Boosting the Mechanical and Thermal Properties of CUG-1A Lunar Regolith Simulant by Spark Plasma Sintering
Abstract
1. Introduction
2. Materials and Methods
2.1. Melting of CUG-1A Lunar Regolith Simulant in Vacuum
2.2. Spark Plasma Sintering (SPS) of CUG-1A Lunar Regolith Simulant
2.3. Characterization
2.4. Mechanical Property Measurements
2.5. Thermal Property Measurements
3. Results and Discussion
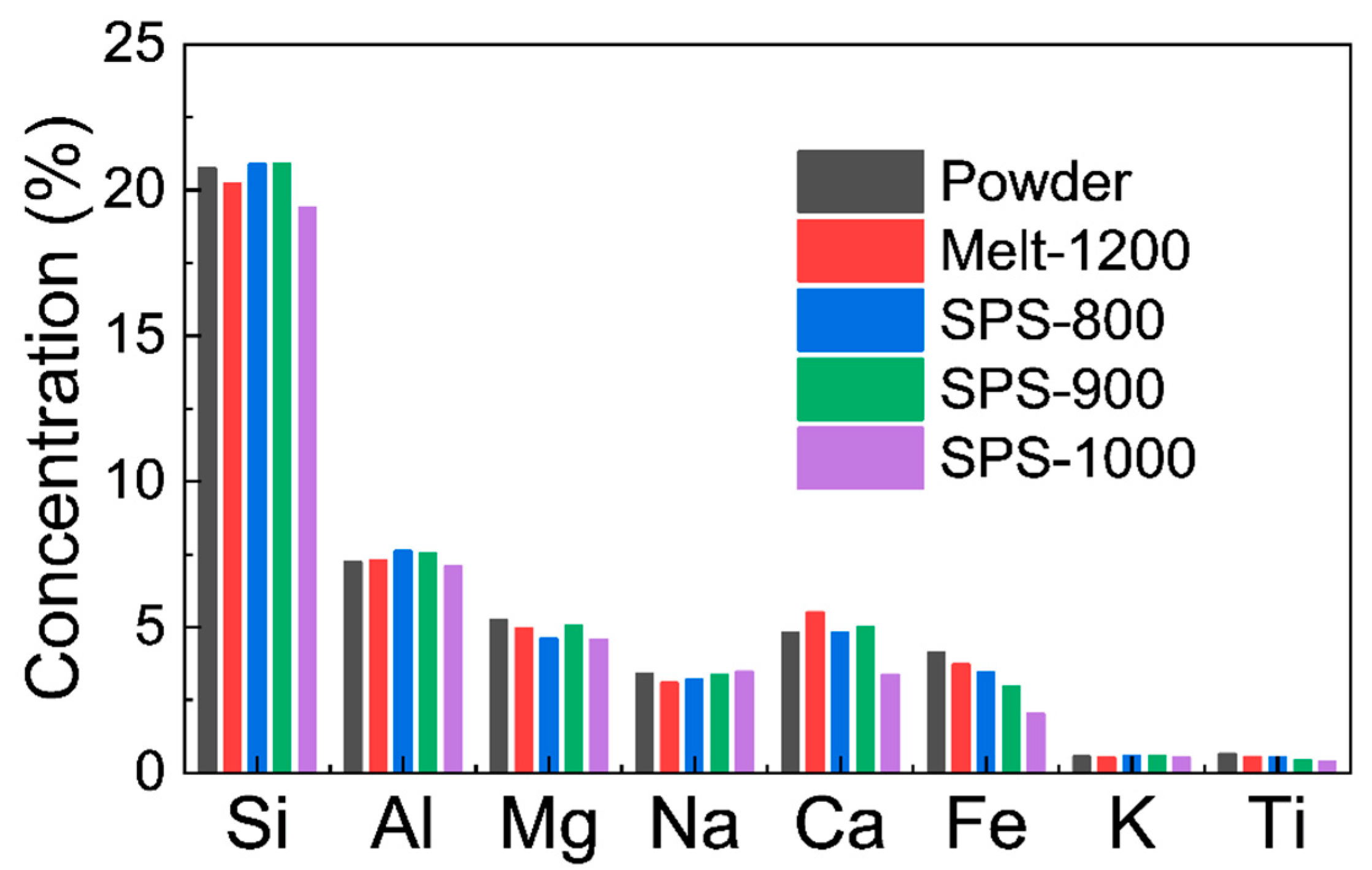
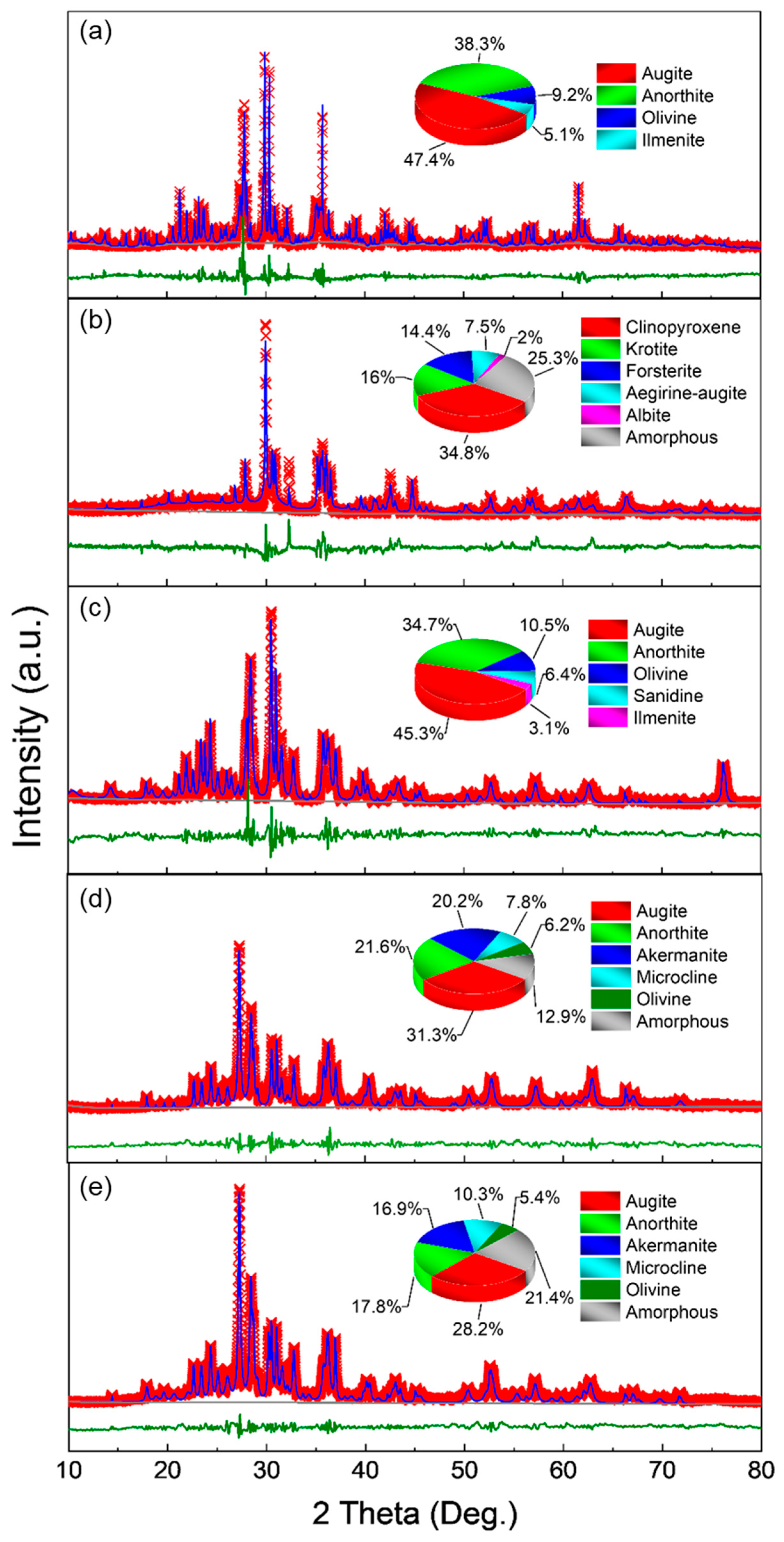
3.1. Morphology and Composition
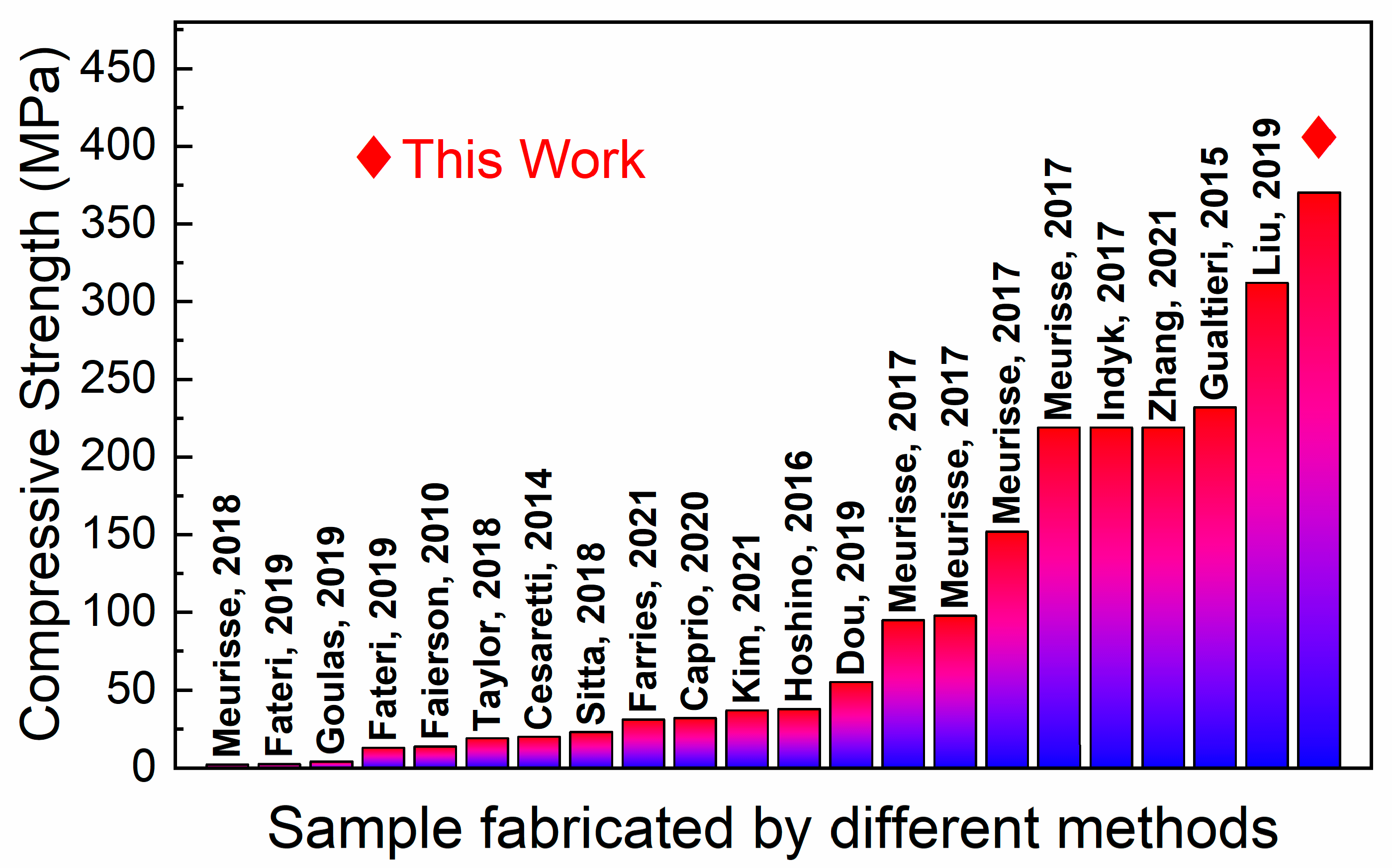
3.2. Mechanical Properties
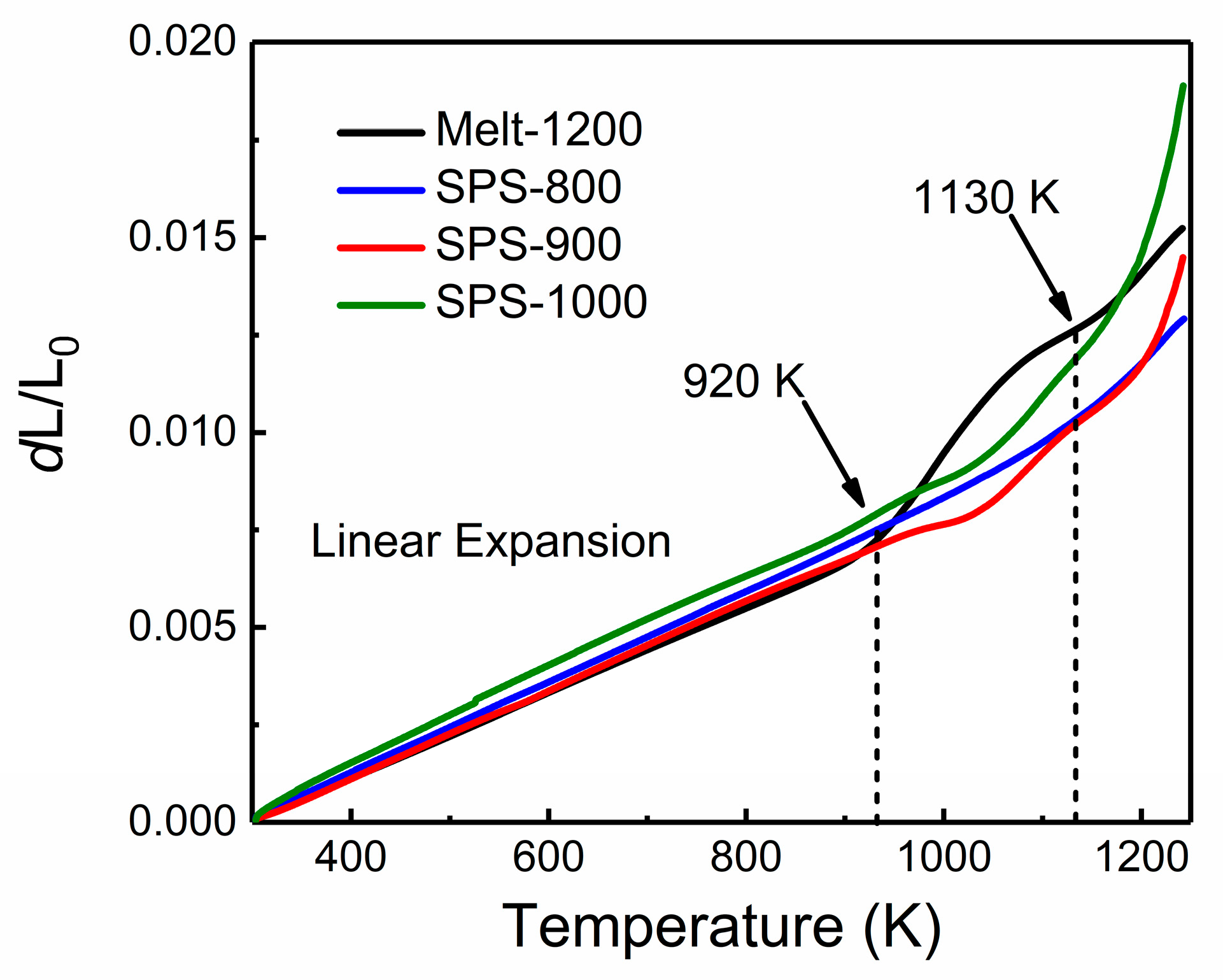
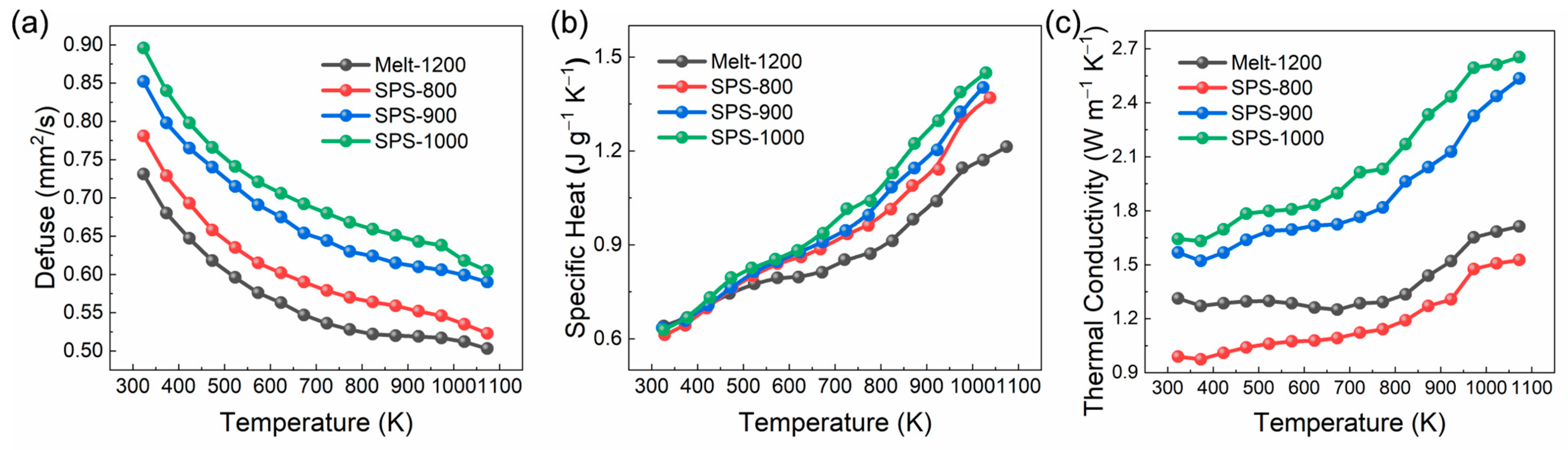
3.3. Thermal Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ruess, F.; Schaenzlin, J.; Benaroya, H. Structural Design of a Lunar Habitat. J. Aerosp. Eng. 2006, 19, 133–157. [Google Scholar] [CrossRef]
- Dou, R.; Tang, W.Z.; Wang, L.; Li, S.; Duan, W.Y.; Liu, M.; Zhang, Y.B.; Wang, G. Sintering of lunar regolith structures fabricated via digital light processing. Ceram. Int. 2019, 45, 17210–17215. [Google Scholar] [CrossRef]
- Liu, M.; Tang, W.; Duan, W.; Li, S.; Dou, R.; Wang, G.; Liu, B.; Wang, L. Digital light processing of lunar regolith structures with high mechanical properties. Ceram. Int. 2019, 45, 5829–5836. [Google Scholar] [CrossRef]
- Zocca, A.; Fateri, M.; Al-Sabbagh, D.; Günster, J. Investigation of the sintering and melting of JSC-2A lunar regolith simulant. Ceram. Int. 2020, 46, 14097–14104. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Ryu, B.H.; Jin, H.; Lee, J.; Shin, H.-S. Microstructural, mechanical, and thermal properties of microwave-sintered KLS-1 lunar regolith simulant. Ceram. Int. 2021, 47, 26891–26897. [Google Scholar] [CrossRef]
- Duke, M.B.; Blair, B.R.; Diaz, J. Lunar resource utilization: Implications for commerce and exploration. Adv. Space Res. 2003, 31, 2413–2419. [Google Scholar] [CrossRef]
