Pressure-Induced Structural Phase Transition and Fluorescence Enhancement of Double Perovskite Material Cs2NaHoCl6
Abstract
1. Introduction
2. Materials and Methods
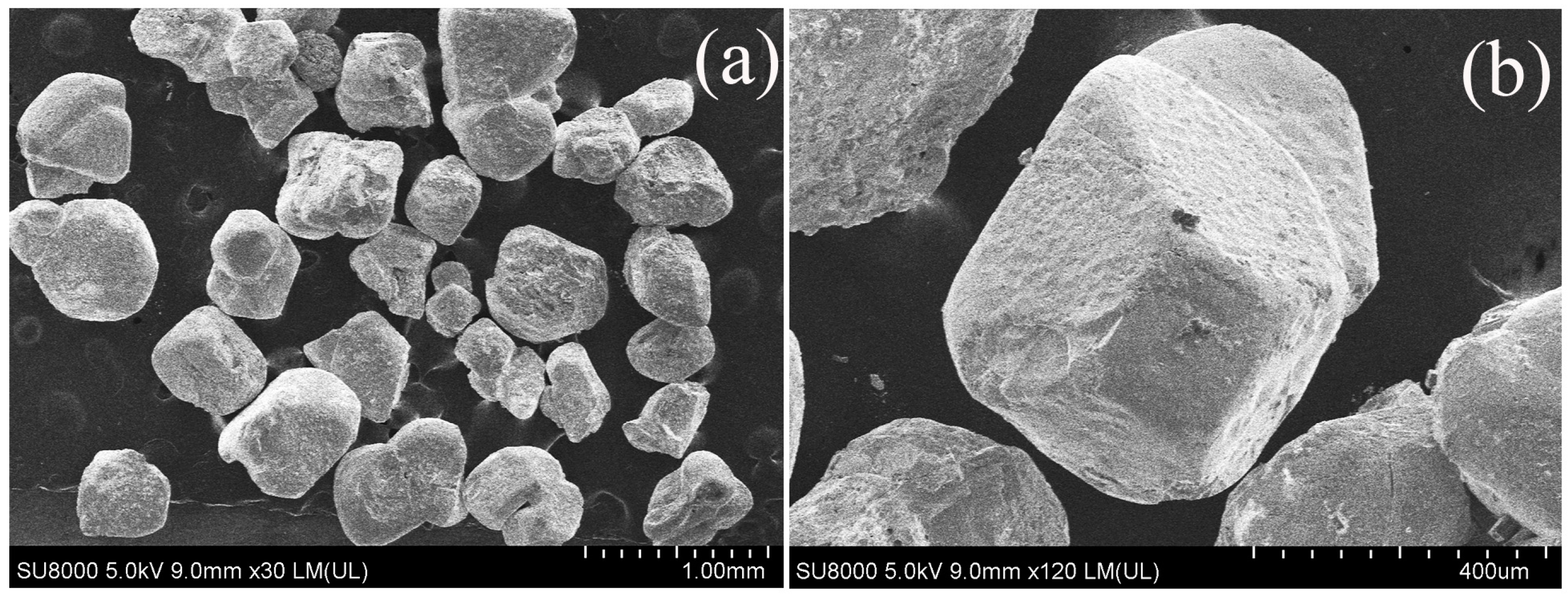
2.1. Sample Preparation
2.2. Experimental Setup
2.3. Experiments
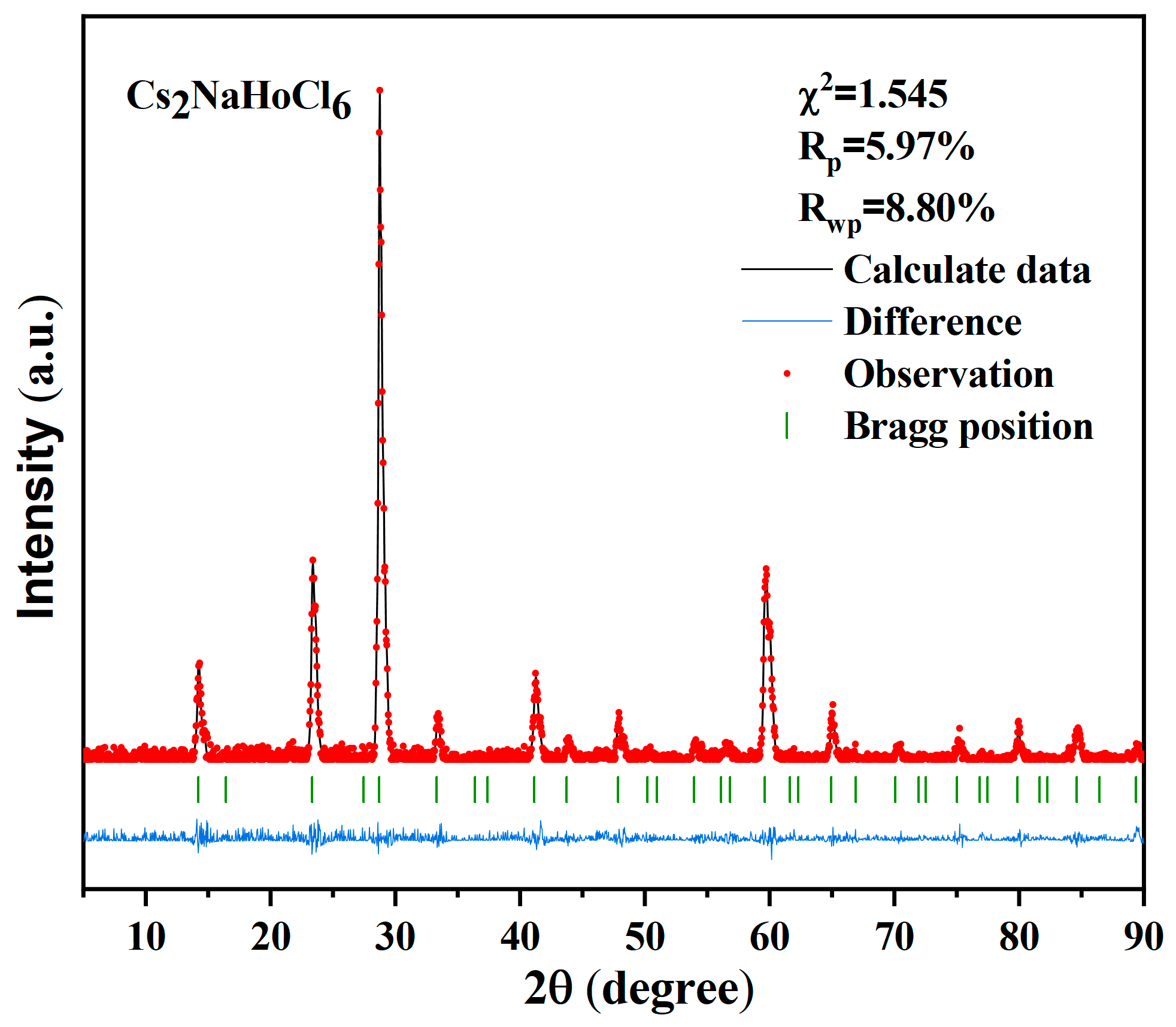
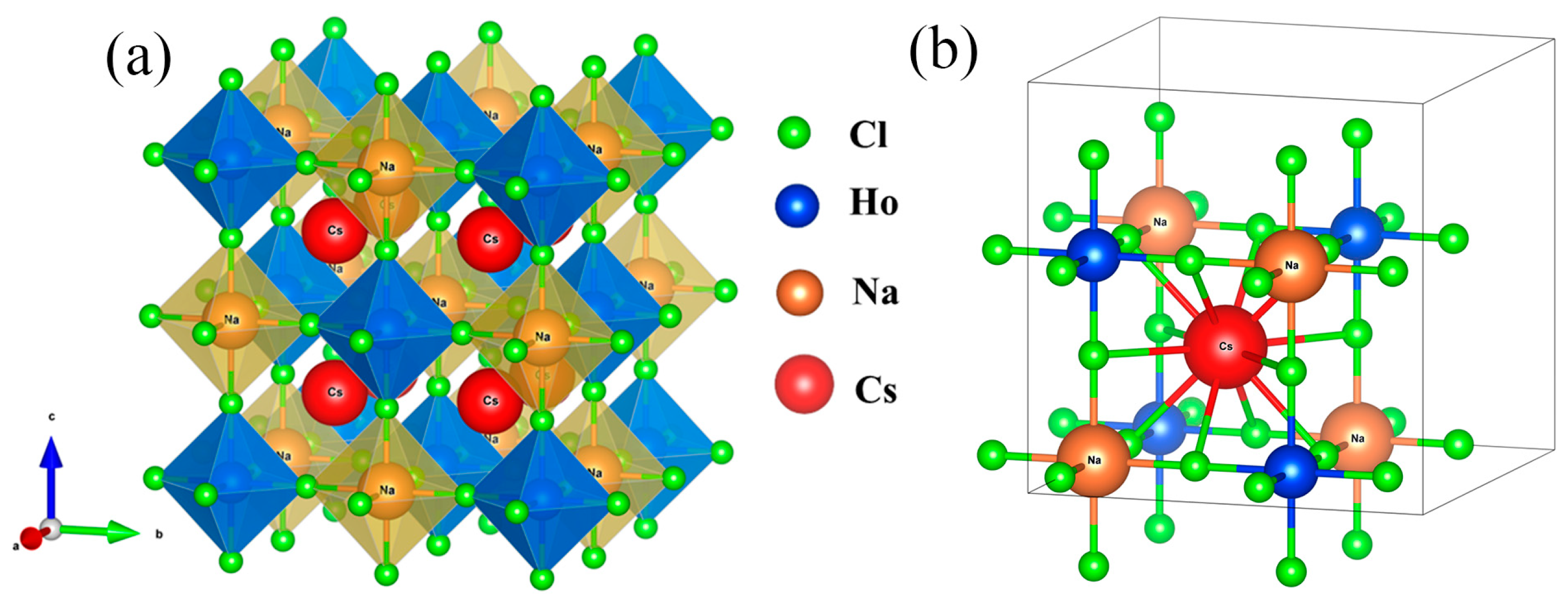
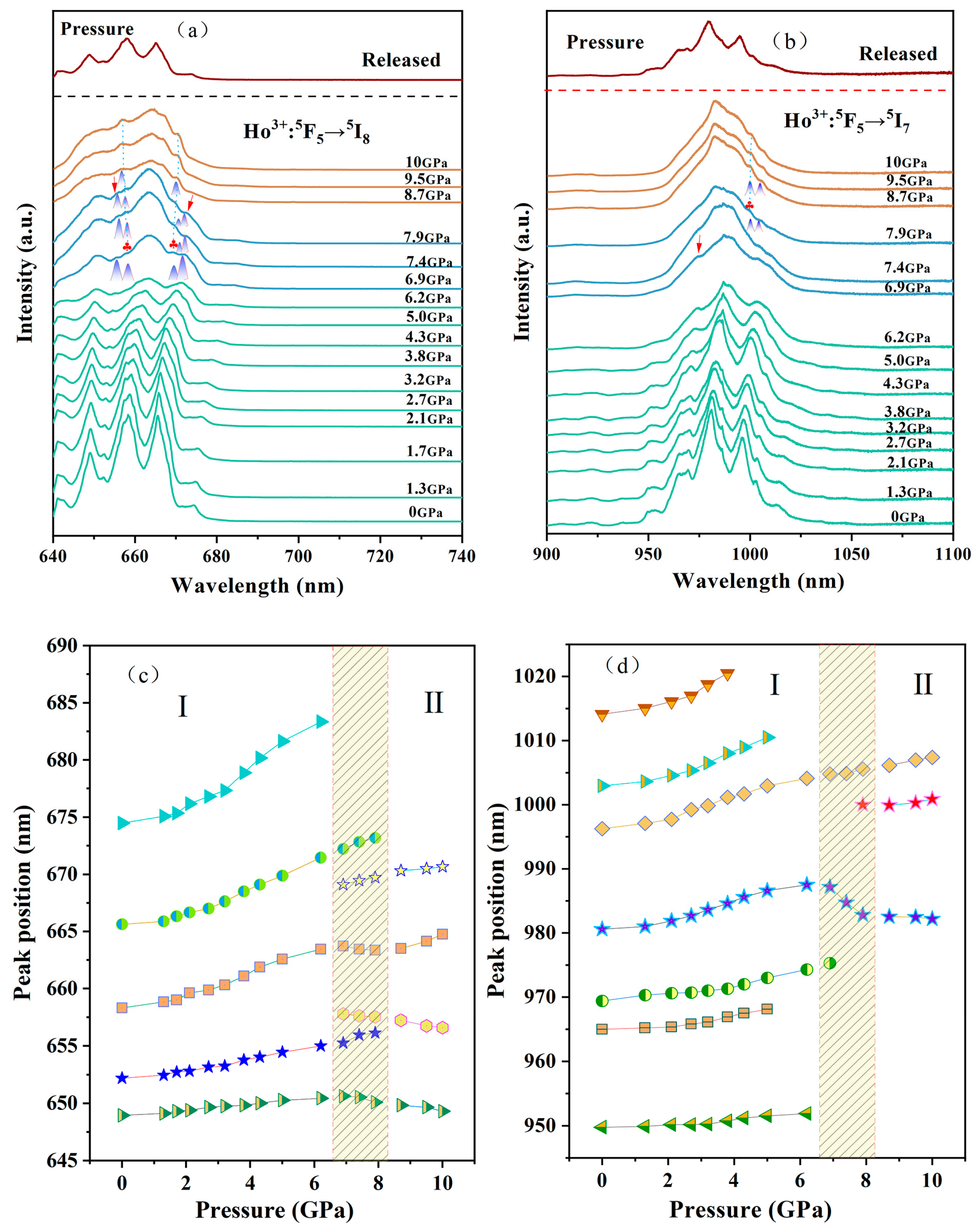
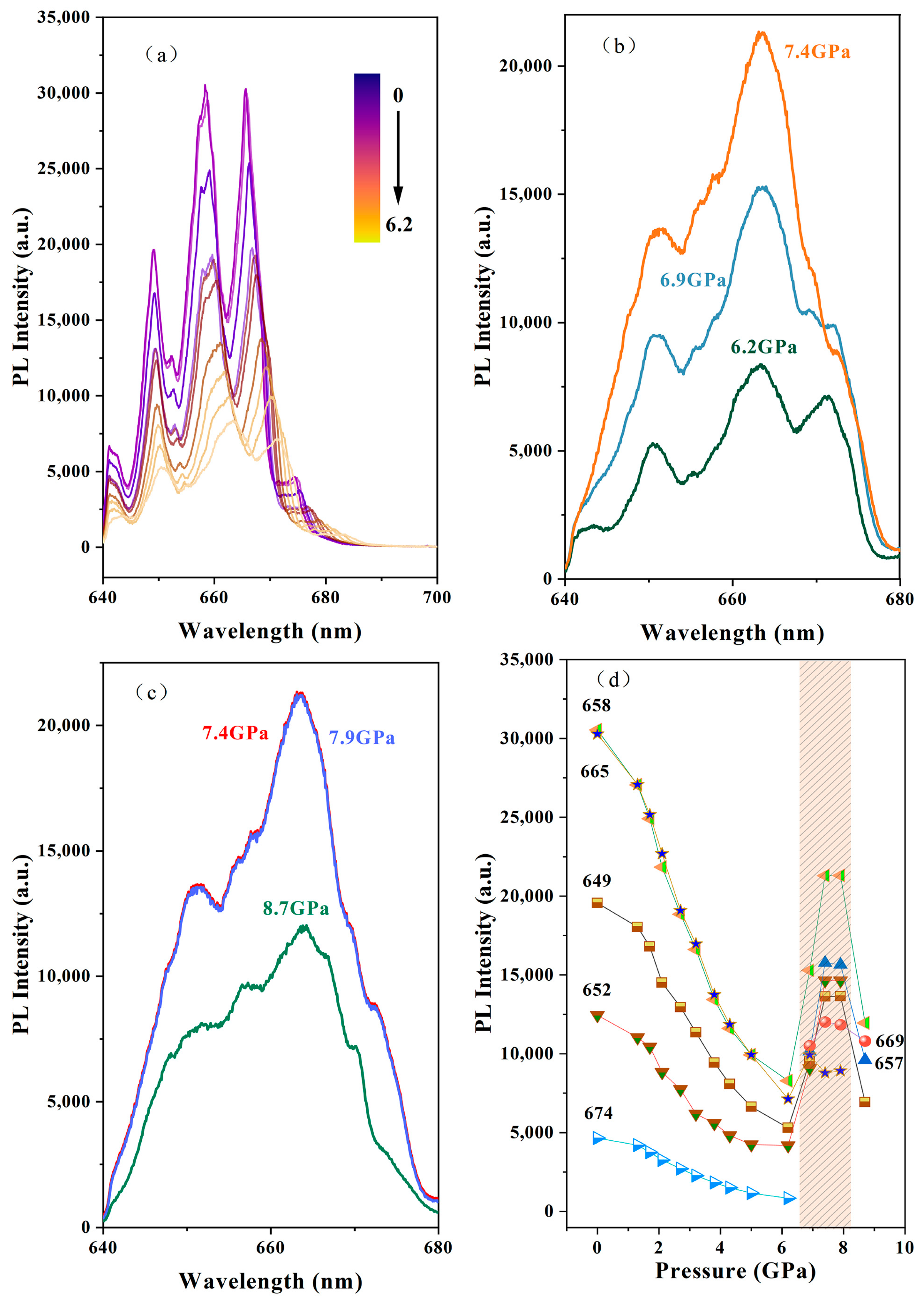
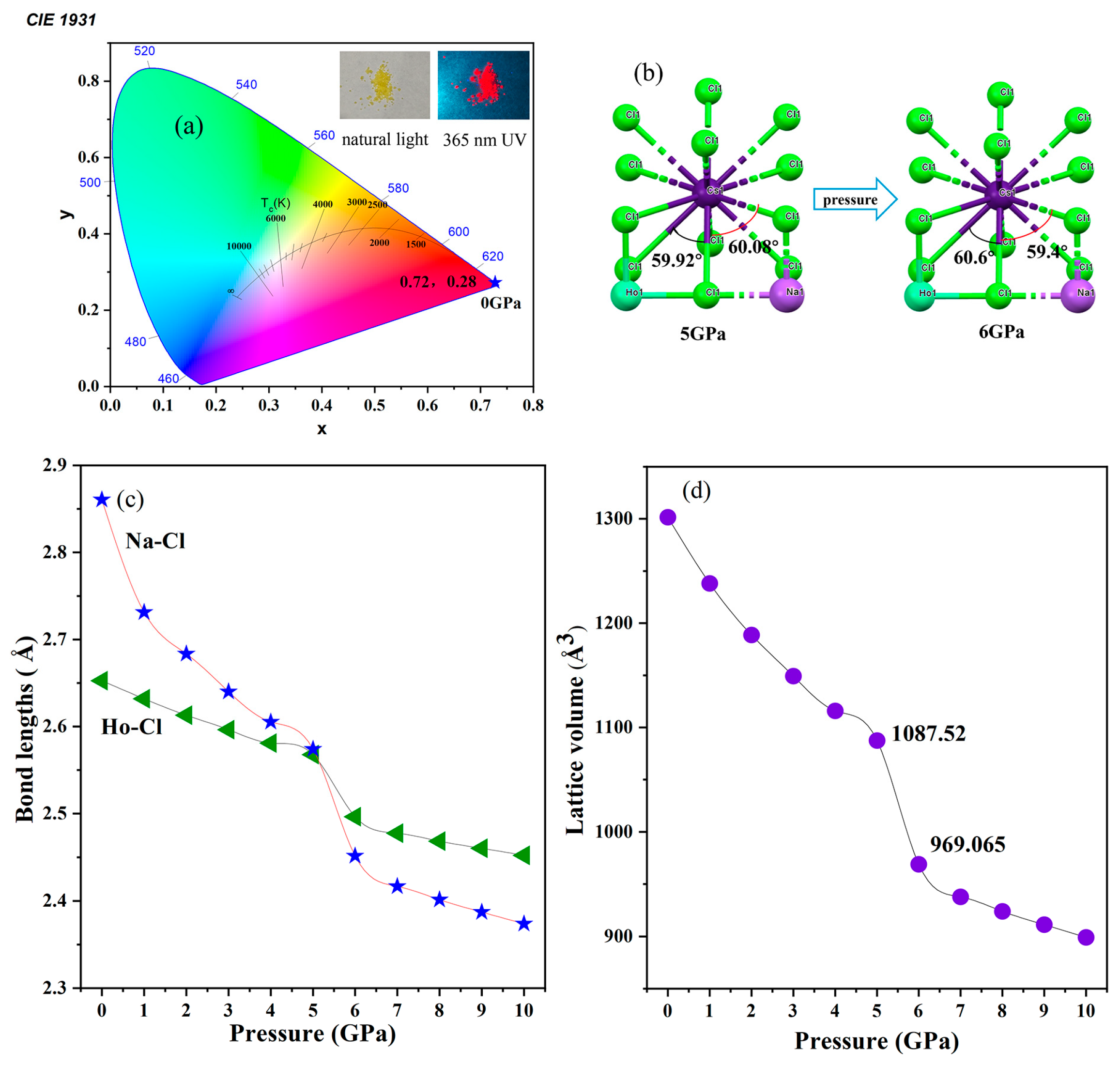
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- López-Fernández, I.; Valli, D.; Wang, C.Y.; Samanta, S.; Okamoto, T.; Huang, Y.T.; Sun, K.; Liu, Y.; Chirvony, V.S.; Patra, A.; et al. Lead-Free Halide Perovskite Materials and Optoelectronic Devices: Progress and Prospective. Adv. Funct. Mater. 2024, 34, 2307896. [Google Scholar] [CrossRef]
- Grätzel, M. The light and shade of perovskite solar cells. Nat. Mater. 2014, 13, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Mcmeekin, D.P.; Sadoughi, G.; Rehman, W.; Eperon, G.E.; Saliba, M.; Hörantner, M.T.; Haghighirad, A.; Sakai, N.; Korte, L.; Rech, B. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 2016, 351, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Karunadasa, H.I. White-light emission from layered halide perovskites. Acc. Chem. Res. 2018, 51, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Di Stasio, F.; Bi, C.; Zhang, J.; Xia, Z.; Shi, Z.; Manna, L. Near-infrared light emitting metal halides: Materials, mechanisms, and applications. Adv. Mater. 2024, 36, 2312482. [Google Scholar] [CrossRef]
- Rao, Z.H.; Zhao, X.J.; Gong, X. Rare-Earth-Based Lead-Free Halide Double Perovskites for Light Emission: Recent Advances and Applications. Adv. Funct. Mater. 2024, 34, 2406424. [Google Scholar] [CrossRef]
- Sun, R.R.; Jia, M.C.; Chen, X.; Zhang, F.; Ma, Z.Z.; Liu, Y.; Zhang, J.B.; Lian, L.Y.; Han, Y.B.; Li, M.Y. Constructing Efficient and Thermostable Red-NIR Emitter via Cross Relaxation and Crystal-Field Engineering of Holmium-Based Perovskite-Type Half Metal. Laser Photonics Rev. 2024, 18, 2301028. [Google Scholar] [CrossRef]
- Wang, Y.S.; Dang, P.P.; Qiu, L.; Zhang, G.D.; Liu, D.J.; Wei, Y.; Lian, H.Z.; Li, G.G.; Cheng, Z.Y.; Lin, J. Multimode luminescence tailoring and improvement of Cs2NaHoCl6 cryolite crystals via Sb3+/Yb3+ alloying for versatile photoelectric applications. Angew. Chem. 2023, 135, e202311699. [Google Scholar] [CrossRef]
