Abstract
We synthesized a series of five novel Mn–salen-based compounds (1a–1c, 2a, 2b) through the reaction between precursor chloride complexes and potassium silver/gold dicyanide. The prepared compounds were structurally and magnetically characterized. Our findings revealed that all the Mn(III) central atoms exhibited an axially elongated coordination polyhedron, leading to the observation of axial magnetic anisotropy as indicated by the negative axial magnetic parameter D, which was determined through fitting the experimental magnetic data and supported by theoretical CASSCF/NEVPT2 calculations. Furthermore, we observed magnetic-exchange interactions only in compounds with a special supramolecular topology involving O–H···O hydrogen-bonded dimers. In these cases, the weak magnetic exchange (J/cm−1 = −0.58(2) in 1b and −0.73(7) in 2b) was mediated by the O–H···O hydrogen bonds. These findings were further supported by BS–DFT calculations, which predicted weak antiferromagnetic exchanges in these complexes and ruled out exchange interactions mediated by diamagnetic cyanido metallo–complex bridges. Additionally, we investigated the observed Ag···π (1b) and Au···Au (2b) interactions using QT–AIM calculations, confirming their non-covalent nature. We compared these results with previously reported Mn–salen-based compounds with metallophilic interactions arising from the presence of the [Ag/Au(CN)2]− bridging units.
1. Introduction
The complexes of Mn(III) with H2salen ligands (H2salen = N,N′-1,2-ethylenebis(salicylimine) are well-known examples of magnetically interesting compounds, which often behave as molecular nanomagnets, so-called Single-Molecule Magnets (SMMs) [1]. The salen-based Mn(III) SMMs almost exclusively refer to compounds involving dimeric [{Mn(salen)}2]2+ units bridged by the phenolic oxygen atoms [2]. However, there are also other magnetically interesting examples in this group of compounds reported in the literature, e.g., SMMs containing only one paramagnetic centre, so-called Single-Ion Magnets (SIMs), or compounds with chain magnetism, so-called Single-Chain Magnets [3,4].
In our previous work on the Mn(III) salen-based compounds [5], we focused our attention on the investigation of weak antiferromagnetic-exchange interactions mediated by O-H···O hydrogen bonds formed in the supramolecular dimers built up from [Mn(salen)(H2O)]+ or [Mn(salen)(CH3OH)]+ subunits. This hydrogen bonding formed between the oxygen atom of the coordinated solvent molecule (Osolv) and phenolic oxygen atom (OPh) of the coordinated Schiff base. We revealed that the strength of the observed exchange interaction is a function of the Osolv···OPh donor···acceptor distance when shorter contact distance ensures stronger antiferromagnetic interaction. We also investigated Mn(III) salen-based complexes with Pt(II) [6] and Pt(IV) [7] thiocyanido or Pt(II) cyanido-bridging [8] metallo-ligands. The obtained results were consistent with the above-mentioned findings—the majority of the prepared compounds formed O-H···O-bound supramolecular dimers and magnetic properties were dominated by the combination of zero-field splitting and weak antiferromagnetic-exchange interactions. With the aim to increase the dimensionality of the prepared Mn(III) salen-based compounds, we decided to investigate another class of bridging noble metal metalloligands, the Au(I) and Ag(I) cyanidometallates. These tend to form metallophilic interactions, so-called numismophilic interactions, i.e., argentophilic and aurophilic interactions [9,10]. These might be of relatively significant strength (7–12 kcal/mol) comparable even with interaction energies reported for hydrogen bonding [10,11].
