Abstract
Two new Tb(III) metal–organic frameworks based on 4,7-dimethylphenanthroline (dmphen) and flexible ligand trans-1,4-cyclohexanedicarboxylate (chdc2−) were synthesized and characterized. Their crystallographic formulae are [Tb2(dmphen)2(H2O)2(chdc)3]·2DMF (1; DMF = N,N-dimethylformamide) and [Tb2(dmphen)2(NO3)2(chdc)2]·2DMF (2). Among some differences in their synthetic conditions, the most important one is apparently the using of terbium(III) nitrate instead of terbium(III) chloride as a metal precursor in the synthesis of 2, providing a nitrate coordination to Tb3+, and its subsequent notable structural differences to 1. Compound 1 was found to have a layered hcb structure with intralayer windows ca. 10 × 8 Å2 in size. Its layer-to-layer packing leaves narrow channels running across these windows, with 18% as a total solvent-accessible volume in the coordination structure. Compound 2 was found to have a layered sql structure with smaller intralayer windows of ca. 8 × 6 Å2 in size. Methyl substituents on the phen ligands do not affect the topology of the framework but seem to have a substantial effect on the packing density, as well as the pore volume of the resulting MOF. A high 18.4% luminescence quantum yield was found for 2. Its emission lifetime of 0.695(12) ms belongs to a typical range for phosphorescent Tb(III)-carboxylate complexes. A quenching of its emission by different nitroaromatic molecules was found. A linear concentration dependence on 3-nitrotoluene and 4-nitro-m-xylene at micromolar concentrations was found during luminescent titration experiments (LOD values ca. 350 nM), suggesting this MOF to be a viable and highly sensitive luminescent sensor for such substrates.
1. Introduction
Metal−organic frameworks (MOFs; or coordination polymers) are based on both inorganic and organic building blocks, affording a practically infinite structural library [1,2,3] with variable properties and applications. Gas storage for fuel tanks [4,5], selective separations [6,7] and luminescent sensing [8,9,10] are recognized as apparently the most promising applications of porous coordination polymers. The development of MOF chemistry strictly adheres to the reticular approach [11,12]. This concept provides the synthesis of a wide range of MOF families [13,14,15], whose porosity, physical properties, and surface chemistry can be tuned predictably by varying the length, geometry, and chemical nature of the organic ligands. Two [16,17,18] or more [19,20] ligand types within the coordination framework may, moreover, result in a fractional tuning of the structural features and functionalities of the coordination networks in different dimensions, while maintaining its topology and main structural motifs.
In MOFs based on rare earth elements (REEs), a two-ligand approach is widely applicable, by using a polytopic linker in combination with diimine chelators, such as 2,2′-bipyridine (bpy) [21,22], 1,10-phenanthroline (phen) [23,24], and other aromatic (N,N)-donor derivatives [6,25,26]. The extended electron-rich aromatic systems in such molecules effectively harvest light photons and transfer the excited energy from the photosensitizer to the highly emissive Ln3+ cation, thus manifesting a well-known “antenna effect”. The other ligand (for example, an anion of di- or polycarboxylic acid) is implicated in bearing a bridging function [27,28], forming a polymeric porous coordination lattice. Our previous experience in a synthesis of bpy- and phen-based REE metal–organic frameworks with aliphatic dicarboxylates has yielded several series of highly emissive and porous luminophores [22,29,30], in which an aliphatic UV-transparent strut bears a purely structure-forming role, with no significant impact on the optical properties of the MOF, allowing a delicate tuning of porosity and luminescence properties in an orthogonal manner. Prominent examples of multi-colored luminophores with close to quantitative quantum yields (QY), very efficient white emitters [22] and luminescent sensors [29] are demonstrated in these series. A present investigation is devoted to the synthesis and study of chemically close Tb3+ trans-1,4-cyclohexanedicarboxylates (chdc2−) with 4,7-dimethyl-1,10-phenanthroline (dmphen) as an antenna ligand, including additional auxochromic methyl groups with an electron–donor effect and possible new adsorption centers for the enhanced sensing properties of analytes containing similar non-polar aliphatic substituents. As a result, we report two new Tb3+-based MOFs with the formulae [Tb2(dmphen)2(H2O)2(chdc)3]·2DMF (1) and [Tb2(dmphen)2(NO3)2(chdc)2]·2DMF (2). For 2, a linear dependence of its emission on different methyl-substituted nitroaromatics, down to micromolar concentrations, was found during luminescent titration experiments, suggesting this MOF to be a viable and highly sensitive luminescent sensor for such substrates.
2. Materials and Methods
2.1. Materials
trans-1,4-cyclohexanedicarboxylic acid (H2chdc, >97.0%) was received from BLD Pharmatech. 4,7-Dimethyl-1,10-phenanthroline (dmphen, 98%) was received from ABCR. Terbium(III,IV) oxide (Tb4O7, >99.98%) was received from ChemCraft. N,N-dimethylformamide (DMF, reagent grade) was received from Vekton. Nitric acid (HNO3, 62% solution in water) was received from Reachem. 2-nitrotoluene (>99.0%) and 3-nitrotoluene (>99.0%) were received from TCI. 4-nitro-m-xylene (99%) was received from Acros Organics. All reagents were used as received without further purification.
2.2. Instruments
FT-IR spectra (in KBr pellets) were obtained using a Bruker Scimitar FTS 2000 device (Billerica, MA, USA). Elemental (CHN) analysis was carried out using a VarioMICROcube analyzer (Elementar Analysensysteme GmbH, Hanau, Germany). Powder X-ray diffraction (PXRD) patterns were acquired using a Bruker D8 Advance diffractometer (Cu-Kα radiation, λ = 1.54178 Å; Billerica, MA, USA). Thermogravimetric analysis was conducted using a Netzsch TG 209 F1 Iris instrument (Selb, Germany) under Ar flow (30 cm3·min−1) and a 10 K∙min−1 heating rate. Photoluminescence spectra of solid samples and dispersions were recorded using a Horiba Jobin Yvon Fluorolog 3 spectrofluorometer (Kyoto, Japan) equipped with a 450 W power ozone-free Xe-lamp, cooled photon detector R928/1860 PFR technologies with refrigerated chamber PC177CE-010, and double grating monochromators. The spectra were corrected for source intensity and detector spectral response by standard correction curves. A set of NanoLED pulsed nanosecond lasers was used for the time-resolved measurements, and a Quanta-φ integrating sphere (Horiba, Kyoto, Japan) was used for the determination of absolute quantum yields (QYs).
2.3. Synthetic Methods
Synthesis of Tb(NO3)3·5H2O. A total of 2.00 g (2.67 mmol) of Tb4O7 and 5.0 mL of water was placed in a glass vial. Then, 3.0 mL (41 mmol) of concentrated HNO3 (62% water solution) was added dropwise, with continuous stirring to prevent foaming the mixture out. After stirring for several hours, the Tb4O7 completely dissolved. Then the obtained solution was transferred to an evaporation bowl and dried at 150 °C to delete any excess of HNO3. After full drying out, the solid was dissolved in water again, and its pH was found to be pH = 5–6, confirming the full elimination of the excess of nitric acid. Then, the solution had to stand for slow evaporation at 90 °C for the crystallization of Tb(NO3)3·5H2O. The yield was close to quantitative.
