The Synthesis and Characterization of CdS Nanostructures Using a SiO2/Si Ion-Track Template
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Surface Morphology
3.2. Optical Properties and CVCs of CdS/SiO2/Si Nanocrystals
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atourki, L.; Vega, E.; Mollar, M.; Marí, B.; Kirou, H.; Bouabid, K.; Ihlal, A. Impact of iodide substitution on the physical properties and stability of cesium lead halide perovskite thin films CsPbBr3−xIx (0 ≤ x ≤ 1). J. Alloys Compd. 2017, 702, 404–409. [Google Scholar] [CrossRef]
- Atourki, L.; Ihalane, K.H.; Bouabid, K.; Elfanaoui, A.; Laanab, L.; Portier, X.; Ihlal, A. Characterisation of nanostructured ZnO grown by linear sweep voltammetry. Sol. Energy Mater. Sol. Cells 2016, 148, 20–24. [Google Scholar] [CrossRef]
- Atourki, L.; Vega, E.; Marí, B.; Mollar, M.; Ait Ahsainec, H.; Bouabida, K.H.; Ihlal, A. Shedding light on the effect of chloride and bromide ions on structural and photoluminescence properties. Appl. Surf. Sci. 2016, 390, 744–750. [Google Scholar] [CrossRef]
- Ouafi, M.; Jaber, B.; Atourki, L.; Bekkari, R.; Laânab, L. Improving UV stability of MAPbI3 perovskite thin films by bromide incorporation. J. Alloys Compd. 2018, 746, 391–398. [Google Scholar] [CrossRef]
- Jaber, B.; Laânab, L. One step synthesis of ZnO nanoparticles in free organic medium: Structural and optical characterisations. Mater. Sci. Semicond. Process. 2014, 27, 446–451. [Google Scholar] [CrossRef]
- Yılmaz, S.; Töreli, S.B.; Polat, İ.; Olgar, M.A.; Tomakin, M.; Bacaksız, E. Enhancement in the optical and electrical properties of CdS thin films through Ga and K co-doping. Mater. Sci. Semicond. Process. 2017, 60, 45–52. [Google Scholar] [CrossRef]
