Metastable Racemic Ibuprofen Supercooled Liquid
Abstract
1. Introduction
| System | Method | IBU Concentration | Dispersion Medium | Ref |
|---|---|---|---|---|
| ASDs | Co-milling | 35% | SBA-15 | [11] |
| Impregnated evaporation | 40.4% 26.8% 47.8% | SBA-15 SBA-16 DIM | [16] | |
| Hot melt extrusion | 30% 30% 30% 30% | RSPO PVPVA 64 Soluplus EC | [17] | |
| Spray drying | 20% | CCAB | [18] | |
| Solvent evaporation | 0.15 w/v | PVP | [6] | |
| Hot melt extrusion | 65% | EPO | [19] | |
| Spray drying; electrospinning; Rotary evaporation | 10% | Cellulose excipients: HPMCAS and HPMCP-HP55 | [20] | |
| Co-milling | 37.7% | SBA-15 | [21] | |
| In situ loading | Mesoporous silica | [22] | ||
| Vacuum capillary wetting and heating | SBA-15 | [12] | ||
| Nanoparticles | Antisolvent | [10] |
2. Materials and Methods
2.1. Materials
2.2. Methods
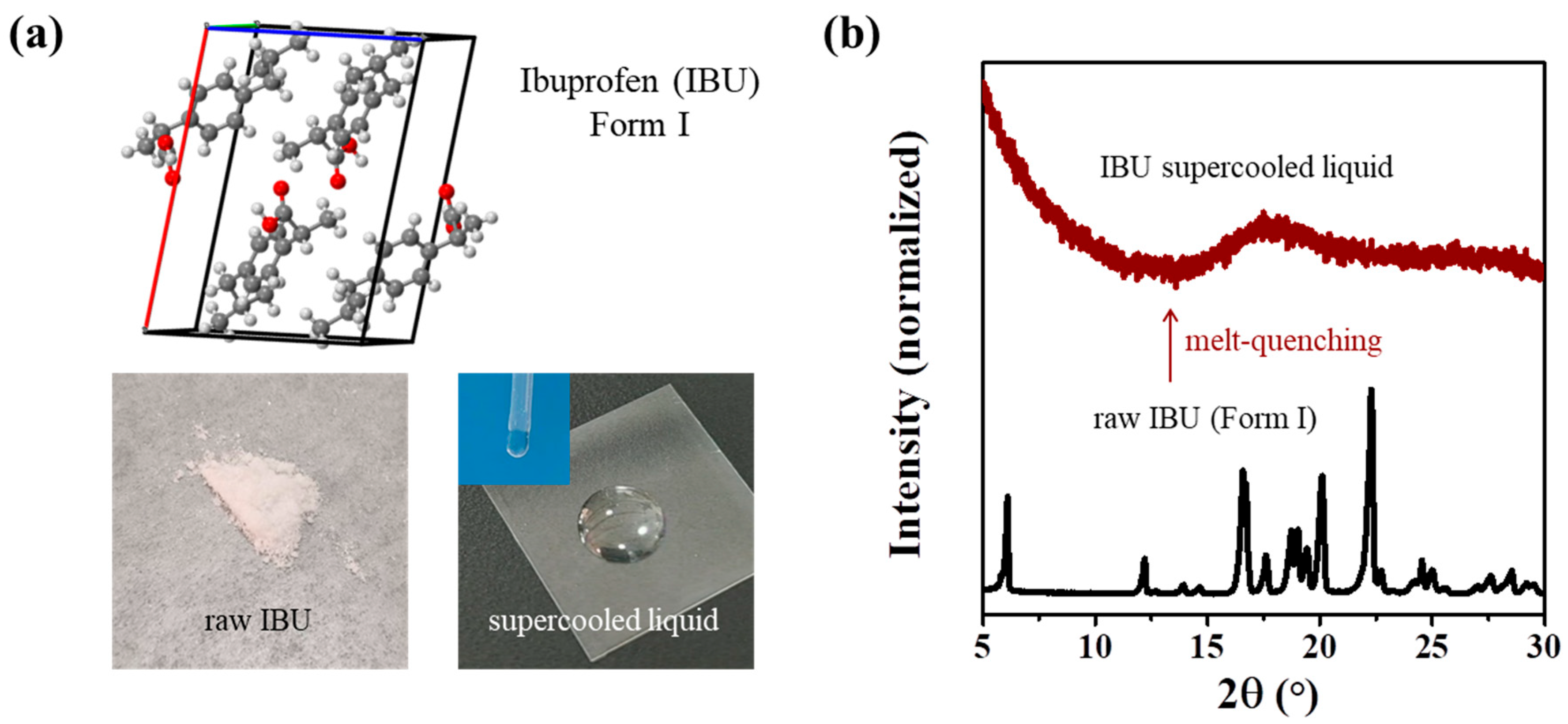
- IBU Form I and supercooled liquid: Raw IBU was in Form I. The IBU supercooled liquid was prepared by quickly quenching the melt of raw IBU from 100 °C to room temperature.