- Yan, T.T.; Zhang, D.D.; Xi, D.Y.; Zhao, Y.; Wang, C.Y.; Jiang, R.; Xu, Y.F. Pressure-Induced Structural Phase Transitions and Photoluminescence Properties of Micro/Nanocrystals HoF3. Inorg. Chem. 2024, 63, 20562–20571. [Google Scholar] [CrossRef]
- Hussain, S.; Rehman, J.U.; Tahir, M.B.; Hussain, A. First-principles study of structural, mechanical, optical, and electronic properties of double perovskite RbBa2Ti3O10 material for photocatalytic applications. Int. J. Hydrogen Energy 2024, 78, 1123–1132. [Google Scholar] [CrossRef]
- Nair, S.S.; Krishnia, L.; Trukhanov, A.; Thakur, P.; Thakur, A. Prospect of double perovskite over conventional perovskite in photovoltaic applications. Ceram. Int. 2022, 48, 34128–34147. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, D.; Yadav, R.S.; Singh, A.K. Recent progress on optical properties of double perovskite phosphors. Prog. Solid State Chem. 2023, 69, 100391. [Google Scholar] [CrossRef]
- Fu, R.; Zhao, W.; Wang, L.; Ma, Z.; Xiao, G.; Zou, B. Pressure-induced emission toward harvesting cold white light from warm white light. Angew. Chem. 2021, 60, 10082–10088. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Yan, T.T.; Xi, D.Y.; Zhang, D.D.; Xu, Y.F.; Niu, S.Q.; Ma, J. High Pressure Study of Pharmaceutical and Energetic Material Hexamethylenetetramine. J. Phys. Chem. C 2023, 127, 17863–17870. [Google Scholar] [CrossRef]