- Antoniadou, M.; Daskalaki, V.M.; Balis, N.; Kondarides, D.I.; Kordulis, C.; Lianos, P. Photocatalysis and photo electrocatalysis using (CdS-ZnS)/TiO2 combined photocatalyst. Appl. Catal. B Environ. 2011, 107, 188–196. [Google Scholar] [CrossRef]
- Sorokin, E.; Klimentov, D.; Frolov, M.P.; Korostelin, Y.V.; Kozlovsky, V.I.; Podmar’kov, Y.P.; Skasyrsky, Y.K.; Sorokina, I.T. Continuous-wave broadly tunable high-power Cr: CdS laser. Appl. Phys. 2014, 117, 1009–1014. [Google Scholar] [CrossRef]
- Bartlome, R.; Strahm, B.; Sinquin, Y.; Feltrin, A.; Ballif, C. Laser applications in thin-film photovoltaics. Appl. Phys. B 2010, 100, 427–436. [Google Scholar] [CrossRef]
- Komarov, F.; Vlasukova, L.A.; Milchanin, O.M.; Gaiduk, P.I.; Yuvchenko, V.N.; Grechnyi, S.S. Ion-beam formation of nanopores and nanoclusters in SiO2. Vacuum 2005, 78, 361–366. [Google Scholar] [CrossRef]
- Akylbekova, A.; Dauletbekova, A.; Baimukhanov, Z.; Vlasukova, L.A.; Usseinov, A.; Saduova, N.; Akilbekov, A.T.; Pankratov, V.A.; Popov, A.I. Annealing Effect on Structural, Optical and Electrophysical Properties of ZnSe Nanocrystals Synthesized into SiO2/Si Ion Track Template. Materials 2024, 17, 4149. [Google Scholar] [CrossRef] [PubMed]
- Aralbayeva, G.; Sarsekhan, G.; Akylbekova, A.; Vlasukova, L.A.; Baimukhanov, Z.; Yuvchenko, V.; Bazarbek, A.-D.; Dauletbekova, A.; Kabdrakhimova, G.; Akilbekov, A.T. The Thermal Stability and Photoluminescence of ZnSeO3 Nanocrystals Chemically Synthesized into SiO2/Si Track Templates. Crystals 2024, 14, 730. [Google Scholar] [CrossRef]