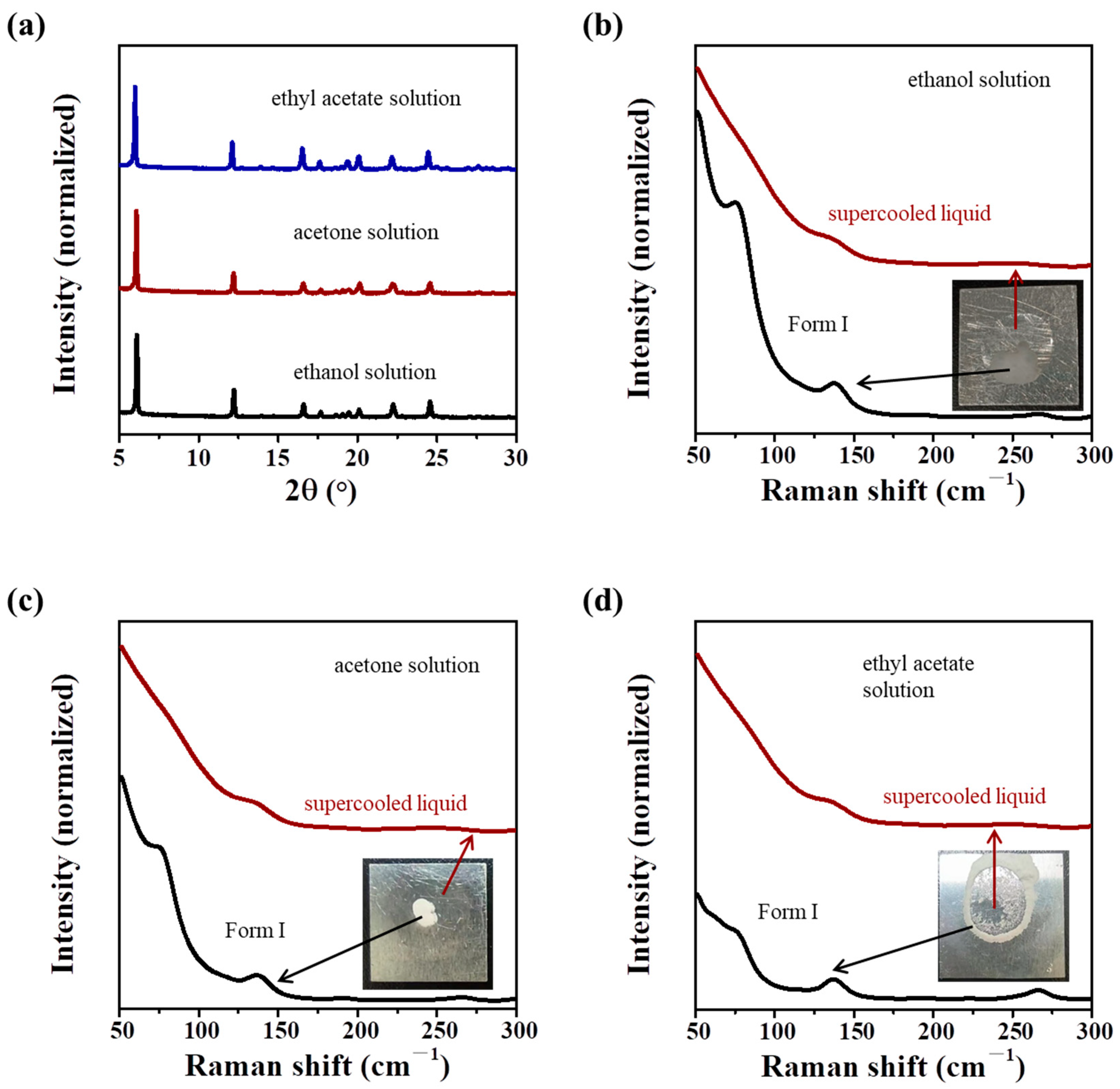
- Evaporation crystallization of IBU solutions: 100 μL of IBU ethanol, acetone, or ethyl acetate solution (0.1 g·mL−1) was dropped on an aluminum plate and evaporated at room temperature. The low-frequency Raman spectra of the samples were tested during the evaporation process.
