Microstructure and Mechanical Properties of TiC/WC-Reinforced AlCoCrFeNi High-Entropy Alloys Prepared by Laser Cladding
Abstract
1. Introduction
2. Experimental Procedure
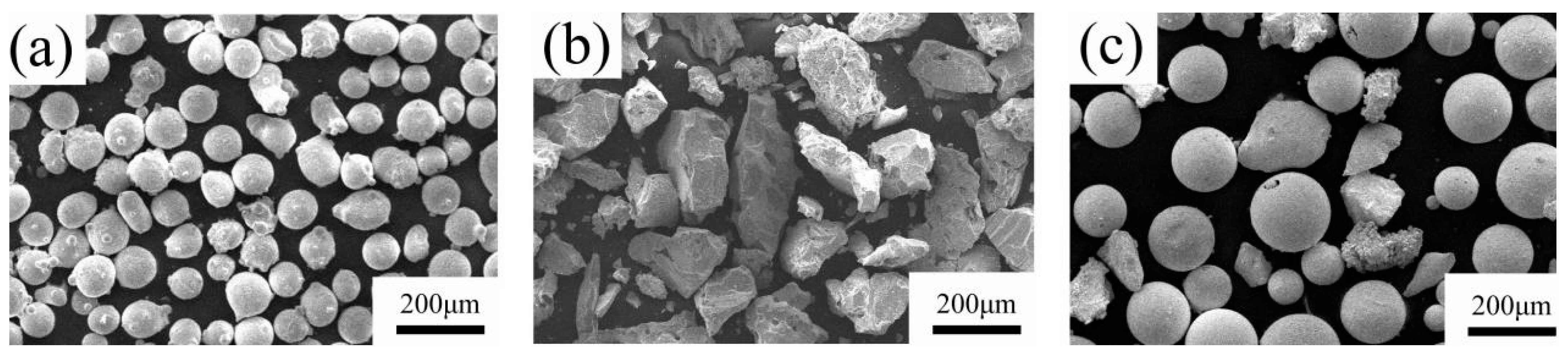
2.1. Raw Powder Preparation and Experimental Process
2.2. Methods
2.3. Test Methods
3. Results and Discussion
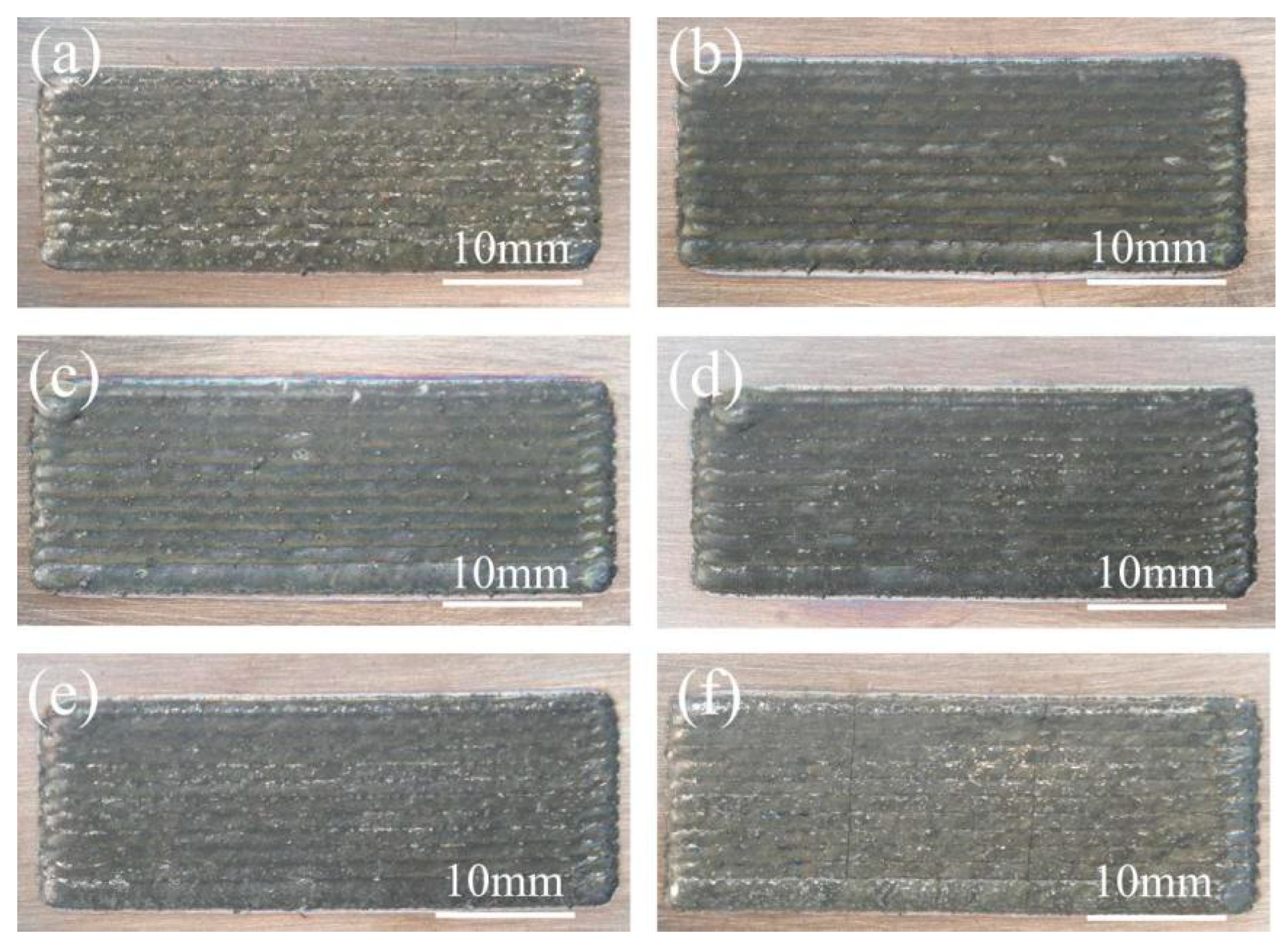
3.1. Macroscopic Morphology of the Coating
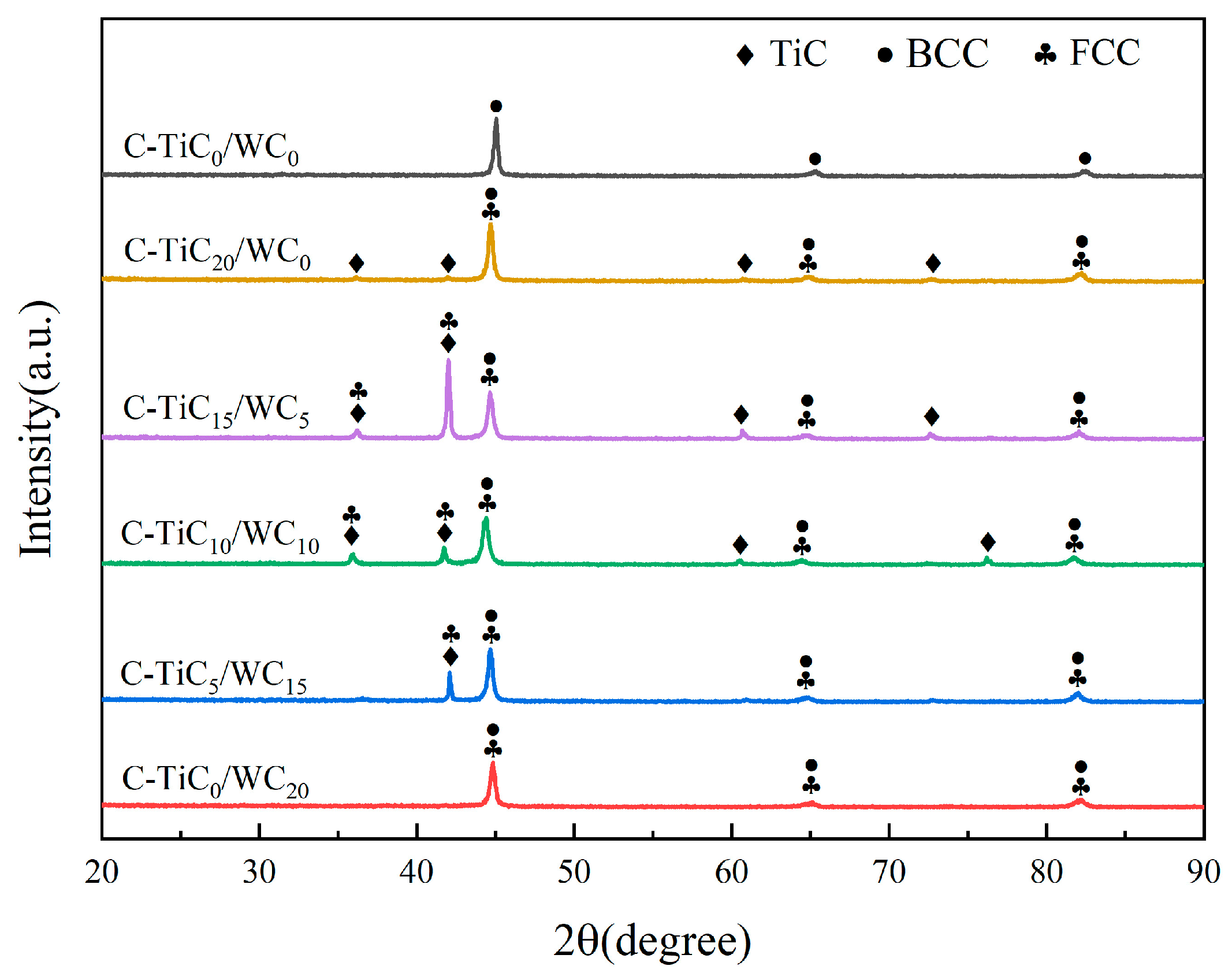
3.2. Phase Composition of the Coating
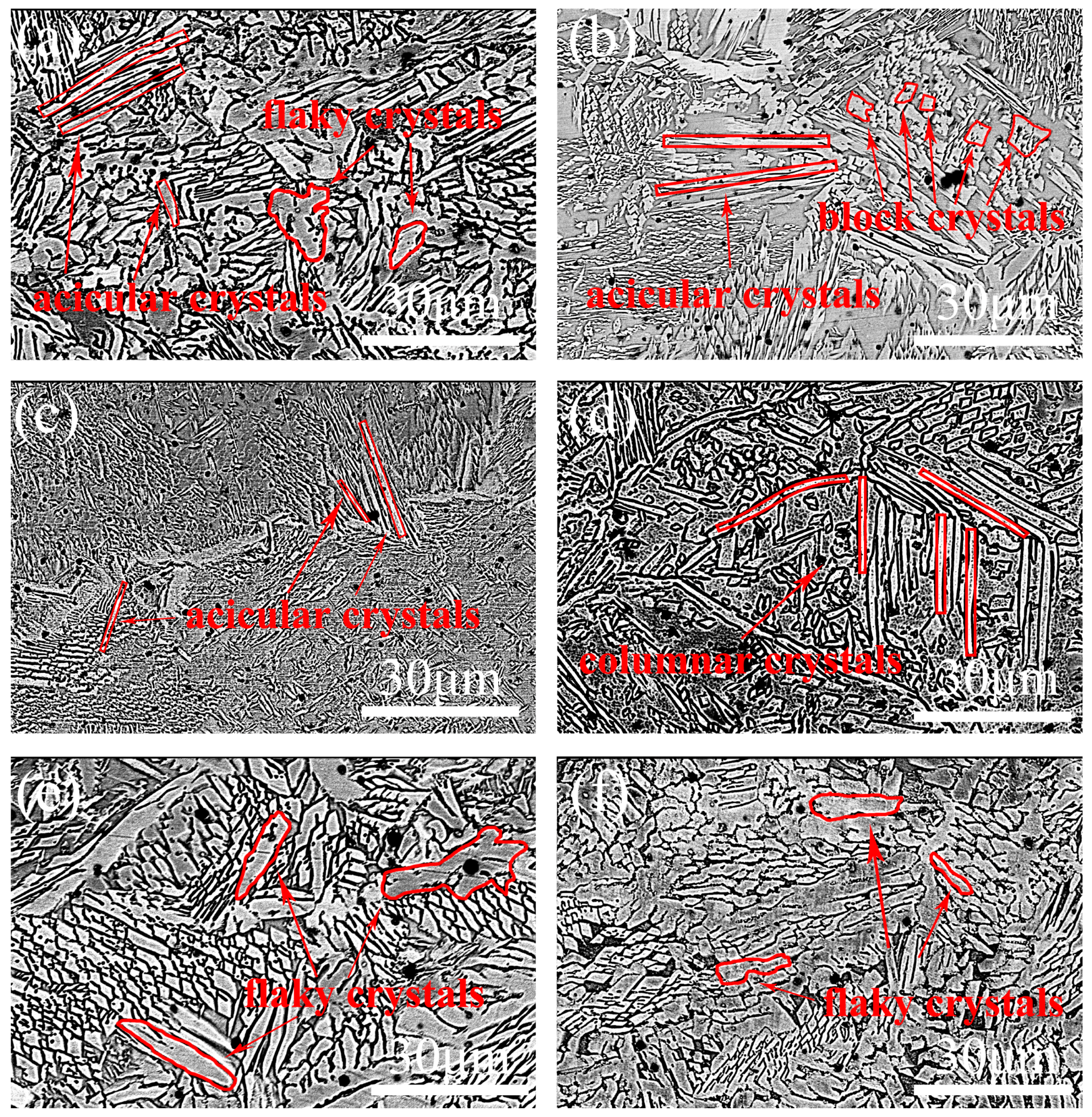
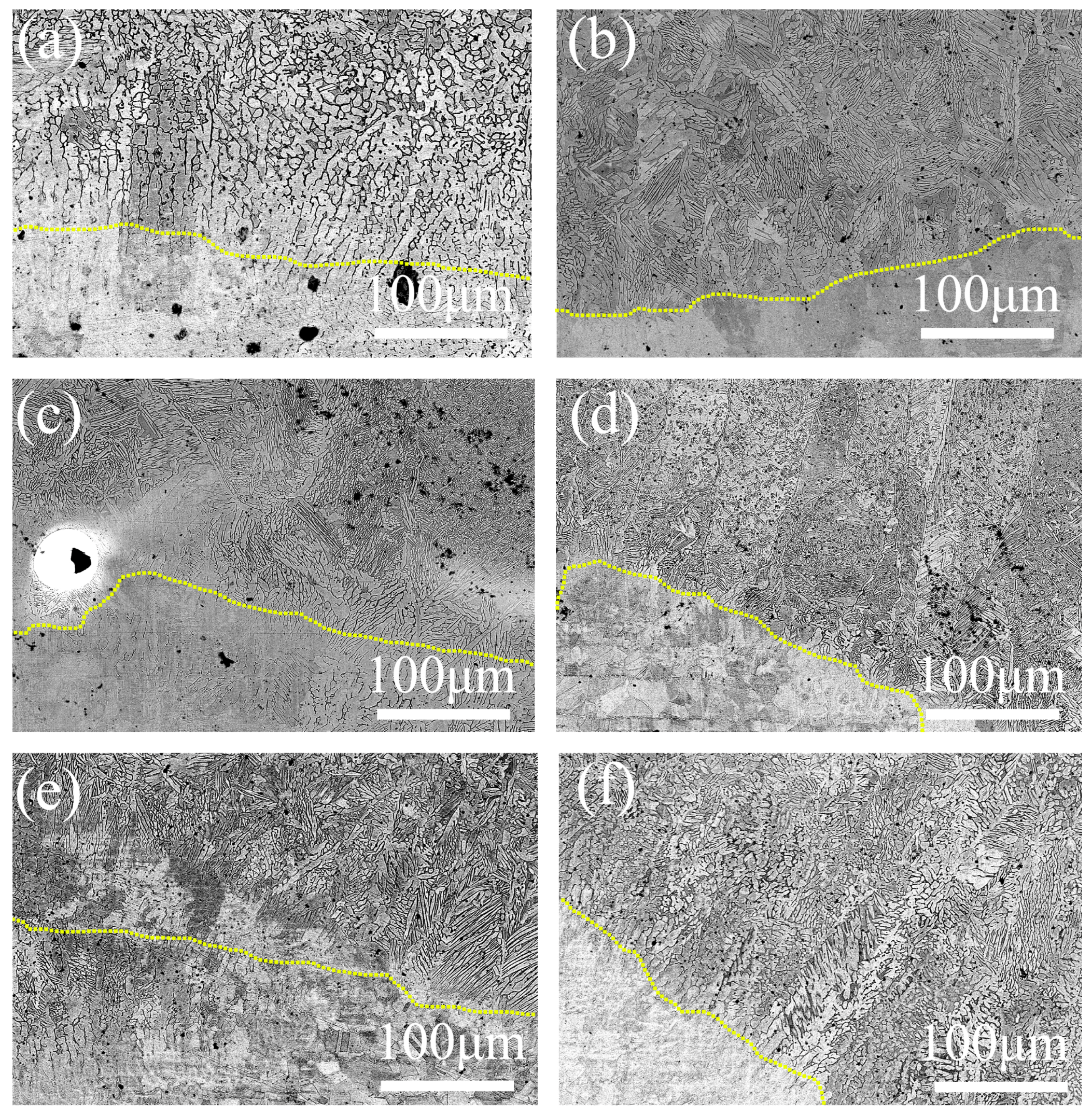
3.3. Microstructure of the Coating
3.4. Microhardness of the Coating
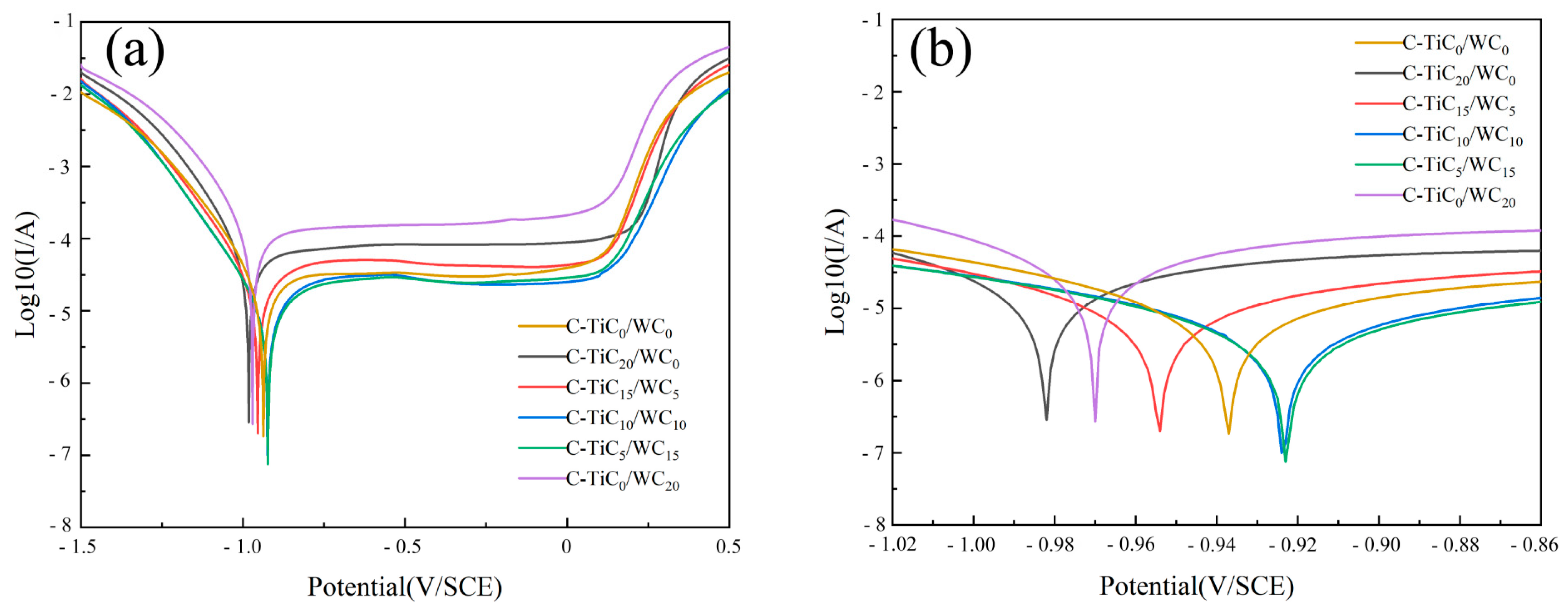
3.5. Corrosion Resistance of the Coating
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: Materials, processes and mechanisms. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Rehman, E.U.; Ullah, S.; Atif, M.; Tariq, A. A review on laser cladding of high-entropy alloys, their recent trends and potential applications. J. Manuf. Process. 2021, 68, 225–273. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chang, S.Y.; Hong, Y.D.; Chen, S.K.; Lin, S.J. Anomalous decrease in X-ray diffraction intensities of Cu-Ni-Al-Co-Cr-Fe-Si alloy systems with multi-principal elements. Mater. Chem. Phys. 2007, 103, 41–46. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Z.; Liu, H.M.; Zhang, Y.B.; Li, H.X.; Shi, C.W.; Liu, P.; Yan, D.F. Recent progress on the microstructure and properties of high entropy alloy coatings prepared by laser processing technology: A review. J. Manuf. Process. 2022, 76, 397–411. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Ren, N.N.; Cheng, N.; Dong, Z.X.; Ma, Q.S. Microstructure evolution and high temperature wear resistance of in-situ synthesized carbides reinforced NiCoFeCrSiMo high entropy alloy coatings fabricated by laser cladding. Surf. Coat. Technol. 2023, 464, 129573. [Google Scholar] [CrossRef]
- Li, W.D.; Xie, D.; Li, D.Y.; Zhang, Y.; Gao, Y.F.; Liaw, P.K. Mechanical behavior of high-entropy alloys. Prog. Mater. Sci. 2021, 118, 100777. [Google Scholar] [CrossRef]
- Li, Z.Z.; Zhao, S.T.; Ritchie, R.O.; Meyers, M.A. Mechanical properties of high-entropy alloys with emphasis on face-centered cubic alloys. Prog. Mater. Sci. 2019, 102, 296–345. [Google Scholar] [CrossRef]
- Guo, W.M.; Ding, N.; Liu, G.Q.; Jing, C.N.; Xu, H.X.; Liu, L.; Xu, N.; Wu, X.F.; He, J.Q.; Zaïri, F. Microstructure evolution of a multi-track AlCoCrFeNi high entropy alloy coating fabricated by laser cladding. Mater. Charact. 2022, 184, 111660. [Google Scholar] [CrossRef]
- Han, B.; Zhang, S.Y.; Zhang, T.M.; Chen, Y.H.; Qin, X.W.; Li, M.Y.; Hu, C.Y.; Wei, M.W.; Xue, X.X. Hardness enhancement mechanism of AlxCoCrFeNiSi high-entropy alloy coatings prepared by laser cladding. Intermetallics 2023, 158, 107909. [Google Scholar] [CrossRef]
- Shang, X.J.; Liu, Q.B.; Guo, Y.X.; Ding, K.L.; Liao, T.H.; Wang, F.P. Nano-TiC reinforced [Cr–Fe4Co4Ni4] Cr3 high-entropy-alloy composite coating fabricated by laser cladding. J. Mater. Res. Technol. 2022, 21, 2076–2088. [Google Scholar] [CrossRef]
- Han, B.; Chen, Y.B.; Tan, C.W.; Jiang, M.; Bi, J.; Feng, J.C.; Chen, X.; Chen, L.J.; Zhang, L.; Liu, X.G.; et al. Microstructure and wear behavior of laser clad interstitial CoCrFeNi high entropy alloy coating reinforced by carbon nanotubes. Surf. Coat. Technol. 2022, 434, 128241. [Google Scholar] [CrossRef]