Only a few Mn(III) salen-based complexes containing the Au(I) and Ag(I) cyanidometallates with determined crystal structures have been reported in the literature. Thus, the knowledge about this interesting group of compounds is rather limited. The reaction between K[Ag(CN)2] and [Mn(L4A)(CH3COO)], as well as H2L4A = N,N′-1,2-ethylenebis(salicylimine), led to the preparation of the polymeric chain complex [{Mn(L4A)}{µ-Ag(CN)2}]n, which does not exhibit argentophilic interactions. Its magnetic properties are typical for single-ion magnetism without any significant exchange interaction mediated between the Mn(III) atoms by the [Ag(CN)2]− bridge [12]. Another example exhibited a combination of the O-H···O-bound supramolecular dimer and argentophilic interactions observed in the crystal structure of the [{Mn(L4B)(H2O)}{µ-Ag(CN)2}] compound (H2L4B= N,N′-1,2-ethylenebis(3-methoxysalicylimine)). Just a few complexes, other than salen based with the [Ag/Au(CN)2]− bridges, have been reported in the literature, e.g., the 1D polymer complexes [{Mn(L)}{µ-Ag(CN)2}{Ag(CN)2}]n, the 2D polymer complexes [{Mn(L)2}{µ-Ag(CN)2}2]n and [{Mn(L)}{µ-Ag(CN)2}]n [13,14], or the 3D polymer complexes [{Mn(L)}{Mn(CN)4(H2O)2}{µ-Ag(CN)2}]n [15], where L = pyridine-4-aldoxime, N,N′-ethylenebis(acetylacetonylideneiminate, or macrocycleligands. In addition, the dinuclear Mn(II) complexes [{Mn(bpy)(H2O)}{µ-Ag(CN)2}][Ag(CN)2] were prepared [16], where bpy = 2,2′-bipyridyl, and all these exhibited weak antiferromagnetic-exchange interactions. To date, there are only one polymeric and one dinuclear