- Farries, K.W.; Visintin, P.; Smith, S.T.; Eyk, P.V. Sintered or melted regolith for lunar construction: State-of-the-art review and future research directions. Constr. Build. Mater. 2021, 296, 123627. [Google Scholar] [CrossRef]
- Fateri, M.; Sottong, R.; Kolbe, M.; Gamer, J.; Sperl, M.; Cowley, A. Thermal properties of processed lunar regolith simulant. Int. J. Appl. Ceram. Technol. 2019, 16, 2419–2428. [Google Scholar] [CrossRef]
- Bonanno, A.; Bernold, L.E. Exploratory Review of Sintered Lunar Soil Based on the Results of the Thermal Analysis of a Lunar Soil Simulant. J. Aerosp. Eng. 2015, 28, 04014114. [Google Scholar] [CrossRef]
- Wilhelm, S.; Curbach, M. Review of possible mineral materials and production techniques for a building material on the moon. Struct. Concr. 2014, 15, 419–428. [Google Scholar] [CrossRef]
- Hasnain, S.M. Review on sustainable thermal energy storage technologies, Part I: Heat storage materials and techniques. Energy Convers. Manag. 1998, 39, 1127–1138. [Google Scholar] [CrossRef]
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renewable Sustainable Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Lin, T.; Love, H.; Stark, D. Physical properties of concrete made with Apollo 16 lunar soil sample. In Proceedings of the Second Conference on Lunar Bases and Space Activities of the 21st Century; NASA: Houston, TX, USA, 1992; p. 483. [Google Scholar]
- Leach, N.; Carlson, A.; Khoshnevis, B.; Thangavelu, M. Robotic Construction by Contour Crafting: The Case of Lunar Construction. Int. J. Archit. Comput. 2012, 10, 423–438. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, J.; Zahiri, B.; Khoshnevis, B. Performance of Sulfur Concrete in Planetary Applications of Contour Crafting, in International Solid Freeform Fabrication Symposium. In Proceedings of the Addictive Manufacturing Conference, Chicago, IL, USA, 13–14 September 2016; pp. 2282–2294. [Google Scholar]
