Considerations for Differences in Melt Growth Kinetics Between II–VI and III–V Compound Crystals
Abstract
1. Introduction
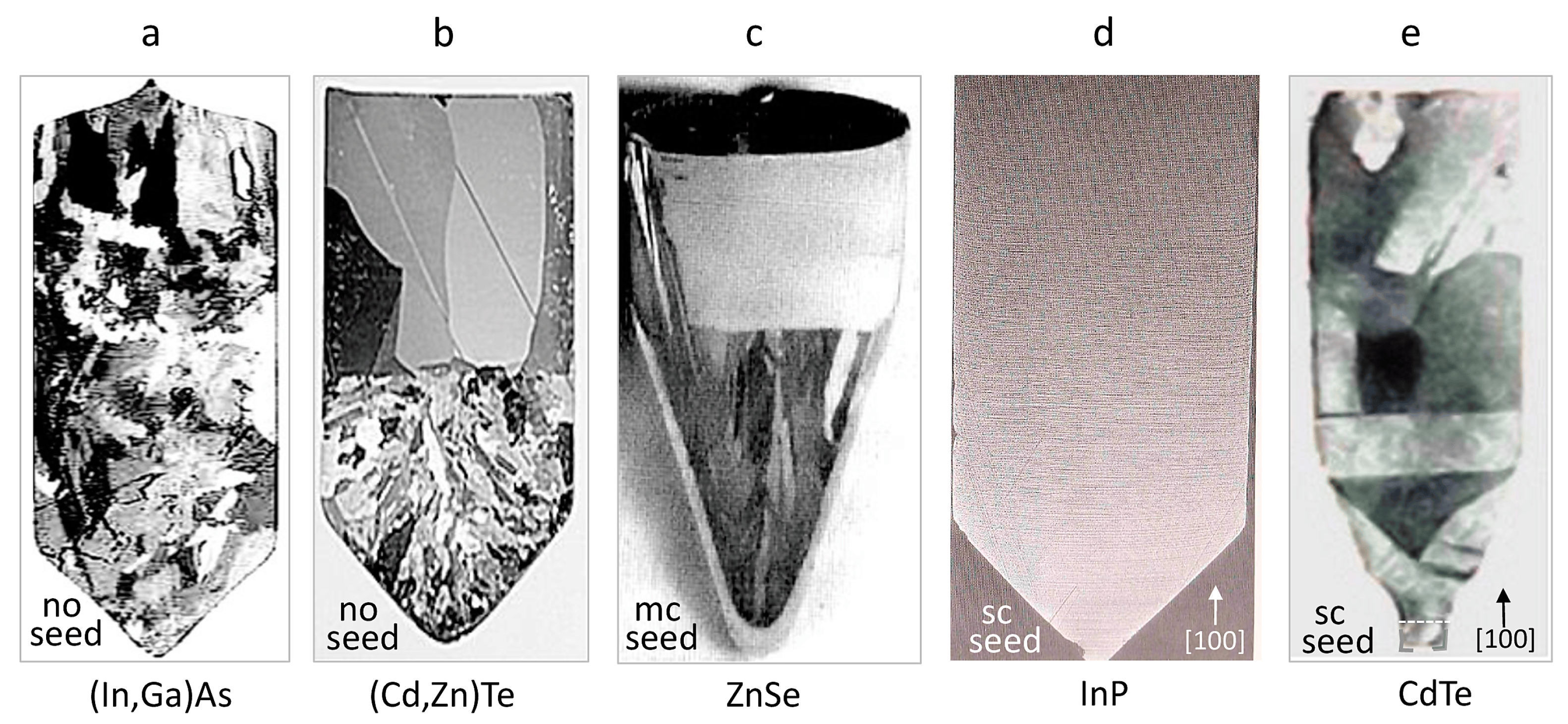
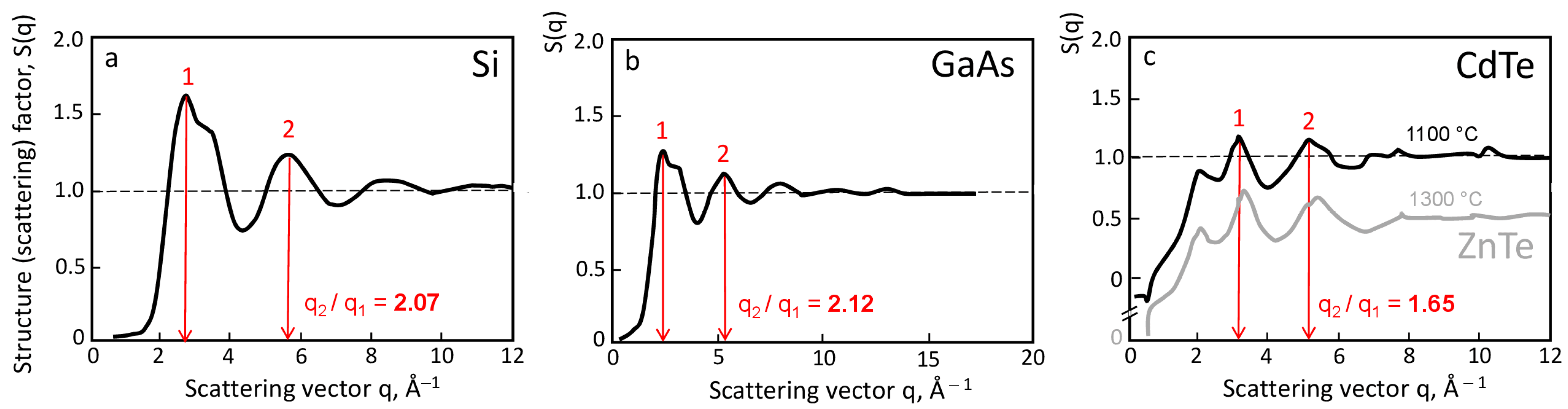
2. Experimental Facts
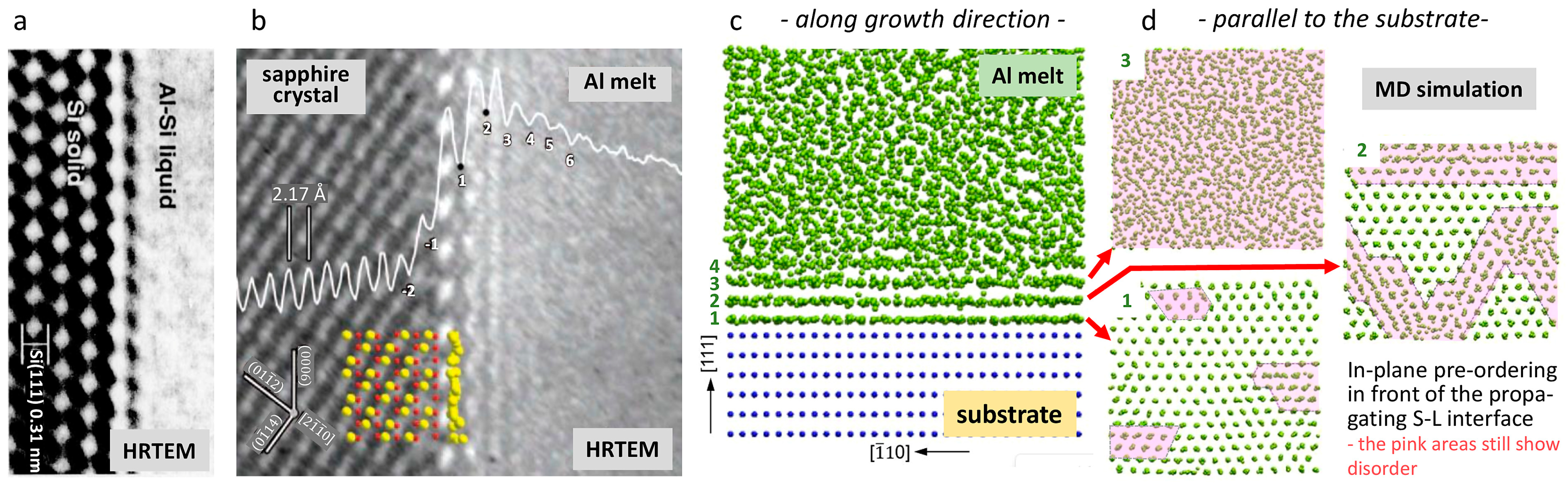
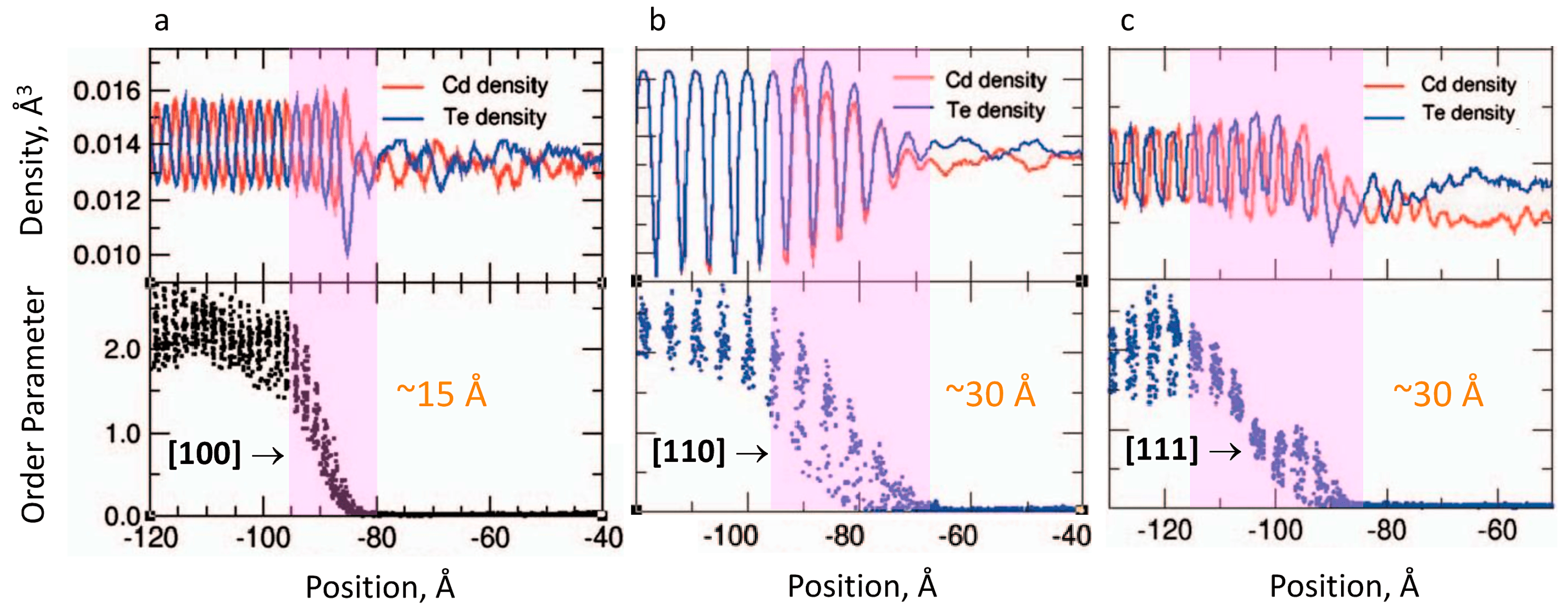
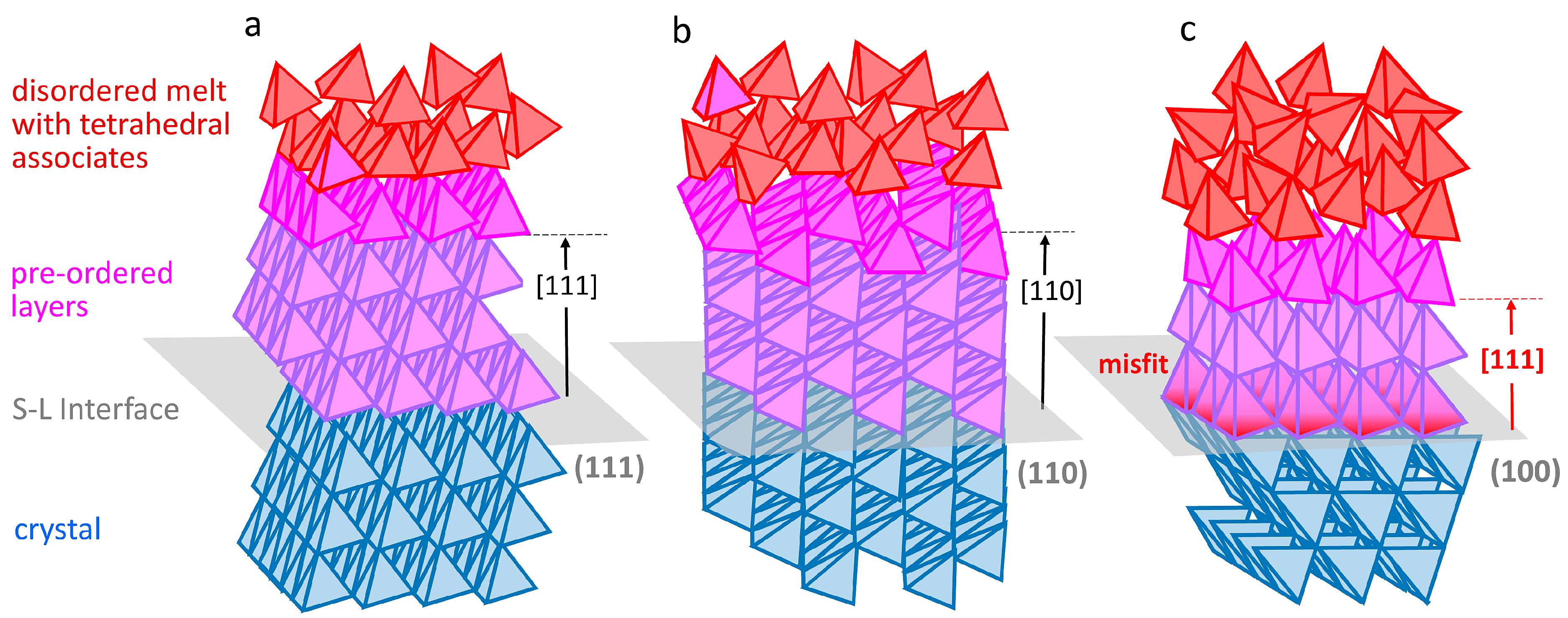
3. Consideration of Melt Pre-Ordering in Front of the Solid–Liquid Interface
4. Selected Consequences for Crystal Growth Methodology
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hashio, K.; Tatsumi, M.; Kato, H.; Kinoshita, K. Directional Solidification of InxGa1−xAs. J. Cryst. Growth 2000, 10, 471–477. [Google Scholar] [CrossRef]
- Kiessling, F.-M.; Büllesfeld, F.; Dropka, N.; Frank-Rotsch, C.; Müller, M.; Rudolph, P. Characterization of mc-Si directionally solidified in travelling magnetic fields. J. Cryst. Growth 2012, 360, 81–86. [Google Scholar] [CrossRef]
- Monberg, E. Bridgman and Related Growth Techniques. In Handbook of Crystal Growth, Growth Techniques; Hurle, D.T.J., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1994; Volume 2A, pp. 99–212. [Google Scholar]
- Rudolph, P.; Matsumoto, F.; Fukuda, T. Studies on interface curvature during vertical Bridgman growth of InP in a flat-bottom container. J. Cryst. Growth 1996, 158, 43–48. [Google Scholar] [CrossRef]
- Casagrande, L.G.; Di Marzio, D.; Lee, M.B.; Larson, D.J.; Dudley, M.; Fanning, T. Vertical Bridgman growth and characterization of large-diameter single-crystal CdTe. J. Cryst. Growth 1993, 128, 576–581. [Google Scholar] [CrossRef]
- Rudolph, P. Fundamental studies on Bridgman growth of CdTe. Progr. Cryst. Grow. Charact. Mat. 1994, 29, 275–381. [Google Scholar] [CrossRef]