- Wen, X.; Cui, X.F.; Jin, G.; Liu, Y.F.; Zhang, Y.; Zhang, X.R.; Liu, E.B.; Tian, H.L.; Fang, Y.C. Corrosion and tribo-corrosion behaviors of nano-lamellar Ni1.5CrCoFe0.5Mo0.1Nbx eutectic high-entropy alloy coatings: The role of dual-phase microstructure. Corros. Sci. 2022, 201, 110305. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, Y.; Zhang, C.Y. Laser cladding of CoCrFeNi high-entropy alloy coatings: Compositional homogeneity towards improved corrosion resistance. Mater. Lett. 2022, 318, 132133. [Google Scholar] [CrossRef]
- Li, H.G.; Huang, Y.J.; Jiang, S.S.; Lu, Y.Z.; Gao, X.Y.; Lu, X.; Ning, Z.L.; Sun, J.F. Columnar to equiaxed transition in additively manufactured CoCrFeMnNi high entropy alloy. Mater. Des. 2021, 197, 109262. [Google Scholar] [CrossRef]
- Wang, C.S.; Li, R.F.; Bi, X.L.; Yuan, W.Y.; Gu, J.Y.; Chen, J.; Yan, M.J.; Zhang, Z.Y. Microstructure and wear resistance property of laser cladded CrCoNi coatings assisted by ultrasonic impact treatment. Mater. Res. Technol. 2023, 22, 853–864. [Google Scholar] [CrossRef]
- Raahgini, C.; Verdi, D. Abrasive wear performance of laser cladded Inconel 625 based metal matrix composites: Effect of the vanadium carbide reinforcement phase content. Surf. Coat. Technol. 2022, 429, 127975. [Google Scholar] [CrossRef]
- An, X.L.; Liu, Q.B.; Zheng, B. Effect of Tungsten carbide on laser cladding of high entropy alloy. High Power Laser Part. Beams 2014, 26, 255–260. (In Chinese) [Google Scholar]