- Skupiński, M.; Johansson, A.; Jarmar, T.; Razpet, A.; Hjort, K.; Boman, M.; Possnert, G.; Jensen, J. Deposition of carbon nanopillar matrix on SiO2 by ion irradiation through a porous aluminium oxide template. Vacuum 2007, 82, 359–362. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Zhang, K.; Gu, S.; Cao, J.; Xia, Y.; Yang, C.; Sun, W.; Zhou, Z. Highly efficient and stable photocatalytic properties of CdS/FeS nanocomposites. New J. Chem. 2020, 44, 14695–14702. [Google Scholar] [CrossRef]
- Rajbongshi, H.; Kalita, D. Morphology-dependent photocatalytic degradation of organic pollutants and antibacterial activity with CdS nanostructures. J. Nanosci. Nanotechnol. 2020, 20, 5885–5895. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Li, W.; Xu, P.; Zhang, M.; Dai, D.; Wang, P.; Tong, L. On-chip single-mode CdS nanowire laser. Sci. Appl. 2020, 9, 42. [Google Scholar] [CrossRef]
- Guo, R.; Yan, A.; Xu, J.; Xu, B.; Li, T.; Liu, X.; Luo, S. Influence of morphology on visible light-induced photocatalytic and bactericidal properties of BiVO4/CdS heterojunctions: Discussion on the mechanism of photocatalysis. J. Alloys Compd. 2020, 817, 153246. [Google Scholar] [CrossRef]
- You, J.; Wang, L.; Bao, W.; Yan, A.; Guo, R. Synthesis and visible light photocatalytic properties of BiOVr/CdS nanomaterials. J. Mater. Sci. 2021, 56, 6732–6744. [Google Scholar] [CrossRef]
- Sagadevan, S.; Pandurangan, K. Synthesis, Structural, Optical and Electrical Properties of Cadmium Sulphide Thin Films by Chemical Bath Deposition Method. Int. J. ChemTech Res. 2014, 7, 3748–3752. [Google Scholar]
- Singh, J.P.; Pal, S.; Nag, A.; Sharma, Y.K. Structural Properties, Energy Interaction, and Applications of CdS Nanomaterial Doped with Rare-Earth Ions. Macromol. Symp. 2024, 413, 2300169. [Google Scholar] [CrossRef]
- Azab, A.A.; Ibrahim, R.S.; Seoudi, R. Investigation of the effect of Mn content on morphology and dielectric properties of CdS nanoparticles. Appl. Phys. A 2024, 130, 294. [Google Scholar] [CrossRef]