- Stability of the IBU supercooled liquid:
- Effect of temperature: 1 g of racemic IBU was heated at 100 °C in a water bath for 10 min to completely melt and crystallize at different temperatures (0 °C, 10 °C, 25 °C, 40 °C, 50 °C).
- Effect of additives: 1 g mixtures of raw IBU and S-IBU/water/ethanol were heated at 100 °C in a water bath for 10 min to completely melt and crystallize at 0 °C. The additive contents (S-IBU/water/ethanol) were 1%, 5%, 10%, 25%, 50%, 75%, and 90%.
- Each experiment was conducted three times in parallel.
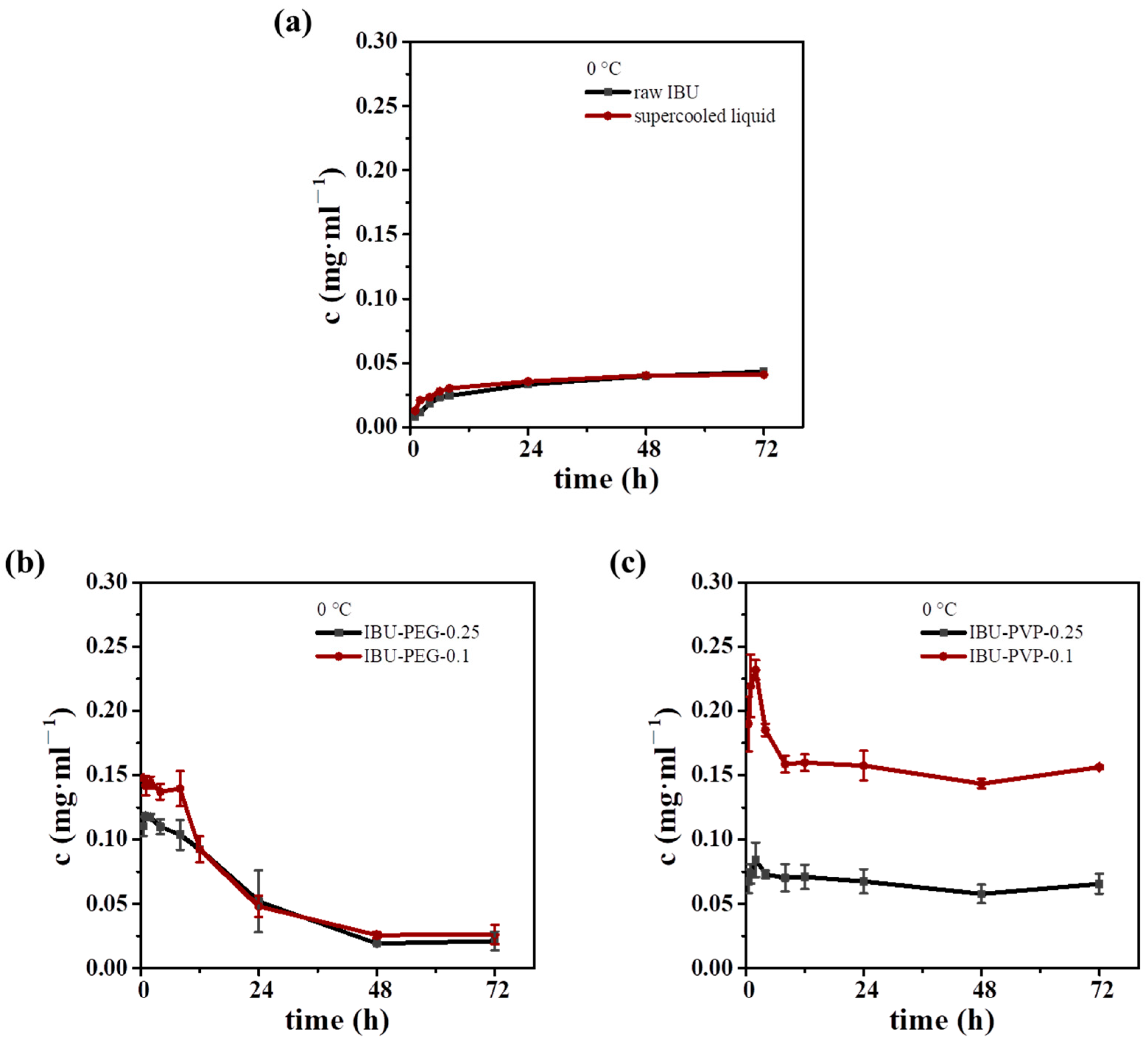
- Preparation of IBU formulation: 1 g mixtures of raw IBU (10%, 25%, 50%, 75%, or 90%) and PEG-200 or PVP were heated at 100 °C in a water bath for 10 min and quenched at 0 °C. The samples were named IBU-PEG/PVP-IBU contents. Each experiment was conducted three times in parallel.