- Gosau, J.-M. Regolith Stabilization and Building Materials for the Lunar Surface. In Earth and Space; ASCE: Reston, VA, USA, 2012; pp. 243–249. [Google Scholar]
- Mueller, R.P.; Gelino, N.J.; Smith, J.D.; Buckles, B.C.; Lippitt, T.; Schuler, J.M.; Nick, A.J.; Nugent, M.W.; Townsend, I.I. Zero Launch Mass Three-Dimensional Print Head. In Earth and Space; ASCE: Reston, VA, USA, 2018; pp. 219–232. [Google Scholar]
- Li, H.; Zhao, W.; Wu, X.; Tang, H.; Li, Q.; Tan, J.; Wang, G. 3D Printing and Solvent Dissolution Recycling of Polylactide–Lunar Regolith Composites by Material Extrusion Approach. Polymers 2020, 12, 1724. [Google Scholar] [CrossRef]
- Buchner, C.; Pawelke, R.H.; Schlauf, T.; Reissner, A.; Makaya, A. A new planetary structure fabrication process using phosphoric acid. Acta Astronaut. 2018, 143, 272–284. [Google Scholar] [CrossRef]
- Song, L.; Xu, J.; Fan, S.; Tang, H.; Li, X.; Liu, J.; Duan, X. Vacuum sintered lunar regolith simulant: Pore-forming and thermal conductivity. Ceram. Int. 2019, 45, 3627–3633. [Google Scholar] [CrossRef]
- Indyk, S.J.; Benaroya, H. A structural assessment of unrefined sintered lunar regolith simulant. Acta Astronaut. 2017, 140, 517–536. [Google Scholar] [CrossRef]
- Meurisse, A.; Beltzung, J.C.; Kolbe, M.; Cowley, A.; Sperl, M. Influence of Mineral Composition on Sintering Lunar Regolith. J. Aerosp. Eng. 2017, 30, 04017014. [Google Scholar] [CrossRef]
- Hoshino, T.; Wakabayashi, S.; Yoshihara, S.; Hatanaka, N. Key Technology Development for Future Lunar Utilization—Block Production Using Lunar Regolith. Trans. Jpn. Soc. Aeronaut. Space Sci. 2016, 14, 35–40. [Google Scholar] [CrossRef]
- Gualtieri, T.; Bandyopadhyay, A. Compressive deformation of porous lunar regolith. Mater. Lett. 2015, 143, 276–278. [Google Scholar] [CrossRef]
