Cortesognoite, CaV2Si2O7(OH)2·H2O, a New Mineral from the Molinello Manganese Mine, Graveglia Valley, Italy
Abstract
1. Introduction
2. Methods
3. Results
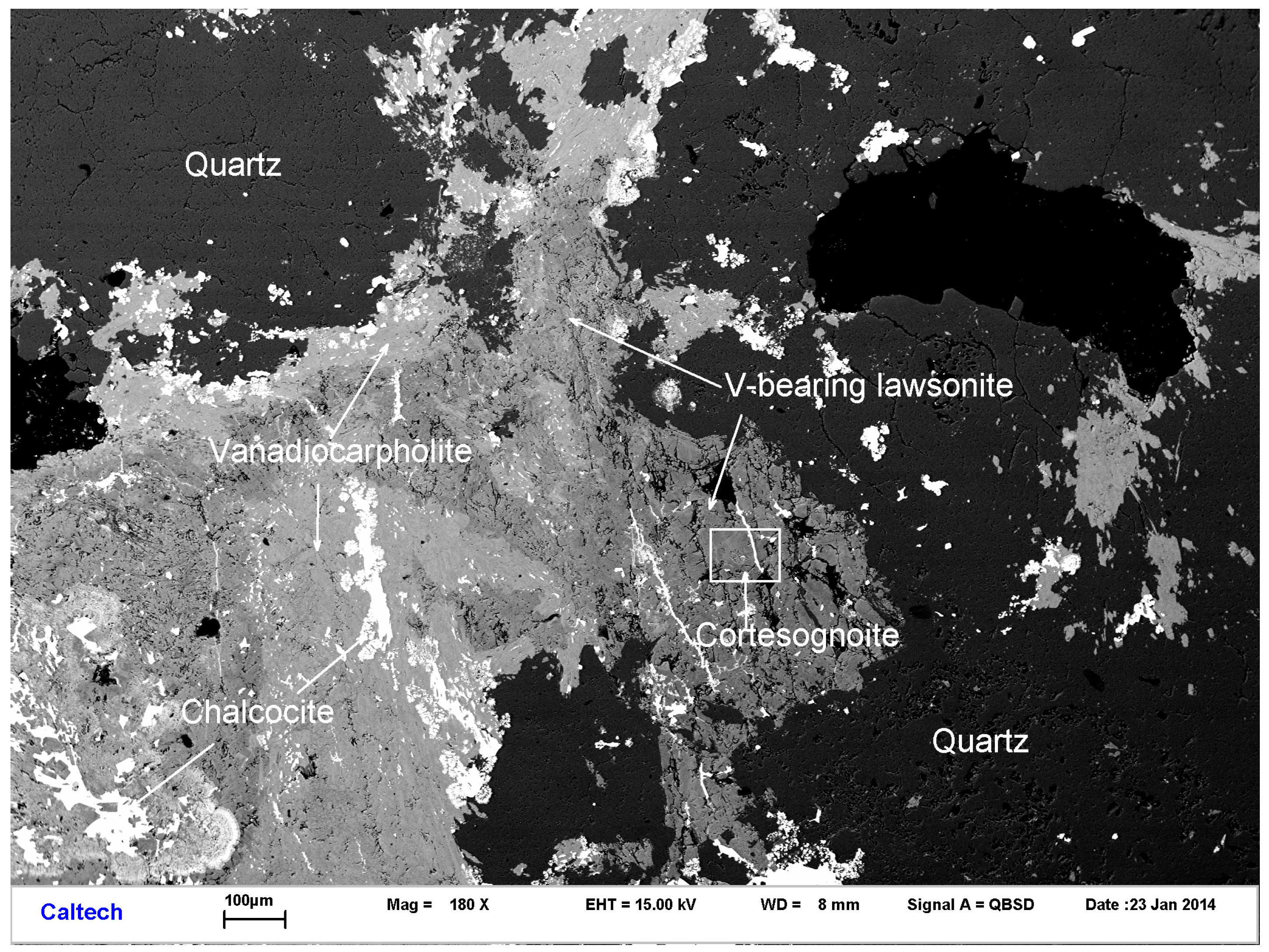
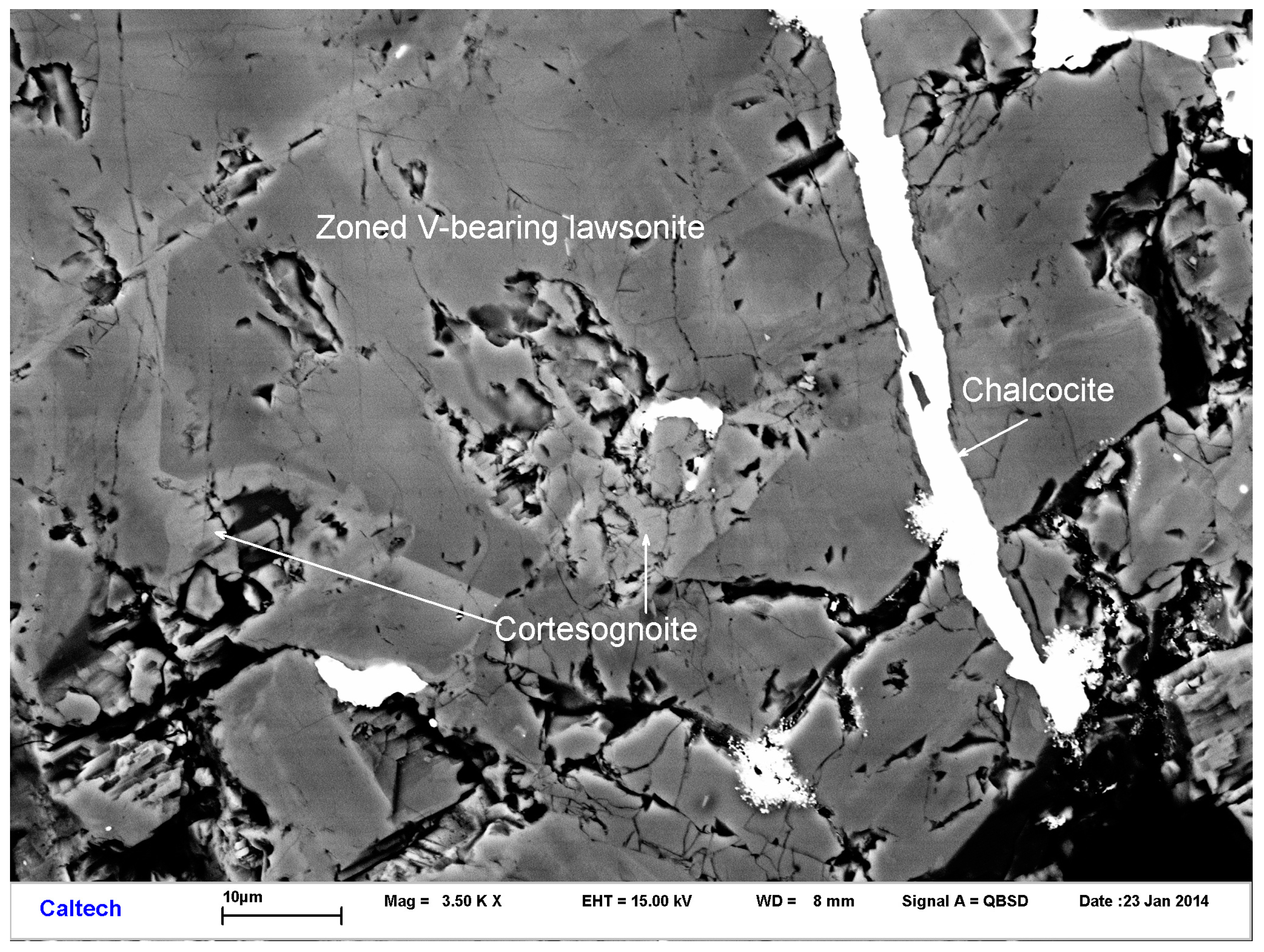
3.1. Occurrence and Physical Properties
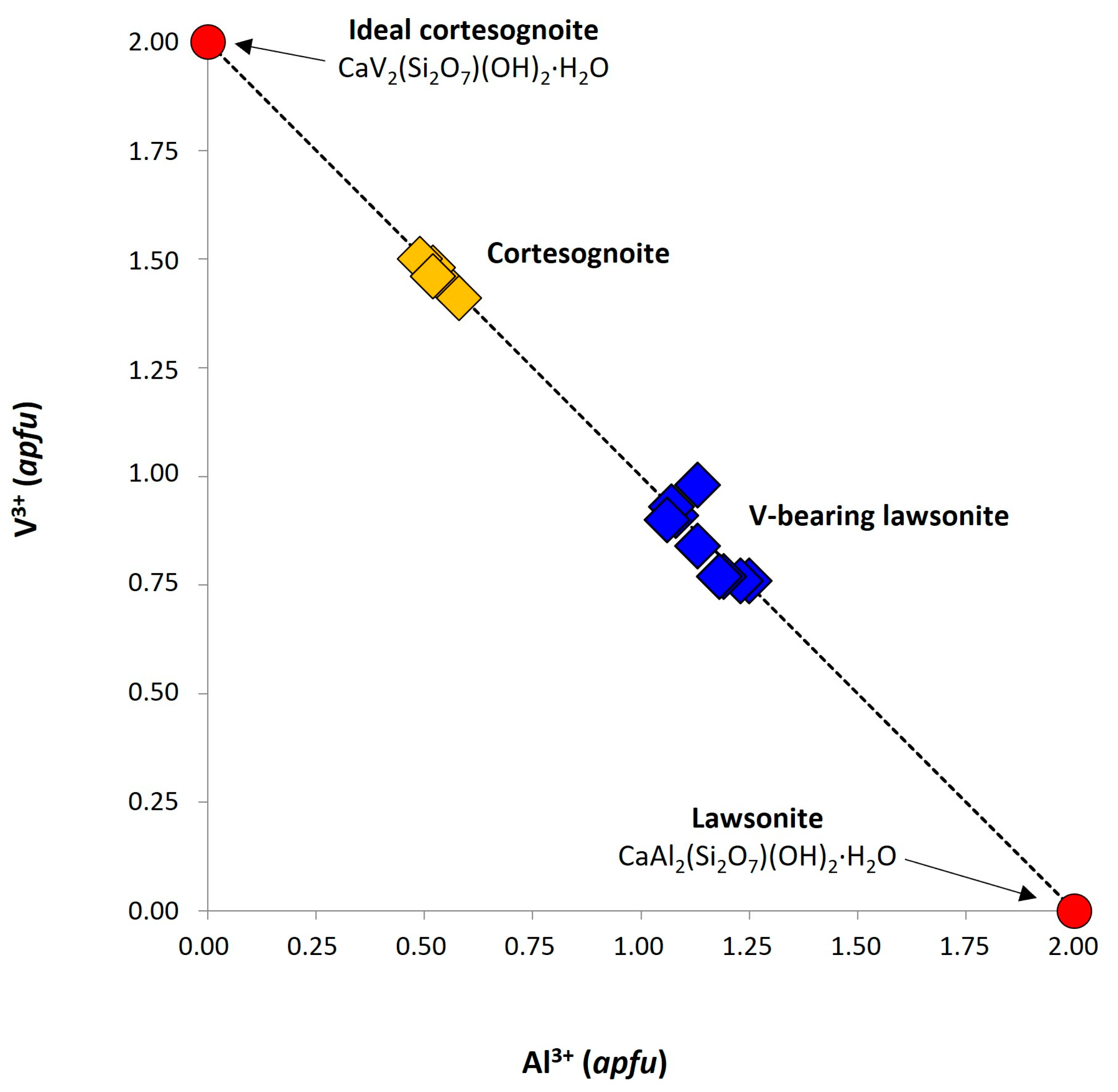