- Ma, Z.W.; Li, F.F.; Zhao, D.L.; Xiao, G.J.; Zou, B. Whether or not emission of Cs4PbBr6 nanocrystals: High-pressure experimental evidence. CCS Chem. 2020, 2, 71–80. [Google Scholar] [CrossRef]
- Ma, Z.W.; Li, Q.; Luo, J.J.; Li, S.R.; Sui, L.Z.; Zhao, D.L.; Yuan, K.J.; Xiao, G.J.; Tang, J.; Quan, Z.W. Pressure-driven reverse intersystem crossing: New path toward bright deep-blue emission of lead-free halide double perovskites. J. Am. Chem. Soc. 2021, 143, 15176–15184. [Google Scholar] [CrossRef]
- Wang, Y.G.; Lü, X.J.; Yang, W.G.; Wen, T.; Yang, L.X.; Ren, X.T.; Wang, L.; Lin, Z.S.; Zhao, Y.S. Pressure-induced phase transformation, reversible amorphization, and anomalous visible light response in organolead bromide perovskite. J. Am. Chem. Soc. 2015, 137, 11144–11149. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Zhang, L.; Wu, L.W.; Yan, J.J.; Lin, Y.; Wang, K.; Mao, W.L.; Zou, B. Pressure-Induced Emission (PIE) and Phase Transition of a Two-dimensional Halide Double Perovskite (BA)4AgBiBr8 (BA=CH3(CH2)3NH3+). Angew. Chem. 2019, 58, 15249–15253. [Google Scholar] [CrossRef]
