Reversible Transformations of Palladium–Indium Intermetallic Nanoparticles upon Repetitive Redox Treatments in H2/O2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
- (1)
- Reduction in H2 at 500 °C for 1 h;
- (2)
- Oxidation in O2 at 300 °C for 1 h;
- (3)
- Reduction in H2 at 50 °C for 1 h;
- (4)
- Reduction in H2 at 100 °C for 1 h;
- (5)
- Reduction in H2 at 150 °C for 1 h;
- (6)
- Reduction in H2 at 200 °C for 1 h;
- (7)
- Reduction in H2 at 250 °C for 1 h;
- (8)
- Reduction in H2 at 300 °C for 1 h;
- (9)
- Reduction in H2 at 350 °C for 1 h;
- (10)
- Reduction in H2 at 400 °C for 1 h;
- (11)
- Reduction in H2 at 450 °C for 1 h;
- (12)
- Reduction in H2 at 500 °C for 1 h.
2.2. X-ray Diffraction
2.3. Diffuse Reflectance Infrared Fourier-Transform Spectroscopy of Adsorbed CO
2.4. X-ray Photoelectron Spectroscopy
3. Results and Discussion
3.1. X-ray Diffraction
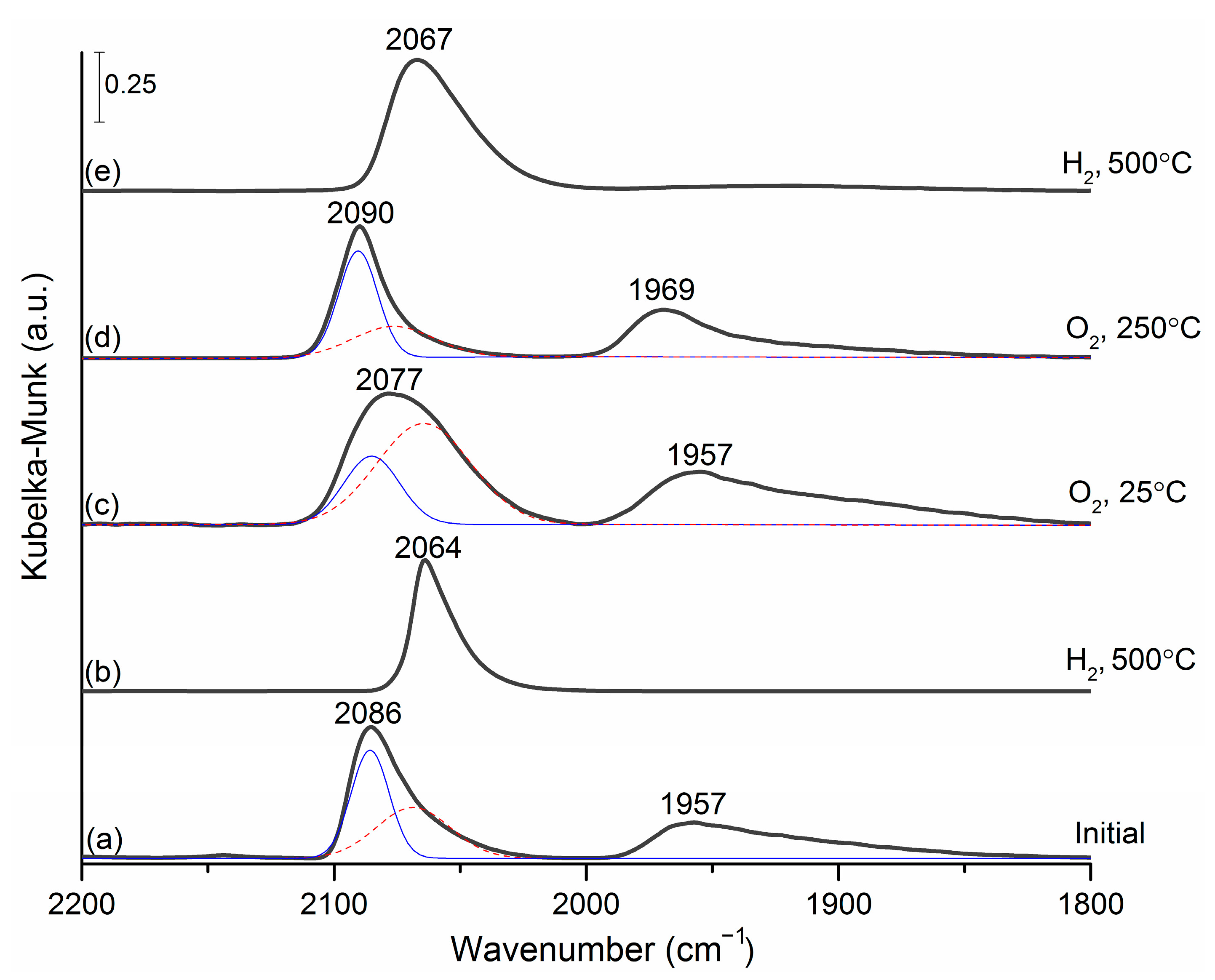
3.2. DRIFT Spectroscopy of Adsorbed CO
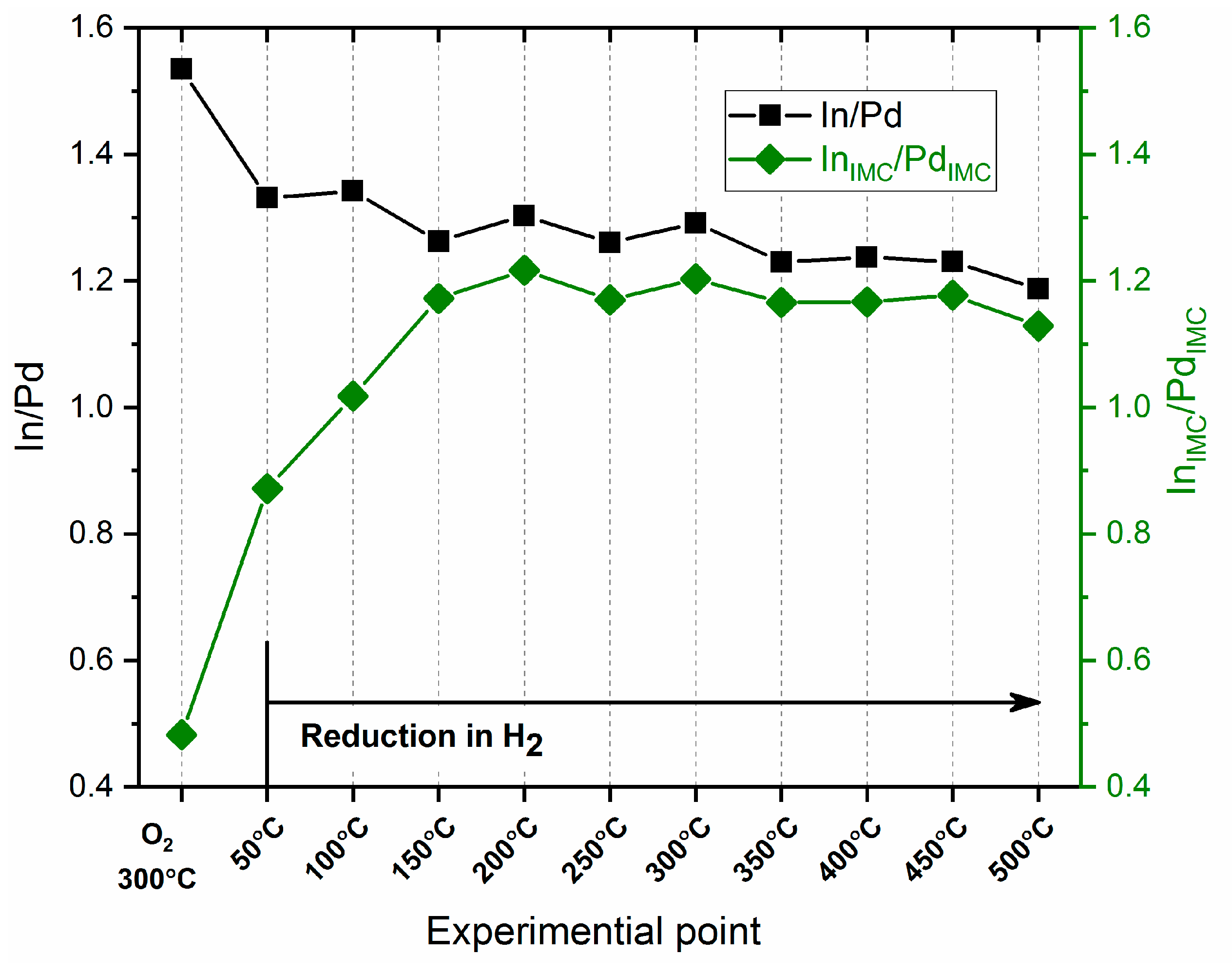
3.3. X-ray Photoelectron Spectroscopy
- (1)
- Reduction of the initial PdIn/Al2O3 catalyst (after prolonged storage in air) in H2 at 500 °C leads to the formation of PdIn intermetallic nanoparticles. XPS data show the presence of only PdIMC and domination of InIMC (with a minor admixture of In oxide) on the catalyst’s surface. At the same time, the InIMC/PdIMC atomic ratio is 1.1, which is actually close to the nominal stoichiometry of the expected PdIn intermetallic phase. This suggestion is also in good agreement with DRIFTS CO data, which show the presence of only linearly adsorbed CO, characterized by a band at 2065 cm−1, indicating that the CO adsorption occurs exclusively on single-atom Pd1 surface sites completely isolated from each other by In atoms and, therefore, suggesting the formation of intermetallic nanoparticles with a regular PdIn structure [38,39,40]. Respective in situ XRD patterns also reveal broadened (110), (200) and (211) peaks of the PdIn 1:1 intermetallic nanoparticles.