- Choi, B.W.; Wadley, H.N.G. In situ studies of Cd1−xZnxTe nucleation and crystal growth. J. Cryst. Growth 2000, 208, 219–230. [Google Scholar] [CrossRef]
- Kakkireni, S.; Murugesan, M.; Montag, B.; McCloy, J. Seeded Crystal Growth of Cd-Zn-Te (CZT) Assisted via Numerical Modelling. In Emerging Materials; Thoutam, L.R., Tayal, S., Ajayan, J., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2022; pp. 103–133. [Google Scholar] [CrossRef]
- Rudolph, P.; Schäfer, N.; Fukuda, T. Crystal Growth of ZnSe from the melt. Mater. Sci. Eng. Rep. 1995, 15, 85–133. [Google Scholar] [CrossRef]
- Feigelson, R.S.; Route, R.K. Improved Techniques for the Growth of High Quality Cadmium Telluride Crystals; Final Rep. CMR-85-3. Center for Mat. Res.; Stanford University: Stanford, CA, USA, 1985; pp. 1–52. [Google Scholar]
- Sundaram, S.K.; Henager, S.K., Jr.; Edwards, D.J.; Schemer-Kohrn, A.L.; Bliss, M.; Riley, B.R. Electron backscatter diffraction analysis of a CZT growth tip from a vertical gradient freeze furnace. J. Cryst. Growth 2011, 329, 12–19. [Google Scholar] [CrossRef]
- Kulakov, M.P.; Kulakovskii, V.D.; Fadeev, A.V. Twinning in ZnSe crystals obtained from melt under pressure. Izv. Akad. Nauk. SSSR Neorgan. Mater. 1976, 12, 1867–1869. [Google Scholar]
- Kikuma, I.; Furukoshi, M. Direct Observation of the 3c-2h Transformation in ZnSe by High-Temperature X-Ray Diffraction. J. Cryst. Growth 1985, 71, 136–140. [Google Scholar] [CrossRef]
- Asahi, T.; Arakawa, A.; Sato, K. Growth of large-diameter ZnTe single crystals by the vertical gradient freezing method. J. Cryst. Growth 2001, 229, 74–78. [Google Scholar] [CrossRef]
- Balbaşı, Ö.B.; Ünal, M.; Genç, A.M.; Çelik, G.; Parlak, M.; Turan, R. Investigation of seeded vertical gradient freeze (VGF) growth of CdZnTe bulk crystals. J. Cryst. Growth 2022, 584, 126573. [Google Scholar] [CrossRef]