- Taylor, L.A.; Meek, T.T. Microwave Sintering of Lunar Soil: Properties, Theory, and Practice. J. Aerosp. Eng. 2005, 18, 188–196. [Google Scholar] [CrossRef]
- Allan, S.M.; Merritt, B.J.; Griffin, B.F.; Hintze, P.E.; Shulman, H.S. High-Temperature Microwave Dielectric Properties and Processing of JSC-1AC Lunar Simulant. J. Aerosp. Eng. 2013, 26, 874–881. [Google Scholar] [CrossRef]
- Fateri, M.; Cowley, A.; Kolbe, M.; Garcia, O.; Sperl, M.; Cristoforetti, S. Localized Microwave Thermal Posttreatment of Sintered Samples of Lunar Simulant. J. Aerosp. Eng. 2019, 32, 04019051. [Google Scholar] [CrossRef]
- Nakamura, T.; Smith, B. Solar thermal system for lunar ISRU applications: Development and field operation at Mauna Kea, HI. In Proceedings of the 49th AIAA Aerospace Sciences Meeting including the New Horizons Forum and Aerospace Exposition, Orlando, FL, USA, 4–7 January 2011; p. 433. [Google Scholar]
- Hintze, P.E.; Quintana, S. Building a Lunar or Martian Launch Pad with In Situ Materials: Recent Laboratory and Field Studies. J. Aerosp. Eng. 2013, 26, 134–142. [Google Scholar] [CrossRef]
- Meurisse, A.; Makaya, A.; Willsch, C.; Sperl, M. Solar 3D printing of lunar regolith. Acta Astronaut. 2018, 152, 800–810. [Google Scholar] [CrossRef]
- Fateri, M.; Meurisse, A.; Sperl, M.; Urbina, D.; Madakashira, H.K.; Govindaraj, S.; Gancet, J.; Imhof, B.; Hoheneder, W.; Waclavicek, R.; et al. Solar Sintering for Lunar Additive Manufacturing. J. Aerosp. Eng. 2019, 32, 04019101. [Google Scholar] [CrossRef]
- Balla, V.K.; Roberson, L.B.; O’Connor, G.W.; Trigwell, S.; Bose, S.; Bandyopadhyay, A. First demonstration on direct laser fabrication of lunar regolith parts. Rapid Prototyp. J. 2012, 18, 451–457. [Google Scholar] [CrossRef]