- Ghosh, B.; Singh, B.K.; Banerjee, P.; Das, S. Nucleation and growth of CBD-CdS thin films on ultrathin aluminium layers and annealing induced doping. Optik 2016, 127, 4413–4417. [Google Scholar] [CrossRef]
- Rami, M.; Benamar, E.; Fahoume, M.; Chraibi, F.; Ennaoui, A. Effect of the cadmium ion source on the structural and optical properties of chemical bath deposited CdS thin films. Solid State Sci. 1999, 1, 179–188. [Google Scholar] [CrossRef]
- Kumar, V. Sb2Se3 Thin-Film Solar Cells via Low-Temperature Thermal Evaporation Technique. Ph.D. Thesis, Universita’ degli studi di Verona, Verona, Italy, 2018. [Google Scholar]
- Aliyev, A.S.; Majidzade1, V.A.; Soltanova1, N.S.; Tagiyev1, D.B.; Fateev, V.N. Some features of electrochemically deposited CdS nanowires. KimyaProblemleri 2018, 2, 178–185. [Google Scholar] [CrossRef]
- Lei, M.; Qian, L.Q.; Hu, Q.R.; Wang, S.L.; Tang, W.H. Fabrication of CdS/Cd-Sn heterostructure nanowires and their photoluminescence property. J. Alloys Compd. 2009, 487, 568–571. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalised gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.K.; Mondal, S.P. Synthesis of CdS nanowires using nanoporousa luminium oxide template. Int. J. Nanofabr. 2008, 2, 583–597. [Google Scholar]
- Il’chuk, G.A.; Kusnezh, V.V.; Rud’, V.Y.; Rud’, Y.V.; Shapowal, P.Y.; Petrus’, R.Y. Photosensitivity of n-CdS/p-CdTe heterojunctions obtained by chemical surface deposition of CdS. Semiconductors 2010, 44, 318–320. [Google Scholar] [CrossRef]
- Xu, H.J.; Li, H.J. Preparation, structural and photoluminescence properties of CdS/silicon nanoporous array. J. Phys. Condens. Matter 2007, 19, 056003. [Google Scholar] [CrossRef]