Synthesis of TbCl3·6H2O. A total of 2.00 g (2.67 mmol) of Tb4O7 and 5.0 mL of water was placed in a glass vial. Then, 4.0 mL (47 mmol) of concentrated HCl (36% water solution) was added dropwise with continuous stirring, to prevent foaming the mixture out. After stirring for several hours, the Tb4O7 completely dissolved. Then, the obtained solution was transferred to an evaporation bowl and dried at 150 °C to delete any excess of HCl. After full drying out, the solid was dissolved in water again, and its pH was found to be pH = 5–6, confirming the full elimination of the excess hydrogen chloride. Then, the solution had to stand for slow evaporation at 90 °C for the crystallization of TbCl3·6H2O. The yield was close to quantitative.
Synthesis of [Tb2(dmphen)2(H2O)2(chdc)3]·2DMF (1). A total of 14.2 mg (0.04 mmol) of TbCl3·5H2O, 16.6 mg (0.08 mmol) of dmphen, and 13.8 mg (0.08 mmol) of H2chdc was mixed in a glass vial, then a mixture of 1.2 mL of DMF and 0.8 mL of water was added. The obtained solution was heated at 80 °C for 3 days. The crystallized light brown precipitate was filtered off, then washed with DMF and dried in air. A single crystal suitable for SCXRD was taken from the mother liquor before filtration. The chemical composition of 1 was determined according to SCXRD data.
Synthesis of [Tb2(dmphen)2(NO3)2(chdc)2]·2DMF (2). A total of 17.4 mg (0.04 mmol) of Tb(NO3)3·5H2O, 16.6 mg (0.08 mmol) of dmphen, and 13.8 mg (0.08 mmol) of H2chdc was mixed in a glass vial, then a mixture of 1.2 mL of DMF and 0.8 mL of water was added. The obtained solution was heated at 80 °C for 3 days. The formed light brown precipitate was filtered off, then washed with DMF and dried in air. Yield: 5.8 mg (22%). The crystal structure of 2 was determined by SCXRD analysis. IR spectrum (KBr, cm−1) main bands: 3082 (w, νCsp2–H), 2940 (m, νCsp3–H), 2855 (w, νCsp3–H), 1682 (s, νC=O), 1586 (s, νCOO−as), 1416 (s, νCOO−s, νNO3−). Elemental analysis data (%), calculated for [Tb2(C14H12N2)2(NO3)2(C8H10O4)2]·2C3H7NO: C, 44.1; H, 4.5; N, 8.0. Found: C, 44.2; H, 4.2; N, 8.0. TG data: 10% weight loss at 155 °C. Calculated for 2DMF: 11%.
Synthesis of 2 microcrystalline dispersion. Powder samples of 2 were prepared by scaling up its synthesis method (2.5-fold multiplication) and carrying out the heating with continuous intensive stirring. The formed light brown precipitate was washed with 10 mL of DMF and then decanted twice. The phase purity of the bulk material was confirmed by PXRD.
Synthesis of [Tb2(dmphen)2(NO3)2(chdc)2]·DMF·3NB·H2O (2NB). The sample of 2 (~10.0 mg) was decanted from DMF in a glass vial and immersed in 1.0 mL of nitrobenzene (NB). The NB was refreshed twice, with a two days interval. After immersion, the obtained solid was filtered and dried in air. Elemental analysis data (%), calculated for [Tb2(C14H12N2)2(NO3)2(C8H10O4)2]·C3H7NO·3C6H5NO2·H2O: C, 47.0; H, 4.1; N, 8.4. Found: C, 47.1; H, 4.3; N, 8.6.
Synthesis of [Tb2(dmphen)2(NO3)2(chdc)2]·DMF·3(2NT)·H2O (22NT). The sample of 2 (~10.0 mg) was decanted from DMF in a glass vial and immersed in 1.0 mL of 2-nitrotoluene (2NT). The 2NT was refreshed twice, with a two days interval. After the immersion, the obtained solid was filtered and dried in air. Elemental analysis data (%), calculated for [Tb2(C14H12N2)2(NO3)2(C8H10O4)2]·C3H7NO·3C7H7NO2·H2O: C, 48.0; H, 4.4; N, 8.2. Found: C, 47.6; H, 4.5; N, 8.3.
Synthesis of [Tb2(dmphen)2(NO3)2(chdc)2]·DMF·3(3NT) (23NT). The sample of 2 (~10.0 mg) was decanted from DMF in a glass vial and immersed in 1.0 mL of 3-nitrotoluene (3NT). The 3NT was refreshed twice, with a two days interval. After the immersion, the obtained solid was filtered and dried in air. Elemental analysis data (%), calculated for [Tb2(C14H12N2)2(NO3)2(C8H10O4)2]·C3H7NO·3C7H7NO2: C, 48.5; H, 4.28; N, 8.3. Found: C, 48.6; H, 4.7; N, 8.3.
Synthesis of [Tb2(dmphen)2(NO3)2(chdc)2]·DMF·3(NMX). The sample of 2 (~10.0 mg) was decanted from DMF in a glass vial and immersed in 1.0 mL of 4-nitro-m-xylene (NMX). The NMX was refreshed twice, with a two days interval. After the immersion, the obtained solid was filtered and dried in air. Elemental analysis data (%), calculated for [Tb2(C14H12N2)2(NO3)2(C8H10O4)2]·3(C8H9NO2)·C3H7NO: C, 49.4; H, 4.5; N, 8.1. Found: C, 49.1; H, 4.8; N, 8.1.
2.4. Single-Crystal X-Ray Diffraction Details
Diffraction data for single crystals of 1 and 2 were acquired using an automated Agilent Xcalibur diffractometer (Santa Clara, California, USA) equipped with an area AtlasS2 detector (graphite monochromator, λ(MoKα) = 0.71073 Å). The integration, absorption correction, and determination of unit cell parameters were performed using the CrysAlisPro [31] program package. The structures were solved by the dual-space algorithm (SHELXT [32]) and refined by the full-matrix least-squares technique (SHELXL [33]) in the anisotropic approximation (except hydrogen atoms). The positions of hydrogen atoms of organic ligands were calculated geometrically and refined in the riding model. The crystallographic data and details of the structure refinements are summarized in Table A1. The CCDC 2385343 (1) and 2385344 (2) entries contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Center at https://www.ccdc.cam.ac.uk/structures/ (Accessed on 25 November 2024).