- Cai, Y.C.; Chen, Y.; Luo, Z.; Gao, F.; Li, L. Manufacturing of FeCoCrNiCux medium-entropy alloy coating using laser cladding technology. Mater. Des. 2017, 133, 91–108. [Google Scholar] [CrossRef]
- Qiu, X.W.; Liu, C.G. Microstructure and properties of Al2CrFeCoCuTiNix high-entropy alloys prepared by laser cladding. J. Alloys Compd. 2013, 553, 216–220. [Google Scholar] [CrossRef]
- Zhang, M.D.; Zhang, L.J.; Fan, J.T.; Li, G.; Liaw, P.K.; Liu, R.P. Microstructure and enhanced mechanical behavior of the Al7Co24Cr21Fe24Ni24 high-entropy alloy system by tuning the Cr content. Mater. Sci. Eng. A 2018, 733, 299–306. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, Y.P.; Kong, J.R.; Zhang, J.J.; Li, T.J. Microstructure and mechanical properties of multi-component AlCrFeNiMox high-entropy alloys. J. Alloys Compd. 2013, 573, 96–101. [Google Scholar] [CrossRef]
- Quan, T.; Guo, W.B.; Qian, C. Study on Friction and Wear Properties of Powder Metallurgy Al1Co25Cr18Fe23Ni23Ta10 eutectic High Entropy alloy. J. Cem. Carbide 2018, 38, 156–164. (In Chinese) [Google Scholar]
- Liu, H.; Gao, Q.; Dai, J.B.; Chen, P.J.; Gao, W.P.; Hao, J.B.; Yang, H.F. Microstructure and high-temperature wear behavior of CoCrFeNiWx high-entropy alloy coatings fabricated by laser cladding. Tribol. Int. 2022, 172, 107574. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Li, J.; Juan, Y.F.; Lu, Z.J.; Jia, W.L. Evolution in microstructure and corrosion behavior of AlCoCrxFeNi high-entropy alloy coatings fabricated by laser cladding. J. Alloys Compd. 2019, 775, 1–14. [Google Scholar] [CrossRef]