- (2)
- The subsequent oxidation of the reduced catalyst in O2 at a temperature of 250–350 °C leads to the decomposition of a significant fraction of the PdIn intermetallic compound. According to XPS data, the fractions of PdIMC and InIMC do not exceed 15–20%. At the same time, oxidized forms of Pd (PdO) and In (InOsurf and In2O3) predominate on the surface. The In/Pd atomic ratio is increased up to ~1.5, indicating the segregation of oxidized In on the surface of intermetallic nanoparticles. At the same time, the InIMC/PdIMC atomic ratio drops down to 0.5, pointing out the depletion of the PdIn phase with In. DRIFTS CO data also indicate the decomposition of the IMC phase with the formation of monometallic palladium particles—the FTIR spectrum is characterized by an asymmetric peak with a maximum at 2090 cm−1 and a broad band at 1980–1950 cm−1. In situ XRD consistently identifies no In- or Pd-containing phases after the oxidative treatment, assuming the complete decomposition of the intermetallic particles to highly dispersed oxide forms, similar to the state of the initial catalyst (exposed to prolonged storage in air).
- (3)
- Reduction of the oxidized PdIn/Al2O3 in H2 at mild temperatures 50–100 °C: The reduction of the oxidized PdIn/Al2O3 catalyst in H2 at 50 °C leads to the full reduction of the Pd component and a partial reduction of the In component with an increase in the PdIn IMC fraction. Indeed, XPS data show the complete disappearance of the PdO state in the Pd3d spectra and increase in the Pd0 and PdIMC fractions after the reduction of the sample at 50 °C. The In3d spectra show an increase in the InIMC fraction and a concerted decrease in the fractions of oxidized In species (InOsurf and In2O3). The In/Pd atomic ratio drops down to ~1.35, indicating a reverse redistribution of the Pd and In components. An increase in the InIMC/PdIMC ratio points out the re-saturation of the PdIn IMC by In atoms. An increase in the reduction temperature up to 100 °C leads to a decrease in the Pd0 fraction and an increase in the PdIMC fraction, and a further reduction of oxidized In states occurs simultaneously. The presence of both Pd0 and Pdalloy on the surface of the PdIn/Al2O3 catalyst after reduction at 50 and 100 °C points out the presence of different types of Pd sites—multi-atomic Pdn centers and single-site Pd1 centers, respectively. It means that the ratio between those sites on the surface could be tuned by selecting a specific reduction temperature below 150 °C. Therefore, a novel strategy to deliberately tune the PdIn nanoparticle surface composition/structure could be suggested, in addition to the post-synthesis modification using an oxidative treatment, as described in [23]. This suggestion is in good agreement with the DRIFTS CO results. The broadening of the band at ~2060–2090 cm−1 corresponds to the formation of both Pd site types (multi-atomic Pdn centers vs. single-site Pd1 centers) on the surface of the PdIn IMC nanoparticles. At 100 °C, the shape of this broad band changes, indicating the redistribution of those two site types on the surface. According to XRD, no In- or Pd-containing phases could be identified. Presumably, the bulk structure of PdIn nanoparticles remains somewhat disordered after the reduction of the sample at such low temperatures (below 100 °C).