- Working curve: Standard solutions with concentrations of 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, and 1.0 mg/mL were prepared by dissolving 100 mg of IBU into 100 mL of 50 vol. % ethanol and further dilutions. The 263 nm absorbance was used to establish the standard curve.
- Dissolution: Raw IBU or IBU dispersions (the IBU content was 25% or 10%) containing 0.2 g of IBU were added to 200 mL of water and kept at 0 or 37 °C in a water bath; 5 mL of supernatant was taken at predetermined time points (0.5, 1, 2, 4, 8, 12, and 24 h) and then filtered (PES membrane, 0.22 μm). Moreover, 2 mL of filtrate was added to 2 mL of ethanol, respectively, which was used for the UV-vis test (UV-vis, Shimadzu UV-2550, Shimadzu, Japan, 200~400 nm) to measure the supernatant’s IBU contents. The dissolution test was performed in triplicate.
- Characterization: Samples were characterized conventionally via powder X-ray diffraction (PXRD, Philips X’Pert Pro, PANalytical, Netherlands, Cu Kα, 40 kV, 30 mA, 5–30°, 4°·min−1), infrared spectrometer (IR, Shimadzu IRAffinity-1S, Shimadzu, Japan, 400–4000 cm−1, 2 cm−1), confocal Raman spectroscopy (Thermo Fisher Scientific, USA, DXR3xi, 532 nm, 40 mW, 0.1 s, 1000 scanning times, 50–3400 cm−1, 50× objective lens), thermogravimetric analysis, and differential scanning calorimetry (DSC-TG, NETZSCH STA 449F3, NETZSCH, Germany, nitrogen, 10 °C·min−1 or 1 °C·min−1).
3. Results
3.1. Preparation of the IBU Supercooled Liquid
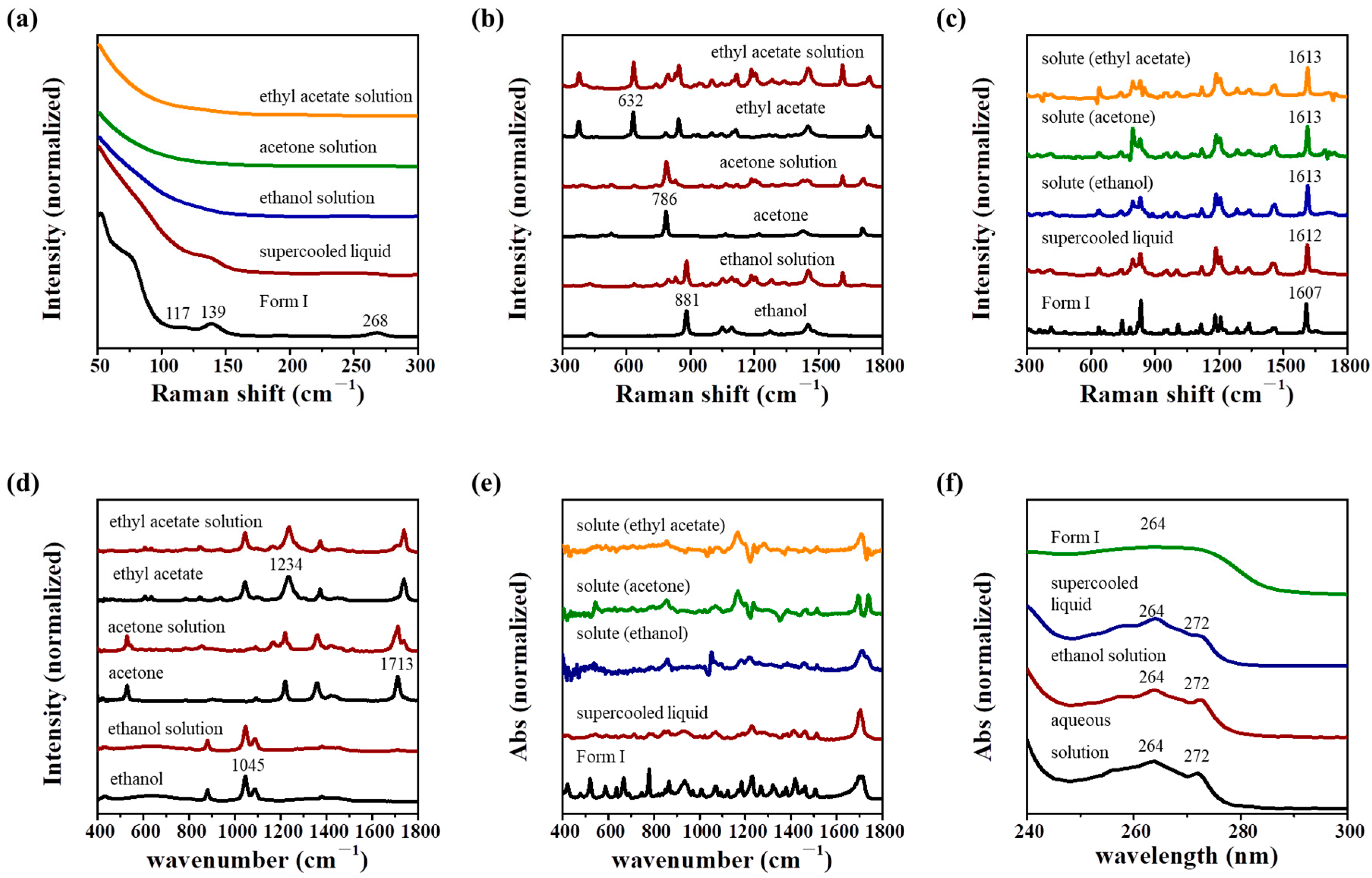
3.2. Structure Similarity Analysis Using Spectroscopy
| Form I | Supercooled Liquid | Solute in Solution | Assignment [27,28] | |||
|---|---|---|---|---|---|---|
| Ethanol | Acetone | Ethyl Acetate | ||||
| MFRS (300–1800 cm−1) | 311 | 304 | τ | |||
| 359 | 352 | |||||
| 413 | 409 | 410 | 409 | 410 | Deformation | |
| 637 | 636 | 636 | 637 | In-plane ring deformation | ||
| 745 | 741 | 740 | 739 | 740 | ||
| 783 | 795 | 797 | 796 | 795 | ||
| 833 | 831 | 830 | 829 | 830 | ||
| 958 | 955 | 956 | 956 | 955 | ||
| 1007 | 1002 | 999 | 1000 | 1003 | Ring breathing | |
| 1115 | 1117 | 1117 | 1118 | 1118 | ||
| 1181 | 1184 | 1185 | 1185 | 1185 | ||
| 1206 | 1206 | 1206 | 1203 | 1204 | ||
| 1283 | 1283 | 1283 | 1283 | 1284 | ||
| 1339 | 1339 | 1340 | 1339 | 1340 | ||
| 1450 | 1448 | 1452 | 1448 | 1451 | ||
| 1462 | 1458 | 1459 | 1459 | 1460 | ||
| 1607 | 1612 | 1613 | 1613 | 1613 | ν asym a C-C in Φ b | |
| IR (400–1800 cm−1) | 714 | 705 | 707 | |||
| 865 | 848, 862 | 853 | ||||
| 1168 | 1168 | 1181 | 1166 | 1164 | ||
| 1229 | 1229 | 1222 | ||||
| 1506 | 1512 | 1514 | 1511 | 1510 | ν asym C-C in Φ | |
| 1711 | 1702 | 1711 | 1706 | |||
| 1733 | 1738 | |||||
3.3. Formation of the IBU Supercooled Liquid During the Evaporation Crystallization Process