3. Results and Discussion
3.1. Synthesis and Crystal Structure
Single crystals of compound [Tb2(dmphen)2(H2O)2(chdc)3]·2DMF (1) were obtained by the reaction between terbium(III) chloride, trans-1,4-cyclohexanedicarboxylic acid (H2chdc), and 4,7-dimethyl-1,10-phenantroline (dmphen) in a mixture of N,N-dimethylformamide (DMF) and water at 80 °C. Compound 1 crystallizes in triclinic symmetry with the P–1 space group and contains one independent Tb atom, which is coordinated by six O atoms of three κ2-carboxylic groups, two N atoms of chelated κ2-dmphen molecule, and one O atom of water molecule. Therefore, the Tb atom adopts a coordination number (CN) 9 with a typical “muffin” polyhedron (Figure 1a). The Tb–O(COO) bond lengths its range from 2.404(2) Å to 2.473(2) Å, Tb–O(H2O) bond length is 2.433(2) Å, and the Tb–N distances are 2.560(3) Å and 2.562(3) Å. Such three-connected metal blocks are bound by chdc linkers to form distorted hexagonal layers with a honeycomb (hcb) topology [34,35] (Figure 1b). Remarkably, two out of three bridging ligands exist in a more common biequatorial (e,e) conformation, while one chdc linker adopts a somewhat shorter biaxial (a,a) conformation, resulting in the decreased symmetry of the intralayer windows and their slightly flattened shape, with a corresponding size of ca. 10 × 8 Å2. These layers have a corrugated shape and are packed closely to each other (Figure 1c). Despite this packing, the windows having a rather large size are stacked to each other, forming a system of interconnected channels with the dimensions of 7 × 4 Å2 (Figure S1) and 6 × 3 Å2 in size, large enough for the possible inclusion of light gases, as well as aromatic molecules. As was found by the PLATON [36] routine, the total solvent accessible volume in the coordination lattice of 1 is 18%. The voids are occupied by the guest DMF molecules. Both the methyl groups of dmphen and the H atoms of coordinated water molecules are directed inward to the voids, providing possible branched intermolecular interactions with both polar and non-polar substrates [37]. Moreover, the notable porosity of 1’s crystal structure, based on a flexible chdc ligand, suggests possible conformation rearrangements during solvent exchange or other external stimuli [38,39,40].
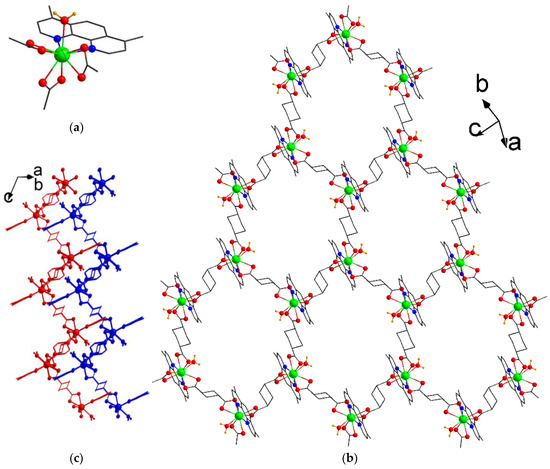
Figure 1.
Coordination environment of Tb(III) in 1 (a) and view of coordination layer (b). Tb atoms are shown in green, O in red, N in blue; H atoms of water molecules are shown in orange. Two adjacent layers in 3D packing of 1 (c). Atoms of independent layers are shown in different colors. The hydrogen atoms of the organic ligands are omitted for clarity.
Single crystals of compound [Tb2(dmphen)2(NO3)2(chdc)2]·2DMF (2) were obtained by the reaction between terbium(III) nitrate, trans-1,4-cyclohexanedicarboxylic acid (H2chdc), and 4,7-dimethyl-1,10-phenantroline (dmphen) in a mixture of N,N-dimethylformamide (DMF) and water at 80 °C. The main difference between the syntheses of these two chemically close compounds is using terbium(III) nitrate for 2’s synthesis instead of chloride as for 1, providing a coordination of NO3− to the metal cation, significantly changing the connectivity and overall structure of the resulting framework. Compound 2 crystallizes in monoclinic symmetry with the P21/c space group and contains one independent Tb atom. Each Tb(III) is coordinated by two O atoms of two μ2-κ1:κ1-carboxylic groups, three O atoms of two μ2-κ1:κ2-carboxylic groups (one of which chelates this Tb atom), two N atoms of κ2-dmphen molecule, and two O atoms of κ2-nitrate anion. The metal center adopts a typical capped square antiprismatic environment (CN = 9). The cap position in the antiprism is a non-chelating O atom of the chelate-bridging (μ2-κ1:κ2-COO) carboxyl group with a Tb–O bond length of 2.5660(17) Å. The Tb–O(COO) bond lengths lie in the range from 2.3292(17) Å to 2.4132(18) Å. The Tb–N distances are 2.551(2) Å and 2.594(2) Å. The Tb–O(NO3) bond lengths are 2.462(2) Å and 2.531(2) Å. The two closest symmetry-equivalent Tb(III) ions are coupled into binuclear blocks with the formulae {Tb2(dmphen)2(NO3)2(OOCR)4} (Figure 2a). These four connected blocks are bound by chdc bridging ligands into tetragonal coordination layers with a square-layered (sql) topology (Figure 2b). The layer windows also have a distorted tetragonal shape, with a size of ca. 8 × 6 Å2. The layers are stacked to each other in an AAAA manner, with partial π-π stacking between phenanthroline molecules from adjacent layers (Figure 2c). Due to such packing, narrow slit-like channels with dimensions of about 7 × 3 Å2 are formed. The crystal structure of 2 resembles the chemically cognate MOF [Tb2(phen)2(NO3)2(chdc)2]·2DMF, prepared from unsubstituted phenanthroline (phen) [29]. Strikingly, the modification of the coordinated phenanthroline ligands by the methyl groups expands the interlayer distance (parameter a) by 1 Å and the unit cell volume by as much as 9% in 2, compared with its non-modified predecessor [Tb2(phen)2(NO3)2(chdc)2]·2DMF. The total solvent accessible volume in the coordination lattice of 2 is also increased to 25% (the interstitial voids in the as-synthesized structure are occupied by guest DMF molecules). Apparently, even small alkyl substituents of the pendant chelate ligands may lead to notable differences in the packing density, as well as in the pore volume, while maintaining the topology of the resulting MOF. The surface of these pores is lined by the methyl groups, nitrate anions, and cyclohexane rings; therefore, some hydrophobicity of compound 2 is to be expected, which should facilitate the exchange and luminescence detection of aromatic guest molecules.
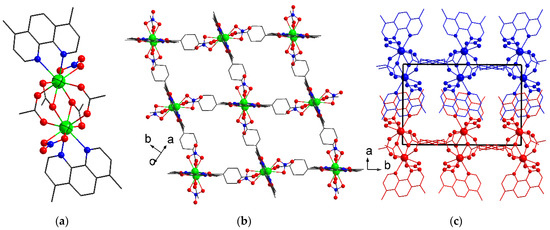
Figure 2.
Binuclear carboxylate block in 2 (a) and view of coordination layer (b). Tb atoms are shown in green, O in red, and N in blue. Two adjacent layers in 3D package of 2 (c). Atoms of independent layers are shown in different colors.