- Lozada-Morales, R.; Zelaya-Angel, O.; Torres-Delgado, G. Photoluminescence in cubic and hexagonal CdS films. Appl. Surf. Sci. 2001, 175–176, 562–566. [Google Scholar] [CrossRef]
- Tao, G.; Meng, G.-W.; Wang, T.-H. Blue luminescence of CdS nanowires synthesised by sulfurization. Chin. Phys. Lett. 2004, 21, 959. [Google Scholar] [CrossRef]
- Levy, L.; Feltin, N.; Ingert, D.; Pileni, M.P. Three Dimensionally Diluted Magnetic Semiconductor Clusters Cd1-yMnyS with a Range of Sizes and Compositions: Dependence of Spectroscopic Properties on the Synthesis Mode. J. Phys. Chem. B 1997, 101, 9153–9160. [Google Scholar] [CrossRef]
- Pan, A.L.; Liu, R.B.; Yang, Q.; Zhu, Y.C.; Zuo, J.; Zou, B.S. Stimulated emission behaviours from Excitons in CdS nanoribbons. J. Phys. Conf. Ser. 2006, 28, 12. [Google Scholar]
- Magde, D.; Mahr, H. Exciton-exciton interaction in CdS, CdSe, and ZnO. Phys. Rev. Lett. 1970, 24, 890. [Google Scholar] [CrossRef]
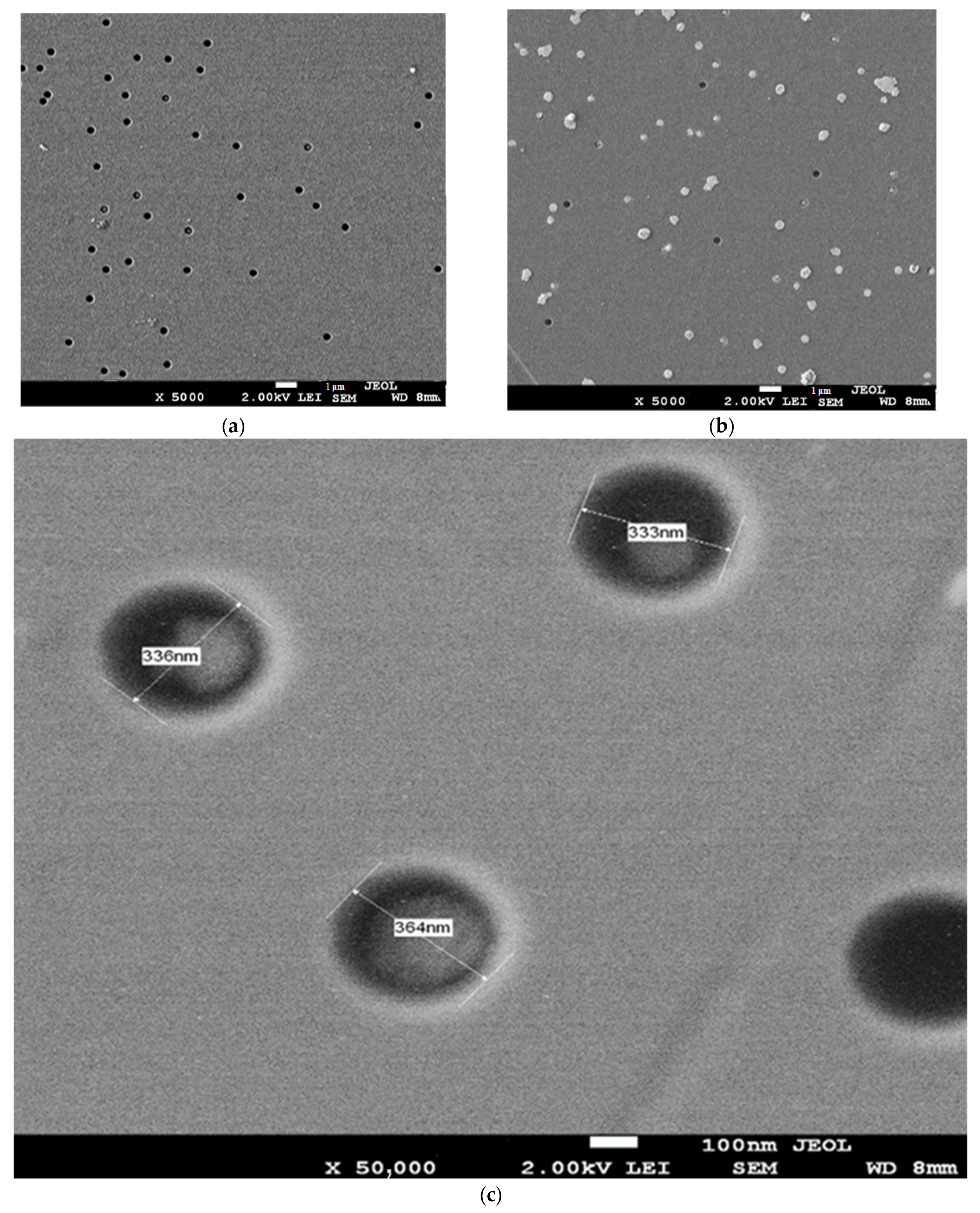

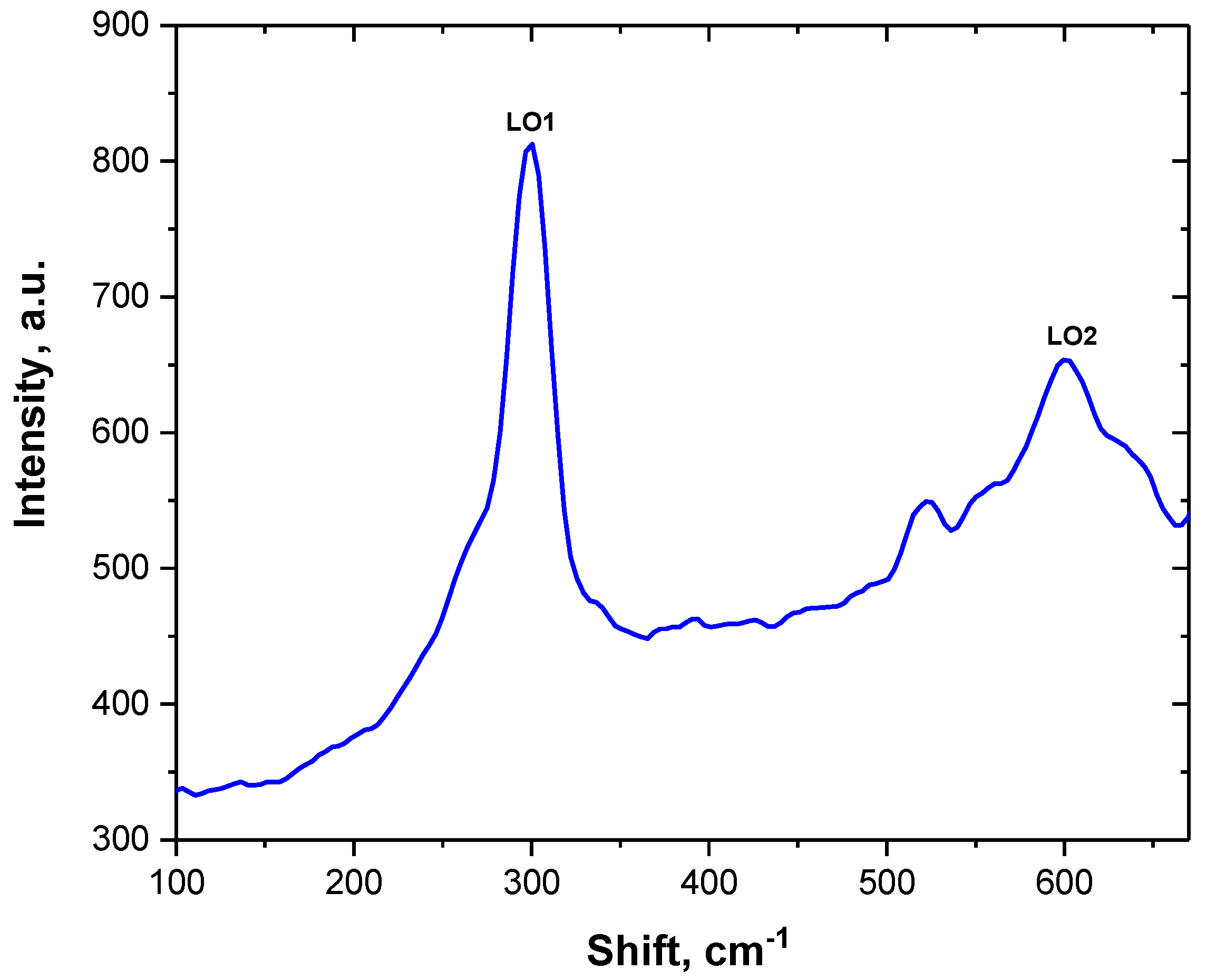
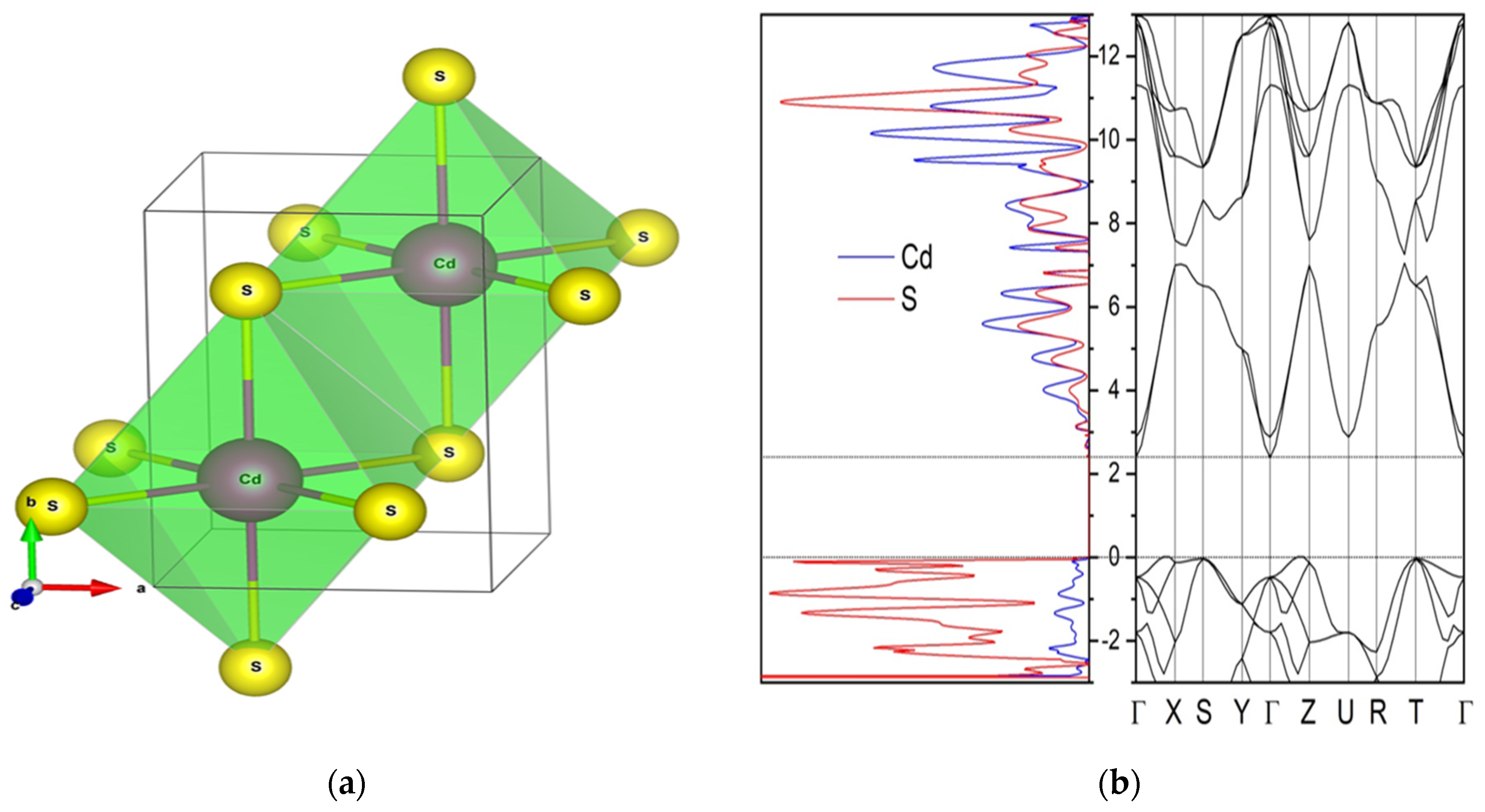


| Type of Structure | Spatial Group | Phase | 2θ° | V (Å3) Volume | Cell Parameter, Å | Full Width at Half Maximum |
|---|---|---|---|---|---|---|
| Orthorhombic (1) | 59: Pmmn | CdS | 61.48 | 55.080 | 3.484591 4.750555 3.327354 | 0.20 0.147 |
| Cubic (1, 2) | 225: Fm-3m | CdO | 65.72 | 104.660 | 4.712610 4.712610 4.712610 | 0.19 0.147 |
| Orthorhombic (3) | 59: Pmmn | CdS | 69.25 65.72 | 57.020 | 3.536320 4.799425 3.359600 | 0.22 0.14 |
| Parameter | This Work, Calc. | This Work, Exp. |
|---|---|---|
| Space group | Pmmn | Pmmn |
| a, Å | 3.96 | 3.98 |
| b, Å | 3.96 | 3.98 |
| c, Å | 5.6 | 5.63 |
| d (Cd-S), Å | 2.8 | 2.5–2.7 |
| V (Å)3 | 87.86 | 83.75 |