- Zhao, S.L.; Xin, D.Q.; Chen, X.Z.; Stasic, J.; Trtica, M.; Siddiquee, A.N.; Mohan, S. Microstructure and enhanced tensile properties of AlCoxCrFeNi high entropy alloys with high Co content fabricated by laser melting deposition. J. Alloys Compd. 2022, 917, 165403. [Google Scholar] [CrossRef]
- Li, Z.T.; Jing, C.N.; Feng, Y.; Wu, Z.L.; Lin, T.; Zhao, J.R. Phase evolution and properties of AlxCoCrFeNi high-entropy alloys coatings by laser cladding. Mater. Today Commun. 2023, 35, 105800. [Google Scholar] [CrossRef]
- Akash, V.; Jyoti, M.; Harshad, N. Influence of WC particle on the metallurgical, mechanical, and corrosion behavior of AlFeCuCrCoNi-WCx high-entropy alloy coatings. J. Mater. Eng. Perform. 2021, 30, 2449–2461. [Google Scholar]
- Ma, Q.; Lu, B.W.; Zhang, Y.M.; Wang, Y.L.; Yan, X.C.; Liu, M.; Zhao, G.R. Crack-free 60 wt% WC reinforced FeCoNiCr high-entropy alloy composite coating fabricated by laser cladding. Mater. Lett. 2022, 324, 132667. [Google Scholar] [CrossRef]
- Li, L.Q.; Liu, D.J.; Chen, Y.B.; Wang, C.M.; Li, F.Q. Electron microscopy study of reaction layers between single-crystal WC particle and Ti–6Al–4V after laser melt injection. Acta Mater. 2009, 57, 3606–3614. [Google Scholar] [CrossRef]
- Yu, K.D.; Zhao, W.; Li, Z.; Guo, N.; Xiao, G.C.; Zhang, H. High-temperature oxidation behavior and corrosion resistance of in-situ TiC and Mo reinforced AlCoCrFeNi-based high entropy alloy coatings by laser cladding. Ceram. Int. 2023, 49, 10151–10164. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Tang, Y.C.; Luo, L.; Luo, L.S.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Microstructure and mechanical properties of CoCrFeNiWx high entropy alloys reinforced by μ phase particles. J. Alloys Compd. 2020, 843, 155997. [Google Scholar] [CrossRef]