3.2. Characterization and Luminescent Properties of [Tb2(dmphen)2(NO3)2(chdc)2]·2DMF (2)
The crystalline compound 2, featuring a higher pore volume and more promising expectations in sensing studies, was additionally characterized by routine methods to confirm its chemical composition and phase purity. Its fundamental luminescence properties were also investigated. The phase purity of 2 was confirmed by PXRD (Figure 3a), and its chemical nature and composition were confirmed by CHN analysis (see Experimental). The IR spectrum of 2 (Figure S2) contains typical absorption bands corresponding to C(sp2)–H bond vibrations in the phenanthroline core (~3080 cm−1 [41,42]); C(sp3)–H bond vibrations in methyl groups of both DMF and dmphen (~2940 cm−1 [43]) and in the cyclohexane ring of chdc (~2860 cm−1); amide C=O stretching (~1680 cm−1); and antisymmetric (~1590 cm−1) and symmetric (~1416 cm−1) carboxylate stretching, the latter apparently overlapping with the nitrate NO bond valence vibrations. According to the TGA (Figure 3b), compound 2 loses solvent at T ≈ 130 °C. After that, no weight loss is observed until T ≈ 360 °C, while a decomposition of the coordination lattice apparently starts. Such thermal stability is comparable to other reported lanthanide(III) MOFs based on 4,7-dimethylphenanthroline [43,44].
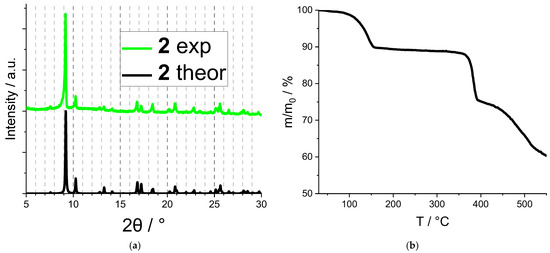
Figure 3.
Experimental PXRD pattern of sample 2 compared to the theoretical one (a). TGA plot of sample 2 (b).
Solid-state luminescent properties were investigated for compound 2, since it features a higher pore volume. The excitation spectrum (Figure 4) contains a very wide multimodal band in the UV region, with a λ shorter than 350 nm, corresponding to the absorption of the π-conjugated organic moiety 4,7-dimethylphenanthroline. Despite this, only Tb3+-centered characteristic emission bands, corresponding to the series of 5D4 → 7FJ (J = 6, 5, 4, 3) [45] transitions, can be found at λmax = 488 nm, 545 nm, 586 nm, and 620 nm in the emission spectrum. Apparently, the dmphen molecule acts as a very effective antenna-type photosensitizer for Tb3+ emission in 2, leaving none of the phen-centered emissions typically expected in the blue region [22,30,46,47]. A photoluminescence quantum yield (QY) for 2 at the abovementioned excitation wavelength was determined as 18.39 ± 0.09%. It is noticeably higher compared to the structurally close compound [Tb2(phen)2(NO3)2(chdc)2]·2DMF [29] based on a non-methylated dmphen analogue, for which a QY = 13.5% has been determined. Such an observation agrees with the known fact that the triplet energy level of 4,7-dmphen is slightly higher than for phen [48], and, therefore, the presence of a methyl substituent increases the efficiency of the energy transfer to the emissive Tb3+ ion closer to the optimal 2500–4000 cm−1 values [49]. Among other reported examples of Tb(III) complexes with 4,7-dimethylphenanthroline, QY values were reported to be 0.34% for [Tb2(dmp)2(ada)3]n·2EtOH·H2O (H2ada = 1,3-adamantanediacetic acid) [43] and 10.30% for [Tb2(dmp)2(H2O)2(PFBA)6]2 (Hpfba = 2,3,4,5,6-pentafluorobenzoic acid) [44]. Such a considerable difference between the measured quantum yields can be explained by the different dimensionalities and modes of packing of the coordination moieties and, partially, by the impact of other strong electron-accepting perfluorinated aromatic acids [50]. The excited state lifetime (τl) for compound 2 was determined as 0.695 ± 0.012 ms. Among other reported examples of Tb(III) complexes with 4,7-dimethylphenanthroline, the examples of measured τl values are 0.59 ms for [Tb2(dmp)2(ada)3]n·2EtOH·H2O [43] and 0.317 ms for [Tb2(dmp)2(H2O)2(PFBA)6]2 [44]. No apparent correlation can be found between the QYs and excited state lifetimes of the examples provided, also implying the impact of different structural factors on these characteristics.
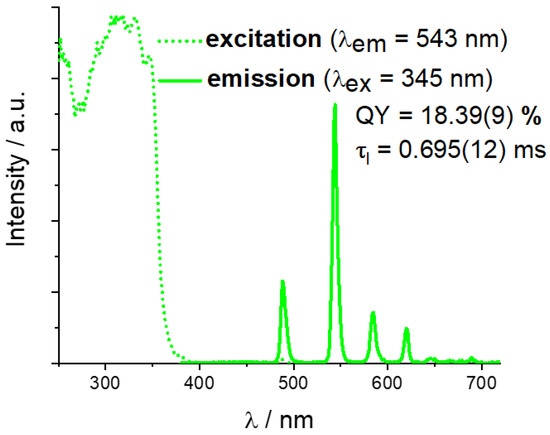
Figure 4.
Solid-state excitation and emission spectra for compound 2.
3.3. Sensing of Nitroaromatic Molecules by [Tb2(dmphen)2(NO3)2(chdc)2]·2DMF (2)
The detection of nitroaromatic compounds is a task that is in demand, since many species of this subclass are well-known explosives [51,52,53]. Along with that, nitroaromatics bear a pronouncedly negative impact on the environment [54], although some widespread examples of related drugs, such as nitrofurazone, nitrofurantoin, and nifurtimox, also exist. MOFs are recognized as promising materials for the luminescent sensing of diverse substrates, including nitro-derivatives, due to their large surface area, structural diversity, and the tunability of both their luminescent characteristics and the nature of their adsorption centers [55,56,57]. The sensing properties of the highly emissive new porous compound 2 were probed using a number of nitroaromatic compounds, namely nitrobenzene (NB), 2-nitrotoluene (2NT), 3-nitrotoluene (3NT), and 4-nitro-m-xylene (NMX), acting as model substrates for nitro-explosives and some common drugs. For the corresponding luminescent titration experiments, the heating in the oven of the synthetic method for 2 was changed to stove-heating with continuous intensive stirring, keeping all other synthetic conditions similar to the initial ones. Such a modification in the preparation technique provided a very thin microcrystalline powder of 2 (see Figure S3), which could form a stable dispersion in DMF for the time required for the luminescence measurements.
As shown on the emission spectra (Figure 5), each of the used nitroaromatic solvents induces a notable gradual quenching of 2 emission upon the incremental addition of analyte at micromolar concentrations. While NB and 3NT give no more than a 50% decrease in the MOF luminescence at concentrations up to 9–10 μM, 2NT and NMX provide a more pronounced quenching with a ca. 3 and 5 times decrease in the emission intensities, respectively.
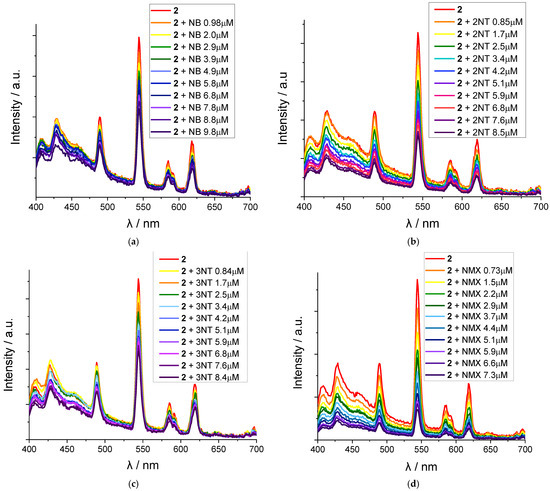
Figure 5.
