Batch Production of Wafer-Scale Monolayer MoS2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Batch Production of Monolayer MoS2 Wafers
2.2. Batch Production of Oxygen-Doped MoS2 (MoS2−xOx) Wafers
2.3. Characterizations of Samples
2.4. Device Fabrication and Measurements
3. Results and Discussion
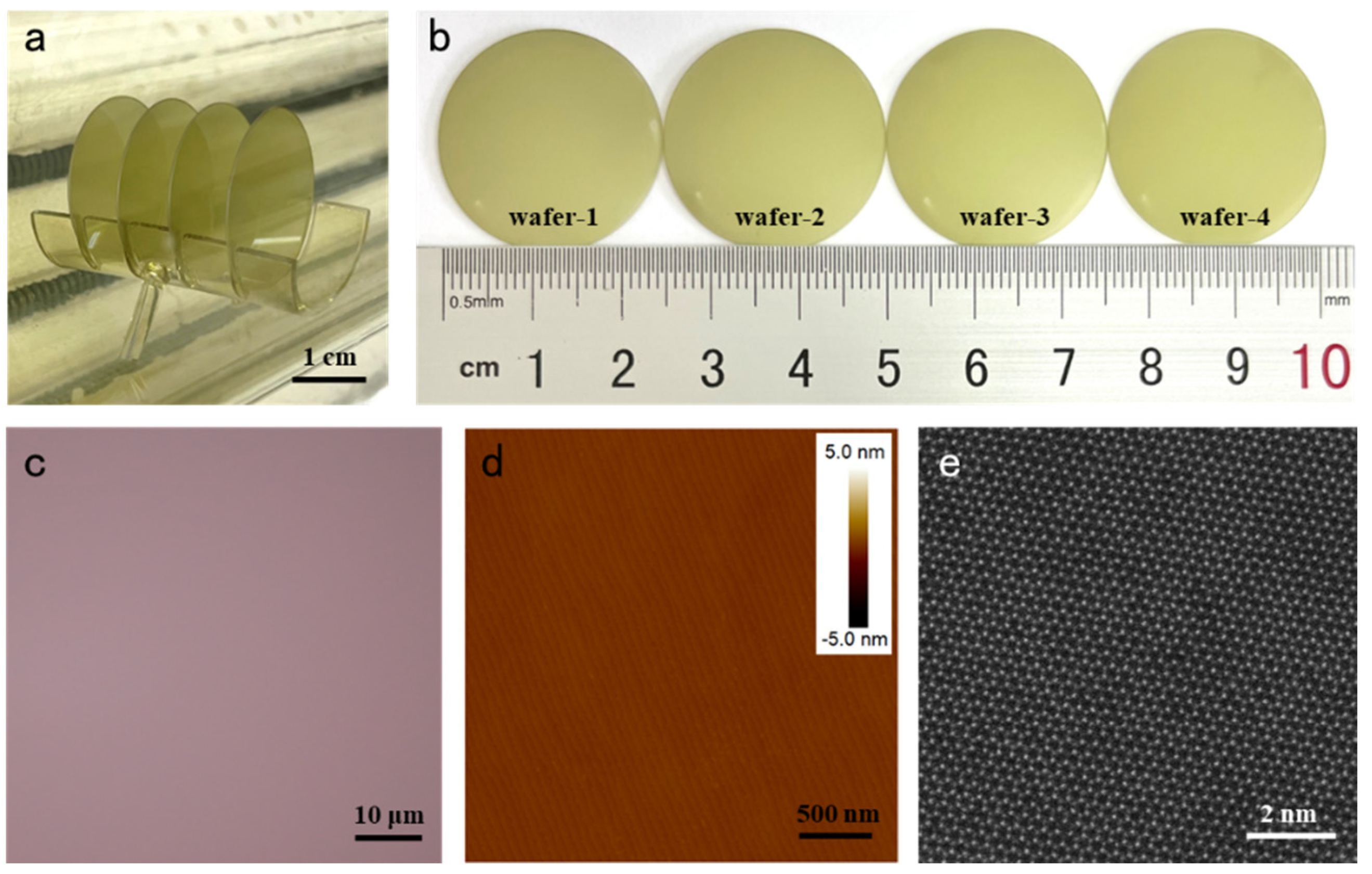
3.1. Morphology Characterization
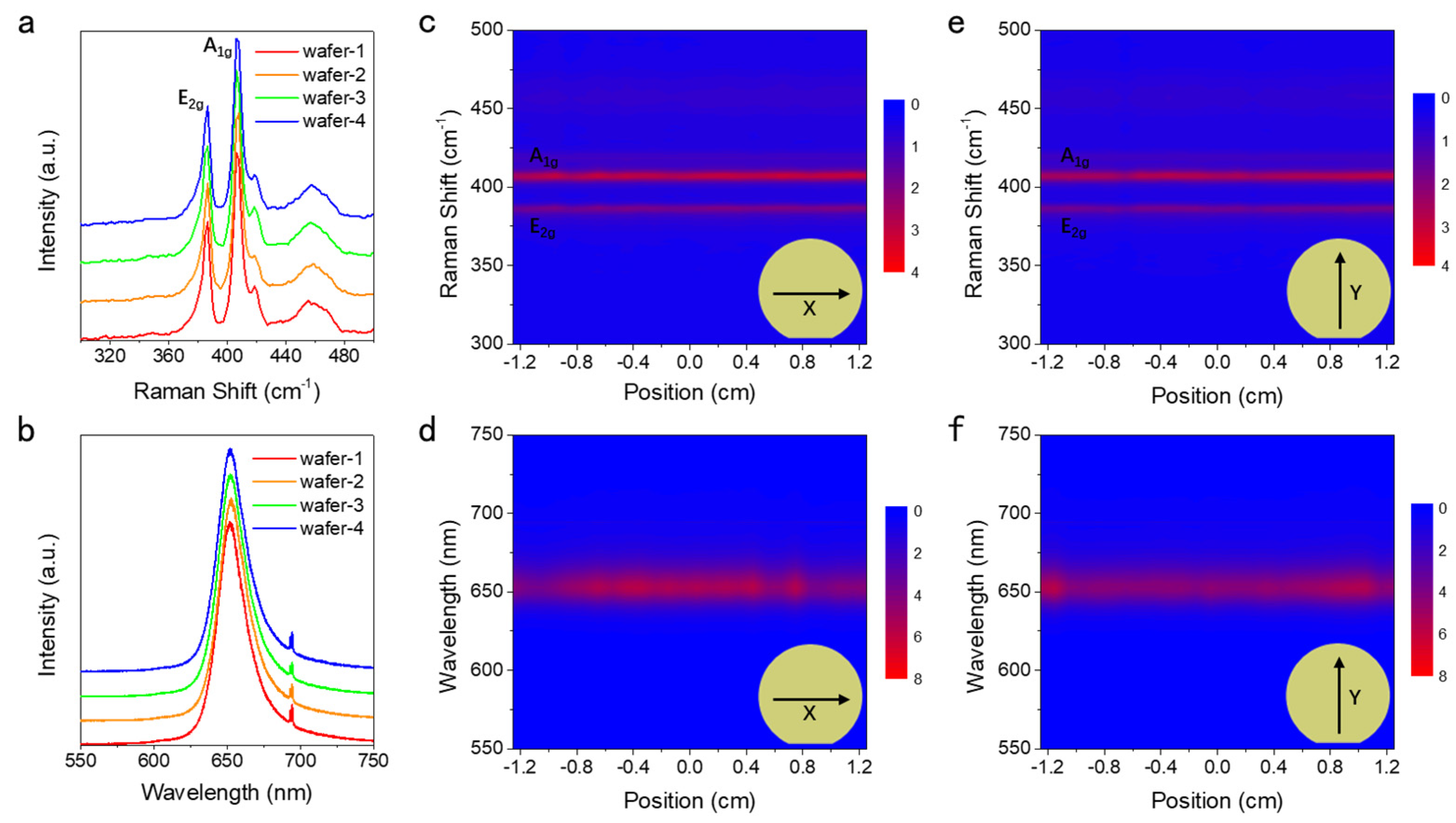
3.2. Optical Spectroscopy and Homogeneity
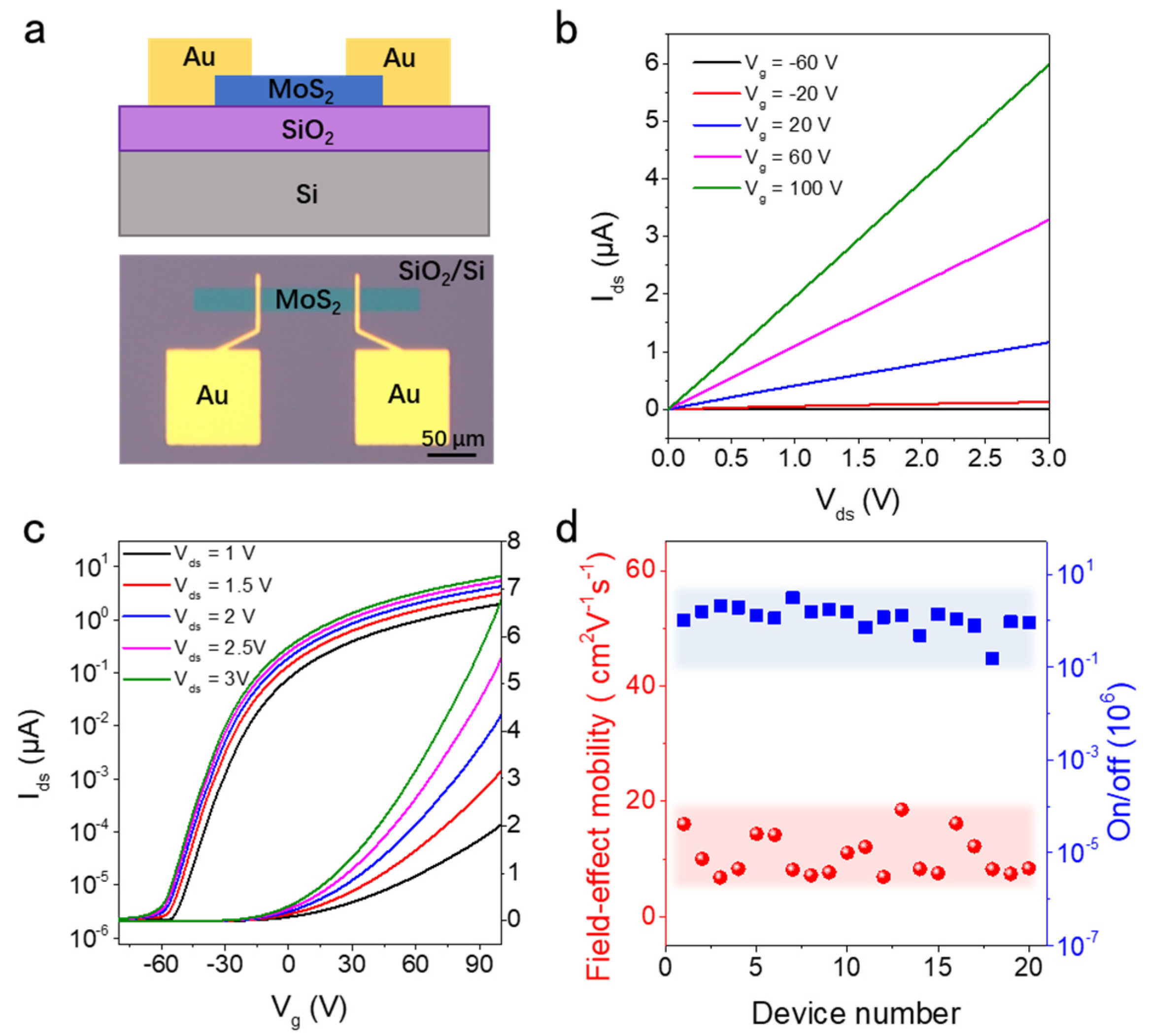
3.3. Electrical Performance of MoS2 Field Effect Transistors (FETs)
3.4. Batch Production of Oxygen Doped MoS2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moore, G.E. Cramming more components onto integrated circuits. Electronics 1965, 38, 114–117. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kwak, J.; Ciobanu, C.V.; Kwon, S.Y. Recent developments in controlled vapor-phase growth of 2D group 6 transition metal dichalcogenides. Adv. Mater. 2019, 31, e1804939. [Google Scholar] [CrossRef]
- Butler, S.Z.; Hollen, S.M.; Cao, L.; Cui, Y.; Gupta, J.A.; Gutierrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.; Ismach, A.F.; et al. Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Emerging device applications for semiconducting two-dimensional transition metal dichalcogenides. ACS Nano 2014, 8, 1102–1120. [Google Scholar] [CrossRef]
- Li, X.M.; Tao, L.; Chen, Z.F.; Fang, H.; Li, X.S.; Wang, X.R.; Xu, J.B.; Zhu, H.W. Graphene and related two-dimensional materials: Structure-property relationships for electronics and optoelectronics. Appl. Phys. Rev. 2017, 4, 021306. [Google Scholar] [CrossRef]