| ρV (g/cm)3 | 5.5 | 5.73 |
| Eg (eV) | 2.39 | 2.42 |
| qeff (Cd/S) | +1.26/−1.26 | - |
| Phase | Wavelength Range of Visible Radiation, λ (nm) | Visible Radiation Energy Range, E (eV) | Impurities | Radiation Spectrum |
|---|---|---|---|---|
| CdS | 490 | 2.53 | VS | Blue |
| CdS | 506 | 2.45 | Green | |
| CdS | 522 | 2.37 | VS | Green |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akylbekova, A.; Mantiyeva, K.; Dauletbekova, A.; Akilbekov, A.; Baimukhanov, Z.; Vlasukova, L.; Aralbayeva, G.; Abdrakhmetova, A.; Bazarbek, A.-D.; Abdihalikova, F. The Synthesis and Characterization of CdS Nanostructures Using a SiO2/Si Ion-Track Template. Crystals 2024, 14, 1091. https://doi.org/10.3390/cryst14121091
Akylbekova A, Mantiyeva K, Dauletbekova A, Akilbekov A, Baimukhanov Z, Vlasukova L, Aralbayeva G, Abdrakhmetova A, Bazarbek A-D, Abdihalikova F. The Synthesis and Characterization of CdS Nanostructures Using a SiO2/Si Ion-Track Template. Crystals. 2024; 14(12):1091. https://doi.org/10.3390/cryst14121091
Chicago/Turabian StyleAkylbekova, Aiman, Kyzdarkhan Mantiyeva, Alma Dauletbekova, Abdirash Akilbekov, Zein Baimukhanov, Liudmila Vlasukova, Gulnara Aralbayeva, Ainash Abdrakhmetova, Assyl-Dastan Bazarbek, and Fariza Abdihalikova. 2024. "The Synthesis and Characterization of CdS Nanostructures Using a SiO2/Si Ion-Track Template" Crystals 14, no. 12: 1091. https://doi.org/10.3390/cryst14121091
APA StyleAkylbekova, A., Mantiyeva, K., Dauletbekova, A., Akilbekov, A., Baimukhanov, Z., Vlasukova, L., Aralbayeva, G., Abdrakhmetova, A., Bazarbek, A.-D., & Abdihalikova, F. (2024). The Synthesis and Characterization of CdS Nanostructures Using a SiO2/Si Ion-Track Template. Crystals, 14(12), 1091. https://doi.org/10.3390/cryst14121091






