013, 10, 4687–4697. [Google Scholar] [CrossRef]
- Harrington, P.J.; Lodewijk, E. Twenty years of naproxen technology. Org. Process Res. Dev. 1997, 1, 72–76. [Google Scholar] [CrossRef]
- ADAPT Research Group. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology 2007, 68, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.M.; Anderson, K.E. Clinical pharmacokinetics of naproxen. Clin. Pharmacokinet. 1997, 32, 268–293. [Google Scholar] [CrossRef] [PubMed]
- Sevelius, H.; Runkel, R.; Segre, E.; Bloomfield, S.S. Bioavailability of naproxen sodium and its relationship to clinical analgestic effects. Br. J. Clin. Pharmacol. 1980, 10, 259–263. [Google Scholar] [CrossRef]
- Calvo, M.V.; Lanao, J.M.; Dominguez-Gil, A. Bioavailability of rectally administered naproxen. Int. J. Pharm. 1987, 38, 117–122. [Google Scholar] [CrossRef]
- Ando, S.; Kikuchi, J.; Fujimura, Y.; Ida, Y.; Higashi, K.; Moribe, K.; Yamamoto, K. Physicochemical characterization and structural evaluation of a specific 2:1 cocrystal of naproxen-nicotinamide. J. Pharm. Sci. 2012, 101, 3214–3221. [Google Scholar] [CrossRef]
- Abbas, N.; Latif, S.; Afzal, H.; Arshad, M.S.; Hussain, A.; Sadeeqa, S.; Bukhari, N.I. Simultaneously improving mechanical, formulation, and in vivo performance of naproxen by co-crystallization. AAPS PharmSciTech 2018, 19, 3249–3257. [Google Scholar] [CrossRef]
- Kim, H.; Jang, S.; Kim, I.W. Enhanced dissolution of naproxen by combining cocrystallization and eutectic formation. Pharmaceutics 2021, 13, 618. [Google Scholar] [CrossRef]
- Kerr, H.E.; Softley, L.K.; Suresh, K.; Hodgkinson, P.; Evans, I.R. Structure and physicochemical characterization of a naproxen-picolinamide cocrystal. Acta Crystallogr. C 2017, 73, 168–175. [Google Scholar] [CrossRef]
- Skorupska, E.; Jeziorna, A.; Potrzebowski, M.J. Thermal solvent-free method of loading of pharmaceutical cocrystals into the pores of silica particles: A case of naproxen/picolinamide cocrystal. J. Phys. Chem. C 2016, 120, 13169–13180. [Google Scholar] [CrossRef]
- Buschmann, H.H.; Sola, C.; Buchholz, J.B.; Bertran, J.C. Co-Crystals of Duloxetine and Co-Crystal Formers for the Treatment of Pain. European Patent EP2123626A1, 25 November 2009. [Google Scholar]
- Latif, S.; Ijaz, Q.A.; Hameed, M.; Shoaib, Q.U.; Fatima, K.; Hussain, A.; Arshad, M.S.; Abbas, N. Improvement of physico-mechanical and pharmacokinetic attributes of naproxen by cocrystallization with L-alanine. J. Drug Deliv. Sci. Technol. 2021, 61, 102236. [Google Scholar] [CrossRef]
- Tumanova, N.; Tumanov, N.; Robeyns, K.; Filinchuk, Y.; Wouters, J.; Leyssens, T. Structural insight into cocrystallization with zwitterionic co-formers: Cocrystals of S-naproxen. CrystEngComm 2014, 16, 8185–8196. [Google Scholar] [CrossRef]
- Kasten, G.; Lobo, L.; Dengale, S.; Grohganz, H.; Rades, T.; Löbmann, K. In vitro and in vivo comparison between crystalline and co-amorphous salts of naproxen-arginine. Eur. J. Pharm. Biopharm. 2018, 132, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Tilborg, A.; Springuel, G.; Norberg, B.; Wouters, J.; Leyssens, T. On the influence of using a zwitterionic coformer for cocrystallization: Structural focus on naproxen-proline cocrystals. CrystEngComm 2013, 15, 3341–3350. [Google Scholar] [CrossRef]