Emission spectra of 2 dispersion in the presence of analytes with the concentrations shown in the legend: nitrobenzene (a); 2-nitrotoluene (b); 3-nitrotoluene (c); and 4-nitro-m-xylene (d).
To check the luminescent titration data to conform the Stern–Volmer equation and to analyze a possible quenching mechanism, a linearization of the most intensive 2 emission band (λmax ≈ 545 nm) dependency on the analyte concentration was carried out. As shown in Figure 6, the intensity–concentration dependencies shown in the Stern–Volmer coordinates are slightly curved for nitrobenzene and 2-nitrotoluence, suggesting the impact of an inner filter effect (IFE) [58,59] on the observed quenching of the MOF emission. However, UV/vis absorption spectra of the nitroaromatics (Figure S4) show a poor absorption of each analyte at the MOF excitation wavelength (λex = 345 nm), along with their pronounced overlapping with the excitation spectrum (see Figure 4) at higher energies. This fact, in turn, implies that resonance energy transfer (FRET) is important in the quenching process. For 3-nitrotoluene, the Stern–Volmer dependency is linear in the micromolar (up to, at least, 10−5 M) range within the measurement error, while for 4-nitro-m-xylene the similar dependency can be divided into two linear regions with the break near C ≈ 3 × 10−5 M, suggesting some change in the NMX adsorption mechanism at these concentrations. According to the PXRD data (Figure S5), all the guest-exchanged samples, obtained after the immersion of 2 in liquid nitroaromatics, retain the initial porous structure of the 2 network, with no significant drift of the unit cell parameters. IR spectra (Figure S6), containing medium absorption bands at 1520–1528 cm−1 (NO2as stretching) and weaker bands at 1348–1353 cm−1 (NO2s stretching), qualitatively confirm the inclusion of nitroaromatics. According to CHN analysis data, ca. 50% of guest DMF can be substituted by each of these analytes, according to the corresponding decrease in the amount of DMF adsorbed, derived from the experimental data. An adsorption of an additional amount of each guest (ca. 2 molecules per formula unit) into 2 can be attributed to the adsorption of the excess of large nitroaromatic molecules on the surface of the crystallites.
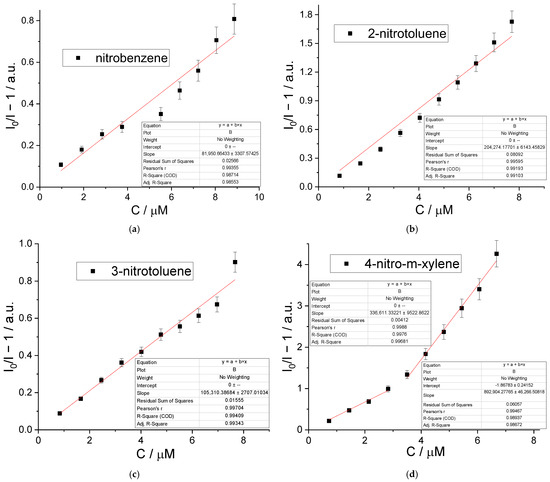
Figure 6.
Stern–Volmer plots for 2 dispersion in the presence of analytes: nitrobenzene (a); 2-nitrotoluene (b); 3-nitrotoluene (c); and 4-nitro-m-xylene (d).
The intensity ratios (I/I0 − 1) vs. the concentrations of nitroaromatics were fitted with the equations I/I0 − 1 = 8.2 × 104∙CNB (R2 = 0.986), I/I0 − 1 = 2.04 × 105∙C2NT (R2 = 0.991), I/I0 − 1 = 1.05 × 105∙C3NT (R2 = 0.993), and I/I0 − 1 = 3.4 × 105∙C4MK (R2 = 0.997). It can be clearly seen that the luminescent titration data for nitrobenzene (NB) and 2-nitrotoluene (2NT) indeed are fitted with lower correlation coefficients compared to 3-nitrotoluene (3NT) and 4-nitro-m-xylene (NMX). Apparently, the linear Stern–Volmer dependency of the 2 emissions on the concentrations of nitroaromatics cannot be determined in the case of NB and 2NT. Nevertheless, these linearization results were also calculated for comparison, showing that such a roughly estimated KSV for 2NT is of the same order of magnitude as for 3NT and NMX, fitting the typical range for other MOF-based nitroaromatics detection systems [56,57]. The hypothetical constant for NB is 1.5–2 orders of magnitude higher than for its methylated derivatives. Such a remarkable difference, along with the poor linearization data for nitrobenzene, can be explained by its smallest molecular size and largest polarity among the analyte selection studied. These properties of the NB molecule can significantly reduce its binding strength to emissive Tb-dmphen blocks, since the largest void volume within a crystal packing of 2 is obviously accessible for the smallest NB molecules. In turn, the incorporation of additional non-polar methyl substituents into the guest analyte can apparently harness its in-pore diffusion due to steric hindrance and provide more well-defined adsorption centers near 4,7-dimethylphenanthroline due to the increase in hydrophobic interactions between the aliphatic moieties.
The data for 3NT and NMX at the concentration ranges up to 8 μM and 3 μM, respectively, are very well fitted by linear Stern–Volmer expression, allowing a limit of detection (LOD) determination as LOD = 3σ/KSV, where σ is a standard deviation of three blank measurements, the reproducibility of which is shown in Figure S7. The calculated LOD values are 0.31 and 0.35 μM for 3NT and NMX, respectively (see also Table S1). Such limits of detection are among the best reported values [60,61,62], only inferior to a few prominent examples from the whole of the lanthanide-based MOFs, such as LOD = 4.14 nM [63] and 13 nM [64] for nitrobenzene. Therefore, compound 2 with an aliphatic decoration of its internal surface can be suggested as an effective sensor for methyl- and, possibly, larger alkyl-substituted nitrobenzenes.
4. Conclusions
In summary, two new Tb(III) metal–organic frameworks based on 4,7-dimethylphenanthroline and flexible ligand trans-1,4-cyclohexcanedicarboxylate were synthesized and characterized. Both compounds were found to have mobile layered structures with significant porosities of the coordination networks, capable of diverse structural rearrangements. A decent luminescence was found for [Tb2(dmphen)2(NO3)2(chdc)2]·2DMF, with a quantum yield of 18.39(9)% and emission lifetime of 0.695(12) ms. A linear dependence of its emission on 3-nitrotoluene and 4-nitro-m-xylene at micromolar concentrations was found during luminescent titration experiments, suggesting this MOF to be a viable and highly sensitive luminescent sensor for such substrates.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst14121026/s1, Figure S1: View along the channels within the packing of 1 coordination layers; Figure S3: PXRD pattern of the 2 microcrystalline powder prepared in dispersion; Figure S4: UV/vis spectra of 10–4 M solutions of individual analytes in DMF; Figure S5: PXRD patterns of the 2NB, 22NT, 23NT and 2NMX samples compared to the theoretical pattern for 2; Figure S6: IR spectra of the 2NB, 22NT, 23NT and 2NMX samples (1200–1600 cm–1 range); Table S1: Stern-Volmer fitting results for 2 sensing tests; Figure S7: Intensities of the strongest Tb3+ emission peak (λem = 544 nm) obtained at repeated measurements.