- Akinwande, D.; Huyghebaert, C.; Wang, C.H.; Serna, M.I.; Goossens, S.; Li, L.J.; Wong, H.P.; Koppens, F.H.L. Graphene and two-dimensional materials for silicon technology. Nature 2019, 573, 507–518. [Google Scholar] [CrossRef]
- Li, N.; Wang, Q.Q.; Shen, C.; Wei, Z.; Yu, H.; Zhao, J.; Lu, X.B.; Wang, G.L.; He, C.L.; Xie, L.; et al. Large-scale flexible and transparent electronics based on monolayer molybdenum disulfide field-effect transistors. Nat. Electron. 2020, 3, 711–717. [Google Scholar] [CrossRef]
- Tang, J.; He, C.L.; Tang, J.S.; Yue, K.; Zhang, Q.T.; Liu, Y.Z.; Wang, Q.Q.; Wang, S.P.; Li, N.; Shen, C.; et al. A reliable all-2D materials artificial synapse for high energy-efficient neuromorphic computing. Adv. Funct. Mater. 2021, 31, 2011083. [Google Scholar] [CrossRef]
- Wu, W.; Wang, L.; Li, Y.; Zhang, F.; Lin, L.; Niu, S.; Chenet, D.; Zhang, X.; Hao, Y.; Heinz, T.F.; et al. Piezoelectricity of single-atomic-layer MoS2 for energy conversion and piezotronics. Nature 2014, 514, 470–474. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Lopez-Sanchez, O.; Lembke, D.; Kayci, M.; Radenovic, A.; Kis, A. Ultrasensitive photodetectors based on monolayer MoS2. Nat. Nanotechnol. 2013, 8, 497–501. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Wang, Q.; Xiao, M.; Zhong, D.; Sun, W.; Zhang, G.; Zhang, Z. Ultrasensitive monolayer MoS2 field-effect transistor based DNA sensors for screening of down syndrome. Nano Lett. 2019, 19, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Liu, D.; Jiang, L.; Ma, Y.X.; Yang, G.L.; Qian, Y.J.; Lei, W.W. Advanced 2D-2D heterostructures of transition metal dichalcogenides and nitrogen-rich nitrides for solar water generation. Nano Energy 2022, 98, 107192. [Google Scholar] [CrossRef]