3.2. Chemical Composition
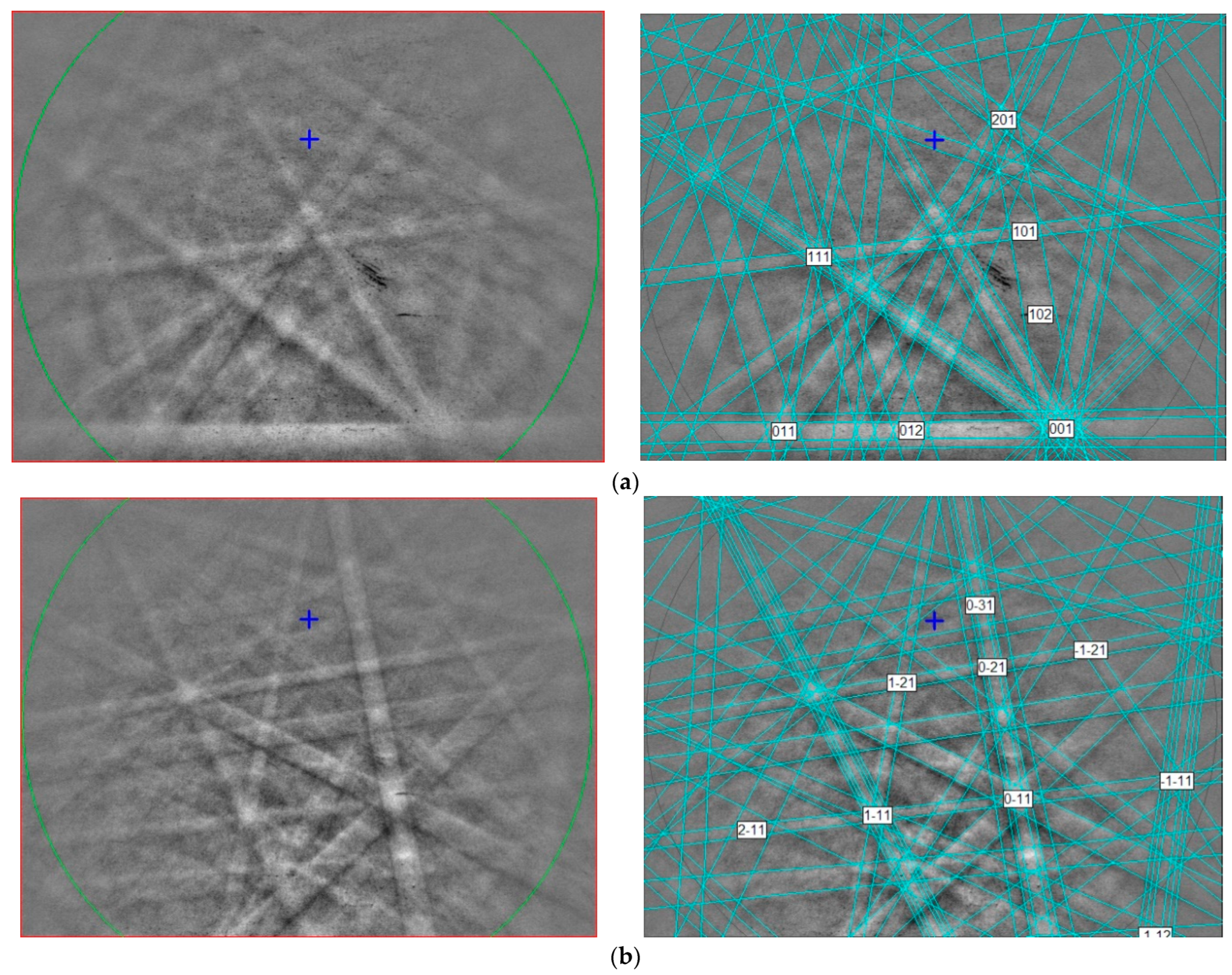
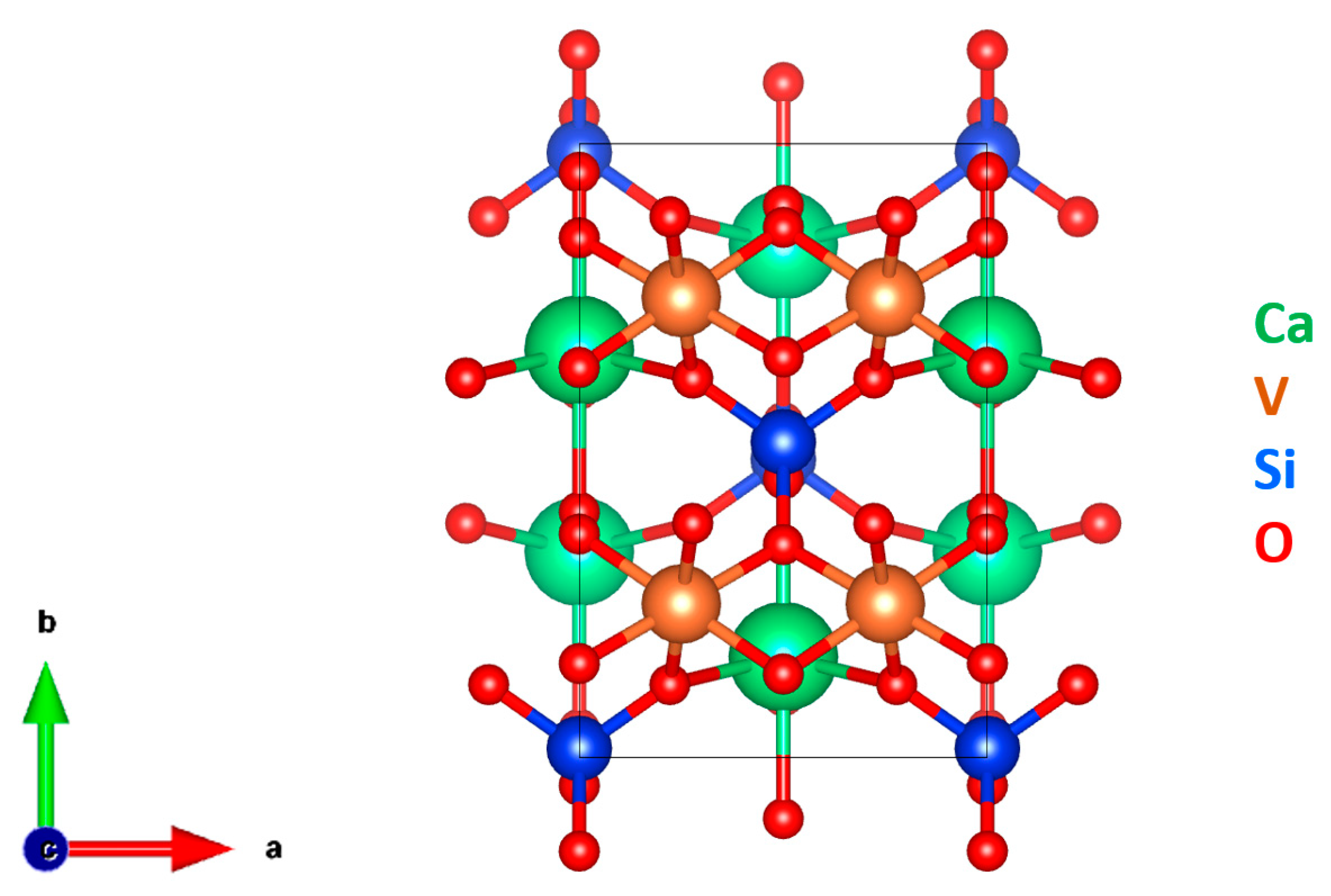
3.3. Crystallography
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, C.; Carbone, C.; Belmonte, D. Cortesognoite, IMA 2014-029. CNMNC Newsletter No. 21, August 2014, page 