Structural Aspects of Pt(η3–P1C2X1C2P2)(Y) Derivative Types
Abstract
:1. Introduction
2. Pt(η3–P1C2X1C2P2)(Y), (X1 = O1,N1, C1, S1, or Si1)
2.1. Pt(η3–P1O1P2)(P3)
2.2. Pt(η3–P1N1P2)(Y), (Y = N2 L, (x1), CL(x9), Cl(x7), P3L(x2))
2.3. Pt(η3–P1C1P2)(Y), (Y = OL (x4), NL(x4), C2L (x9), Cl (x12), Br (x2))
2.4. Pt(η3–P1S1P2)(Y), (Y = CH3 (x1), Cl (x2), P3Ph3 (x2), I (x1))
2.5. Pt(η3–P1Si1P2)(Y), (Y = H (x2), OL (x1), NL (x1), CL (x1), Cl (x5), P3L (x1))
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| But2P(C11H16N3)PBut2 | (N,N’-3,3,5 triethylpyridine-2,6-(1H,2H)-diylidene)bis(di-t-butyl(phosphinusamidato) |
| But2P(C12H9)PBut2 | (1,3-bis(di-t-butylphosphinomethyl)-2-naphtyl |
| But2P(C7H6N)PBut2 | (6-((di-t-butylphosphino)methyl-2-((di-t-butyl phosphino)methylene)-1,2-dihydropyridine-1-yl) |
| But2P(C7H7N)PBut2 | (2,6-bis(di-t-butylphosphino)methyl)pyridine |
| (CF3)2P(C8H7)P(CF3)2 | (2,6-bis(bis(trifluoromethyl)phosphinomethyl) phenyl) |
| (η2–C18H28)P(C7H6N)P(η1–C18H24) | (2-((5,7-di-t-butyl-3,3-dimethyl-2,3-dihydro-14-phosphindol-1-yl)methylene)-6-(((2,4,6-tri-t-butylphenylphosphino)methyl)-1,2-dihydro pyridinyl) undecachloro-carba-undecaborane |
| cy2P(C6H4)Si(Me)(C6H4)Pcy2 | ((methylsilanediyl)di-2,1-phenylene)bis(dicyclohexylphosphine)) |
| Pcy3 | tricyclohexylphosphino |
| Ph2P(C12H8N)PPh2 | (2,2’-bis (diphenylphosphino)diphenylamido) |
| Ph2P(C14H7)PPh2 | (1,8-bis(diphenylphosphino)-9-anthryl) |
| Ph2P(C14H7O2)PPh2 | (1,8-bis(diphenylphosphino)-9-hydroxy-10-oxo-9,10-dihydroanthracen-9-yl) |
| Ph2P(C15H12O)PPh2 | ((9,9-dimethyl-9H-xanthene-4,5-diyl)bis (diphenylphosphine)-diphenyl(2-pyridyl) phosphine |
| Ph2P(C18H19O8)PPh2 | (2,6-bis(1-(diphenylphosphino)-3-methoxy-2-(methoxycarbonyl)-3-oxopropylphenyl) |
| Ph2P(C20H11O2)PPh2 | (13,16-bis(diphenylphosphino)-3,6-dihydroxypentacyclo [6.6.6.O2,7.O9,14.O15,20]icosa-2,4,6,9,11,13,15,17,19-nonaen-1-yl) |
| Ph2P(C20H13O4)PPh2 | (3,13-bis(diphenylphosphino)-15,16-bis(methoxy-carbonyl)tetracyclo[6.6.6.2 O2,7.O9,14]hexadeca-2,4,6,9,11,13,15-heptaen-1-yl) |
| Ph2P(C23H28S)PPh2 | ((9,9-dimethyl-2,7-bis(t-butyl)-9H-thioxantene-4,5-diyl)bis(diphenylphosphine) |
| Ph2P(C6H4)S(=O)(C6H4)PPh2 | ((sulfinydi-2,1-phenylene)bis(diphenylphosphine) |
| Ph2P(C8H7)PPh2 | (2,6-bis((diphenylphosphino)methyl)phenyl) |
| Ph2P(CH2)2S(CH2)2PPh2 | (bis(2-(diphenylphosphino)ethyl)sulfide) |
| PPh3 | triphenylphosphine |
| Pri2P (C20H11)PPri2 | (3,13-bis(diisopropylphosphino)-pentacyclo [6.6.6.O2,7.O9,14.O15,20]icosa-2,4,6,9,11,13,15,17,19-heptaen-1-yl) |
| Pri2P(C12H6F2N)PPri2 | (2-(diisopropylphosphino)-N-(2-(diisopropyl-phosphino)-4-fluorophenyl)-4-fluoroanilinato) |
| Pri2P(C12H7F2N)PPri2 | (2-(diisopropylphosphino)-N-(2-(diisopropyl-phosphino)-4-fluorophenyl)-4-fluoroaniline) |
| Pri2P(C14H12N)PPh2 | (bis(2-(di-isopropylphosphino)-4-methyl-phenyl)(2-((diphenylphosphino)-4-methyl-phenyl)amide) |
| Pri2P(C6H4)Si(C12H46P)(C6H4)PPri2 | (tris(2-(diisopropylphosphino)phenyl)silyl) |
| Pri2P(C6H4)Si(H)(C6H4)PPri2 | ((silanediyldi-2,1-phenylene)bis(diiso-propyl phosphine)) |
References
- Rosenberg, B.; Van Camp, L.; Trasko, Y.E.; Mansour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Ashiq, M.; Danish, M.; Mohsin, M.; Bari, S.; Mukhtar, F. Chemistry of Platinum and Palladium Metal Complexes in Homogeneous and Heterogeneous Catalysis: A Mini Review. Int. J. Sci. Basic. Appl. Res. 2013, 7, 50–61. Available online: http://gssrr.org/index.php?journal=JournalOfBasicAndApplied&page=article&op=view&path%5B%5D=1134 (accessed on 25 May 2023).
- Chaaban, M.; Zhou, C.; Lin, H.; Chyic, B.; Ma, B. Platinum(ii) binuclear complexes: Molecular structures, photophysical properties, and applications. J. Mater. Chem. C 2019, 7, 5910–5924. [Google Scholar] [CrossRef]
- Santos, T.M.R.; Andolpho, G.A.; Tavares, C.A.; Gonçalves, M.A.; Ramalho, T.C. Improving the Path to Obtain Spectroscopic Parameters for the PI3K—(Platinum Complex) System: Theoretical Evidences for Using 195Pt NMR as a Probe. Magnetochemistry 2023, 9, 89. [Google Scholar] [CrossRef]
- Horiuchi, S.; Umakoshi, K. Recent advances in pyrazolato-bridged homo- and heterometallic polynuclear platinum and palladium complexes. Coord. Chem. Rev. 2023, 476, 214924. [Google Scholar] [CrossRef]
- Melník, M.; Mikuš, P. Organomonophosphines in PtP2Cl2 Derivatives: Structural Aspects. Rev. Inorg. Chem. 2015, 35, 179–189. [Google Scholar] [CrossRef]
- Melník, M.; Mikuš, P. Organodiphosphines in PtP2X2 (X = As, Ge or Te) Derivatives—Structural Aspects. Main. Group. Chem. 2020, 43, 132–137. [Google Scholar] [CrossRef]
- Melník, M.; Mikuš, P. Distortion Isomers of Cis-PtP2X2 and Cis-PtP2XY Derivatives—Structural Aspects. Rev. Inorg. Chem. 2020, 40, 153–165. [Google Scholar] [CrossRef]
- Albrecht, M.; van Koten, G. Platinum Group Organometallics Based on “Pincer” Complexes: Sensors, Switches, and Catalysts. Angew. Chem. Int. Ed. 2001, 40, 3750. [Google Scholar] [CrossRef]
- van der Boom, M.E.; Milstein, D. Cyclometalated Phosphine-Based Pincer Complexes: Mechanistic Insight in Catalysis, Coordination, and Bond Activation. Chem. Rev. 2003, 103, 1759. [Google Scholar] [CrossRef]
- Rybtchinski, B.; Milstein, D. Metal Insertion into C−C Bonds in Solution. Angew. Chem. Int. Ed. 1999, 38, 870. [Google Scholar] [CrossRef]
- Singleton, J.T. The Uses of Pincer Complexes in Organic Synthesis. Tetrahedron 2003, 59, 1837–1857. [Google Scholar] [CrossRef]
- Vigalok, A.; Milstein, D. Advances in metal chemistry of quinonoid compounds: New types of interactions between metals and aromatics. Acc. Chem. Res. 2001, 34, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Milstein, D. Challenging metal-based transformations. From single-bond activation to catalysis and metallaquinonoids. Pure Appl. Chem. 2003, 75, 445–460. [Google Scholar] [CrossRef]