- Sato, K.; Seki, Y.; Matsuda, Y.; Oda, O. Recent developments in II–VI substrates. J. Cryst. Growth 1999, 197, 413–422. [Google Scholar] [CrossRef]
- Wang, T.; Jie, W.; Xu, Y.; Zha, G. Characterization of CdZnTe crystal grown by bottom-seeded Bridgman and Bridgman accelerated crucible rotation techniques. Trans. Nonferrous Met. Soc. China 2009, 19, s622–s625. [Google Scholar] [CrossRef]
- Xu, Y.; Jie, W.; Sellin, P.J.; Wang, T.; Fu, L.; Zha, G.; Veeramani, P. Characterization of CdZnTe Crystals Grown Using a Seeded Modified Vertical Bridgman Method. IEEE Trans. Nucl. Sci. 2009, 56, 2808–2813. [Google Scholar] [CrossRef]
- Chao, X.; Juan, Z.; Changhe, Z.; Shangshu, L.; Ruiyun, S. Advancements in Iso-Diameter seeded growth of CdZnTe crystals: A numerical modeling approach and applications in liquid phase Epitaxy. J. Cryst. Growth 2024, 633, 127670. [Google Scholar] [CrossRef]
- Mühlberg, M.; Rudolph, P.; Laasch, M.; Treser, E. The correlation between superheating and supercooling in CdTe melts during unseeded Bridgman growth. J. Cryst. Growth 1993, 128, 571–577. [Google Scholar] [CrossRef]
- Rudolph, P.; Umetsu, K.; Koh, H.J.; Fukuda, T. Correlation between ZnSe Crystal Growth Conditions from Melt and Generation of Large-Angle Grain Boundaries and Twins. Jpn. J. Appl. Phys. 1994, 33, 1991–1994. [Google Scholar] [CrossRef]
- Rudolph, P.; Koh, H.J.; Schäfer, N.; Fukuda, T. The crystal perfection depends on the superheating of the mother phase too—Experimental facts and speculations on the “melt structure” of semiconductor compounds. J. Cryst. Growth 1996, 166, 578–582. [Google Scholar] [CrossRef]