3.4. Stability of the IBU Supercooled Liquid
3.5. IBU Formulation Inspired by the IBU Supercooled Liquid
3.6. Dissolution Behavior of IBU Phases
4. Discussion
4.1. Disadvantage of the Supercooled Liquid in Drug Formulation
4.2. Distinguish Between the Glassy State and the Supercooled Liquid
4.3. Stability of the IBU Supercooled Liquid
4.4. Inspiration for the Design of IBU Formulations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tomar, D.; Singh, P.K.; Hoque, S.; Modani, S.; Sriram, A.; Kumar, R.; Madan, J.; Khatri, D.; Dua, K. Amorphous systems for delivery of nutraceuticals: Challenges opportunities. Crit. Rev. Food Sci. Nutr. 2022, 62, 1204–1221. [Google Scholar] [CrossRef] [PubMed]
- Berthier, L.; Reichman, D.R. Modern computational studies of the glass transition. Nat. Rev. Phys. 2023, 5, 102–116. [Google Scholar] [CrossRef]
- Li, R.; Lin, D.; Roos, Y.H.; Miao, S. Glass transition, structural relaxation and stability of spray-dried amorphous food solids: A review. Dry. Technol. 2019, 37, 287–300. [Google Scholar] [CrossRef]
- Mather, L. Do the pharmacodynamics of the nonsteroidal anti-inflammatory drugs suggest a role in the management of postoperative pain? Drugs 1992, 44, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Akay, S.; Öztürk, S.; Kalderis, D.; Kayan, B. Degradation, solubility and chromatographic studies of ibuprofen under high temperature water conditions. Chemosphere 2021, 277, 130307. [Google Scholar] [CrossRef] [PubMed]
- Ivone, M.; Denora, N.; D’Amico, V.; Mareczek, L.; Mueller, L.K.; Arduino, I.; Ambruosi, A.; Lopedota, A.A. Microbeads produced by prilling/vibration technique: A new way to use polyvinyl alcohol in pediatric and veterinary formulations. J. Drug Deliv. Sci. Technol. 2024, 99, 105974. [Google Scholar] [CrossRef]
- Uddin, A.; Halder, S.; Deb, N.; Das, H.; Shuma, M.L.; Hasan, I.; Shill, M.C.; Haider, S.S. Impact of methods of preparation on mechanical properties, dissolution behavior, and tableting characteristics of ibuprofen-loaded amorphous solid dispersions. Adv. Pharmacol. Pharm. Sci. 2024, 2024, 2303942. [Google Scholar] [CrossRef]
- Williams, P.A.; Hughes, C.E.; Harris, K.D.M. New insights into the preparation of the low-melting polymorph of racemic ibuprofen. Cryst. Growth Des. 2012, 12, 5839–5845. [Google Scholar] [CrossRef]
- Dudognon, E.; Correia, N.T.; Danède, F.; Descamps, M. Solid-solid transformation in racemic ibuprofen. Pharm. Res. 2013, 30, 81–89. [Google Scholar] [CrossRef]
- Melzig, S.; Finke, J.H.; Schilde, C.; Kwade, A. Formation of long-term stable amorphous ibuprofen nanoparticles via antisolvent melt precipitation (AMP). Eur. J. Pharm. Biopharm. 2018, 131, 224–231. [Google Scholar] [CrossRef]