- Poletti, M.G.; Fiore, G.; Gili, F.; Mangherini, D.; Battezzati, L. Development of a new high entropy alloy for wear resistance: FeCoCrNiW0.3 and FeCoCrNiW0.3 + 5 at.% of C. Mater. Des. 2017, 115, 247–254. [Google Scholar] [CrossRef]
- Chen, Y.H.; Sun, S.W.; Zhang, T.M.; Zhou, X.W.; Li, S.H. Effects of post-weld heat treatment on the microstructure and mechanical properties of laser-welded NiTi/304SS joint with Ni filler. Mater. Sci. Eng. A 2020, 771, 138545. [Google Scholar] [CrossRef]
- Fang, J.X.; Ma, G.Z.; Tian, H.L.; Li, S.B.; Huang, H.S.; Liu, Y.; Jiang, Y.L.; Liu, B. Transformation-induced strain of a low transformation temperature alloy with high hardness during laser metal deposition. J. Manuf. Process. 2021, 68, 1585–1595. [Google Scholar] [CrossRef]
- Wang, K.L.; Zhu, J.P.; Wang, H.L.; Yang, K.J.; Zhu, Y.M.; Qing, Y.B.; Ma, Z.; Gao, L.H.; Liu, Y.B.; Wei, S.H.; et al. Air plasma-sprayed high-entropy (Y0.2Yb0.2Lu0.2Eu0.2Er0.2)3Al5O12 coating with high thermal protection performance. J. Adv. Ceram. 2022, 11, 1571–1582. [Google Scholar] [CrossRef]
- Zhang, R.; Gu, X.Y.; Gong, H.T.; Gu, X.P.; Zhao, X.H. Effect of Nb content on microstructure and properties of FeCoNi2CrMnV0.5Nbx high-entropy alloy coatings by laser cladding. J. Mater. Res. Technol. 2022, 21, 3357–3370. [Google Scholar] [CrossRef]
- Li, Y.T.; Fu, H.G.; Ma, T.J.; Wang, K.M.; Yang, X.J.; Lin, J. Microstructure and wear resistance of AlCoCrFeNi-WC/TiC composite coating by laser cladding. Mater. Charact. 2022, 194, 112479. [Google Scholar] [CrossRef]