801. Min. Mag. 2014, 78, 797–804. [Google Scholar]
- Cortesogno, L.; Lucchetti, G.; Penco, A.M. Le mineralizzazioni a manganese nei diaspri delle ofioliti liguri: Mineralogia e genesi. Rend. Soc. Ital. Min. Pet. 1979, 35, 151–197. [Google Scholar]
- Kampf, A.R.; Roberts, A.C.; Venance, K.E.; Carbone, C.; Belmonte, D.; Dunning, G.E.; Walstrom, R.E. Cerchiaraite-(Fe) and cerchiaraite-(Al), two new barium cyclosilicate chlorides from Italy and California, USA. Min. Mag. 2013, 77, 69–80. [Google Scholar] [CrossRef]
- Kampf, A.R.; Carbone, C.; Belmonte, D.; Nash, B.P.; Chiappino, L.; Castellaro, F. Alpeite, Ca4Mn3+2Al2(Mn3+Mg)(SiO4)2(Si3O10)(V5+O4)(OH)6, a new ardennite-group mineral from Italy. Eur. J. Mineral. 2017, 29, 907–914. Available online: https://pubs.geoscienceworld.org/eurjmin/article-abstract/29/5/907/525356/Alpeite-Ca4Mn3-2Al2-Mn3-Mg-SiO4-2-Si3O10-V5-O4-OH (accessed on 20 August 2023).