- (4)
- Reduction of the oxidized PdIn/Al2O3 in H2 at temperatures 150–500 °C: According to XPS data, the reduction at 150 °C leads to the full reduction of In oxides and also to the complete disappearance of Pd0, indicating the complete restoration of the PdIn IMC structure on the surface of nanoparticles. The Pd/In atomic ratio just slightly drops to ~1.2, indicating the further redistribution of Pd and In components. The InIMC/PdIMC ratio approaches ~1.2, pointing out the formation of the IMC with the stoichiometry close to the PdIn phase. A further increase in the reduction temperature affords no noticeable change in the XPS data. DRIFTS CO supports the mechanism of the nearly complete restoration of the PdIn IMC structure on the surface after the reduction of the PdIn/Al2O3 catalyst at a temperature above 150 °C. The complete disappearance of bands in a range of 2000–1800 cm−1, which are associated with multiple CO bonds, at 200 °C point to the formation of the IMC PdIn phase on the catalyst surface. A further increase in temperature leads only to a small shift in the linear adsorption band maximum to 2064 cm−1, confirming the formation of the intermetallic PdIn phase with the single-atom Pd1 sites. According to in situ XRD data, the formation of the intermetallic PdIn particles upon a reduction treatment starts at 235 °C and continues up to 350 °C. Such a difference in temperature ranges needed for the restoration of the IMC PdIn structure, as retrieved through surface-sensitive (XPS and DRIFTS CO) and bulk (XRD) techniques allow us to suggest that the recovery of the IMC structure on the surface of PdIn nanoparticles occurs at a lower temperature of ~150 °C; the complete transformation into regularly ordered bulk PdIn phase requires temperatures as high as ~350 °C.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, A.; Liu, X.Y.; Mou, C.-Y.; Zhang, T. Understanding the synergistic effects of gold bimetallic catalysts. J. Catal. 2013, 308, 258–271. [Google Scholar] [CrossRef]
- Bukhtiyarov, V.I.; Slin’ko, M.G. Metallic nanosystems in catalysis. Russ. Chem. Rev. 2001, 70, 147–159. [Google Scholar] [CrossRef]
- Venezia, A. Synergetic effect of gold in Au/Pd catalysts during hydrodesulfurization reactions of model compounds. J. Catal. 2003, 215, 317–325. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Xu, Q. Recent progress in synergistic catalysis over heterometallic nanoparticles. J. Mater. Chem. 2011, 21, 13705. [Google Scholar] [CrossRef]
- Wu, J.; Shan, S.; Luo, J.; Joseph, P.; Petkov, V.; Zhong, C.-J. PdCu Nanoalloy Electrocatalysts in Oxygen Reduction Reaction: Role of Composition and Phase State in Catalytic Synergy. ACS Appl. Mater. Interfaces 2015, 7, 25906–25913. [Google Scholar] [CrossRef] [PubMed]
- Kovnir, K.; Osswald, J.; Armbrüster, M.; Teschner, D.; Weinberg, G.; Wild, U.; Knop-Gericke, A.; Ressler, T.; Grin, Y.; Schlögl, R. Etching of the intermetallic compounds PdGa and Pd3Ga7: An effective way to increase catalytic activity? J. Catal. 2009, 264, 93–103. [Google Scholar] [CrossRef]
- García-Trenco, A.; Regoutz, A.; White, E.R.; Payne, D.J.; Shaffer, M.S.P.; Williams, C.K. PdIn intermetallic nanoparticles for the Hydrogenation of CO2 to Methanol. Appl. Catal. B Environ. 2018, 220, 9–18. [Google Scholar] [CrossRef]
- Shesterkina, A.A.; Kustov, L.M.; Strekalova, A.A.; Kazansky, V.B. Heterogeneous iron-containing nanocatalysts—Promising systems for selective hydrogenation and hydrogenolysis. Catal. Sci. Technol. 2020, 10, 3160–3174. [Google Scholar] [CrossRef]
- Chen, M.; Kumar, D.; Yi, C.-W.; Goodman, D.W. The Promotional Effect of Gold in Catalysis by Palladium-Gold. Science 2005, 310, 291–293. [Google Scholar] [CrossRef]
- Tao, F.; Zhang, S.; Nguyen, L.; Zhang, X. Action of bimetallic nanocatalysts under reaction conditions and during catalysis: Evolution of chemistry from high vacuum conditions to reaction conditions. Chem. Soc. Rev. 2012, 41, 7980. [Google Scholar] [CrossRef]