- Jing, X.L.; Zhou, D.L.; Sun, R.; Zhang, Y.; Li, Y.C.; Li, X.D.; Li, Q.J.; Song, H.W.; Liu, B.B. Enhanced Photoluminescence and Photoresponsiveness of Eu3+ Ions-Doped CsPbCl3 Perovskite Quantum Dots under High Pressure. Adv. Funct. Mater. 2021, 31, 2100930. [Google Scholar] [CrossRef]
- Li, W.T.; Ren, X.T.; Huang, Y.W.; Yu, Z.H.; Mi, Z.Y.; Tamura, N.; Li, X.D.; Peng, F.; Wang, L. Phase transformation and fluorescent enhancement of ErF3 at high pressure. Solid State Commun. 2016, 242, 30–35. [Google Scholar] [CrossRef][Green Version]
- Gong, C.; Li, Q.; Liu, R.; Hou, Y.; Wang, J.; Dong, X.; Liu, B.; Yang, X.; Yao, Z.; Tan, X.; et al. Structural phase transition and photoluminescence properties of YF3 and YF3:Eu3+ under high pressure. Phys. Chem. Chem. Phys. 2013, 15, 19225–19931. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Mookherjee, M.; Clapp, S.; Chariton, S.; Prakapenka, V.B. High-pressure Raman scattering and X-ray diffraction study of kaolinite, Al2Si2O5(OH)4. Appl. Clay Sci. 2023, 245, 107144. [Google Scholar] [CrossRef]
- Carpenella, V.; Ripanti, F.; Stellino, E.; Fasolato, C.; Nucara, A.; Petrillo, C.; Malavasi, L.; Postorino, P. High-pressure behavior of δ-phase of formamidinium lead iodide by optical spectroscopies. J. Phys. Chem. C 2023, 127, 2440–2447. [Google Scholar] [CrossRef]