- Kampf, A.R.; Rossman, G.R.; Ma, C.; Belmonte, D.; Biagioni, C.; Castellaro, F.; Chiappino, L. Ramazzoite, [Mg8Cu12(PO4)(CO3)4(OH)24(H2O)20][(H0.33SO4)3(H2O)36], the first mineral with a polyoxometalate cation. Eur. J. Mineral. 2018, 30, 827–834. [Google Scholar] [CrossRef]
- Kolitsch, U.; Merlino, S.; Belmonte, D.; Carbone, C.; Cabella, R.; Lucchetti, G.; Ciriotti, M.E. Lavinskyite-1M, K(LiCu)Cu6(Si4O11)2(OH)4, the monoclinic MDO equivalent of lavinskyite-2O (formerly lavinskyite), from the Cerchiara manganese mine, Liguria, Italy. Eur. J. Mineral. 2018, 30, 811–820. [Google Scholar] [CrossRef]
- Biagioni, C.; Belmonte, D.; Carbone, C.; Cabella, R.; Demitri, N.; Perchiazzi, N.; Kampf, A.R.; Bosi, F. Isselite, Cu6(SO4)(OH)10(H2O)4·H2O, a new mineral species from Eastern Liguria, Italy. Min. Mag. 2020, 84, 653–661. [Google Scholar] [CrossRef]
- Bonatti, E.; Zerbi, M.; Kay, R.; Rydell, H. Metalliferous deposits from the Apennine ophiolites: Mesozoic equivalents of modern deposits from oceanic spreading centers. Geol. Soc. Am. Bull. 1976, 87, 83–94. [Google Scholar] [CrossRef]
- Cabella, R.; Lucchetti, G.; Marescotti, P. Mn-ores from Eastern Liguria ophiolitic sequences (“Diaspri di Monte Alpe” Formation, Northern Apennines, Italy). Trends Mineral. 1998, 2, 1–17. [Google Scholar]
- Gramaccioli, C.M.; Griffin, W.L.; Mottana, A. Tiragalloite, Mn4[AsSi3O12(OH)], a new mineral and the first example of arsenatotrisilicate. Am. Mineral. 1980, 65, 947–952. [Google Scholar]
- Gramaccioli, C.M.; Griffin, W.L.; Mottana, A. Medaite, Mn6[VSi5O18(OH)], a new mineral and the first example of vanadatopentasilicate ion. Am. Mineral. 1982, 67, 85–89. [Google Scholar]
- Armstrong, J.T. CITZAF: A package of correction programs for the quantitative electron beam X-ray analysis of thick polished materials, thin films, and particles. Microbeam Anal. 1995, 4, 177–200. [Google Scholar]
- Ma, C.; Rossman, G.R. Barioperovskite, BaTiO3, a new mineral from the Benitoite Mine, California. Am. Mineral. 2008, 93, 154–157. [Google Scholar] [CrossRef]
- Ma, C.; Rossman, G.R. Tistarite, Ti2O3, a new refractory mineral from the Allende meteorite. Am. Mineral. 2009, 94, 841–844. [Google Scholar] [CrossRef]
- Libowitzky, E.; Armbruster, T. Low-temperature phase transitions and the role of hydrogen bonds in lawsonite. Am. Mineral. 1995, 80, 1277–1285. [Google Scholar] [CrossRef]
- Cabella, R.; Gaggero, L.; Lucchetti, G. Isothermal-isobaric mineral equilibria in braunite-, rhodonite-, johannsenite-, calcite-bearing assemblages from Northern Apennine metacherts (Italy). Lithos 1991, 27, 149–154. [Google Scholar] [CrossRef]
- Basso, R.; Cabella, R.; Lucchetti, G.; Martinelli, A.; Palenzona, A. Vanadiocarpholite, Mn2+V3+Al(Si2O6)(OH)4, a new mineral from the Molinello mine, northern Apennines, Italy. Eur. J. Mineral. 2005, 17, 501–507. [Google Scholar] [CrossRef]
- Carbone, C.; Basso, R.; Cabella, R.; Martinelli, A.; Grice, J.D.; Lucchetti, G. Mcalpineite from the Gambatesa mine, Italy, and redefinition of the species. Am. Mineral. 2013, 98, 1899–1905. [Google Scholar] [CrossRef]
- Bindi, L.; Carbone, C.; Cabella, R.; Lucchetti, G. Bassoite, SrV3O7·4H2O, a new mineral from Molinello mine, Val Graveglia, eastern Liguria, Italy. Min. Mag. 2011, 75, 2677–2686. [Google Scholar] [CrossRef]