- Jensen, C.M. Iridium PCP pincer complexes: Highly active and robust catalysts for novel homogeneous aliphatic dehydrogenations. Chem. Commun. 1999, 2443–2449. [Google Scholar] [CrossRef]
- Gandelman, M.; Konstantinovski, L.; Rozenberg, H.; Milstein, D. Interplay between solvent and counteranion stabilization of highly unsaturated rhodium(III) complexes: Facile unsaturation-induced dearomatization. Chem. Eur. J. 2003, 9, 2595–2602. [Google Scholar] [CrossRef]
- Melník, M.; Mikuš, P. Tetradentate organophosphines in Pt(η4–A4L) (A = P4, P3Si), P2X2 (X2 = N2, S2, C2), (PX3) (X3 = N3, N2O): Structural aspects. Main Group Met. Chem. 2021, 44, 270–280. [Google Scholar] [CrossRef]
- Melník, M.; Mikuš, P. Heterotridentate organophosphines in Pt(κ3–P1C1P2)(X), (X=H, OL, NL, CL, Cl, Br or I) and Pt(κ3–P1P2C1)(Cl) derivatives structural aspects. J. Mol. Struct. 2023, 1276, 134768. [Google Scholar] [CrossRef]
- Lenero, K.Q.A.; Guari, Y.; Kamer, P.C.J.; van Leeuwen, P.W.N.M.; Donnadiere, B.; Sabo-Etienne, S.; Chaudret, B.; Lutz, M.; Spek, A.L. Heterolytic activation of dihydrogen by platinum and palladium complexes. Dalton Trans. 2013, 42, 6495–6512. [Google Scholar] [CrossRef]
- Liang, L.C.; Lin, J.M.; Lee, W.Y. Benzene C–H Activation by Platinum(Ii) Complexes of Bis(2-Diphenylphosphinophenyl)Amide. Chem. Commun. 2005, 19, 2462–2464. [Google Scholar] [CrossRef]
- Feller, M.; Ben-Ari, E.; Iron, M.A.; Diskin-Posner, Y.; Leitus, G.; Shimon, L.J.W.; Konstantinovski, L.; Milstein, D. Cationic, Neutral, and Anionic PNP Pd II and Pt II Complexes: Dearomatization by Deprotonation and Double-Deprotonation of Pincer Systems. Inorg. Chem. 2010, 49, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, S.; Matta, A.; Valles, H.; Bracken, J.B.; Miranda, M.; Yoo, J.; Hahn, C. Addition to Alkynes Promoted by a Dicationic Platinum(II) Complex. Organometallics 2011, 30, 6446–6457. [Google Scholar] [CrossRef]
- Hahn, C. Structural Investigations of Platinum(II) Styrene and Styryl Complexes and Mechanistic Study of Vinylic Deprotonation. Organometallics 2010, 29, 1331–1338. [Google Scholar] [CrossRef]
- DeMott, J.C.; Bhuvanesh, N.; Ozerov, O.V. Frustrated Lewis Pair-like Splitting of Aromatic C–H Bonds and Abstraction of Halogen Atoms by a Cationic [(FPNP)Pt]+. Species. Chem. Sci. 2013, 4, 642–649. [Google Scholar] [CrossRef]
- Cucciolito, M.E.; D’Amora, A.; Tuzi, A.; Vitagliano, A. Catalytic Hydroarylation of Olefins Promoted by Dicationic Platinum(II) and Palladium(II) Complexes. The Interplay of C−C Bond Formation and M−C Bond Cleavage. Organometallics 2007, 26, 5216–5223. [Google Scholar] [CrossRef]
- Lansing, R.B.; Goldberg, K.I.; Kemp, R.A. Unsymmetrical RPNPR′ Pincer Ligands and Their Group 10 Complexes. Dalton Trans. 2011, 40, 8950–8958. [Google Scholar] [CrossRef]
- Sacco, A.; Vasapollo, G.; Nobile, C.F.; Piergiovanni, A.; Pellinghelli, M.A.; Lanfranchi, M. Syntheses and Structures of 2-Diphenylphosphinomethylenide-6-Diphenylphosphinomethylenepyridine Complexes of Palladium(II) and Platinum(II); Crystal Structures of [PtCl2-(CHPPH2)-6-(CH2PPh2)Pyridine] and [Pd(COOMe)2-(CHPPh2)-6-(CH2PPh2)Pyridine]. J. Organomet. Chem. 1988, 356, 397–409. [Google Scholar] [CrossRef]
- Takeuchi, K.; Taguchi, H.; Tanigawa, I.; Tsujimoto, S.; Matsuo, T.; Tanaka, H.; Yoshizawa, I.K.; Ozawa, F. A Square-Planar Complex of Platinum(0). Angew. Chem. Int. Ed. Engl. 2016, 55, 15347–15350. [Google Scholar] [CrossRef]
- Taguchi, H.; Chang, Y.H.; Takeuchi, K.; Ozawa, F. Catalytic Synthesis of an Unsymmetrical PNP-Pincer-Type Phosphaalkene Ligand. Organometallics 2015, 34, 1589–1596. [Google Scholar] [CrossRef]
- Adams, J.J.; Arulsamy, N.; Roddick, D.M. Acceptor Pincer Coordination Chemistry of Platinum: Reactivity Properties of (CF3 PCP)Pt(L) + (L = NC5F5, C2H4). Organometallics 2009, 28, 1148–1157. [Google Scholar] [CrossRef]
- Arunachalampillai, A.; Loganathan, N.; Wendt, O.F. Carboxylation of Pincer PCP Platinum Methoxide Complexes under Formation of Metalla Carbonates. Polyhedron 2012, 32, 24–29. [Google Scholar] [CrossRef]
- Musa, S.; Shpruhman, A.; Gelman, D. New PC(sp3)P pincer complexes of platinum and palladium. J. Organomet. Chem. 2012, 699, 92–95. [Google Scholar] [CrossRef]
- De-Botton, S.; Romm, R.; Bensoussan, G.; Hitrik, M.; Musa, S.; Gelman, D. Coordination Versatility of P-Hydroquinone-Functionalized Dibenzobarrelene-Based PC(Sp3)P Pincer Ligands. Dalton Trans. 2016, 45, 16040–16046. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.X.; Yang, X.Y.; Tay, W.S.; Li, Y.; Pullarkat, S.A.; Xu, K.; Hirao, H.; Leung, P.H. Computational and Carbon-13 NMR Studies of Pt–C Bonds in P–C–P Pincer Complexes. Dalton Trans. 2016, 45, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.J.; Lau, A.; Arulsamy, N.; Roddick, D.M. Synthesis and Platinum Coordination Chemistry of the Perfluoroalkyl Acceptor Pincer Ligand, 1,3-(CH2P(CF3)2)2C6H4. Inorg. Chem. 2007, 46, 11328–11334. [Google Scholar] [CrossRef]
- Vuzman, D.; Poverenov, E.; Diskin-Posner, Y.; Leitus, G.; Shimon, L.J.W.; Milstein, D. Reactivity and Stability of Platinum(Ii) Formyl Complexes Based on PCP-Type Ligands. The Significance of Sterics. Dalton Trans. 2007, 48, 5692–5700. [Google Scholar] [CrossRef]
- Schwartsburd, L.; Poverenov, E.; Shimon, L.J.W.; Milstein, D. Naphthyl-Based PCP Platinum Complexes. Nucleophilic Activation of Coordinated CO and Synthesis of a Pt(II) Formyl Complex. Organometallics 2007, 26, 2931–2936. [Google Scholar] [CrossRef]
- Spek, A.L.; Dani, P.; van Koten, G. Crystal Structure of [Pt{η3-Ph2P(C8H7)PPh2}(η1-C12H19N2)]. Private Communication CCDB, IXICEX (2004). Available online: https://www.ccdc.cam.ac.uk/ (accessed on 28 April 2023).