- Akahama, Y.; Kawamura, H. High-pressure Raman spectroscopy of diamond anvils to 250 GPa: Method for pressure determination in the multimegabar pressure range. J. Appl. Phys. 2004, 96, 3748–3751. [Google Scholar] [CrossRef]
- Milstein, T.J.; Roh, J.Y.D.; Jacoby, L.M.; Crane, M.J.; Sommer, D.E.; Dunham, S.T.; Gamelin, D.R. Ubiquitous Near-Band-Edge Defect State in Rare-Earth-Doped Lead-Halide Perovskites. Chem. Mater. 2022, 34, 3759–3769. [Google Scholar] [CrossRef]
- Yu, O.Y.; Jiang, X.X.; Jiang, F.; Li, L.H.; Zhao, H.P.; Zhang, C.; Zheng, M.; Zheng, W.H.; Jiang, Y.; Zhu, X.L.; et al. Light-Soaking Induced Optical Tuning in Rare Earth-Doped All-Inorganic Perovskite. Adv. Funct. Mater. 2022, 32, 2107086. [Google Scholar] [CrossRef]
- Shah, S.a.A.; Sayyad, M.H.; Sun, J.; Guo, Z. Recent advances and emerging trends of rare-earth-ion doped spectral conversion nanomaterials in perovskite solar cells. J. Rare Earths 2022, 40, 1651–1667. [Google Scholar] [CrossRef]
- Xia, W.R.; Pei, Z.P.; Leng, K.; Zhu, X.H. Research Progress in Rare Earth-Doped Perovskite Manganite Oxide Nanostructures. Nanoscale Res. Lett. 2020, 15, 9. [Google Scholar] [CrossRef]