- Ellert, O.G.; Tsodikov, M.V.; Nikolaev, S.A.; Novotortsev, V.M. Bimetallic nanoalloys in heterogeneous catalysis of industrially important reactions: Synergistic effects and structural organization of active components. Russ. Chem. Rev. 2014, 83, 718–732. [Google Scholar] [CrossRef]
- Venezia, A.M.; Liotta, L.F.; Deganello, G.; Schay, Z.; Horváth, D.; Guczi, L. Catalytic CO oxidation over pumice supported Pd–Ag catalysts. Appl. Catal. A Gen. 2001, 211, 167–174. [Google Scholar] [CrossRef]
- Sneka-Płatek, O.; Kaźmierczak, K.; Jędrzejczyk, M.; Sautet, P.; Keller, N.; Michel, C.; Ruppert, A.M. Understanding the influence of the composition of the Ag Pd catalysts on the selective formic acid decomposition and subsequent levulinic acid hydrogenation. Int. J. Hydrogen Energy 2020, 45, 17339–17353. [Google Scholar] [CrossRef]
- Sápi, A.; Rajkumar, T.; Kiss, J.; Kukovecz, Á.; Kónya, Z.; Somorjai, G.A. Metallic Nanoparticles in Heterogeneous Catalysis. Catal. Lett. 2021, 151, 2153–2175. [Google Scholar] [CrossRef]
- Ponec, V. Alloy catalysts: The concepts. Appl. Catal. A Gen. 2001, 222, 31–45. [Google Scholar] [CrossRef]
- Liu, P.; Nørskov, J.K. Ligand and ensemble effects in adsorption on alloy surfaces. Phys. Chem. Chem. Phys. 2001, 3, 3814–3818. [Google Scholar] [CrossRef]
- Markov, P.V.; Bukhtiyarov, A.V.; Mashkovsky, I.S.; Smirnova, N.S.; Prosvirin, I.P.; Vinokurov, Z.S.; Panafidin, M.A.; Baeva, G.N.; Zubavichus, Y.V.; Bukhtiyarov, V.I.; et al. PdIn/Al2O3 Intermetallic Catalyst: Structure and Catalytic Characteristics in Selective Hydrogenation of Acetylene. Kinet. Catal. 2019, 60, 842–850. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Tao, F.; Grass, M.E.; Zhang, Y.; Butcher, D.R.; Renzas, J.R.; Liu, Z.; Chung, J.Y.; Mun, B.S.; Salmeron, M.; Somorjai, G.A. Reaction-Driven Restructuring of Rh-Pd and Pt-Pd Core-Shell Nanoparticles. Science 2008, 322, 932–934. [Google Scholar] [CrossRef]
- McCue, A.J.; Anderson, J.A. Recent advances in selective acetylene hydrogenation using palladium containing catalysts. Front. Chem. Sci. Eng. 2015, 9, 142–153. [Google Scholar] [CrossRef]
- Løvvik, O.M. Surface segregation in palladium based alloys from density-functional calculations. Surf. Sci. 2005, 583, 100–106. [Google Scholar] [CrossRef]
- Chen, Z.-X.; Neyman, K.M.; Rösch, N. Theoretical study of segregation of Zn and Pd in Pd–Zn alloys. Surf. Sci. 2004, 548, 291–300. [Google Scholar] [CrossRef]
- Wu, J.B.; Shi, R.P.; Qin, Z.F.; Liu, H.; Li, Z.K.; Zhu, H.Q.; Zhao, Y.X.; Wang, J.G. Selective oxidation of methanol to methyl formate over bimetallic Au-Pd nanoparticles supported on SiO2. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 2019, 47, 780–790. [Google Scholar] [CrossRef]
- Zafeiratos, S.; Piccinin, S.; Teschner, D. Alloys in catalysis: Phase separation and surface segregation phenomena in response to the reactive environment. Catal. Sci. Technol. 2012, 2, 1787. [Google Scholar] [CrossRef]
- McCue, A.J.; Gibson, A.; Anderson, J.A. Palladium assisted copper/alumina catalysts for the selective hydrogenation of propyne, propadiene and propene mixed feeds. Chem. Eng. J. 2016, 285, 384–391. [Google Scholar] [CrossRef]
- McCue, A.J.; Anderson, J.A. CO induced surface segregation as a means of improving surface composition and enhancing performance of CuPd bimetallic catalysts. J. Catal. 2015, 329, 538–546. [Google Scholar] [CrossRef]
- Smirnova, N.S.; Markov, P.V.; Baeva, G.N.; Rassolov, A.V.; Mashkovsky, I.S.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Panafidin, M.A.; Zubavichus, Y.V.; Bukhtiyarov, V.I.; et al. CO-induced segregation as an efficient tool to control the surface composition and catalytic performance of PdAg3/Al2O3 catalyst. Mendeleev Commun. 2019, 29, 547–549. [Google Scholar] [CrossRef]