- Hughes, R.P.; Williamson, A.; Incarvito, C.D.; Rheingold, A.L. Synthesis and Molecular Structure of a Perfluoroalkyl Complex of Platinum Containing a PCP Pincer Ligand. Organometallics 2001, 20, 4741–4744. [Google Scholar] [CrossRef]
- Albrecht, M.; Dani, P.; Lutz, M.; Spek, A.L.; van Koten, G. Transcyclometalation Processes with Late Transition Metals: C-aRyl−H Bond Activation via Noncovalent C−H···Interactions. J. Am. Chem. Soc. 2000, 122, 11822–11833. [Google Scholar] [CrossRef]
- Olsson, D.; Arunachalampillai, A.; Wendt, O.F. Synthesis and Characterisation of PCsp3P Phosphine and Phosphinite Platinum(Ii) Complexes. Cyclometallation and Simple Coordination. Dalton Trans. 2007, 46, 5427–5433. [Google Scholar] [CrossRef]
- Poverenov, E.; Leitus, G.; Shimon, L.J.W.; Milstein, D. C-Metalated Diazoalkane Complexes of Platinum Based on PCP- and PCN-Type Ligands. Organometallics 2005, 24, 5937–5944. [Google Scholar] [CrossRef]
- Fischer, S.; Wendt, O.F. {2,6-Bis[(Diphenylphosphino)Methyl]phenyl} chloroplatinum(II). Acta Cryst. Sect. E Struct. Rep. Online 2004, 60, m69–m70. [Google Scholar] [CrossRef]
- Hu, J.; Xu, H.; Nguyen, M.H.; Yip, J.H.K. Photooxidation of a Platinum-Anthracene Pincer Complex: Formation and Structures of Pt II -Anthrone and -Ketal Complexes. Inorg. Chem. 2009, 48, 9684–9692. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Tay, W.S.; Li, Y.; Pullarkat, S.A.; Leung, P.H. Versatile Syntheses of Optically Pure PCE Pincer Ligands: Facile Modifications of the Pendant Arms and Ligand Backbones. Organometallics 2015, 34, 1582–1588. [Google Scholar] [CrossRef]
- Azerraf, C.; Gelman, D. New Shapes of PC(Sp3)P Pincer Complexes. Organometallics 2009, 28, 6578–6584. [Google Scholar] [CrossRef]
- Williams, B.S.; Dani, P.; Lutz, M.; Spek, A.L.; van Koten, G. Development of the First P-Stereogenic PCP Pincer Ligands, Their Metallation by Palladium and Platinum, and Preliminary Catalysis. Helv. Chim. Acta 2001, 84, 3519–3530. [Google Scholar] [CrossRef]
- Suess, D.L.M.; Peters, J.C. Late-Metal Diphosphinosulfinyl S(O)P 2 Pincer-Type Complexes. Organometallics 2012, 31, 5213–5222. [Google Scholar] [CrossRef]
- Siah, S.Y.; Leung, P.H.; Mok, K.F. Palladium(II) and Platinum(II) Complexes with a Novel P—S(O)—P Tridentate Ligand. Polyhedron 1994, 13, 3253–3255. [Google Scholar] [CrossRef]
- Andreasen, L.V.; Hazell, A.; Wernberg, O. [Bis(2-Diphenylphosphinoethyl) Sulfide-κ3P,S,P′](Triphenylphosphine-κP)Platinum(II) Diperchlorate Acetone Solvate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2002, 58, m385–m387. [Google Scholar] [CrossRef]
- Emslie, D.J.H.; Harrington, L.E.; Jenkins, H.A.; Robertson, C.M.; Britten, J.F. Group 10 Transition-Metal Complexes of an Ambiphilic PSB-Ligand: Investigations into η3(BCC)-Triarylborane Coordination. Organometallics 2008, 27, 5317–5325. [Google Scholar] [CrossRef]
- Suh, H.W.; Balcells, D.; Edwards, A.J.; Guard, L.M.; Hazari, N.; Mader, E.A.; Mercado, B.Q.; Repisky, M. Understanding the Solution and Solid-State Structures of Pd and Pt PSiP Pincer-Supported Hydrides. Inorg. Chem. 2015, 54, 11411–11422. [Google Scholar] [CrossRef] [PubMed]
- Mitton, S.J.; McDonald, R.; Turculet, L. Synthesis and Characterization of Neutral and Cationic Platinum(II) Complexes Featuring Pincer-like Bis(Phosphino)Silyl Ligands: Si−H and Si−Cl Bond Activation Chemistry. Organometallics 2009, 28, 5122–5136. [Google Scholar] [CrossRef]
- Korshin, E.E.; Leitus, G.; Shimon, L.J.W.; Konstantinovski, L.; Milstein, D. Silanol-Based Pincer Pt(II) Complexes: Synthesis, Structure, and Unusual Reactivity. Inorg. Chem. 2008, 47, 7177–7189. [Google Scholar] [CrossRef] [PubMed]
- DeMott, J.C.; Gu, W.; McCulloch, B.J.; Herbert, D.E.; Goshert, M.D.; Walensky, J.R.; Zhou, J.; Ozerov, O.V. Silyl–Silylene Interplay in Cationic PSiP Pincer Complexes of Platinum. Organometallics 2015, 34, 3930–3933. [Google Scholar] [CrossRef]