- Dorogokupets, P.I.; Oganov, A.R. Equations of state Cu and Ag and revised ruby pressure scale. Dokl. Earth Sci. 2003, 391A, 854–857. [Google Scholar]
- Shen, G.; Smith, J.S.; Kenney-Benson, C.; Klotz, S. Calibration of ruby (Cr3+:Al2O3) and Sm2+:SrFCl luminescence lines from the melting of mercury: Constraints on the initial slopes. High Press. Res. 2021, 41, 175–183. [Google Scholar] [CrossRef]
- Carnall, W.T.; Goodman, G.L.; Rajnak, K.; Rana, R. A systematic analysis of the spectra of the lanthanides doped into single crystal LaF3. J. Chem. Phys. 1989, 90, 3443–3457. [Google Scholar] [CrossRef]
- Kaminskii, A.A. Laser Crystals: Their Physics and Properties; Springer: Berlin/Heidelberg, Germany, 2013; Volume 14. [Google Scholar]
- Shen, G.Y.; Mao, H.K. High-pressure studies with x-rays using diamond anvil cells. Rep. Prog. Phys. 2016, 80, 016101. [Google Scholar] [CrossRef] [PubMed]
- Syassen, K. Ruby under pressure. High Press. Res. 2008, 28, 75–126. [Google Scholar] [CrossRef]
| Empirical Formula | Cl24 Cs8.10 Ho4 Na3.80 | |
|---|---|---|
| Formula weight | 2674.45 | |
| Temperature | 296(2) K | |
| Wavelength | 0.71073 Å | |
| Crystal system | Cubic | |
| Space group | Fm-3m | |
| Unit cell dimensions | a = 10.7173(8) Å | a = 90°. |
| b = 10.7173(8) Å | b = 90°. | |
| c = 10.7173(8) Å | g = 90°. | |