- Heinrich, B.H.; Lluis, S.C.; Jordi, B.B.; Carles, C.B.J.; Ramon, P.; Nicolas, T. Co-Crystals of Tramadol and NSAIDs. Japan Pantent JP5645830B2, 24 December 2014. [Google Scholar]
- Manoj, K.; Tamura, R.; Takahashi, H.; Tsue, H. Crystal engineering of homochiral molecular organization of naproxen in cocrystals and their thermal phase transformation studies. CrystEngComm 2014, 16, 5811–5819. [Google Scholar] [CrossRef]
- Nechipadappu, S.K.; Trivedi, D.R. Structural and physicochemical characterization of pyridine derivative salts of anti-inflammatory drugs. J. Mol. Struct. 2017, 1141, 64–74. [Google Scholar] [CrossRef]
- Rajurkar, V.G.; Gite, R.D.; Ghawate, V.B. Development of naproxen cocrystal formation: An efficient approach to enhance aqueous solubility. Anal. Chem. Lett. 2015, 5, 229–238. [Google Scholar] [CrossRef]
- Fu, Q.; Lu, H.D.; Xie, Y.F.; Liu, J.Y.; Han, Y.; Gong, N.B.; Guo, F. Salt formation of two BCS II drugs (indomethacin and naproxen) with (1R,2R)-1,2-diphenylethylenediamine: Crystal structures, solubility and thermodynamics analysis. J. Mol. Struct. 2019, 1185, 281–289. [Google Scholar] [CrossRef]
- Xing, C.; Chen, T.; Wang, L.; An, Q.; Jin, Y.; Yang, D.; Zhang, L.; Du, G.; Lu, Y. Two novel co-crystals of naproxen: Comparison of stability, solubility and intermolecular interaction. Pharmaceuticals 2022, 15, 807. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y. A novel drug-drug salt of naproxen and vortioxetine: Synthesis, characterization, and solubility study. J. Chem. 2023, 2023, 6678252. [Google Scholar] [CrossRef]
- Yuliandra, Y.; Izadihari, R.; Rosaini, H.; Zaini, E. Multicomponent crystals of mefenamic acid-tromethamine with improved dissolution rate. J. Res. Pharm. 2019, 23, 988–996. [Google Scholar] [CrossRef]
- Bruni, G.; Berbenni, V.; Maggi, L.; Mustarelli, P.; Friuli, V.; Ferrara, C.; Pardi, F.; Castagna, F.; Girella, A.; Milanese, C. Multicomponent crystals of gliclazide and tromethamine: Preparation, physico-chemical, and pharmaceutical characterization. Drug Dev. Ind. Pharm. 2018, 44, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Bookwala, M.; Thipsay, P.; Ross, S.; Zhang, F.; Bandari, S.; Repka, M.A. Preparation of a crystalline salt of indomethacin and tromethamine by hot melt extrusion technology. Eur. J. Pharm. Biopharm. 2018, 131, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.; Coyle, S.; Andrew, J. The trometamol salt of fosfomycin: Microbiological evaluation. Eur. Urol. 1987, 13, 69–75. [Google Scholar] [CrossRef]
- Fitriani, L.; Firdaus, W.A.; Sidadang, W.; Rosaini, H.; Putra, O.D.; Oyama, H.; Uekusa, H.; Zaini, E. Improved solubility and dissolution rate of ketoprofen by the formation of multicomponent crystals with tromethamine. Crystals 2022, 12, 275. [Google Scholar] [CrossRef]
- Rossi, P.; Paoli, P.; Milazzo, S.; Chelazzi, L.; Giovannoni, M.P.; Guerrini, G.; Lenco, A.; Valleri, M.; Conti, L. A combined crystallographic and computational study on dexketoprofen trometamol dehydrate salt. Crystals 2020, 10, 659. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Penningtom, W.T. Diamond-visual crystal structure information system. J. Appl. Crystallogr. 1999, 32, 1028–1029. [Google Scholar] [CrossRef]