- Dumcenco, D.; Ovchinnikov, D.; Marinov, K.; Lazic, P.; Gibertini, M.; Marzari, N.; Sanchez, O.L.; Kung, Y.C.; Krasnozhon, D.; Chen, M.W.; et al. Large-area epitaxial monolayer MoS2. ACS Nano 2015, 9, 4611–4620. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.P.; Ma, D.L.; Han, G.F.; Zhang, Y.; Ji, Q.Q.; Gao, T.; Sun, J.Y.; Song, X.J.; Li, C.; Zhang, Y.S.; et al. Controllable growth and transfer of monolayer MoS2 on Au foils and its potential application in hydrogen evolution reaction. ACS Nano 2014, 8, 10196–10204. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Sun, Y.; Wu, N.; Mendes, R.G.; Chen, L.; Xu, Z.; Zhang, T.; Rummeli, M.H.; Rellinghaus, B.; Pohl, D.; et al. Direct growth of MoS2/h-BN heterostructures via a sulfide-resistant alloy. ACS Nano 2016, 10, 2063–2070. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, W.; Lu, A.Y.; Fang, W.; Lee, Y.H.; Hsu, A.L.; Kim, S.M.; Kim, K.K.; Yang, H.Y.; Li, L.J.; et al. Van der Waals epitaxy of MoS2 layers using graphene as growth templates. Nano Lett. 2012, 12, 2784–2791. [Google Scholar] [CrossRef]
- Yu, H.; Yang, Z.; Du, L.; Zhang, J.; Shi, J.; Chen, W.; Chen, P.; Liao, M.; Zhao, J.; Meng, J.; et al. Precisely aligned monolayer MoS2 epitaxially grown on h-BN basal plane. Small 2017, 13, e1603005. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Chen, W.; Tian, X.Z.; Liu, D.H.; Cheng, M.; Xie, G.B.; Yang, W.; Yang, R.; Bai, X.D.; et al. Scalable growth of high-quality polycrystalline MoS2 monolayers on SiO2 with tunable grain sizes. ACS Nano 2014, 8, 6024–6030. [Google Scholar] [CrossRef]