- Bindi, L.; Carbone, C.; Belmonte, D.; Cabella, R.; Bracco, R. Weissite from Gambatesa mine, Val Graveglia, Liguria, Italy: Occurrence, composition and determination of the crystal structure. Min. Mag. 2013, 77, 475–483. [Google Scholar] [CrossRef]
- Biagioni, C.; Belmonte, D.; Carbone, C.; Cabella, R.; Zaccarini, F.; Balestra, C. Arsenmedaite, Mn2+6As5+Si5O18(OH), the arsenic analogue of medaite, from the Molinello mine, Liguria, Italy: Occurrence and crystal structure. Eur. J. Mineral. 2019, 31, 117–126. [Google Scholar] [CrossRef]
- Cortesogno, L.; Galli, M. Tronchi fossili nei diaspri della Liguria orientale. Ann. Mus. Civ. Stor. Nat. Genova 1974, 80, 142–156. [Google Scholar]
- Ghiso, G.; Messiga, B. Volborthite in Liguria. Min. Mag. 1976, 40, 794–796. [Google Scholar] [CrossRef]
- Pan, Y.; Fleet, M.E. Vanadium-rich minerals of the pumpellyite group from the Hemlo gold deposit, Ontario. Canad. Mineral. 1992, 30, 153–162. [Google Scholar]
- Marchig, V.; Gundlach, H.; Möller, P.; Schley, F. Some geochemical indicators for discrimination between diagenetic and hydrothermal metalliferous sediments. Mar. Geol. 1982, 50, 241–256. [Google Scholar] [CrossRef]
- Trefry, J.H.; Metz, S. Role of hydrothermal precipitates in the geochemical cycling of vanadium. Nature 1989, 342, 531–533. [Google Scholar] [CrossRef]
- Brugger, J.; Liu, W.; Etschmann, B.; Mei, Y.; Sherman, D.M.; Testemale, D. A review of the coordination chemistry of hydrothermal systems, or do coordination changes make ore deposits? Chem. Geol. 2016, 447, 219–253. [Google Scholar] [CrossRef]
- Evans, H.T., Jr.; Garrels, R.M. Thermodynamic equilibria of vanadium in aqueous systems as applied to the interpretation of the Colorado Plateau ore deposits. Geochim. Cosmochim. Acta 1958, 15, 131–149. [Google Scholar] [CrossRef][Green Version]
- Huang, J.-H.; Huang, F.; Evans, L.; Glasauer, S. Vanadium: Global (bio)geochemistry. Chem. Geol. 2015, 417, 68–89. [Google Scholar] [CrossRef]
- Breit, G.N.; Wanty, R.B. Vanadium accumulation in carbonaceous rocks: A review of geochemical controls during deposition and diagenesis. Chem. Geol. 1991, 91, 83–97. [Google Scholar] [CrossRef]
- Wanty, R.B.; Goldhaber, M.B. Thermodynamics and kinetics of reactions involving vanadium in natural systems: Accumulation of vanadium in sedimentary rocks. Geochim. Cosmochim. Acta 1992, 56, 1471–1483. [Google Scholar] [CrossRef]
- Pommer, A.M. Reduction of quinquevalent vanadium solutions by wood and lignite. Geochim. Cosmochim. Acta 1957, 13, 20–27. [Google Scholar] [CrossRef]
- Balestra, C. Sistematica ligure 2014. Prie—Not. Mineral. Ligure 2014, 10, 35–45. (In Italian) [Google Scholar]
- Götze, J.; Möckel, R.; Langhof, N.; Hengst, M.; Klinger, M. Silicification of wood in the laboratory. Ceram. Silikáty 2008, 52, 268–277. [Google Scholar]
- Matysová, P.; Götze, J.; Leichmann, J.; Škoda, R.; Strnad, L.; Drahota, P.; Grygar, T.M. Cathodoluminescence and LA-ICP-MS chemistry of silicified wood enclosing wakefieldite—REEs and V migration during complex diagenetic evolution. Eur. J. Mineral. 2016, 28, 869–887. [Google Scholar] [CrossRef]