- Goulas, A.; Harris, R.A.; Friel, R.J. Additive manufacturing of physical assets by using ceramic multicomponent extra-terrestrial materials. Addit. Manuf. 2016, 10, 36–42. [Google Scholar] [CrossRef]
- Fateri, M.; Gebhardt, A. Process Parameters Development of Selective Laser Melting of Lunar Regolith for On-Site Manufacturing Applications. Int. J. Appl. Ceram. Technol. 2015, 12, 46–52. [Google Scholar] [CrossRef]
- Sitta, L.A.; Lavagna, M. 3D printing of Moon highlands regolith simulant. In Proceedings of the 69th International Astronautical Congress (IAC 2018), Bremen, Germany, 1–5 October 2018; pp. 1–7. [Google Scholar]
- Goulas, A.; Binner, J.G.; Engstrøm, D.S.; Harris, R.A.; Friel, R.J. Mechanical behaviour of additively manufactured lunar regolith simulant components. Proc. Inst. Mech. Eng. Part L 2019, 233, 1629–1644. [Google Scholar] [CrossRef]
- Caprio, L.; Demir, A.G.; Previtali, B.; Colosimo, B.M. Determining the feasible conditions for processing lunar regolith simulant via laser powder bed fusion. Addit. Manuf. 2020, 32, 101029. [Google Scholar] [CrossRef]
- Zhang, X.; Khedmati, M.; Kim, Y.-R.; Shin, H.-S.; Lee, J.; Kim, Y.-J.; Cui, B. Microstructure evolution during spark plasma sintering of FJS-1 lunar soil simulant. J. Am. Ceram. Soc. 2020, 103, 899–911. [Google Scholar] [CrossRef]
- Phuah, X.L.; Wang, H.; Zhang, B.; Cho, J.; Zhang, X.; Wang, H. Ceramic Material Processing Towards Future Space Habitat: Electric Current-Assisted Sintering of Lunar Regolith Simulant. Materials 2020, 13, 4128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gholami, S.; Khedmati, M.; Cui, B.; Kim, Y.-R.; Kim, Y.-J.; Shin, H.-S.; Lee, J. Spark plasma sintering of a lunar regolith simulant: Effects of parameters on microstructure evolution, phase transformation, and mechanical properties. Ceram. Int. 2021, 47, 5209–5220. [Google Scholar] [CrossRef]