- Moutamenni, B.; Tabary, N.; Mussi, A.; Dhainaut, J.; Ciotonea, C.; Fadel, A.; Paccou, L.; Dacquin, J.-P.; Guinet, Y.; Hédoux, A. Milling-assisted loading of drugs into mesoporous silica carriers: A green and simple method for obtaining tunable customized drug delivery. Pharmaceutics 2023, 15, 390. [Google Scholar] [CrossRef] [PubMed]
- Brás, A.R.; Merino, E.G.; Neves, P.D.; Fonseca, I.M.; Dionísio, M.; Schönhals, A.; Correia, N.T. Amorphous ibuprofen confined in nanostructured silica materials: A dynamical approach. J. Phys. Chem. C 2011, 115, 4616–4623. [Google Scholar] [CrossRef]
- Wiedenbeck, E.; Kovermann, M.; Gebauer, D.; Cölfen, H. Liquid metastable precursors of ibuprofen as aqueous nucleation intermediates. Angew. Chem. Int. Ed. 2019, 58, 19103–19109. [Google Scholar] [CrossRef] [PubMed]
- Tsarfati, Y.; Biran, I.; Wiedenbeck, E.; Houben, L.; Cölfen, H.; Rybtchinski, B. Continuum crystallization model derived from pharmaceutical crystallization mechanisms. ACS Cent. Sci. 2021, 7, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Codan, L.; Casillo, S.; Bäbler, M.U.; Mazzotti, M. Phase diagram of a chiral substance exhibiting oiling out. 2. Racemic compound forming ibuprofen in water. Cryst. Growth Des. 2012, 12, 5298–5310. [Google Scholar] [CrossRef]
- Quevedo, G.P.; Celis, A.C.; Ordonez, C.V.; Martinez, M.L.O. SBA-type mesoporous materials with cylindrical and spherical structures for the controlled loading and release of ibuprofen. J. Sol-Gel Sci. Technol. 2017, 85, 486–494. [Google Scholar] [CrossRef]
- Chen, L.; Hu, E.; Shen, P.; Qian, S.; Heng, W.; Zhang, J.; Gao, Y.; Wei, Y. Development of amorphous solid dispersion sustained-release formulations with polymer composite matrix-regulated stable release plateaus. Pharm. Res. 2024, 41, 1233–1245. [Google Scholar] [CrossRef]
- Marks, J.A.; Nichols, B.L.B.; Mosquera-Giraldo, L.I.; Yazdi, S.T.; Taylor, L.S.; Edgar, K.J. 6-Carboxycellulose acetate butyrate: Effectiveness as an amorphous solid dispersion polymer. Mol. Pharm. 2024, 21, 4589–4602. [Google Scholar] [CrossRef]
- Tian, Y.; Jacobs, E.; Jones, D.S.; McCoy, C.P.; Wu, H.; Andrews, G.P. The design and development of high drug loading amorphous solid dispersion for hot-melt extrusion platform. Int. J. Pharm. 2020, 586, 119545. [Google Scholar] [CrossRef]
- Ziaee, A.; O’Dea, S.; Howard-Hildige, A.; Padrela, L.; Potter, C.; Iqbal, J.; Albadarin, A.B.; Walker, G.; O’Reilly, E.J. Amorphous solid dispersion of ibuprofen: A comparative study on the effect of solution based techniques. Int. J. Pharm. 2019, 572, 118816. [Google Scholar] [CrossRef]
- Malfait, B.; Correia, N.T.; Mussi, A.; Paccou, L.; Guinet, Y.; Hédoux, A. Solid-state loading of organic molecular materials within mesoporous silica matrix: Application to ibuprofen. Microporous Mesoporous Mater. 2019, 277, 203–207. [Google Scholar] [CrossRef]