- Brost, R.D.; Bruce, G.C.; Joslin, F.L.; Stobart, S.R. Phosphinoalkylsilyl Complexes. 12. Stereochemistry of the Tridentate Bis(Diphenylphosphinopropyl)Silyl (BiPSi) Framework: Complexation That Introduces “Face Discrimination” at Coordinatively Unsaturated Metal Centers. X-Ray Crystal and Molecular Structure. Organometallics 1997, 16, 5669–5680. [Google Scholar] [CrossRef]
- Bachechi, F. X-Ray Structural Analysis of NiII, PdII, and PtII Complexes with the Potentially Tridentate Ligand 1,3-Bis(Diphenylphosphinomethyl)Benzene, 1,3-C6H4(CH2PPh2)2. Struct. Chem. 2003, 14, 263–269. [Google Scholar] [CrossRef]
- Tsay, C.; Mankad, N.P.; Peters, J.C. Four-Coordinate, Trigonal Pyramidal Pt(II) and Pd(II) Complexes. J. Am. Chem. Soc. 2010, 132, 13975–13977. [Google Scholar] [CrossRef]
- Takaya, J.; Iwasawa, N. Reaction of Bis(o-Phosphinophenyl)Silane with M(PPh3)4(M = Ni, Pd, Pt): Synthesis and Structural Analysis of H2-(Si–H) Metal(0) and Pentacoordinate Silyl Metal(Ii) Hydride Complexes of the Ni Triad Bearing a PSiP-Pincer Ligand. Dalton Trans. 2011, 40, 8814–8821. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural Variation in Copper(i) Complexes with Pyridylmethylamide Ligands: Structural Analysis with a New Four-Coordinate Geometry Index, Τ4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef]


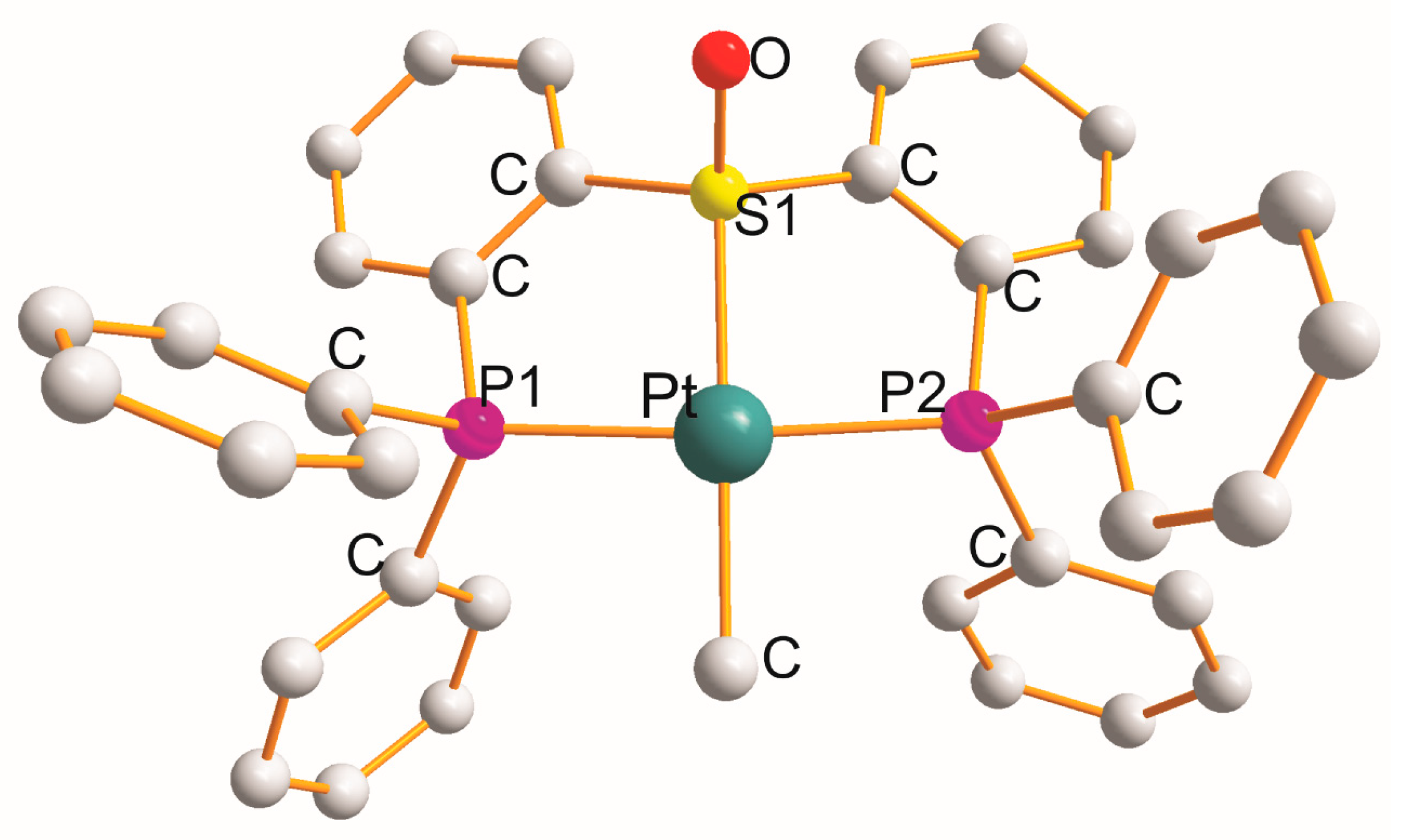
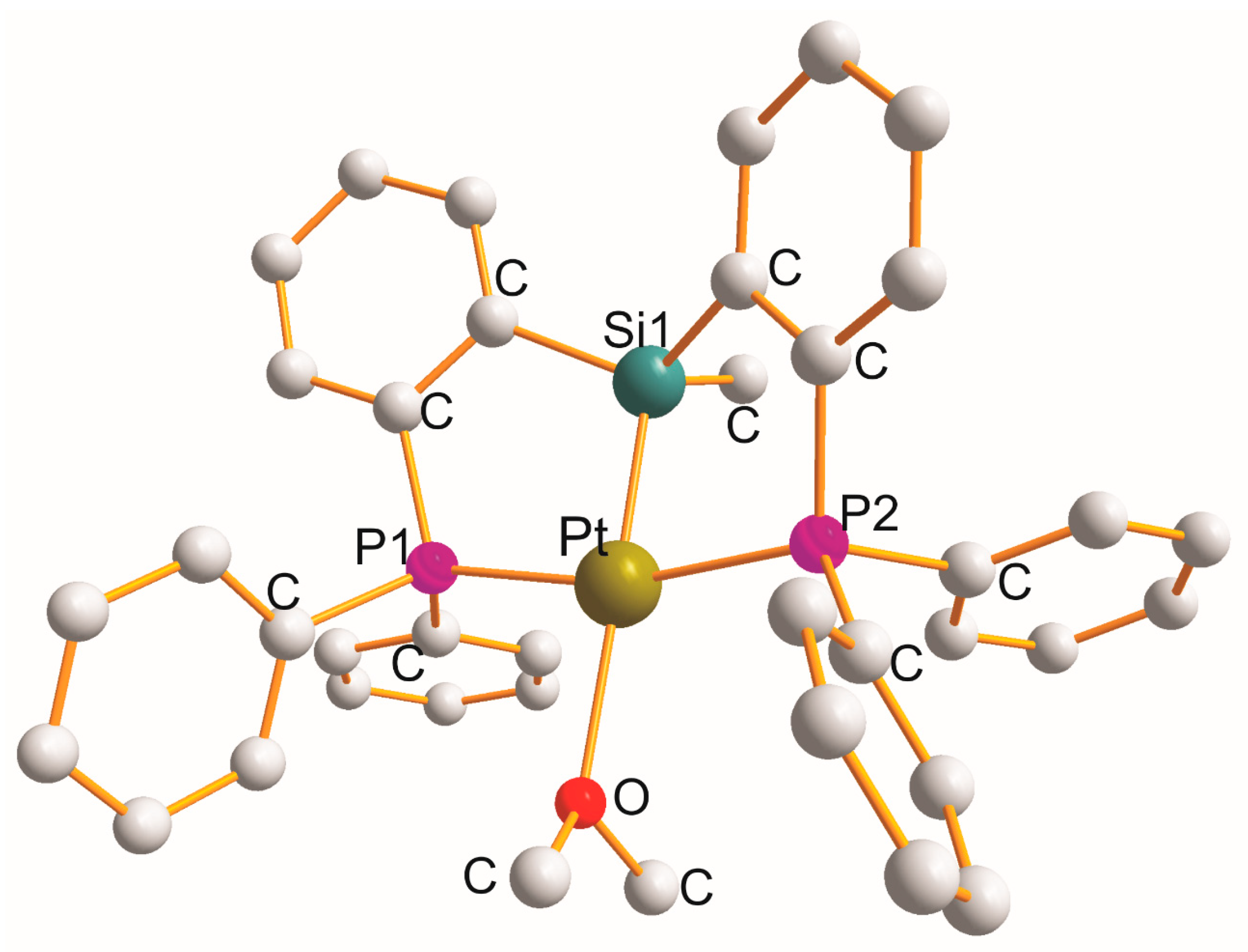
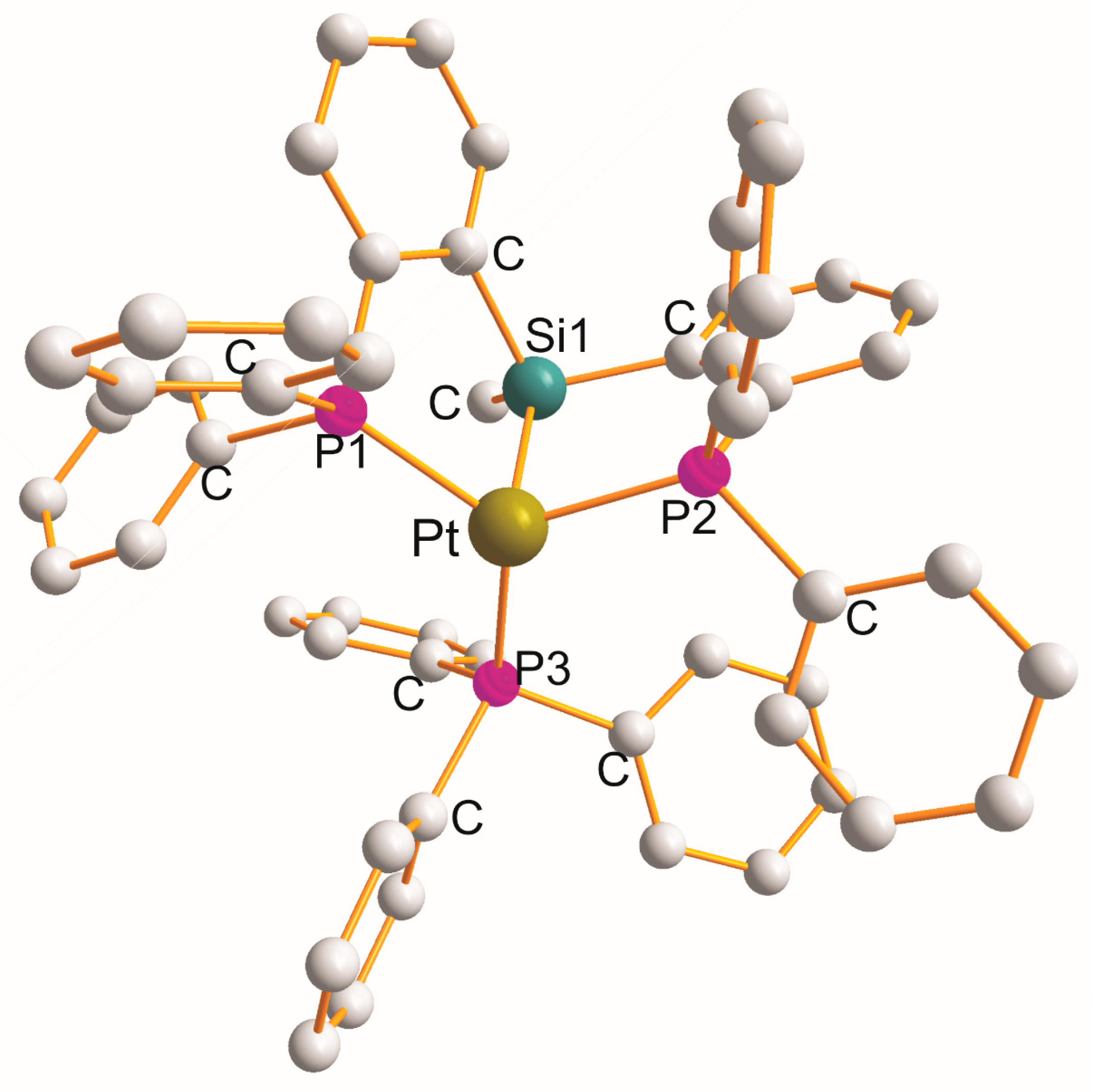
| Complex | Cryst. cl. Space gr. Z | a (Å) b (Å) c (Å) | α (°) β (°) γ (°) | Chromophore (Chelate Rings) τ4 b | Pt-L c (Å) | L-Pt-L c (˚) | Ref. |
|---|---|---|---|---|---|---|---|
| [Pt{η3-Ph2P(C15H12O) PPh2} {η1-P3(C5H4N) (Ph)2}].2CF3SO3.0.5H2O (at 150 K) | m C2/c 4 | 42.762(0) 12.161(0) 23.995(0) | 121.60(0) | PtP2OP (P1C2O1C2P2) 0.164 | P1,P2 2.302(-,11) O1 2.189 P3 2.239 | P1,2,O1 81.6(-,7) d P1,P2 162.2 P1,2,P3 98.5(-,1,7) O1,P3 174.7(2) | [19] |
| [Pt{η3-Ph2P (C12H8N). PPh2-P1,N1,P2}(py)]. CF3SO3.toluene | m P21/n 4 | 15.114(0) 17.695(0) 16.901(0) | 105.98(0) | PtP2NN (P1C2N1C2P2) 0.125 | P1 2.294 P2 2.273 N1 2.024 py, N2 2.056 | P1,2,N1 83.3(-,7) d P1,P2 166.6 P1,2,N2 96.1(-,3.0) N1,N2 175.6 | [20] |
| [Pt{η3-Ph2P(C2H8N)PPh2-P1,N1,P2}(CH3)] | or Fdd2 4 | 9.961(0) 18.601(0) 32.725(0) | PtP2NC (P1C2N1C2P2) 0.110 | P1 2.274 P2 2.274 N1 2.093 C 2.110 | P1,2,N1 82.3 d P1,P2 164.6 P1,2,C 97.7 N1,C 180.0 | [20] | |
| [Pt{η3-But2P(C7H7N) PBut2-P1,N1,P2} (CH3)]Cl.CHCl3 (at 100 K) | tr P 2 | 14.569(0) 15.447(0) 16.237(0) | 114.73(0) 99.12(0) 96.40(0) | PtP2NC (P1C2N1C2P2) 0.128 | P1 2.286 P2 2.301 N1 2.108 C 2.057 | P1,2,N1 84.0 d P1,P2 164.4 P1,2,C 96.1(-,1) N1,C 177.5 | [21] |
| [Pt{η3-But2P(C7H7N)PBut2-P1,N1,P2} (CH3)]Cl.CHCl3 (at 100 K) | m P21/c 8 | 10.976(2) 15.479(3) 30.094(3) | 90.71(3) | PtP2NC (P1C2N1C2P2) 0.120 | P1 2.295 P2 2.282 N1 2.089 C 2.105 | P1,2,N1 83.2(-,4) d P1,P2 164.6 N1,N2 96.6(-,5) N1,C 178.3 | [21] |
| [Pt{η3-Ph2P(C7H7N) PPh2-P1,N1,P2}{C(=O) Et}].BF4.0.5CH2Cl2 (at 100 K) | m P21/n 4 | 18.173(3) 9.960(1) 21.092(4) | 114.94(0) | PtP2NC (P1C2N1C2P2) 0.130 | P1 2.279 P2 2.284 N1 2.131 C 2.001 | P1,2,N1 81.8(-,4) d P1,P2 163.6 P1,2,C 98.1(-,1.8) N1,C 178.0 | [22] |
| [Pt{η3-Ph2P(C7H7N) PPh2-P1,N1,P2} (CH2CHO)].BF4 (at 110 K) | tr P 2 | 9.198(1) 10.688(1) 16.421(1) | 99.77(0) 100.04(0) 97.95(0) | PtP2NC (P1C2N1C2P2) 0.112 | P1 2.269 P2 2.303 N1 2.112 C 2.132 | P1,2,N1 83.0(-,2) d P1,P2 165.9 P1,2,C 97.0(-,1.7) N1,C 178.1 | [22] |
| [Pt{η3-Ph2P(C7H7N)PPh2-P1,N1,P2} (CH = CHPh)].BF4 | tr P 2 | 12.534(8) 17.101(8) 17.919(8) | 70.04(0) 82.64(0) 78.66(0) | PtP2NC (P1C2N1C2P2) 0.135 | P1 2.289 P2 2.309 N1 2.125 C 2.005 | P1,2,N1 82.3(-,6) d P1,P2 164.3 P1,2,C 97.6(-,1.2) N1,C 176.6 | [23] |
| [Pt{η3-Pri2P(C12H7F2N)PPri2-P1,N1,P2} (C6H4F)].B(C6F4)4 (at 110 K) | m P21/c 4 | 15.495(2) 18.673(3) 22.444(2) | 125.95(0) | PtP2NC (P1C2N1C2P2) 0.171 | P1 2.286 P2 2.272 N1 2.126 C 2.015 | P1,2,N1 83.0(-,1.2) P1,P2 163.4 P1,2,C 96.5(-,2.0) N1,C 172.3 | [24] |
| [Pt{η3-Pri2P(C12H7F2N)PPri2-P1,N1,P2} (p-tol)].B(C6F5)4 (at 110 K) | m P21/n 4 | 15.655(1) 18.868(1) 18.242(1) | 98.76(0) | PtP2NC (P1C2N1C2P2) 0.225 | P1 2.288 P2 2.270 N1 2.183 C 2.070 | P1,2,N1 83.9(-,8) P1,P2 161.4 P1,2,C 97.4(-,3.5) N1,C 166.8 | [24] |