- Mustoe, G.E. Silicification of wood: An overview. Minerals 2023, 13, 206. [Google Scholar] [CrossRef]
- Mills, S.J.; Bindi, L.; Cadoni, M.; Kampf, A.R.; Ciriotti, M.E.; Ferraris, G. Paseroite, PbMn2+(Mn2+,Fe2+)2(V5+,Ti,Fe3+,□)18O38, a new member of the crichtonite group. Eur. J. Mineral. 2012, 24, 1061–1067. [Google Scholar] [CrossRef]
- Garrels, R.M.; Pommer, A.M. Some quantitative aspects of the oxidation and reduction of the ores. In Geochemistry and Mineralogy of the Colorado Plateau Uranium Ores; Geol. Surv. Prof. Paper 320; Garrels, R.M., Larsen, E.S., Eds.; United States Government Printing Office: Washington, DC, USA, 1959; pp. 157–164. [Google Scholar]
- Weeks, A.D. Mineralogy and geochemistry of vanadium in the Colorado Plateau. J. Less-Common Met. 1961, 3, 443–450. [Google Scholar] [CrossRef]
- Meunier, J.D. The composition and origin of vanadium-rich clay minerals in Colorado Plateau Jurassic sandstones. Clay Clay Miner. 1994, 42, 391–401. [Google Scholar] [CrossRef]
- Cabella, R.; Cortesogno, L.; Gaggero, L. Hydrothermal contribution to cherts deposition in Northern Apennines: A preliminary report. Ofioliti 1994, 19, 367–376. [Google Scholar]
- Marescotti, P.; Cabella, R. Significance of chemical variations in a chert sequence of the “Diaspri di Monte Alpe” Formation (Val Graveglia, Northern Apennine, Italy). Ofioliti 1996, 21, 139–144. [Google Scholar]
- Huebner, J.S.; Flohr, M.J.K.; Grossman, J.N. Chemical fluxes and origin of a manganese carbonate—Oxide—Silicate deposit in bedded chert. Chem. Geol. 1992, 100, 93–118. [Google Scholar] [CrossRef]
- Abernathy, M.J.; Schaefer, M.V.; Ramirez, R.; Garniwan, A.; Lee, I.; Zaera, F.; Polizzotto, M.L.; Ying, S.C. Vanadate retention by iron and manganese oxides. ACS Earth Space Chem. 2002, 6, 2041–2052. [Google Scholar] [CrossRef] [PubMed]

| Constituent | wt% | Range | SD | Probe Standard |
|---|---|---|---|---|
| SiO2 | 34.33 | 34.05–34.65 | 0.23 | anorthite |
| V2O3 | 31.38 | 30.48–32.02 | 0.59 | V2O5 |
| CaO | 15.80 | 15.69–15.93 | 0.10 | anorthite |
| Al2O3 | 7.69 | 7.11–8.50 | 0.51 | anorthite |
| MnO | 0.14 | 0.09–0.21 | 0.05 | Mn2SiO4 |
| FeO | 0.09 | 0.06–0.11 | 0.02 | fayalite |
| MgO | 0.06 | 0.06–0.07 | 0.00 | forsterite |
| TiO2 | 0.02 | 0.01–0.05 | 0.01 | TiO2 |
| H2O * | 10.29 | |||
| Total | 99.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Carbone, C.; Belmonte, D. Cortesognoite, CaV2Si2O7(OH)2·H2O, a New Mineral from the Molinello Manganese Mine, Graveglia Valley, Italy. Crystals 2023, 13, 1295. https://doi.org/10.3390/cryst13091295
Ma C, Carbone C, Belmonte D. Cortesognoite, CaV2Si2O7(OH)2·H2O, a New Mineral from the Molinello Manganese Mine, Graveglia Valley, Italy. Crystals. 2023; 13(9):1295. https://doi.org/10.3390/cryst13091295
Chicago/Turabian StyleMa, Chi, Cristina Carbone, and Donato Belmonte. 2023. "Cortesognoite, CaV2Si2O7(OH)2·H2O, a New Mineral from the Molinello Manganese Mine, Graveglia Valley, Italy" Crystals 13, no. 9: 1295. https://doi.org/10.3390/cryst13091295
APA StyleMa, C., Carbone, C., & Belmonte, D. (2023). Cortesognoite, CaV2Si2O7(OH)2·H2O, a New Mineral from the Molinello Manganese Mine, Graveglia Valley, Italy. Crystals, 13(9), 1295. https://doi.org/10.3390/cryst13091295