- Wan, M.M.; Li, Y.Y.; Yang, T.; Zhang, T.; Sun, X.D.; Zhu, J.H. In situ loading of drugs into mesoporous silica SBA-15. Chem.-Eur. J. 2016, 22, 6294–6301. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, J.; Korter, T.M.; Bērziņš, K.R.; McGoverin, C.M.; Gordon, K.C.; Fraser-Miller, S.J. Characterization of variably substituted hydroxyapatites using low-frequency Raman spectroscopy. Cryst. Growth Des. 2023, 23, 5748–5761. [Google Scholar] [CrossRef]
- Chen, F.; Yang, C.; Cheng, X.; Fan, Y.; Chen, X.; Ren, S.; Xue, R. Explanation for the selective crystallization from inosine solutions using mid-frequency Raman difference spectra analysis. RSC Adv. 2022, 12, 18301–18306. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liang, C.; Li, Y.; Xiao, W.; Niu, Y.; Jin, H.; Xue, R.; Chen, F. Explanation and prediction of the selective crystallization of boscalid by mid-frequency Raman difference spectroscopy analysis. CrystEngComm 2023, 25, 3548–3555. [Google Scholar] [CrossRef]
- Ren, S.; Nian, F.; Chen, X.; Xue, R.; Chen, F. Routes of theophylline monohydrate dehydration process proposed by mid-frequency Raman difference spectra. J. Pharm. Sci. 2023, 112, 2863–2868. [Google Scholar] [CrossRef]
- Logacheva, K.; Gergelezhiu, P.; Raksha, E.; Savostina, L.; Arzumanyan, G.; Eresko, A.; Malakhov, S.; Mamatkulov, K.; Ponomareva, O.; Belushkin, A.; et al. Vibrational spectroscopic features of ibuprofen and ketoprofen: IR and Raman spectroscopy combined with DFT calculations. Phys. Part. Nucl. Lett. 2024, 21, 839–842. [Google Scholar] [CrossRef]
- Jubert, A.; Legarto, M.L.; Massa, N.E.; Tévez, L.L.; Okulik, N.B. Vibrational and theoretical studies of non-steroidal anti-inflammatory drugs ibuprofen [2-(4-isobutylphenyl)propionic acid]; Naproxen [6-methoxy-α-methyl-2-naphthalene acetic acid] and Tolmetin acids [1-methyl-5-(4-methylbenzoyl)-1H-pyrrole-2-acetic acid]. J. Mol. Struct. 2006, 783, 34–51. [Google Scholar] [CrossRef]
- Phaechamud, T.; Tuntarawongsa, S. Transformation of eutectic emulsion to nanosuspension fabricating with solvent evaporation and ultrasonication technique. Int. J. Nanomed. 2016, 11, 2855. [Google Scholar] [CrossRef][Green Version]
- Rashid, A.; White, E.T.; Howes, T.; Litster, J.D.; Marziano, I. Effect of solvent composition and temperature on the solubility of ibuprofen in aqueous tthanol. J. Chem. Eng. Data 2014, 59, 2699–2703. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Q.; Xu, Z.; Yang, Q.; Hao, G.; Liu, M.; Zeng, Z. Recent progress on crystal nucleation of amorphous solid dispersion. Cryst. Growth Des. 2024, 24, 8655–8666. [Google Scholar] [CrossRef]