| [Pt{η3-Ph2P(C7H7N)PPh2-P1,N1,P2} (C11H15O3)].BF4 | m P21/c 4 | 18.286(2) 11.617(3) 20.683(1) | 114.68(1) | PtP2NC (P1C2N1C2P2) 0.130 | P1 2.263 P2 2.282 N1 2.097 C 2.157 | P1,2,N1 83.0(-,9) P1,P2 164.9 P1,2,C 97.1(-,1.6) N1,C 176.6 | [25] |
| [Pt{η3-Ph2P(C12H8N)PPh2}(Cl)].5C6H6 | m P21/n 4 | 17.378(0) 12.705(0) 25.555(0) | 104.58(0) | PtP2NCl (P1C2N1C2P2) 0.107 | P1,2 2.277(-,7) N1 2.024 Cl 2.318 | P1,2,N1 83.7(-,1) P1,P2 167.3 P1,2,Cl 96.3(-,1.9) N1,Cl 177.5 | [20] |
| [Pt{η3-But2P(C7H6N)PBut2}(Cl)] (at 120 K) | or Pna21 4 | 22.499(0) 8.107(0) 14.161(0) | PtP2NCl (P1C2N1C2P2) 0.102 | P1,2 2.296(-,15) N1 2.021 Cl 2.333 | P1,2,N1 84.6(-,3) P1,P2 169.2 P1,2,Cl 95.3(-,4) N1,Cl 176.5 | [21] | |
| [Pt{η3-But2P(C7H7N)PBut2}(Cl)]Cl | trg P3 6 | 18.631(3) 14.821(3) | PtP2NCl (P1C2N1C2P2) 0.092 | P1,2 2.302(-,1) N1 2.030 Cl 2.307 | P1,2,N1 84.2(-,3) P1,P2 168.2 P1,2,Cl 95.8(-,5) N1,Cl 178.9 | [21] | |
| [Pt{η3-Pri2P(C12H6F2N)PPri2} (Cl)].CHB11Cl11 (at 110 K) | m P21/c 4 | 19.446(20) 15.820(18) 15.256(15) | 107.78(1) | PtP2NCl (P1C2N1C2P2) 0.151 | P1 2.285 P2 2.304 N1 1.987 Cl 2.297 | P1,2,N1 84.2(-,6) P1,P2 166.7 P1,2,Cl 95.8(-,1.9) N1,Cl 171.9 | [24] |
| [Pt{η3-Ph2P(C14H12N)PPri2}(Cl)].0.5C6H6 (at 183 K) | m P21/c 4 | 9.702(0) 11.526(0) 28.499(1) | 100.73(0) | PtP2NCl (P1C2N1C2P2) 0.120 | P1,2 2.278(-,5) N1 2.028 Cl 2.321 | P1,2,N1 83.2(-,1.2) d P1,P2 164.0 P1,2,Cl 96.7(-,1.2) N1,Cl 179.2 | [26] |
| [Pt{η3-Ph2P(C14H12N) P(cy)2} (Cl)].C6H6 (at 183 K) | tr P 2 | 11.322(0) 11.735(0) 15.501(0) | 93.81(0) 110.18(0) 93.00(0) | PtP2NCl (P1C2N1C2P2) 0.135 | P1,2 2.282(-,9) N1 2.047 Cl 2.322 | P1,2,N1 82.9(-,1) P1,P2 164.8 P1,2,Cl 97.2(-,2.0) N1,Cl 176.1 | [26] |
| [Pt{η3-Ph2P(CH2)(C5H4N)(CH2)Ph2P-P1,N1,P2}(Cl)] | m P21/n 4 | 15.915(5) 19.337(8) 8.861(6) | 98.94(2) | PtP2NCl (P1C2N1C2P2) 0.089 | P1,2 2.285(3,1) N1 2.2008(7) Cl 2.312(3) | P1,2,N1 84.6(2,4) d P1,P2 169.0(2) P1,2,Cl 95.5(1,9) N1,Cl 178.5(2) | [27] |
| [Pt{η3-(η1-C24H44)P(C7H6N)P(η1-C24H44)}(PPh3)].2CH2Cl2 (at 103 K) | tr P 2 | 13.341(2) 18.981(4) 30.668(6) | 93.86(0) 90.89(0) 98.03 (0) | PtP2NP (P1C2N1C2P2) 0.199 | P1,2 2.317(-,8) N1 2.082(3) P3 2.270 | P1,2,N1 80.9(-,6) d P1,P2 158.7 P1,2,P3 98.3(-,2.0) N1, P3 173.0 | [28] |
| [Pt{η3-(η2-C18H28)P(C7H6N)P(η1-C18H29)}(Pcy3)] (at 103 K) | m P21/c 4 | 17.281(2) 11.881(1) 28.514(4) | 104.32(0) | PtP2NP (P1C2N1C2P2) 0.209 | P1 2.326 P2 2.389 N1 2.073 P3 2.284 | P1,2,N1 82.7(-,1.1) d P2,N1 162.9 P1,P198.0(-,5.8) N1, P3 167.5 | [29] |
| Complex | Cryst. cl. Space gr. Z | a (Å) b (Å) c (Å) | α (°) β (°) γ (°) | Chromophore (Chelate Rings) τ4 b | Pt-L c (Å) | L-Pt-L c (°) | Ref. |
|---|---|---|---|---|---|---|---|
| [Pt{η3-(CF3)2P(C8H7)P(CF3)2}. (H2O)]SbF6 (at 150 K) | m C2/c 4 | 41.924(0) 10.457(0) 22.512(0) | 110.54(0) | PtP2CO (P1C2C1C2P2) 0.143 | P1,2 2.245 C1 1.995 H2O 2.156 | P1,2,C1 81.7(-,2) d P1,P2 163.3 P1,2,O 98.3(-,3.9) C1,O 176.3 | [30] |
| [Pt{η3-Ph2P(C8H7) PPh2}(H2O)]CF3SO3 (at 165 K) | tr Pī 2 | 9.644(1) 13.885(1) 14.012(3) | 119.08(1) 93.43(1) 104.59(1) | PtP2CO (P1C2C1C2P2) 0.146 | P1,2 2.295(-,3) C1 1.995 H2O 2.156 | P1,2,C1 82.0(-,6) d P1,P2 163.7 P1,2,O 98.1(-,2.7) C1,O 176.6 | [31] |
| [Pt{η3-Ph2P(C8H7)PPh2} (OMe)].0.5C6H6 | tr Pī 2 | 8.824(2) 12.517(1) 14.712(2) | 91.94(1) 104.96(1) 104.20(1) | PtP2CO (P1C2C1C2P2) 0.120 | P1,2 2.266(-,4) C1 2.053 MeO 2.086 | P1,2,C1 83.7(-,7) d P1,P2 166.6 P1,2,O 98.8(-,6.8) C1,O 176.5 | [31] |
| [Pt{(η3-Pri2)P(C20H11) P(Pri2)}(OOCCF3)] (at 173 K) | or Fdd2 4 | 29.241(1) 39.275(2) 11.361(0) | PtP2CO (P1C2C1C2P2) 0.181 | P1,2 2.289(-,1) C1 2.045 O 2.139 | P1,2,C1 85.8(-,1) d P1,P2 157.0 P1,2,O 94.2(-,2.5) C1,O 177.4 | [32] | |
| [Pt{η3-(CF3)2P(C8H7)P (CF3)2}(NC5F5)]B(C6F5)4 (at 150 K) | m P21/c 4 | 13.932(1) 17.137(2) 19.213(0) | 96.29(0) | PtP2CN (P1C2C1C2P2) 0.138 | P1,2 2.242(-,2) C1 2.030 N 2.173 | P1,2,C1 80.6(-,1) d P1,P2 161.3 P1,2,N 99.3(-,5) C1,N 179.2 | [30] |