- Bukhtiyarov, A.V.; Panafidin, M.A.; Prosvirin, I.P.; Mashkovsky, I.S.; Markov, P.V.; Rassolov, A.V.; Smirnova, N.S.; Baeva, G.N.; Rameshan, C.; Rameshan, R.; et al. Boosting the activity of PdAg2/Al2O3 supported catalysts towards the selective acetylene hydrogenation by means of CO-induced segregation: A combined NAP XPS and mass-spectrometry study. Appl. Surf. Sci. 2022, 604, 154497. [Google Scholar] [CrossRef]
- Van Spronsen, M.A.; Daunmu, K.; O’Connor, C.R.; Egle, T.; Kersell, H.; Oliver-Meseguer, J.; Salmeron, M.B.; Madix, R.J.; Sautet, P.; Friend, C.M. Dynamics of Surface Alloys: Rearrangement of Pd/Ag(111) Induced by CO and O2. J. Phys. Chem. C 2019, 123, 8312–8323. [Google Scholar] [CrossRef]
- Zhu, B.; Thrimurthulu, G.; Delannoy, L.; Louis, C.; Mottet, C.; Creuze, J.; Legrand, B.; Guesmi, H. Evidence of Pd segregation and stabilization at edges of AuPd nano-clusters in the presence of CO: A combined DFT and DRIFTS study. J. Catal. 2013, 308, 272–281. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Goodman, D.W. CO Oxidation over AuPd(100) from Ultrahigh Vacuum to Near-Atmospheric Pressures: The Critical Role of Contiguous Pd Atoms. J. Am. Chem. Soc. 2009, 131, 5734–5735. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Henkelman, G. CO Adsorption-Driven Surface Segregation of Pd on Au/Pd Bimetallic Surfaces: Role of Defects and Effect on CO Oxidation. ACS Catal. 2013, 3, 2541–2546. [Google Scholar] [CrossRef]
- Strømsheim, M.D.; Svenum, I.-H.; Mahmoodinia, M.; Boix, V.; Knudsen, J.; Venvik, H.J. Segregation dynamics of a Pd-Ag surface during CO oxidation investigated by NAP-XPS. Catal. Today 2022, 384–386, 265–273. [Google Scholar] [CrossRef]
- Zhou, C.; Ngan, H.T.; Lim, J.S.; Darbari, Z.; Lewandowski, A.; Stacchiola, D.J.; Kozinsky, B.; Sautet, P.; Boscoboinik, J.A. Dynamical Study of Adsorbate-Induced Restructuring Kinetics in Bimetallic Catalysts Using the PdAu(111) Model System. J. Am. Chem. Soc. 2022, 144, 15132–15142. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sui, Z.; Zhu, Y.; Zhou, X.; Chen, D. Selective Hydrogenation of Acetylene over Pd-In/Al2O3 Catalyst: Promotional Effect of Indium and Composition-Dependent Performance. ACS Catal. 2017, 7, 7835–7846. [Google Scholar] [CrossRef]
- Osswald, J.; Giedigkeit, R.; Jentoft, R.E.; Armbrüster, M.; Girgsdies, F.; Kovnir, K.; Ressler, T.; Grin, Y.; Schlögl, R. Palladium-gallium intermetallic compounds for the selective hydrogenation of acetylene. Part I: Preparation and structural investigation under reaction conditions. J. Catal. 2008, 258, 210–218. [Google Scholar] [CrossRef]
- Osswald, J.; Kovnir, K.; Armbrüster, M.; Giedigkeit, R.; Jentoft, R.E.; Wild, U.; Grin, Y.; Schlögl, R. Palladium-gallium intermetallic compounds for the selective hydrogenation of acetylene. Part II: Surface characterization and catalytic performance. J. Catal. 2008, 258, 219–227. [Google Scholar] [CrossRef]
- Armbrüster, M. Intermetallic compounds in catalysis—A versatile class of materials meets interesting challenges. Sci. Technol. Adv. Mater. 2020, 21, 303–322. [Google Scholar] [CrossRef]
- Wencka, M.; Hahne, M.; Kocjan, A.; Vrtnik, S.; Koželj, P.; Korže, D.; Jagličić, Z.; Sorić, M.; Popčević, P.; Ivkov, J.; et al. Physical properties of the InPd intermetallic catalyst. Intermetallics 2014, 55, 56–65. [Google Scholar] [CrossRef]
- Furukawa, S.; Endo, M.; Komatsu, T. Bifunctional Catalytic System Effective for Oxidative Dehydrogenation of 1-Butene and n -Butane Using Pd-Based Intermetallic Compounds. ACS Catal. 2014, 4, 3533–3542. [Google Scholar] [CrossRef]
- Mashkovsky, I.S.; Smirnova, N.S.; Markov, P.V.; Baeva, G.N.; Bragina, G.O.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Stakheev, A.Y. Tuning the surface structure and catalytic performance of PdIn/Al2O3 in selective liquid-phase hydrogenation by mild oxidative-reductive treatments. Mendeleev Commun. 2018, 28, 603–605. [Google Scholar] [CrossRef]