- Yu, H.; Liao, M.; Zhao, W.; Liu, G.; Zhou, X.J.; Wei, Z.; Xu, X.; Liu, K.; Hu, Z.; Deng, K.; et al. Wafer-scale growth and transfer of highly-oriented monolayer MoS2 continuous films. ACS Nano 2017, 11, 12001–12007. [Google Scholar] [CrossRef]
- Wang, Q.; Li, N.; Tang, J.; Zhu, J.; Zhang, Q.; Jia, Q.; Lu, Y.; Wei, Z.; Yu, H.; Zhao, Y.; et al. Wafer-scale highly oriented monolayer MoS2 with large domain sizes. Nano Lett. 2020, 20, 7193–7199. [Google Scholar] [CrossRef]
- Kim, T.; Mun, J.; Park, H.; Joung, D.; Diware, M.; Won, C.; Park, J.; Jeong, S.H.; Kang, S.W. Wafer-scale production of highly uniform two-dimensional MoS2 by metal-organic chemical vapor deposition. Nanotechnology 2017, 28, 18LT01. [Google Scholar] [CrossRef]
- Aljarb, A.; Cao, Z.; Tang, H.L.; Huang, J.K.; Li, M.; Hu, W.; Cavallo, L.; Li, L.J. Substrate lattice-guided seed formation controls the orientation of 2D transition-metal dichalcogenides. ACS Nano 2017, 11, 9215–9222. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, S.; Pan, S.; Tang, B.; Liang, Y.; Zhao, X.; Zhang, Z.; Shi, J.; Huan, Y.; Shi, Y.; et al. Epitaxial growth of centimeter-scale single-crystal MoS2 monolayer on Au(111). ACS Nano 2020, 14, 5036–5045. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ovchinnikov, D.; Deiana, D.; Unuchek, D.; Kis, A. Suppressing nucleation in metal-organic chemical vapor deposition of MoS2 monolayers by alkali metal halides. Nano Lett. 2017, 17, 5056–5063. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, P.; Jiang, S.; Zhang, Z.; Huan, Y.; Xie, C.; Hong, M.; Shi, J.; Zhang, Y. Na-assisted fast growth of large single-crystal MoS2 on sapphire. Nanotechnology 2019, 30, 034002. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, J.; Zhang, J.; Gu, L.; Yang, Z.; Li, X.; Yu, H.; Zhu, X.; Yang, R.; Shi, D.; et al. Oxygen-assisted chemical vapor deposition growth of large single-crystal and high-quality monolayer MoS2. J. Am. Chem. Soc. 2015, 137, 15632–15635. [Google Scholar] [CrossRef]
- Lin, Z.; Zhao, Y.; Zhou, C.; Zhong, R.; Wang, X.; Tsang, Y.H.; Chai, Y. Controllable growth of large-size crystalline MoS2 and resist-free transfer assisted with a Cu thin film. Sci. Rep. 2015, 5, 18596. [Google Scholar] [CrossRef]
- Li, H. Microfabrication techniques for producing freestanding multi-dimensional microstructures. Microsyst. Technol. 2015, 22, 223–237. [Google Scholar] [CrossRef]
- Li, M.; Li, W.H.; Zhang, J.; Alici, G.; Wen, W. A review of microfabrication techniques and dielectrophoretic microdevices for particle manipulation and separation. J. Phys. D Appl. Phys. 2014, 47, 063001. [Google Scholar] [CrossRef]
- Zhang, X.; Han, W.P.; Wu, J.B.; Milana, S.; Lu, Y.; Li, Q.Q.; Ferrari, A.C.; Tan, P.H. Raman spectroscopy of shear and layer breathing modes in multilayer MoS2. Phys. Rev. B 2013, 87, 115413. [Google Scholar] [CrossRef]
- Li, H.; Zhu, X.; Tang, Z.K.; Zhang, X.H. Low-temperature photoluminescence emission of monolayer MoS2 on diverse substrates grown by CVD. J. Lumin. 2018, 199, 210–215. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.Y.; Galli, G.; Wang, F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef]
- Liu, L.; Qiu, H.; Wang, J.; Xu, G.; Jiao, L. Atomic MoS2 monolayers synthesized from a metal-organic complex by chemical vapor deposition. Nanoscale 2016, 8, 4486–4490. [Google Scholar] [CrossRef]