- Adachi, S. Properties of Group-IV, III–V and II–VI Semiconductors; John Wiley & Sons, Ltd.: Chichester, UK, 2005. [Google Scholar] [CrossRef]
- Merad, A.E.; Kanoun, M.B.; Merad, G.; Cibert, J.; Aourag, H. Full-potential investigation of the electronic and optical properties of stressed CdTe and ZnTe. Mater. Chem. Phys. 2005, 92, 333–339. [Google Scholar] [CrossRef]
- Pattanaik, A.; Tripathy, S.K.; Naik, P.; Meher, D.K. Structural and elastic properties of binary semiconductors from energy gaps. Appl. Phys. 2021, 127, 14. [Google Scholar] [CrossRef]
- Jordan, A.S. A Theory of Regular Associated Solution Applied to the Liquidus Curves of the Zn-Te and Cd-Te System. Metall. Trans. 1970, 1, 239–249. [Google Scholar] [CrossRef]
- Weiser, K. Pressure-Temperature-Composition (P-T-x) Diagrams. In Compound Semiconductors, Preparation of III–V Compounds; Willardson, R.K., Goering, K.H.L., Eds.; Reinhold: New York, NY, USA, 1962; Volume 1, pp. 472–481. [Google Scholar]
- Gaspard, J.P.; Raty, J.Y.; Céolin, R.; Bellissent, R. Local orders in II–VI liquid compounds. J. Non-Cryst. Solids 1996, 205–207, 75–78. [Google Scholar] [CrossRef]
- Godlevsky, V.V.; Jain, M.; Derby, J.J.; Chelikowsky, J.R. First principles calculations of liquid CdTe at temperatures above and below the melting point. Phys. Rev. 1999, 60, 8640–8649. [Google Scholar] [CrossRef]
- Dalgic, S.; Colakogullari, M. The structural properties of the liquid CdTe based on Reverse Monte Carlo modelling. J. Cryst. Growth 2007, 353, 1936–1940. [Google Scholar] [CrossRef]
- Gaspard, J.P. Structure of Melt and Liquid Alloys. In Handbook of Crystal Growth, 2nd ed.; Fundamentals: Thermodynamics and Kinetics; Nishinaga, T., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume IA, pp. 401–443. [Google Scholar] [CrossRef]