| [Pt{η3-Ph2P(C20H13O4)PPh2}. (N≡CCH3)]BF4 (at 173 K) | or Pca21 6 | 21.587(3) 8.904(1) 21.327(3) | PtP2CN (P1C2C1C2P2) 0.191 | P1,2 2.303(-,1) C1 1.892 N 2.088 | P1,2,C1 85.9(-,1.9) d P1,P2 156.4 P1,2,N 93.5 C1,N 176.8 | [32] | |
| [Pt{η3-Ph2P(C20H11O2)PPh2}. (NC5H5)]Cl.(NC5H5)3 | m P21/c 4 | 12.892(6) 15.613(8) 26.900(10) | 101.48(1) | PtP2CN (P1C2C1C2P2) 0.138 | P1,2 2.275(-,2) C1 2.021 N 2.111 | P1,2,C1 81.0 d P1,P2 162.0 P1,2,N 98.8 C1,N 178.5 | [33] |
| [Pt{η3-Ph2P(C20H11O2)PPh2}. (N≡CCH3)]BF4.CH2Cl2 (at 173 K) | m P21/c 4 | 14.711(1) 15.920(1) 17.987(1) | 92.15(0) | PtP2CN (P1C2C1C2P2) 0.202 | P1,2 2.293(-,5) C1 2.062 N 2.056 | P1,2,C1 85.7(-,3) d P1,P2 157.4 P1,2,N 97.3(-,3) C1,N 174.0 | [33] |
| [Pt{η3-Ph2P(C24H19O2)PPh2}(CN)] (at 103 K) | tg P212121 4 | 9.492(0) 9.492 (0) 13.030(1) | PtP2C2 (P1C2C1C2P2) 0.140 | P1,2 2.286 C1 2.029 NC 2.062 | P1,2,C1 80.1 d P1,P2 160.1 P1,2,C 99.2 C1,C 180.0 | [34] | |
| [Pt{η3-(CF3)2P(C8H7)P. (CF3)2}(CO)]SbF6 | tr Pī 2 | 11.770(1) 13.950(1) 14.529(1) | 91.31(0) 100.81(0) 91.90(0) | PtP2C2 (P1C2C1C2P2) 0.125 | P1,2 2.256 C1 1.969 OC 2.053 | P1,2,C1 81.6(-,3) d P1,P2 163.4 P1,2,C 98.3(-,3) C1,C 179.0 | [35] |
| [Pt{η3-(CF3)2P(C8H7)P. (CF3)2}(CH3)] | m P21/c 8 | 14.732(0) 16.189(0) 17.737(0) | 113.42(3) | PtP2C2 (P1C2C1C2P2) 0.153 | P1,2 2.203(-,2) C1 2.089 H3C 2.148 | P1,2,C1 81.0(-,5) d P1,P2 161.0 P1,2,C 98.9(-,4) C1,C 177.3 | [35] |
| [Pt{η3-Pri2P(C8H7)PPri2}(CO)]. CF3SO30.5C6H6 (at 120 K) | m P21/c 4 | 13.430(3) 15.667(3) 15.472(3) | 112.73(3) | PtP2C2 (P1C2C1C2P2) 0.117 | P1,2 2.305(-,2) C1 2.048 C 2.053 | P1,2,C1 82.7(-,5) d P1,P2 165.2 P1,2,C 97.3(-,9) C1,C 178.2 | [36] |
| [Pt{η3-But2P(C8H7)PBut2}(η1-CHOMe)].CF3SO3.thf (at 120 K) | m P21/c 4 | 15.824(0) 11.375(0) 20.093(0) | 97.46(0) | PtP2C2 (P1C2C1C2P2) 0.123 | P1,2 2.302(-,1) C1 2.081 C 1.986 | P1,2,C1 81.9(-,3) d P1,P2 163.5 P1,2,C 98.0(-,1.1) C1,C 179.2 | [34] |
| [Pt{η3-But2P(C12H9)PBut2} (CO)]BF4 (at 120 K) | or Pna21 8 | 12.097(2) 13.150(3) 38.514(8) | PtP2C2 (P1C2C1C2P2) 0.102 | P1,2 2.311(-,4) C1 2.074 OC 1.903 | P1,2,C1 83.1(-,1) d P1,P2 166.3 P1,2,C 96.8(-,2) C1,C 179.3 | [37] | |
| [Pt{η3-Ph2P(C8H7)PPh2}(η1-C12H19N2)] (at 150 K) | m P21/c 4 | 17.576(3) 12.436(2) 18.593(5) | 114.34(1) | PtP2C2 (P1C2C1C2P2) 0.146 | P1,2 2.269(-,2) C1 2.142 C 2.091 | P1,2,C1 81.0(-,2) d P1,P2 162.0 P1,2,C 99.0(-,3.1) C1,C 177.5 | [38] |
| [Pt{η3-Ph2P(C8H7)PPh2}(η1-C3F7)] (at 150 K) | m P21/c 4 | 10.192(3) 18.106(8) 17.018(3) | 91.01(1) | PtP2C2 (P1C2C1C2P2) 0.143 | P1,2 2.279(-,2) C1 2.072 C 2.186 | P1,2,C1 81.4(-,2) d P1,P2 162.8 P1,2,C 98.4(-,5) C1,C 177.0 | [39] |
| [Pt{η3-Ph2P(C8H7)PPh2} (η1-C12H21N2)]2BF4 (at 150 K) | m C2 2 | 12.887(0) 15.901(0) 12.177(0) | 121.54(0) | PtP2C2 (P1C2C1C2P2) 0.133 | P1,2 2.275 C1 2.084 C 2.096 | P1,2,C1 80.6 d P1,P2 161.2 P1,2,C 99.4 C1,C 180.0 | [40] |
| [Pt{η3-Ph2P (C20H11O2P)Ph2}Cl]. (CH3CN)4 (at 150 K) | tr Pī 2 | 12.485(1) 14.669(2) 15.038(2) | 118.03(0) 106.22 (0) 90.30(0) | PtP2CCl (P1C2C1C2P2) 0.232 | P1,2 2.265 C1 2.086 Cl 2.394 | P1,2,C1 85.4(-,3) d P1, P2 155.4 P1,2,Cl 96.2(-,6) C1,Cl 172.0 | [33] |
| [Pt{η3-Ph2P(C24H19O2)PPh2}Cl] (at 103 K) | m P21 4 | 11.798(3) 26.540(2) 12.725(1) | 96.64(0) | PtP2CCl (P1C2C1C2P2) 0.138 | P1,2 2.276(-,1) C1 2.022 Cl 2.388 | P1,2,C1 82.7(-,1.0) d P1, P2 163.9 P1,2,Cl 97.4(-,4) C1,Cl 176.4 | [34] |
| [Pt{η3-(CF3)2P(C8H7)P (CF3)2}Cl]1.5(C6H6) (at 173 K) | m P21/c 4 | 10.111(0) 19.270(0) 13.082(0) | 102.86(0) | PtP2CCl (P1C2C1C2P2) 0.156 | P1,2 2.233(-,5) C1 2.037 Cl 2.370 | P1,2,C1 80.9(-,3) d P1, P2 161.0 P1,2,Cl 99.2(-,5) C1,Cl 176.9 | [35] |
| [Pt{η3-But2P(C8H7)PBut2}Cl] (at 120 K) | or P212121 8 | 12.965(3) 13.853(3) 14.642(3) | PtP2CCl (P1C2C1C2P2) 0.094 | P1,2 2.288(-,3) C1 2.017 Cl 2.407 | P1,2,C1 83.7(-,1) d P1, P2 167.3 P1,2,Cl 96.3(-,64) C1,Cl 179.2 | [36] | |
| [Pt{η3-But2P(C12H9)PBut2}Cl] (at 120 K) | m P21/n 4 | 16.195(3) 10.440(2) 17.588(4) | 109.21(3) | PtP2CCl (P1C2C1C2P2) 0.097 | P1,2 2.287(-,6) C1 2.016 Cl 2.431 | P1,2,C1 83.9(-,4) d P1, P2 167.7 P1,2,Cl 96.1(-,6) C1,Cl 178.7 | [37] |