- Shichalin, O.; Belov, A.; Buravlev, I.; Kolodeznikov, E.; Fedorets, A.; Lembikov, A.; Zolotnikov, S.; Maiorov, V.; Nozdrachev, E.; Ruslan, A.; et al. Additive manufacturing development of construction materials for a lunar base via spark plasma sintering of volcanic rocks using in-situ resource utilization concept. Constr. Build. Mater. 2024, 442, 137553. [Google Scholar] [CrossRef]
- Militky, J.; Kovacic, V. Ultimate Mechanical Properties of Basalt Filaments. Text. Res. J. 1996, 66, 225–229. [Google Scholar] [CrossRef]
- Schultz, R.A. Limits on strength and deformation properties of jointed basaltic rock masses. Rock Mech. Rock Eng. 1995, 28, 1–15. [Google Scholar] [CrossRef]
- Xin-xinga, H.; Longa, X.; Juna, H.; Qia, H.; Ruia, G.; Ganga, Y. Lunar soil simulant development and Lunar soil simulant CUG-1A. Geol. Sci. Technol. Inf. 2011, 30, 137–142. [Google Scholar]
- Qian, Y.; Xiao, L.; Yin, S.; Zhang, M.; Head, J.W. The regolith properties of the Chang’e-5 landing region and the ground drilling experiments using lunar regolith simulants. Icarus 2019, 337, 113508. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Wang, C.; Gao, T.; Wang, C.; Wang, Q.; Gu, J.; Chen, X.; Yao, W. Melting properties of lunar regolith simulant for in-situ construction. Adv. Space Res. 2024; in press. [Google Scholar] [CrossRef]
- Keszthelyi, L.; Self, S. Some physical requirements for the emplacement of long basaltic lava flows. J. Geophys. Res. Solid Earth 1998, 103, 27447–27464. [Google Scholar] [CrossRef]
- Harris, A.J.L.; Allen, J.S., III. One-, two- and three-phase viscosity treatments for basaltic lava flows. J. Geophys. Res. Solid Earth 2008, 113, B09212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Zhang, G.; Dong, K.; Deng, X.; Gao, X.; Yang, Y.; Xiao, Y.; Bai, X.; Liang, K.; et al. Size, morphology, and composition of lunar samples returned by Chang’E-5 mission. Sci. China Phys. Mech. Astron. 2021, 65, 229511. [Google Scholar] [CrossRef]