- Shen, L.; Zhao, Y.; Li, Y.L.; Wu, H.; Zhu, H.G.; Xie, Z.H. Synergistic strengthening of FeCrNiCo high entropy alloys via micro-TiC and nano-SiC particles. Mater. Today Commun. 2021, 26, 101729. [Google Scholar] [CrossRef]
- Hemmati, I.; Ocelik, V.; Csach, K.; de Hosson, J.T.M. Microstructure and Phase Formation in a Rapidly Solidified Laser-Deposited Ni-Cr-B-Si-C Hardfacing Alloy. Metall. Mater. Trans. A 2014, 45, 878–892. [Google Scholar] [CrossRef]
- Shao, J.Y.; Yu, G.; He, X.L.; Li, S.X.; Chen, R.; Zhao, Y. Grain size evolution under different cooling rate in laser additive manufacturing of superalloy. Opt. Laser Technol. 2019, 119, 105662. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, Z.K.; Wang, H.; Li, K.Y.; Shi, W.Q.; Jiao, T.W. Effect of WC Content on Microstructure and Properties of CoCrFeNi HEA Composite Coating on 316L Surface via Laser Cladding. Materials 2023, 16, 2706. [Google Scholar] [CrossRef]
- Luo, F.Y.; Shi, W.Q.; Xiong, Z.Y.; Huang, J. Microstructure and properties analysis of AlCoCrFeNi high-entropy alloy/iron-based amorphous composite coatings prepared by laser cladding. J. Non-Cryst. Solids 2024, 624, 122732. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, Z.K.; Li, K.Y.; Shi, W.Q.; Zhao, Y.; He, M.Y. Microstructures and Mechanical Properties of an AlCoCrNiFe HEA/WC Reinforcing Particle Composite Coating Prepared by Laser Cladding. Materials 2022, 15, 8020. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Cheng, G.; Wu, W.; Zhang, Y. A study on the corrosion behavior of carbon steel exposed to a H2S-containing NH4Cl medium. J. Mater. Eng. Perform. 2018, 27, 2492–2504. [Google Scholar] [CrossRef]