| [Pt{η3-But2P(C8H7)PBut2}Cl] | m P21/n 4 | 12.018(0) 14.803(0) 15.728(0) | 100.79(0) | PtP2CCl (P1C2C1C2P2) 0.120 | P1,2 2.305(-,3) C1 2.065 Cl 2.434 | P1,2,C1 83.8(-,5) d P1, P2 167.3 P1,2,Cl 96.1(-,1) C1,Cl 175.6 | [41] |
| Pt{η3-Pri2P(C8H7)PPri2}Cl] (at 120 K) | tr Pī 2 | 11.148(2) 13.935(3) 14.683(3) | 78.16(3) 82.35(3) 89.33(3) | PtP2CCl (P1C2C1C2P2) 0.105 | P1,2 2.279(-,4) C1 2.006 Cl 2.436 | P1,2,C1 83.6(-,2) d P1, P2 167.4 P1,2,Cl 96.1(-,1) C1,Cl 177.7 | [42] |
| [Pt{η3-Ph2P(C8H7)PPh2}Cl] | m P21/n 4 | 10.290(2) 16.117(3) 15.173(3) | 105.47(3) | PtP2CCl (P1C2C1C2P2) 0.130 | P1,2 2.277(-,2) C1 2.002 Cl 2.383 | P1,2,C1 81.6(-,6) d P1,P2 163.1 P1,2,Cl 98.4(-,1.1) C1,Cl 178.5 | [43] |
| [Pt{η3-Ph2P(C14H7)PPh2}Cl]. CH3CN (at 223 K) | m P21/c 4 | 12.773(0) 17.780(0) 14.72448(0) | 96.54(0) | PtP2CCl (P1C2C1C2P2) 0.107 | P1,2 2.268(-,2) C1 1.991 Cl 2.391 | P1,2,C1 83.7(-,1) d P1,P2 167.3 P1,2,Cl 96.2(-,1.7) C1,Cl 177.5 | [44] |
| [Pt{η3-Ph2P(C13H7O2)PPh2} Cl].CH3CN (at 223 K) | or Pbca 8 | 18.768(0) 16.966(0) 21.649(0) | PtP2CCl (P1C2C1C2P2) 0.138 | P1,2 2.266(-,1) C1 2.050 Cl 2.397 | P1,2,C1 84.6(-,7) d P1,P2 166.3 P1,2,Cl 95.8(-,2) C1,Cl 174.3 | [44] | |
| [Pt{η3-Ph2P(C18H19O8) PPh2}Cl]CH2Cl2 (at 103 K) | or P212121 8 | 10.432(1) 17.092(2) 24.423(3) | PtP2CCl (P1C2C1C2P2) 0.163 | P1,2 2.277(-,5) C1 2.006 Cl 2.385 | P1,2,C1 81.0(-,1) d P1,P2 162.0 P1,2,Cl 99.0(-,5) C1,Cl 174.7 | [45] | |
| Pt{η3-Pri2P(C20H11)PPri2}Cl]. (CH3CN)2 | m P21/n 4 | 14.622(0) 15.048(1) 15.642(1) | 96.24(0) | PtP2CCl (P1C2C1C2P2) 0.171 | P1,2 2.284(-,1) C1 2.064 Cl 2.392 | P1,2,C1 86.0(-,2) d P1,P2 156.2 P1,2,Cl 94.0(-,2) C1,Cl 179.53 | [46] |
| [Pt{η3-Ph2P(C8H7)PPh2}Br] | m P21/n 4 | 10.127(2) 14.776(2) 19.023(3) | 91.01(1) | PtP2CBr (P1C2C1C2P2) 0.138 | P1,2 2.272(-,14) C1 2.023 Br 2.468 | P1,2,C1 82.4(-,1) d P1,P2 164.8 P1,2,Br 97.7(-,6) C1,Br 175.8 | [47] |
| [Pt{η3-But2P(C6H5N2)PBut2}(Br)] (at 150 K) | m C2 4 | 15.517(0) 13.055(0) 15.383(0) | 118.78(0) | PtP2CBr (P1C2C1C2P2) 0.140 | P1 2.290 P2 2.030 Br 2.466 | P1,2,C1 82.5 d P1,P2 165.0 P1,2,Br 97.5 C1,Br 175.2 | [40] |
| Complex | Cryst. cl. Space gr. Z | a (Å) b (Å) c (Å) | α (°) β (°) γ (°) | Chromophore (Chelate Rings) τ4 b | Pt-L c (Å) | L-Pt-L c (˚) | Ref. |
|---|---|---|---|---|---|---|---|
| [Pt{η3-Ph2P(C6H4) S(=O)(C6H4)PPh2} (CH3)]PF6.CH3CN (at 100 K) | m P21/n 4 | 8.936(2) 16.722(4) 25.078(6) | 95.72(1) | PtP2SC (P1C2S1C2P2) 0.102 | P1,2 2.273(-,3) S1 2.268 H3C 2.093 | P1,2,S1 86.6(-,1) d P1,P2 167.0 P1,2,C 93.2(-,3) S1,C 178.2 | [48] |
| [Pt{η3-Ph2P(C6H4)S(=O)(C6H4)PPh2}(Cl)].PF6.CH3CN | m P21/c 4 | 13.360(0) 15.308(0) 18.787(0) | 106.11(0) | PtP2SCl (P1C2S1C2P2) 0.140 | P1,2 2.307 S1 2.192 Cl 2.316 | P1,2,S 84.6 P1,P2 162.7 P1,2,Cl 94.8 S1,Cl 177.6 | [48] |
| [Pt{η3-Ph2P(CH2)2 S(=O)(CH2)2PPh2}(Cl)].ClO4 | tr P 2 | 9.460(2) 12.079(3) 13.834(3) | 93.53(2) 103.85(2) 104.22(2) | PtP2SCl (P1C2S1C2P2) 0.135 | P 2.319(2,0) S1 2.182(2) Cl 2.318(1) | P1,2,S1 85.6 P1,P2 164.4 P1,2,Cl 95.8 S1,Cl 176.6 | [49] |
| [Pt{η3-Ph2P(C6H4) S(=O)(C6H4)PPh2}(PPh3)].0.5(CH2Cl2)(at 100 K) | tr P 2 | 11.295(5) 11.469(1) 17.269(1) | 86.45(1) 88.54(1) 77.08(1) | PtP2SP (P1C2S1C2P2) 0.143 | P1,2 2.273(-,3) S1 2.313 Ph3P3 2.281 | P1,2,S1 86.0(-,3) d P1,P2 162.4 P1,2,P3 94.7 S1,P3 177.5 | [48] |
| [Pt{η3-Ph2P(CH2)2S (CH2)2}PPh2}(PPh3)].2.ClO4 Me2CO (at 120 K) | or Pnma 4 | 15.698(3) 15.337(3) 19.957(4) | PtP2SP (P1C2S1C2P2) 0.143 | P1,2 2.309(-,1) S1 2.343 Ph3P3 2.289 | P1,2,S1 81.3 d P1,P2 161.6 P1,2,P3 98.7 S1,P3 178.3 | [50] | |
| [Pt{η3-Ph2P(C23H28S)PPh2}(I)](I).1.74 CH2Cl2(at 173 K) | tr P 2 | 9.845(0) 15.277(0) 17.264(0) | 84.94(0) 84.03(0) 89.18(0) | PtP2Si (P1C2S1C2P2) 0.120 | P1,2 2.311(-,1) S1 2.252 I 2.510 | P1,2,S1 84.0(-,3) d P1,P2 165.4 P1,2,I 96.7 S, I 177.7 | [51] |
| [Pt{η3-cyh2P(C6H4)Si(Me). (C6H4)Pcyh2}(H)].0.5 pentane (at 150 K) | m C2/c 4 | 24.426(0) 16.300(0) 39.968(3) | 105.39(0) | PtP2SiH (P1C2Si1C2P2) 0.179 | P1,2 2.254(-,4) Si1 2.326 H 1.486 | P1,2,Si1 85.3(-,0) d P1, P2 159.5 P1,2,H 94.7(-,4.7) Si1, H 175.3 | [52] |