- Li, C.; Hu, H.; Yang, M.-F.; Pei, Z.-Y.; Zhou, Q.; Ren, X.; Liu, B.; Liu, D.; Zeng, X.; Zhang, G.; et al. Characteristics of the lunar samples returned by the Chang’E-5 mission. Natl. Sci. Rev. 2022, 9, nwab188. [Google Scholar] [CrossRef]
- Faierson, E.J.; Logan, K.V.; Stewart, B.K.; Hunt, M.P. Demonstration of concept for fabrication of lunar physical assets utilizing lunar regolith simulant and a geothermite reaction. Acta Astronaut. 2010, 67, 38–45. [Google Scholar] [CrossRef]
- Taylor, S.L.; Jakus, A.E.; Koube, K.D.; Ibeh, A.J.; Geisendorfer, N.R.; Shah, R.N.; Dunand, D.C. Sintering of micro-trusses created by extrusion-3D-printing of lunar regolith inks. Acta Astronaut. 2018, 143, 1–8. [Google Scholar] [CrossRef]
- Cesaretti, G.; Dini, E.; De Kestelier, X.; Colla, V.; Pambaguian, L. Building components for an outpost on the Lunar soil by means of a novel 3D printing technology. Acta Astronaut. 2014, 93, 430–450. [Google Scholar] [CrossRef]
- Ray, C.S.; Reis, S.T.; Sen, S.; O’Dell, J.S. JSC-1A lunar soil simulant: Characterization, glass formation, and selected glass properties. J. Non-Cryst. Solids 2010, 356, 2369–2374. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Zhao, S.; Song, J.; Yao, W.; Wang, W.; Zou, Z.; Yang, M. Melting and Rapid Solidification of Lunar Regolith Particles Returned by Chang’E-5 Mission. Research 2024, 7, 0486. [Google Scholar] [CrossRef]
- Schreiner, S.S.; Dominguez, J.A.; Sibille, L.; Hoffman, J.A. Thermophysical property models for lunar regolith. Adv. Space Res. 2016, 57, 1209–1222. [Google Scholar] [CrossRef]
- Sakatani, N.; Ogawa, K.; Iijima, Y.-I.; Honda, R.; Tanaka, S. Experimental study for thermal conductivity structure of lunar surface regolith: Effect of compressional stress. Icarus 2012, 221, 1180–1182. [Google Scholar] [CrossRef]