- Bukhtiyarov, A.V.; Panafidin, M.A.; Prosvirin, I.P.; Smirnova, N.S.; Markov, P.V.; Baeva, G.N.; Mashkovsky, I.S.; Bragina, G.O.; Rameshan, C.; Gerasimov, E.Y.; et al. Deliberate control of the structure-specific active sites in PdIn bimetallic catalysts using adsorbate induced segregation effects. Appl. Surf. Sci. 2023, 608, 155086. [Google Scholar] [CrossRef]
- Bukhtiyarov, A.V.; Panafidin, M.A.; Chetyrin, I.A.; Prosvirin, I.P.; Mashkovsky, I.S.; Smirnova, N.S.; Markov, P.V.; Zubavichus, Y.V.; Stakheev, A.Y.; Bukhtiyarov, V.I. Intermetallic Pd-In/HOPG model catalysts: Reversible tuning the surface structure by O2-induced segregation. Appl. Surf. Sci. 2020, 525, 146493. [Google Scholar] [CrossRef]
- Panafidin, M.A.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Chetyrin, I.A.; Yu Klyushin, A.; Knop-Gericke, A.; Smirnova, N.S.; Markov, P.V.; Mashkovsky, I.S.; Zubavichus, Y.V.; et al. A mild post-synthesis oxidative treatment of Pd-In/HOPG bimetallic catalysts as a tool of their surface structure fine tuning. Appl. Surf. Sci. 2022, 571, 151350. [Google Scholar] [CrossRef]
- Varga, E.; Pusztai, P.; Oszkó, A.; Baán, K.; Erdőhelyi, A.; Kónya, Z.; Kiss, J. Stability and Temperature-Induced Agglomeration of Rh Nanoparticles Supported by CeO 2. Langmuir 2016, 32, 2761–2770. [Google Scholar] [CrossRef]
- Piminov, P.A.; Baranov, G.N.; Bogomyagkov, A.V.; Berkaev, D.E.; Borin, V.M.; Dorokhov, V.L.; Karnaev, S.E.; Kiselev, V.A.; Levichev, E.B.; Meshkov, O.I.; et al. Synchrotron Radiation Research and Application at VEPP-4. Phys. Procedia 2016, 84, 19–26. [Google Scholar] [CrossRef]
- Aulchenko, V.M.; Evdokov, O.V.; Kutovenko, V.D.; Pirogov, B.Y.; Sharafutdinov, M.R.; Titov, V.M.; Tolochko, B.P.; Vasiljev, A.V.; Zhogin, I.A.; Zhulanov, V.V. One-coordinate X-ray detector OD-3M. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2009, 603, 76–79. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Lutterotti, L. Total pattern fitting for the combined size-strain-stress-texture determination in thin film diffraction. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 334–340. [Google Scholar] [CrossRef]
- Available online: http://xpspeak.software.informer.com/4.1/ (accessed on 31 August 2023).
- Moulder, J.F.; Stckle, W.F.; Sobol, P.E.; Bomben, K.D.; Chastain, J. Standard XPS spectra of the elements. In Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., King, R.C., Eds.; Perkin-Elmer: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Lorenz, H.; Turner, S.; Lebedev, O.I.; Van Tendeloo, G.; Klötzer, B.; Rameshan, C.; Pfaller, K.; Penner, S. Pd–In2O3 interaction due to reduction in hydrogen: Consequences for methanol steam reforming. Appl. Catal. A Gen. 2010, 374, 180–188. [Google Scholar] [CrossRef]
- Langford, J.I.; Wilson, A.J.C. Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 1978, 11, 102–113. [Google Scholar] [CrossRef]
- Iwasa, N.; Mayanagi, T.; Ogawa, N.; Sakata, K.; Takezawa, N. New catalytic functions of Pd–Zn, Pd–Ga, Pd–In, Pt–Zn, Pt–Ga.pdf. Catal. Lett. 1998, 54, 119–123. [Google Scholar] [CrossRef]
- Zhou, R.-S.; Snyder, R.L. Structures and transformation mechanisms of the η, γ and θ transition aluminas. Acta Crystallogr. Sect. B Struct. Sci. 1991, 47, 617–630. [Google Scholar] [CrossRef]
- Lear, T.; Marshall, R.; Antonio Lopez-Sanchez, J.; Jackson, S.D.; Klapötke, T.M.; Bäumer, M.; Rupprechter, G.; Freund, H.-J.; Lennon, D. The application of infrared spectroscopy to probe the surface morphology of alumina-supported palladium catalysts. J. Chem. Phys. 2005, 123, 174706. [Google Scholar] [CrossRef]
- Ye, J.; Ge, Q.; Liu, C. Effect of PdIn bimetallic particle formation on CO2 reduction over the Pd–In/SiO2 catalyst. Chem. Eng. Sci. 2015, 135, 193–201. [Google Scholar] [CrossRef]
- Wu, Z.; Wegener, E.C.; Tseng, H.T.; Gallagher, J.R.; Harris, J.W.; Diaz, R.E.; Ren, Y.; Ribeiro, F.H.; Miller, J.T. Pd-In intermetallic alloy nanoparticles: Highly selective ethane dehydrogenation catalysts. Catal. Sci. Technol. 2016, 6, 6965–6976. [Google Scholar] [CrossRef]