Author Contributions
Conceptualization, P.A.D. and D.N.D.; methodology, P.A.D. and D.N.D.; validation, P.A.D. and V.P.F.; formal analysis, A.A.R.; investigation, A.A.O. (synthesis, characterization, graphing), A.A.R. (luminescence measurements), P.A.D. (single-crystal XRD); resources, D.N.D. and V.P.F.; data curation, P.A.D. and V.P.F.; writing—original draft preparation, P.A.D. and A.A.O.; writing—review and editing, D.N.D. and V.P.F.; visualization, P.A.D. and A.A.O.; supervision, P.A.D.; project administration, D.N.D.; funding acquisition, D.N.D. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the Russian Science Foundation, project No. 23-13-00310.
Data Availability Statement
The CCDC 2385343 (1) and 2385344 (2) entries contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Center at https://www.ccdc.cam.ac.uk/structures/ (accessed on 25 November 2024).
Conflicts of Interest
There are no conflicts of interest to declare.
Appendix A. Crystal Data

Table A1.
Single-crystal X-ray diffraction and structure refinement details.
Table A1.
Single-crystal X-ray diffraction and structure refinement details.
| Parameter | 1 | 2 |
|---|---|---|
| Chemical formula | C58H72N6O16Tb2 | C50H58N8O16Tb2 |
| Mr, g/mol | 1427.05 | 1344.88 |
| Crystal system | Triclinic, | Monoclinic |
| Space group | P¯1 | P21/c |
| a, Å | 10.4566(4) | 13.3993(4) |
| b, Å | 10.6951(4) | 17.2194(4) |
| c, Å | 14.3877(4) | 13.3169(4) |
| Temperature, K | 291 | 292 |
| α, ° | 109.538(3) | 90 |
| β, ° | 97.979(3) | 119.658(4) |
| γ, ° | 104.222(3) | 90 |
| V, Å3 | 1426.26(9) | 2670.05(16) |
| Z | 1 | 2 |
| D(calc.), g·cm−3 | 1.661 | 1.673 |
| μ, mm−1 | 2.54 | 2.70 |
| F(000) | 720 | 1344 |
| Crystal size, mm | 0.30 × 0.26 × 0.20 | 0.22 × 0.20 × 0.06 |
| θ range for data collection, ° | 2.07 ≤ θ ≤ 25.24 | 2.11 ≤ θ ≤ 25.24 |
| Index ranges | −12 ≤ h ≤ 12; −12 ≤ k ≤ 12; −17 ≤ l ≤ 17 | −16 ≤ h ≤ 16; −19 ≤ k ≤ 20; −16 ≤ l ≤ 16 |
| No. of reflections: measured/independent/observed [I > 2σ(I)] | 16,175/5226/4726 | 24,435/4894/4378 |
| Rint | 0.0379 | 0.0293 |
| Goodness-of-fit on F2 | 1.035 | 1.049 |
| Final R indices [I > 2σ(I)] | R1 = 0.0259; wR2 = 0.0633 | R1 = 0.0192; wR2 = 0.0445 |
| Final R indices [all data] | R1 = 0.0317; wR2 = 0.0655 | R1 = 0.0234; wR2 = 0.0463 |
| Largest diff. peak, hole, e/Å3 | 0.91, −0.54 | 0.71, −0.40 |
References
- Yaghi, O.M.; Jiang, H.; Alezi, D.; Eddaoudi, M. A reticular chemistry guide for the design of periodic solids. Nat. Rev. Mater. 2021, 6, 466–487. [Google Scholar] [CrossRef]
- Ha, J.; Lee, J.H.; Moon, H.R. Alterations to secondary building units of metal–organic frameworks for the development of new functions. Inorg. Chem. Front. 2020, 7, 12–27. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, L.; Ke, T.; Hu, J.; Suo, X.; Cui, X.; Xing, H. Selective sorting of hexane isomers by anion-functionalized metal-organic frameworks with optimal energy regulation. Nat. Commun. 2024, 15, 2620. [Google Scholar] [CrossRef]
- Yuvaraj, A.R.; Jayarama, A.; Sharma, D.; Nagarkar, S.S.; Duttagupta, S.P.; Pinto, R. Role of metal-organic framework in hydrogen gas storage: A critical review. Int. J. Hydrogen Energy 2024, 59, 1434–1458. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Zhu, F.; Liu, J.; Wan, X.; Liu, R.; Liu, X.; Shang, J.-X.; Yu, R.; Feng, Q.; et al. Mg-MOF-74 Derived Defective Framework for Hydrogen Storage at Above-Ambient Temperature Assisted by Pt Catalyst. Adv. Sci. 2024, 11, 2401868. [Google Scholar] [CrossRef]
- Xie, X.-J.; Zeng, H.; Lu, W.; Li, D. Metal–organic frameworks for hydrocarbon separation: Design, progress, and challenges. J. Mater. Chem. A 2023, 11, 20459–20469. [Google Scholar] [CrossRef]
- Firooz, S.K.; Armstrong, D.W. Metal-organic frameworks in separations: A review. Anal. Chim. Acta 2022, 1234, 340208. [Google Scholar] [CrossRef]
- Yang, G.-L.; Jiang, X.-L.; Xu, H.; Zhao, B. Applications of MOFs as Luminescent Sensors for Environmental Pollutants. Small 2021, 17, 2005327. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kataria, R. MOFs as versatile scaffolds to explore environmental contaminants based on their luminescence bustle. Sci. Total Environ. 2024, 926, 172129. [Google Scholar] [CrossRef]
- Khatua, S.; Biswas, P. Flexible Luminescent MOF: Trapping of Less Stable Conformation of Rotational Isomers, In Situ Guest-Responsive Turn-Off and Turn-On Luminescence and Mechanistic Study. ACS Appl. Mater. Interfaces 2020, 12, 22335–22346. [Google Scholar] [CrossRef]
- Blake, A.J.; Champness, N.R.; Hubberstey, P.; Li, W.-S.; Withersby, M.A.; Schröder, M. Inorganic crystal engineering using self-assembly of tailored building-blocks. Coord. Chem. Rev. 1999, 183, 117–138. [Google Scholar] [CrossRef]
- Férey, G. Building Units Design and Scale Chemistry. J. Solid State Chem. 2000, 152, 37–48. [Google Scholar] [CrossRef]