- Bergman, C.; Bichara, C.; Chieux, P.; Gaspard, J.P. On The Atomic Structure Of Liquid GaAs. J. Phys. Colloq. 1985, 46, C8-97. [Google Scholar] [CrossRef]
- Shcherbak, L. Pre-transition phenomena in CdTe near the melting point. J. Cryst. Growth 1999, 197, 397–405. [Google Scholar] [CrossRef]
- Crocco, J.; Bensalah, H.; Zheng, H.; Carcelén, V.; Diéguez, E. Influence of SiC pedestal in the growth of 50 mm CZT by Vertical gradient freeze method. J. Cryst. Growth 2012, 360, 92–94. [Google Scholar] [CrossRef]
- Zappettini, A. Cadmium Telluride and Cadmium Zinc Telluride. In Single Crystals of Electronic Materials—Growth and Properties; Fornari, R., Ed.; Woodhead Publishing, imprint Elsevier: Duxford, UK, 2019; pp. 273–301. [Google Scholar] [CrossRef]
- Kikuma, I.; Sekine, M.; Furukoshi, M. Growth of ZnSe Crystals Free From Rod-Like Low Angle Grain Boundaries from the Melt Under Argon Pressure. J. Cryst. Growth 1986, 75, 609–612. [Google Scholar] [CrossRef]
- Moskvin, P.; Khodakovskya, V.; Rashkovetskyi, L.; Stronski, A. Polyassociative model of A2B6 semiconductor melt and p-T-x equilibria in Cd–Hg–Te system. J. Cryst. Growth 2008, 210, 2617–2626. [Google Scholar] [CrossRef]
- Shiraki, H.; Funaki, M.; Ando, Y.; Tachibana, A.; Kominami, S.; Ohno, R. THM growth and characterization of 100 mm diameter CdTe single crystals. IEEE Trans. Nucl. Sci. 2009, 56, 1717–1723. [Google Scholar] [CrossRef]
- Mackenzie, J.; Kumar, F.J.; Chen, H. Advancements in THM-Grown CdZnTe for Use as Substrates for HgCdTe. J. Electron. Mat. 2013, 42, 3129–3132. [Google Scholar] [CrossRef]