- Sakatani, N.; Ogawa, K.; Iijima, Y.-I.; Arakawa, M.; Tanaka, S. Compressional stress effect on thermal conductivity of powdered materials: Measurements and their implication to lunar regolith. Icarus 2016, 267, 1–11. [Google Scholar] [CrossRef]

| SiO2 | Al2O3 | FeO | CaO | MgO | TiO2 | K2O | P2O5 | Na2O | MnO | LOI | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CUG-1A | 48.32 | 16.01 | 12.5 | 7.39 | 6.95 | 2.38 | 2.12 | 0.54 | 0.19 | 0.15 | 0.19 | 99.8 |
| Sample | Average Particle Density (g/cm3) | Density (g/cm3) | Porosity (%) | Compressive Strength (MPa) | Flexural Strength (MPa) | Mohs Hardness |
|---|---|---|---|---|---|---|
| Melt-1200 | 2.94 | 2.81 | 4.5 | 180.5 ± 9.5 | 35.3 ± 12.5 | 6 |
| SPS-800 | 2.08 | 29.3 | 80.2 ± 10.8 | 13.5 ± 6.8 | 3 | |
| SPS-900 | 2.90 | 1.4 | 210.5 ± 12.7 | 48.1 ± 15.3 | 6 | |
| SPS-1000 | 2.93 | 0.3 | 370.2 ± 22.3 | 81.4 ± 17.6 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, X.; Chen, X.; Wang, C.; Yu, Y.; Jia, Y.; Yao, W. Boosting the Mechanical and Thermal Properties of CUG-1A Lunar Regolith Simulant by Spark Plasma Sintering. Crystals 2024, 14, 1022. https://doi.org/10.3390/cryst14121022
Liu Y, Zhang X, Chen X, Wang C, Yu Y, Jia Y, Yao W. Boosting the Mechanical and Thermal Properties of CUG-1A Lunar Regolith Simulant by Spark Plasma Sintering. Crystals. 2024; 14(12):1022. https://doi.org/10.3390/cryst14121022
Chicago/Turabian StyleLiu, Yiwei, Xian Zhang, Xiong Chen, Chao Wang, Yaolun Yu, Yi Jia, and Wei Yao. 2024. "Boosting the Mechanical and Thermal Properties of CUG-1A Lunar Regolith Simulant by Spark Plasma Sintering" Crystals 14, no. 12: 1022. https://doi.org/10.3390/cryst14121022
APA StyleLiu, Y., Zhang, X., Chen, X., Wang, C., Yu, Y., Jia, Y., & Yao, W. (2024). Boosting the Mechanical and Thermal Properties of CUG-1A Lunar Regolith Simulant by Spark Plasma Sintering. Crystals, 14(12), 1022. https://doi.org/10.3390/cryst14121022