- Millange, F.; Walton, R.I. MIL-53 and its Isoreticular Analogues: A Review of the Chemistry and Structure of a Prototypical Flexible Metal-Organic Framework. Isr. J. Chem. 2018, 58, 1019–1035. [Google Scholar] [CrossRef]
- Winarta, J.; Shan, B.; Mcintyre, S.M.; Ye, L.; Wang, C.; Liu, J.; Mu, B. A Decade of UiO-66 Research: A Historic Review of Dynamic Structure, Synthesis Mechanisms, and Characterization Techniques of an Archetypal Metal–Organic Framework. Cryst. Growth Des. 2020, 20, 1347–1362. [Google Scholar] [CrossRef]
- Mai, Z.; Liu, D. Synthesis and Applications of Isoreticular Metal–Organic Frameworks IRMOFs-n (n = 1, 3, 6, 8). Cryst. Growth Des. 2019, 19, 7439–7462. [Google Scholar] [CrossRef]
- Kitaura, R.; Seki, K.; Akiyama, G.; Kitagawa, S. Porous Coordination-Polymer Crystals with Gated Channels Specific for Supercritical Gases. Angew. Chem. Int. Ed. 2003, 42, 428–431. [Google Scholar] [CrossRef]
- Chun, H.; Dybtsev, D.N.; Kim, H.; Kim, K. Synthesis, X-ray Crystal Structures, and Gas Sorption Properties of Pillared Square Grid Nets Based on Paddle-Wheel Motifs: Implications for Hydrogen Storage in Porous Materials. Chem. Eur. J. 2005, 11, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- Dybtsev, D.N.; Yutkin, M.P.; Samsonenko, D.G.; Fedin, V.P.; Nuzhdin, A.L.; Bezrukov, A.A.; Bryliakov, K.P.; Talsi, E.P.; Belosludov, R.V.; Mizuseki, H.; et al. Modular, Homochiral, Porous Coordination Polymers: Rational Design, Enantioselective Guest Exchange Sorption and Ab Initio Calculations of Host–Guest Interactions. Chem. Eur. J. 2010, 16, 10348–10356. [Google Scholar] [CrossRef]
- Lysova, A.A.; Samsonenko, D.G.; Kovalenko, K.A.; Nizovtsev, A.S.; Dybtsev, D.N.; Fedin, V.P. A Series of Mesoporous Metal-Organic Frameworks with Tunable Windows Sizes and Exceptionally High Ethane over Ethylene Adsorption Selectivity. Angew. Chem. Int. Ed. 2020, 59, 20561–20567. [Google Scholar] [CrossRef]
- Lysova, A.A.; Samsonenko, D.G.; Dorovatovskii, P.V.; Lazarenko, V.A.; Khrustalev, V.N.; Kovalenko, K.A.; Dybtsev, D.N.; Fedin, V.P. Tuning the Molecular and Cationic Affinity in a Series of Multifunctional Metal–Organic Frameworks Based on Dodecanuclear Zn(II) Carboxylate Wheels. J. Am. Chem. Soc. 2019, 141, 17260–17269. [Google Scholar] [CrossRef]
- Manna, K.; Sutter, J.P.; Natarajan, S. Turn-off luminescence sensing, white light emission and magnetic studies of two-dimensional lanthanide MOFs. Dalton Trans. 2023, 52, 18449–18463. [Google Scholar] [CrossRef] [PubMed]
- Demakov, P.A.; Ryadun, A.A.; Dorovatovskii, P.V.; Lazarenko, V.A.; Samsonenko, D.G.; Brylev, K.A.; Fedin, V.P.; Dybtsev, D.N. Intense multi-colored luminescence in a series of rare-earth metal–organic frameworks with aliphatic linkers. Dalton Trans. 2021, 50, 11899–11908. [Google Scholar] [CrossRef] [PubMed]
- Pagis, C.; Ferbinteanu, M.; Rothenberg, G.; Tanase, S. Lanthanide-Based Metal Organic Frameworks: Synthetic Strategies and Catalytic Applications. ACS Catal. 2016, 6, 6063–6072. [Google Scholar] [CrossRef]
- Luo, A.-Y.; Lan, B.-L.; Shao, B.; Lu, X.-M.; Lan, Y.-F.; Liao, Y.-Z.; Zhang, Z. A 2D mixed-lanthanide metal–organic framework as dual-emitting luminescent sensor for ratiometric detection of tetracycline and nitrophenols. J. Mol. Struct. 2024, 1295, 136734. [Google Scholar] [CrossRef]
- Wang, S.; Macreadie, L.K.; Hanton, L.R. Pillared lanthanide metal organic frameworks with sinusoidal channels formed from bent mixed-donor phenanthroline based ligands of different length. CrystEngComm 2024, 26, 5541–5549. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Lu, L.-P.; Zhu, M.-L.; Si-Si Feng, S.-S. Self-assembly of lanthanide(iii) coordination polymers from a bifunctional 2-(pyridin-2-yl)-1H-imidazole-4,5-dicarboxylate ligand with the assistance of oxalate: Syntheses, structures, luminescence, and magnetic properties. CrystEngComm 2017, 19, 1953–1964. [Google Scholar] [CrossRef]
- Janicki, R.; Mondry, A.; Starynowicz, P. Carboxylates of rare earth elements. Coord. Chem. Rev. 2017, 340, 98–133. [Google Scholar] [CrossRef]
- Gorai, T.; Schmitt, W.; Gunnlaugsson, T. Highlights of the development and application of luminescent lanthanide based coordination polymers, MOFs and functional nanomaterials. Dalton Trans. 2021, 50, 770–784. [Google Scholar] [CrossRef]
- Demakov, P.A.; Vasileva, A.A.; Volynkin, S.S.; Ryadun, A.A.; Samsonenko, D.G.; Fedin, V.P.; Dybtsev, D.N. Cinnamal Sensing and Luminescence Color Tuning in a Series of Rare-Earth Metal−Organic Frameworks with Trans-1,4-cyclohexanedicarboxylate. Molecules 2021, 26, 5145. [Google Scholar] [CrossRef]
- Demakov, P.A.; Ryadun, A.A.; Fedin, V.P. Aliphatic-Bridged Early Lanthanide Metal–Organic Frameworks: Topological Polymorphism and Excitation-Dependent Luminescence. Inorganics 2022, 10, 163. [Google Scholar] [CrossRef]
- CrysAlisPro 1.171.38.46. Rigaku Oxford Diffraction: The Woodlands, TX, USA, 2015. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 13 October 2023).