- Prokesch, M.; Soldner, S.A.; Sundaram, A.G. CdZnTe detectors for gamma spectroscopy and x-ray photon counting at 250 × 106 photons/(mm2 s). J. Appl. Phys. 2018, 24, 044503. [Google Scholar] [CrossRef]
- Zhou, B.; Jie, W.; Wang, T.; Yin, L.; Yang, F.; Zhang, B.; Xi, S.; Dong, J. Modification of growth interface of CdZnTe crystals in THM process by ACRT. J. Cryst. Growth 2018, 483, 281–284. [Google Scholar] [CrossRef]
- Vijayakumar, P.; Amaladass, E.P.; Ganesan, K.; Sarguna, R.M.; Roy, V.; Ganesamoorthy, S. Development of travelling heater method for growth of detector grade CdZnTe single crystals. Mat. Sci. Semicond. Proc. 2024, 169, 107897. [Google Scholar] [CrossRef]
- Kaplan, W.D.; Kauffmann, Y. Structural Order in Liquids Induced by Interfaces with Crystals. Annu. Rev. Mater. Res. 2006, 36, 1–48. [Google Scholar] [CrossRef]
- Freitas, R.; Reed, E.J. Uncovering the effects of interface-induced ordering of liquid on crystal growth using machine learning. Nat. Commun. 2020, 11, 3260. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Tsukimoto, S.; Muto, S.; Saka, H. Direct observation of the atomic structure in a solid–liquid interface. Microsc. Microanal. 2000, 6, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Gandman, M.; Kauffmann, Y.; Koch, C.T.; Kaplan, W.D. Direct Quantification of Ordering at a Solid-Liquid Interface Using Aberration Corrected Transmission Electron Microscopy. Phys. Rev. Lett. 2013, 110, 086106. [Google Scholar] [CrossRef]
- Schülli, T.U.; Daudin, R.; Renaud, G.; Vaysset, A.; Geaymond, O.; Pasture, A. Substrate-enhanced supercooling in AuSi eutectic droplets. Nature 2010, 464, 1174–1177. [Google Scholar] [CrossRef]
- Zhao, J.; Tang, Z.; Kelton, K.F.; Liu, C.T.; Liaw, P.K.; Inoue, A.; Shen, X.; Pan, S.; Johnson, M.L.; Chen, G.; et al. Evolution of the atomic structure of a supercooled Zr55Cu35Al10 liquid. Intermetallics 2017, 82, 53–58. [Google Scholar] [CrossRef]