| [Pt{η3-cyh2P(C6H4)Si (Me)(C6H4)Pcyh2}(H)].1.25 pentane (at 93 K) | m C2/c 4 | 29.595(0) 17.388(0) 33.970(2) | 99.64(0) | PtP2SiH (P1C2Si1C2P2) 0.158 | P1,2 2.262(0,2) Si1 2.336 H 1.527 | P1,2,Si1 84.2(-,0) d P1, P2 162.0 P1,2,H 94.6(-,7) Si1, H 175.6 | [52] |
| [Pt{η3-Ph2P(C6H4)Si(Me) (C6H4)PPh2}(OEt2)].{B(C6F5)3 (CH2Ph)}.OEt2 | tr P 2 | 14.718(1) 14.957(1) 15.402(1) | 100.40(0) 103.42(0) 99.60(0) | PtP2SiO (P1C2Si1C2P2) 0.115 | P1,2 2.299(-,6) Si1 2.276 O 2.282 | P1,2,Si1 83.9(-,1.0) d P1, P2 165.5 P1,2, O 96.1(-,2.3) Si1, O 178.4 | [53] |
| [Pt{η3-cyh2P(C6H4)Si (Me)(C6H4)Pcyh2}(Ph)].OEt2 (at 173 K) | tr P 2 | 13.506(5) 14.057(5) 14.950(0) | 116.38(0) 93.48(0) 112.52(0) | PtP2SiC (P1C2Si1C2P2) 0.156 | P1,2 2.279(-,2) Si1 2.324 C 2.139 | P1,2,Si1 83.7(-,1) d P1, P2 162.7 P1,2, C 96.8(-,2) Si1, C 175.1 | [53] |
| [Pt{η3-Ph2P(C6H4)Si(Me)(C6H4)PPh2} (CH2Ph)].CH2Cl2(at 193 K) | tr P 2 | 13.586(1) 16.908(1) 19.771(2) | 89.73(0) 76.03(0) 67.88(0) | PtP2SiC (P1C2Si1C2P2) 0.151 | P1,2 2.260 Si1 2.356 C 2.201 | P1,2, Si1 82.6(-,3) d P1, P2 163.0 P1,2, C 97.0(-,1) Si1, Cl 175.7 | [53] |
| [Pt{η3-Pri2P(C6H4)Si(OH) (C6H4)PPri2}(CO)].B(C6F5)4 (at 120 K) | tr P 2 | 13.658(0) 15.098(0) 15.201(0) | 112.54(0) 94.60(0) 116.83(0) | PtP2SiC (P1C2Si1C2P2) 0.179 | P1,2 2.325(-,3) Si1 2.365 OC 1.994 | P1,2, Si1 82.1(-,2) d P1, P2 161.2 P1,2, C 98.3(-,1.1) Si1, C 173.4 | [54] |
| [Pt{η3-Pri2P(C6H4)Si(H)(C6H4)PPri2}(mes)] (at 110 K) | or P212121 6 | 8.186(0) 17.908(0) 21.795(1) | PtP2SiC (P1C2Si1C2P2) 0.171 | P1,2 2.286(-,2) Si1 2.312 C 2.154 | P1,2, Si1 83.4(-,2) d P1, P2 159.0 P1,2, C 98.4(-,1.1) Si1, C 177.0 | [55] | |
| [Pt{η3-Ph2P(C6H4)Si(Me)(C6H4)PPh2} (Cl)] (at 173 K) | m P4/c 4 | 9.911(1) 13.656(1) 23.845(3) | 97.90(0) | PtP2SiCl (P1C2Si1C2P2) 0.250 | P1,2 2.261 Si1 2.278 Cl 2.437 | P1,2, Si1 84.9(-,6) d P1, P2 155.4 P1,2, Cl 97.0(-,1) Si1, Cl 169.3 | [53] |
| [Pt{η3-Ph2P(C6H4)Si(Me)(C6H4)PPh2} (AlCl3)].2(C6H5F) (at 193 K) | or P212121 4 | 16.111(1) 16.989(1) 17.437(1) | PtP2SiCl (P1C2Si1C2P2) 0.181 | P1,2 2.289(-,0) Si1 2.285 Cl 2.438 | P1,2,Si1 84.7(-,1) d P1, P2 164.2 P1,2, Cl 96.8(2,3) Si1, Cl 170.2 | [53] | |
| [Pt{η3-Pri2P(C6H4)Si(OH)(C6H4)PPri2}(Cl)] (at 120 K) | m P21/c 4 | 8.465(0) 18.246(0) 16.810(0) | 94.98(0) | PtP2SiCl (P1C2Si1C2P2) 0.163 | P1,2 2.292(-,3) Si1 2.277 Cl 2.469 | P1,2, Si1 84.2(-,1) d P1,P2 161.7 P1,2,Cl 96.3(-,1.2) Si1,Cl 175.1 | [54] |
| [Pt{η3-Pri2P(C6H4)Si(H)(C6H4)Pcyh2}(Cl)] (at 110 K) | m P21/n 4 | 12.420(8) 13.735(8) 15.539(10) | 99.74(0) | PtP2SiCl (P1C2Si1C2P2) 0.146 | P1,2 2.296 Si1 2.276 Cl 2.452 | P1,2, Si1 84.2(-,2) d P1, P2 162.4 P1,2, Cl 95.9(-,2.8) Si1, Cl 177.1 | [55] |
| [Pt{η3-cyh2P(C6H4)Si(Me) (C6H4)Pcyh2}(Cl)] (at 153 K) | m P21/c 4 | 13.104(3) 16.579(3) 17.770(4) | 108.97(3) | PtP2SiCl (P1C2Si1C2P2) 0.140 | P1,2 2.293 Si1 2.279 Cl 2.460 | P1,2, Si1 84.7(-,1) d P1, P2 162.2 P1,2, Cl 95.4(-,1.8) Si1, Cl 178.0 | [56] |
| Donor Atoms | α-X1-Pt-Y (°) | β-P1-Pt-P2 (°) | τ4 |
|---|---|---|---|
| X1(S)-Pt-(H)Y | 162.2 | 175.6 | 0.151 |
| X1(S)-Pt-(B)Y | 164.9 | 175.5 | 0.138 |
| X1(S)-Pt-(S)Y | 164.3 | 175.5 | 0.128 |
| X1(H)-Pt-(H)Y | 166.8 | 176.2 | 0.054 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melník, M.; Mikušová, V.; Mikuš, P. Structural Aspects of Pt(η3–P1C2X1C2P2)(Y) Derivative Types. Crystals 2023, 13, 1340. https://doi.org/10.3390/cryst13091340
Melník M, Mikušová V, Mikuš P. Structural Aspects of Pt(η3–P1C2X1C2P2)(Y) Derivative Types. Crystals. 2023; 13(9):1340. https://doi.org/10.3390/cryst13091340
Chicago/Turabian StyleMelník, Milan, Veronika Mikušová, and Peter Mikuš. 2023. "Structural Aspects of Pt(η3–P1C2X1C2P2)(Y) Derivative Types" Crystals 13, no. 9: 1340. https://doi.org/10.3390/cryst13091340
APA StyleMelník, M., Mikušová, V., & Mikuš, P. (2023). Structural Aspects of Pt(η3–P1C2X1C2P2)(Y) Derivative Types. Crystals, 13(9), 1340. https://doi.org/10.3390/cryst13091340