| Volume | 1231.0(3) Å3 | |
| Z | 1 | |
| Density (calculated) | 3.608 Mg/m3 | |
| Absorption coefficient | 13.603 mm−1 | |
| F (000) | 1163 | |
| Crystal size | 0.120 × 0.100 × 0.080 mm3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, T.; Liu, L.; Xi, D.; Sun, L.; Jin, D.; Li, H. Pressure-Induced Structural Phase Transition and Fluorescence Enhancement of Double Perovskite Material Cs2NaHoCl6. Crystals 2024, 14, 1006. https://doi.org/10.3390/cryst14111006
Yan T, Liu L, Xi D, Sun L, Jin D, Li H. Pressure-Induced Structural Phase Transition and Fluorescence Enhancement of Double Perovskite Material Cs2NaHoCl6. Crystals. 2024; 14(11):1006. https://doi.org/10.3390/cryst14111006
Chicago/Turabian StyleYan, Tingting, Linan Liu, Dongyang Xi, Lei Sun, Dinghan Jin, and Han Li. 2024. "Pressure-Induced Structural Phase Transition and Fluorescence Enhancement of Double Perovskite Material Cs2NaHoCl6" Crystals 14, no. 11: 1006. https://doi.org/10.3390/cryst14111006
APA StyleYan, T., Liu, L., Xi, D., Sun, L., Jin, D., & Li, H. (2024). Pressure-Induced Structural Phase Transition and Fluorescence Enhancement of Double Perovskite Material Cs2NaHoCl6. Crystals, 14(11), 1006. https://doi.org/10.3390/cryst14111006