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Glasby, L.T.; Cordiner, J.L.; Cole, J.C.; Moghadam, P.Z. Topological Characterization of Metal–Organic Frameworks: A Perspective. Chem. Mater. 2024, 36, 9013–9030. [Google Scholar] [CrossRef] [PubMed]
- Noa, F.M.A.; Abrahamsson, M.; Ahlberg, E.; Cheung, O.; Göb, C.R.; Christine JMcKenzie, C.J.; Öhrström, L. A unified topology approach to dot-, rod-, and sheet-MOFs. Chem 2021, 7, 2491–2512. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUUEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. 2015, 71, 9–18. [Google Scholar] [CrossRef]
- He, H.; Sun, Q.; Gao, W.; Perman, J.A.; Sun, F.; Zhu, G.; Aguila, B.; Forrest, K.; Space, B.; Ma, S. A Stable Metal–Organic Framework Featuring a Local Buffer Environment for Carbon Dioxide Fixation. Angew. Chem. Int. Ed. 2018, 57, 4657–4662. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, Y.; Wang, W.; Yang, H.; Hong, A.N.; Bu, X.; Feng, P. Simultaneous Control of Flexibility and Rigidity in Pore-Space-Partitioned Metal–Organic Frameworks. J. Am. Chem. Soc. 2023, 145, 10980–10986. [Google Scholar] [CrossRef]
- Demakov, P.A. Properties of Aliphatic Ligand-Based Metal–Organic Frameworks. Polymers 2023, 15, 2891. [Google Scholar] [CrossRef]
- Murdock, C.R.; Hughes, B.C.; Lu, Z.; Jenkins, D.M. Approaches for synthesizing breathing MOFs by exploiting dimensional rigidity. Coord. Chem. Rev. 2014, 258–259, 119–136. [Google Scholar] [CrossRef]
- Xiaobo Yu, X.; Chang, W.; Cai, Z.; Cilin Yu, C.; Lai, L.; Zhou, Z.; Li, P.; Yang, Y.; Zeng, C. Hg2+ detection and information encryption of new [1+1] lanthanide cluster. Talanta 2024, 266, 125105. [Google Scholar] [CrossRef]
- Saleh, M.I.; Choo, M.Y.; Chan, T.W.; Razali, M.R. Effect of second ligand on the luminescence of Samarium (III) dibenzoylmethane complexes: Syntheses, crystal structures, thermal analysis and luminescence study. J. Chem. Sci. 2015, 127, 2241–2249. [Google Scholar] [CrossRef]
- Zhao, Z.-P.; Zheng, K.; Li, H.-R.; Zeng, C.-H.; Zhong, S.; Weng Ng, S.; Zheng, Y.; Chen, Y. Structure variation and luminescence of 3D, 2D and 1D lanthanide coordination polymers with 1,3-adamantanediacetic acid. Inorg. Chim. Acta 2018, 482, 340–346. [Google Scholar] [CrossRef]
- Yu, X.; Chang, W.; Zhang, H.; Cai, Z.; Yang, Y.; Zeng, C. Visual and Real-Time Monitoring of Cd2+ in Water, Rice, and Rice Soil with Test Paper Based on [2 + 2] Lanthanide Clusters. Inorg. Chem. 2023, 62, 6387–6396. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, C.; Drozdov, A.; Belousov, Y. Coordination Compounds of Lanthanides as Materials for Luminescent Turn Off Sensors. In Rare Earth Elements—Emerging Advances, Technology Utilization, and Resource Procurement; IntechOpen: London, UK, 2022; Chapter 1; pp. 1–31. [Google Scholar]
- Bencini, A.; Lippolis, V. 1,10-Phenanthroline: A versatile building block for the construction of ligands for various purposes. Coord. Chem. Rev. 2010, 254, 2096–2180. [Google Scholar] [CrossRef]
- Sanzhenakova, E.A.; Smirnova, K.S.; Pozdnyakov, I.P.; Lider, E.V. Structural Features and Photoluminescence of Coordination Compounds Obtained in the Lanthanide(III) Acetate–1,10-Phenanthroline System in the Presence of 5-Phenyltetrazole. J. Struct. Chem. 2024, 65, 786–797. [Google Scholar] [CrossRef]
- Klein, A.; McInnes, E.J.L.; Kaim, W. Organometallic platinum(II) complexes of methyl-substituted phenanthrolines. J. Chem. Soc. Dalton Trans. 2002, 2371–2378. [Google Scholar] [CrossRef]
- Latva, M.; Takalo, H.; Mukkala, V.-M.; Matachescu, C.; Rodríguez-Ubis, J.C.; Kankare, J. Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield. J. Lumin. 1997, 75, 149–169. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Chistyakov, A.S.; Razgonyaeva, G.A.; Voronina, J.K.; Varaksina, E.A.; Taydakov, I.V.; Sidorov, A.A.; Eremenko, I.L. Synthesis, Structure, and Photoluminescent Properties of Zn2+, Mn2+, Cd2+, Eu3+, and Tb3+ Complexes with 4-Allyl-2,3,5,6-Tetrafluorobenzoic Acid Anions and 1,10-Phenanthroline. J. Struct. Chem. 2024, 65, 362–380. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.; Li, J. Luminescent Metal–Organic Frameworks for Chemical Sensing and Explosive Detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef]
- Gole, B.; Bar, A.K.; Mukherjee, P.S. Multicomponent Assembly of Fluorescent-Tag Functionalized Ligands in Metal–Organic Frameworks for Sensing Explosives. Chem. Eur. J. 2014, 20, 13321–13336. [Google Scholar] [CrossRef]
- Sohn, H.; Sailor, M.J.; Magde, D.; Trogler, W.C. Detection of Nitroaromatic Explosives Based on Photoluminescent Polymers Containing Metalloles. J. Am. Chem. Soc. 2003, 125, 3821–3830. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Kang, D.W. Hazardous nitroaromatic explosives detection by emerging porous solid sensors. Coord. Chem. Rev. 2023, 492, 215279. [Google Scholar] [CrossRef]
- Haj-Yahya, A.; Kouskouki, D.; Margellou, A.G.; Andreou, E.K.; Armatas, G.S.; Lazarides, T. Functionalised Al(III) metal organic frameworks for fluorescence sensing of nitroaromatic vapours. J. Mater. Chem. C 2024, 12, 8014–8023. [Google Scholar] [CrossRef]
- Zhao, Y.-W.; Xue, B.; Zhang, N.; Guo, L.E.; Zhu, S.-Y.; Zhang, X.-M. Single-component rare-earth-free white light-emitting metal–organic framework towards nitroaromatic explosive sensing and dye adsorption. Inorg. Chem. Front. 2024, 11, 4826–4834. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, X.; Liu, J.; Dong, Z.; Wang, F.; Wang, X. White-Light-Emitting Ln-MOF as Luminescent Probe for Selective Detection of Nitroaromatic Compounds. Z. Anorg. Allg. Chem. 2024, 650, e202400084. [Google Scholar] [CrossRef]
- Panigrahi, S.K.; Mishra, A.K. Inner filter effect in fluorescence spectroscopy: As a problem and as a solution. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100318. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y.-L.; Wang, J.-H. Inner filter effect-based fluorescent sensing systems: A review. Anal. Chim. Acta 2018, 999, 13–26. [Google Scholar] [CrossRef]
- Sathiyan, G.; Venkatesan, G.; Ramasamy, S.K.; Lee, J.; Barathi, S. Recent progress in triazine-based fluorescent probes for detecting hazardous nitroaromatic compounds. J. Environ. Chem. Eng. 2024, 12, 112804. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, F.; Zhou, J.; Zhao, X. Luminescent Metal-Organic Frameworks for Nitroaromatic Compounds Detection. Comments Inorg. Chem. 2021, 41, 100–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Day, G.; Wang, X.; Yang, X.; Zhou, H.-C. Luminescent sensors based on metal-organic frameworks. Coord. Chem. Rev. 2018, 354, 28–45. [Google Scholar] [CrossRef]
- Chen, X.-L.; Shang, L.; Liu, L.; Yang, H.; Cui, H.-L.; Wang, J.-J. A highly sensitive and multi-responsive Tb-MOF fluorescent sensor for the detection of Pb2+, Cr2O72−, B4O72−, aniline, nitrobenzene and cefixime. Dyes Pigm. 2021, 196, 109809. [Google Scholar] [CrossRef]
- Guo, H.; Wu, N.; Xue, R.; Liu, H.; Li, L.; Wang, M.-Y.; Yao, W.-Q.; Li, Q.; Yang, W. Multifunctional Ln-MOF luminescent probe displaying superior capabilities for highly selective sensing of Fe3+ and Al3+ ions and nitrotoluene. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124094. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).