- Liu, K.K.; Zhang, W.; Lee, Y.H.; Lin, Y.C.; Chang, M.T.; Su, C.Y.; Chang, C.S.; Li, H.; Shi, Y.; Zhang, H.; et al. Growth of large-area and highly crystalline MoS2 thin layers on insulating substrates. Nano Lett. 2012, 12, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wei, Z.; Wang, Q.; Wang, Y.; Han, B.; Li, X.; Huang, B.; Liao, M.; Liu, J.; Li, N.; et al. In situ oxygen doping of monolayer MoS2 for novel electronics. Small 2020, 16, e2004276. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Tang, J.; Li, X.; Chi, Z.; Wang, Y.; Wang, Q.; Han, B.; Li, N.; Huang, B.; Li, J.; et al. Wafer-scale oxygen-doped MoS2 monolayer. Small Methods 2021, 5, e2100091. [Google Scholar] [CrossRef]
- Kang, N.; Paudel, H.P.; Leuenberger, M.N.; Tetard, L.; Khondaker, S.I. Photoluminescence quenching in single-layer MoS2 via oxygen plasma treatment. J. Phys. Chem. C 2014, 118, 21258–21263. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Sun, X.; Cai, Y.; Liang, Y.; Zhang, Z. Batch Production of Wafer-Scale Monolayer MoS2. Crystals 2023, 13, 1275. https://doi.org/10.3390/cryst13081275
Wei Z, Sun X, Cai Y, Liang Y, Zhang Z. Batch Production of Wafer-Scale Monolayer MoS2. Crystals. 2023; 13(8):1275. https://doi.org/10.3390/cryst13081275
Chicago/Turabian StyleWei, Zheng, Xingdong Sun, Yongqing Cai, Yao Liang, and Zhihua Zhang. 2023. "Batch Production of Wafer-Scale Monolayer MoS2" Crystals 13, no. 8: 1275. https://doi.org/10.3390/cryst13081275
APA StyleWei, Z., Sun, X., Cai, Y., Liang, Y., & Zhang, Z. (2023). Batch Production of Wafer-Scale Monolayer MoS2. Crystals, 13(8), 1275. https://doi.org/10.3390/cryst13081275