- Stakheev, A.Y.; Smirnova, N.S.; Krivoruchenko, D.S.; Baeva, G.N.; Mashkovsky, I.S.; Yakushev, I.A.; Vargaftik, M.N. Single-atom Pd sites on the surface of Pd–In nanoparticles supported on γ-Al2O3: A CO-DRIFTS study. Mendeleev Commun. 2017, 27, 515–517. [Google Scholar] [CrossRef]
- Rameshan, C.; Lorenz, H.; Mayr, L.; Penner, S.; Zemlyanov, D.; Arrigo, R.; Haevecker, M.; Blume, R.; Knop-Gericke, A.; Schlögl, R.; et al. CO2-selective methanol steam reforming on In-doped Pd studied by in situ X-ray photoelectron spectroscopy. J. Catal. 2012, 295, 186–194. [Google Scholar] [CrossRef]
- Burueva, D.B.; Kovtunov, K.V.; Bukhtiyarov, A.V.; Barskiy, D.A.; Prosvirin, I.P.; Mashkovsky, I.S.; Baeva, G.N.; Bukhtiyarov, V.I.; Stakheev, A.Y.; Koptyug, I.V. Selective Single-Site Pd−In Hydrogenation Catalyst for Production of Enhanced Magnetic Resonance Signals using Parahydrogen. Chem. A Eur. J. 2018, 24, 2547–2553. [Google Scholar] [CrossRef]
- Zemlyanov, D.; Aszalos-Kiss, B.; Kleimenov, E.; Teschner, D.; Zafeiratos, S.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R.; Gabasch, H.; Unterberger, W.; et al. In situ XPS study of Pd(111) oxidation. Part 1: 2D oxide formation in 10−3mbar O2. Surf. Sci. 2006, 600, 983–994. [Google Scholar] [CrossRef]
- Sen, P.; Kar, D.; Laha, R.; Ananthan, M.R.; Kasiviswanathan, S. Electrical conduction in gold nanoparticles embedded indium oxide films: A crossover from metallic to insulating behavior. J. Phys. Condens. Matter 2019, 31, 505702. [Google Scholar] [CrossRef] [PubMed]
- McGuire, G.E.; Schweitzer, G.K.; Carlson, T.A. Study of Core Electron Binding Energies in Some Group IIIA, VB, and VIB Compounds. Inorg. Chem. 1973, 12, 2450–2453. [Google Scholar] [CrossRef]
- McGuirk, G.M.; Ledieu, J.; Gaudry, É.; de Weerd, M.-C.; Fournée, V. Surface structures of In-Pd intermetallic compounds. I. Experimental study of In thin films on Pd(111) and alloy formation. J. Chem. Phys. 2014, 141, 084702. [Google Scholar] [CrossRef]
- Neumann, M.; Teschner, D.; Knop-Gericke, A.; Reschetilowski, W.; Armbrüster, M. Controlled synthesis and catalytic properties of supported In–Pd intermetallic compounds. J. Catal. 2016, 340, 49–59. [Google Scholar] [CrossRef]
- Pancotti, A.; de Siervo, A.; Carazzolle, M.F.; Landers, R.; Nascente, P.A.P. Ultra-thin films of In on Pd(111) characterized by X-ray photoelectron diffraction. Thin Solid Films 2019, 688, 137442. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukhtiyarov, A.V.; Panafidin, M.A.; Prosvirin, I.P.; Smirnova, N.S.; Markov, P.V.; Baeva, G.N.; Mashkovsky, I.S.; Bragina, G.O.; Vinokurov, Z.S.; Zubavichus, Y.V.; et al. Reversible Transformations of Palladium–Indium Intermetallic Nanoparticles upon Repetitive Redox Treatments in H2/O2. Crystals 2023, 13, 1356. https://doi.org/10.3390/cryst13091356
Bukhtiyarov AV, Panafidin MA, Prosvirin IP, Smirnova NS, Markov PV, Baeva GN, Mashkovsky IS, Bragina GO, Vinokurov ZS, Zubavichus YV, et al. Reversible Transformations of Palladium–Indium Intermetallic Nanoparticles upon Repetitive Redox Treatments in H2/O2. Crystals. 2023; 13(9):1356. https://doi.org/10.3390/cryst13091356
Chicago/Turabian StyleBukhtiyarov, Andrey V., Maxim A. Panafidin, Igor P. Prosvirin, Nadezhda S. Smirnova, Pavel V. Markov, Galina N. Baeva, Igor S. Mashkovsky, Galina O. Bragina, Zakhar S. Vinokurov, Yan V. Zubavichus, and et al. 2023. "Reversible Transformations of Palladium–Indium Intermetallic Nanoparticles upon Repetitive Redox Treatments in H2/O2" Crystals 13, no. 9: 1356. https://doi.org/10.3390/cryst13091356
APA StyleBukhtiyarov, A. V., Panafidin, M. A., Prosvirin, I. P., Smirnova, N. S., Markov, P. V., Baeva, G. N., Mashkovsky, I. S., Bragina, G. O., Vinokurov, Z. S., Zubavichus, Y. V., Bukhtiyarov, V. I., & Stakheev, A. Y. (2023). Reversible Transformations of Palladium–Indium Intermetallic Nanoparticles upon Repetitive Redox Treatments in H2/O2. Crystals, 13(9), 1356. https://doi.org/10.3390/cryst13091356