- Modupeola, D.; Patricia, P.; Ntombi, M.; Sisa, P.; Samson, A.; Olufemi, A. The comparative study of the microstructural and corrosion behaviour of laser-deposited high entropy alloys. J. Alloys Compd. 2021, 866, 158777. [Google Scholar]


| Cr | Ni | Mn | Mo | Si | Fe |
|---|---|---|---|---|---|
| 16–18 | 10–14 | 2 | 2–3 | 1.5 | Bal. |
| Al | Co | Cr | Fe | Ni |
|---|---|---|---|---|
| 11.09 | 23.34 | 20.19 | 21.72 | 23.53 |
| Materials | Ecorr (V/SCE) | Icorr (A/cm2) |
|---|---|---|
| C-TiC0/WC0 | −0.937 | 1.831 × 10−7 |
| C-TiC20/WC0 | −0.982 | 2.848 × 10−7 |
| C-TiC15/WC5 | −0.954 | 2.006 × 10−7 |
| C-TiC10/WC10 | −0.924 | 9.874 × 10−8 |
| C-TiC5/WC15 | −0.923 | 7.508 × 10−8 |
| C-TiC0/WC20 | −0.970 | 2.695 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Shi, W.; Huang, J. Microstructure and Mechanical Properties of TiC/WC-Reinforced AlCoCrFeNi High-Entropy Alloys Prepared by Laser Cladding. Crystals 2024, 14, 83. https://doi.org/10.3390/cryst14010083
Zhu Z, Shi W, Huang J. Microstructure and Mechanical Properties of TiC/WC-Reinforced AlCoCrFeNi High-Entropy Alloys Prepared by Laser Cladding. Crystals. 2024; 14(1):83. https://doi.org/10.3390/cryst14010083
Chicago/Turabian StyleZhu, Zhikai, Wenqing Shi, and Jiang Huang. 2024. "Microstructure and Mechanical Properties of TiC/WC-Reinforced AlCoCrFeNi High-Entropy Alloys Prepared by Laser Cladding" Crystals 14, no. 1: 83. https://doi.org/10.3390/cryst14010083
APA StyleZhu, Z., Shi, W., & Huang, J. (2024). Microstructure and Mechanical Properties of TiC/WC-Reinforced AlCoCrFeNi High-Entropy Alloys Prepared by Laser Cladding. Crystals, 14(1), 83. https://doi.org/10.3390/cryst14010083