- Patel, N.G.; Serajuddin, A.T.M. Moisture sorption by polymeric excipients commonly used in amorphous solid dispersion and its effect on glass transition temperature: I. Polyvinylpyrrolidone and related copolymers. Int. J. Pharm. 2022, 616, 121532. [Google Scholar] [CrossRef] [PubMed]
- Lapuk, S.; Ponomareva, M.; Ziganshin, M.; Larionov, R.; Mukhametzyanov, T.; Schick, C.; Lounev, I.; Gerasimov, A. Some aspects of the glass transition of polyvinylpyrrolidone depending on the molecular mass. Phys. Chem. Chem. Phys. 2023, 25, 10706–10714. [Google Scholar] [CrossRef] [PubMed]
- Constantin, J.G.; Schneider, M.; Corti, H.R. Glass transition temperature of saccharide aqueous solutions estimated with the free volume/percolation model. J. Phys. Chem. B 2016, 120, 5047–5055. [Google Scholar] [CrossRef] [PubMed]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric amorphous solid dispersions: A review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef]
- Ojarinta, R.; Saarinen, J.; Strachan, C.J.; Korhonen, O.; Laitinen, R. Preparation and characterization of multi-component tablets containing co-amorphous salts: Combining multimodal non-linear optical imaging with established analytical methods. Eur. J. Pharm. Biopharm. 2018, 132, 112–126. [Google Scholar] [CrossRef]
- Guinet, Y.; Paccou, L.; Hédoux, A. Co-amorphous versus deep eutectic solvents formulations for transdermal administration. Pharmaceutics 2023, 15, 1710. [Google Scholar] [CrossRef]


| MFRDS | a. d. × 103 | s. d. × 103 |
|---|---|---|
| Form I with supercooled liquid | 50 (3) | 104 (9) |
| Form I with solute in ethanol | 57 (5) | 114 (11) |
| Form I with solute in acetone | 64 (4) | 135 (11) |
| Form I with solute in ethyl acetate | 61 (6) | 120 (12) |
| Supercooled liquid with solute in ethanol | 19 (1) | 32 (1) |
| Supercooled liquid with solute in acetone | 26 (1) | 53 (1) |
| Supercooled liquid with solute in ethyl acetate | 26 (1) | 45 (1) |
| Solute in ethanol with solute in acetone | 21 (1) | 48 (1) |
| Solute in ethanol with solute in ethyl acetate | 19 (1) | 36 (1) |
| Solute in acetone with solute in ethyl acetate | 27 (1) | 53 (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Xiao, W.; Ren, S.; Xue, R.; Chen, F. Metastable Racemic Ibuprofen Supercooled Liquid. Crystals 2024, 14, 1037. https://doi.org/10.3390/cryst14121037
Li T, Xiao W, Ren S, Xue R, Chen F. Metastable Racemic Ibuprofen Supercooled Liquid. Crystals. 2024; 14(12):1037. https://doi.org/10.3390/cryst14121037
Chicago/Turabian StyleLi, Tuanjia, Wangchuan Xiao, Shizhao Ren, Rongrong Xue, and Fenghua Chen. 2024. "Metastable Racemic Ibuprofen Supercooled Liquid" Crystals 14, no. 12: 1037. https://doi.org/10.3390/cryst14121037
APA StyleLi, T., Xiao, W., Ren, S., Xue, R., & Chen, F. (2024). Metastable Racemic Ibuprofen Supercooled Liquid. Crystals, 14(12), 1037. https://doi.org/10.3390/cryst14121037