- Men, H.; Fan, Z. Prenucleation Induced by Crystalline Substrates. Metall. Mater. Trans. A 2018, 49, 2766–2777. [Google Scholar] [CrossRef]
- Mikheev, L.V.; Chernov, A.A. Mobility of a diffuse simple crystal-melt interface. J. Cryst. Growth 1991, 112, 591–596. [Google Scholar] [CrossRef]
- Henager, C.H., Jr.; Morris, J.R. Atomistic simulation of CdTe solid-liquid coexistence equilibria. Phys. Rev. 2009, 80, 245309. [Google Scholar] [CrossRef]
- Zhou, X.W.; Ward, D.K.; Wong, X.W.; Doty, F.P. Melt-Growth Dynamics in CdTe Crystals. Phys. Rev. Lett. 2012, 108, 245503. [Google Scholar] [CrossRef]
- Durose, K.; Russell, G. Twinning in CdTe. J. Cryst. Growth 1990, 101, 246–250. [Google Scholar] [CrossRef]
- Rogalski, A. HgCdTe Photodetectors. In Mid-Infrared Optoelectronics. Materials, Devices, and Applications; Tournié, E., Laurent, C., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 235–335. [Google Scholar] [CrossRef]
- Saucedo, E.; Rudolph, P.; Dieguez, E. Modified Bridgman growth of CdTe crystals. J. Cryst. Growth 2008, 310, 2067–2071. [Google Scholar] [CrossRef]
- Hobgood, H.M.; Swanson, B.W.; Thomas, R.N. Czochralski growth of CdTe and CdMnTe from liquid encapsulated melts. J. Cryst. Growth 1987, 85, 510–520. [Google Scholar] [CrossRef]
- Neubert, M.; Rudolph, P. Growth of semi-insulating GaAs crystals in low temperature gradients by using the vapour pressure controlled Czochralski method (VCz). Progr. Cryst. Growth Charact. Mat. 2001, 43, 119–185. [Google Scholar] [CrossRef]
- Ivanov, Y.I. Preparation of CdTe and CdZnTe Single-Crystalline Ingots 100 mm in Diameter by a Modified Obreimov–Shubnikov Method. Russ. J. Inorg. Chem. 2015, 60, 1816–1823. [Google Scholar] [CrossRef]