| NAP-EDA | NAP-TRIS | |
|---|---|---|
| Empirical formula | 2(C14H13O3)•C2H10N2 | C14H13O3•C4H12NO3 |
| Molecule weight | 520.61 | 351.39 |
| Temperature (K) | 293(2) | 293(2) |
| Crystal system | Monoclinic | Orthorhombic |
| space group | I2 | P212121 |
| a (Å) | 12.5633(12) | 6.3167(10) |
| b (Å) | 6.0077(8) | 8.5152(17) |
| c (Å) | 36.747(4) | 33.133(5) |
| α (°) | 90 | 90 |
| β (°) | 99.720(9) | 90 |
| γ (°) | 90 | 90 |
| Volume (Å3) | 2733.7(5) | 1782.2(5) |
| Z | 4 | 4 |
| Density (g/cm3) | 1.265 | 1.310 |
| R1/wR2 [I > 2σ(I)] | 0.0709/0.1273 | 0.0618/0.0918 |
| GOF | 0.940 | 0.991 |
| Larg peak and hole (e/Å3) | 0.164/−0.190 | 0.183/−0.200 |
| CCDC | 2,391,216 | 2,306,212 |
| Medium | Compound | Concentration of NAP (mg/L) | IDR (mg/(cm2·min)) | Final pH in Solubility Experiments |
|---|---|---|---|---|
| water | NAP | 49.6 ± 2.2 | 0.0096 | 6.88 |
| NAP-EDA | 3732.5 ± 57.0 (×75.2) | 0.2093 (×21.8) | 6.88 | |
| NAP-TRIS | 19,720.2 ± 399.2 (×397.5) | 1.6710 (×174.0) | 6.72 | |
| pH 6.86 | NAP | 2771.2 ± 37.8 | 0.3074 | 6.78 |
| NAP-EDA | 5921.8 ± 157.30 (×2.1) | 0.7339 (×2.3) | 6.82 | |
| NAP-TRIS | 17,218.1 ± 357.2 (×6.2) | 3.4899 (×11.3) | 6.82 |
| Drug | T1/2 (h) | Tmax (h) | Cmax (μg/mL) | AUC0→24 (h*μg/mL) | MRT0→t (h) | F (%) |
|---|---|---|---|---|---|---|
| NAP | 24.9 | 4.0 | 17.2 | 269.9 | 16.2 | 100 |
| NAP-TRIS | 28.8 | 4.0 | 18.3 | 319.9 | 16.6 | 119 |
| Compound | D-H⋯A | d(D-H) | d(H⋯A) | d(D⋯A) | <(DHA) | Symmetry Code |
|---|---|---|---|---|---|---|
| NAP-EDA | N1+-H1A⋯O1 | 0.88 | 1.93 | 2.732(10) | 152 | −x − 1, y + 1, −z |
| N1+-H1B⋯O5 | 0.88 | 1.91 | 2.791(10) | 176 | x, y + 1, z | |
| N1+-H1C⋯O4 | 0.87 | 1.89 | 2.757(10) | 173 | −x − 2, y + 1, −z | |
| N2+-H2A⋯O5 | 0.87 | 2.00 | 2.786(10) | 150 | x, y, z | |
| N2+-H2B⋯O1 | 0.87 | 1.83 | 2.702(10) | 177 | −x − 1, y, −z | |
| N2+-H2C⋯O2 | 0.87 | 1.90 | 2.753(10) | 164 | x, y, z | |
| NAP-TRIS | N1+-H1B⋯O4 | 0.89 | 2.07 | 2.950(4) | 169 | x − 1/2, −y − 3/2, −z |
| N1+-H1A⋯O1 | 0.89 | 1.86 | 2.746(4) | 170 | x + 1/2, −y − 3/2, −z | |
| N1+-H1C⋯O6 | 0.89 | 2.01 | 2.896(4) | 174 | x − 1/2, −y − 1/2, −z | |
| O4-H4⋯O1 | 0.82 | 1.83 | 2.644(3) | 168 | x, y, z | |
| O5-H5A⋯O2 | 0.82 | 1.81 | 2.621(3) | 172 | x, y + 1, z | |
| O6-H6⋯O5 | 0.82 | 1.89 | 2.685(3) | 162 | x + 1, y, z |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-R.; Wu, B.-L.; Han, J.-J.; Li, J.-Q. Simultaneous Improvement in Dissolution Behavior and Oral Bioavailability of Naproxen via Salt Formation. Crystals 2024, 14, 1104. https://doi.org/10.3390/cryst14121104
Zhang X-R, Wu B-L, Han J-J, Li J-Q. Simultaneous Improvement in Dissolution Behavior and Oral Bioavailability of Naproxen via Salt Formation. Crystals. 2024; 14(12):1104. https://doi.org/10.3390/cryst14121104
Chicago/Turabian StyleZhang, Xian-Rui, Bao-Lin Wu, Jing-Jing Han, and Jin-Qing Li. 2024. "Simultaneous Improvement in Dissolution Behavior and Oral Bioavailability of Naproxen via Salt Formation" Crystals 14, no. 12: 1104. https://doi.org/10.3390/cryst14121104
APA StyleZhang, X.-R., Wu, B.-L., Han, J.-J., & Li, J.-Q. (2024). Simultaneous Improvement in Dissolution Behavior and Oral Bioavailability of Naproxen via Salt Formation. Crystals, 14(12), 1104. https://doi.org/10.3390/cryst14121104