- Asahi, T.; Yabe, T.; Sato, K.; Arakawa, A. Growth of large diameter ZnTe single crystals by the LEK method. J. Alloys Compd. 2004, 33, 651–653. [Google Scholar] [CrossRef]
- Pavlyuk, M.D.; Sukhanov, E.A.; Zykov, M.P.; Volchkov, I.S.; Kanevskii, V.M.; Subbotin, I.A.; Podurets, K.M.; Pavlyuk, B.F.; Ivanov, Y.M. Mathematical Simulation of the Process of Growing a CdTe Single Crystal by the Obreimov–Shubnikov Method. Phys. Solid State 2020, 62, 1–7. [Google Scholar] [CrossRef]
- Crocco, J.; Franc, J.; Zazvorka, J.; Hlidek, P.; Dieguez, E.; Babentsov, V.; Sochinskyi, M.V.; James, R.B. Semi-Insulated Cd1−xZnxTe Grown by the Vertical Gradient Freeze Method. IEEE Trans. Nucl. Sci. 2012, 59, 1516–1521. [Google Scholar] [CrossRef]
- Itani, K.; Murakami, K.; Yamada, K. Compound Semiconductor and Method for Producing the Same. U.S. Patent US11552174B2, 1 October 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudolph, P. Considerations for Differences in Melt Growth Kinetics Between II–VI and III–V Compound Crystals. Crystals 2024, 14, 1067. https://doi.org/10.3390/cryst14121067
Rudolph P. Considerations for Differences in Melt Growth Kinetics Between II–VI and III–V Compound Crystals. Crystals. 2024; 14(12):1067. https://doi.org/10.3390/cryst14121067
Chicago/Turabian StyleRudolph, Peter. 2024. "Considerations for Differences in Melt Growth Kinetics Between II–VI and III–V Compound Crystals" Crystals 14, no. 12: 1067. https://doi.org/10.3390/cryst14121067
APA StyleRudolph, P. (2024). Considerations for Differences in Melt Growth Kinetics Between II–VI and III–V Compound Crystals. Crystals, 14(12), 1067. https://doi.org/10.3390/cryst14121067