manganese(III) Schiff base compounds bridged by the dicyanidosilver(I) anion reported in the literature [{Mn(L4a)}{µ-Ag(CN)2}]n and [{Mn(L4b)(H2O)}{µ-Ag(CN)2}] where L4a2− = N,N′-1,2-ethylene-bis(salicylideneiminate) dianion or L4b2− = N,N′-1,2-ethylene-bis(3-methoxysalideneiminate) dianion, which have weak antiferromagnetic-exchange interactions [12,17].
Here, we report synthesis and crystal structures of five new Mn(III) complexes with salen-based ligands (Scheme 1) and [Ag(CN)2]− or [Au(CN)2]− bridging metallo ligands. We discuss their crystal structures with special focus on non-covalent interactions with Ag/Au atoms. We analyzed selected non-covalent interactions using the Quantum Theory of Atoms in Molecules (QT–AIM). We also provide analysis of measured DC magnetic data supported by ab initio calculations.
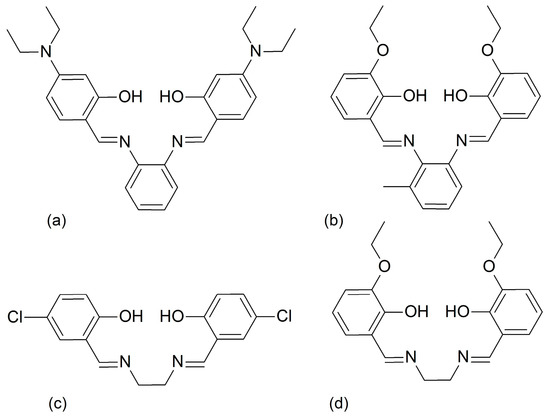
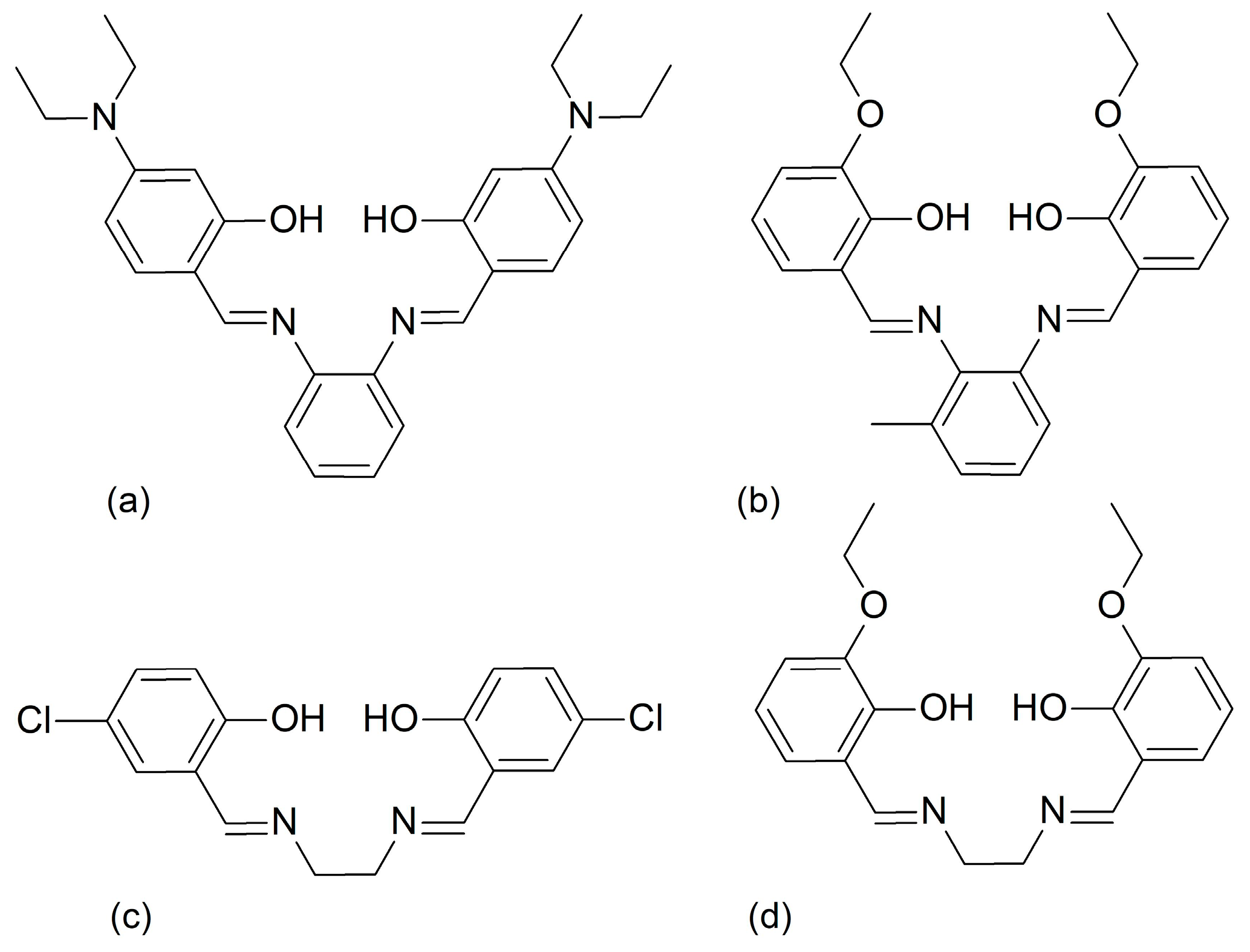
Scheme 1.
Schematic representations of the tetradentate H2L4 Schiff base ligands: H2L41 (a), H2L42 (b), H2L43 (c), and H2L44 (d).
2. Experimental Section
2.1. Synthesis
All the starting chemicals were of analytical reagent grade and were used as received. K[Ag(CN)2], K[Au(CN)2], MnCl2⋅4H2O, and triethylamine (Et3N) solvents, as well as the organic compounds ethane-1,2-diamine, 3-methylbenzene-1,2-diamine, benzene-1,2-diamine, 3-ethoxy-2-hydroxybenzaldehyde, 5-chloro-2-hydroxybenzaldehyde, and 4-aminodiethylene-2-hydroxybenzaldehyde, were obtained from commercial sources.
The Schiff base ligands (H2L41, H2L42, H2L43 and H2L4) and the manganese(III) precursor complexes [Mn(L41)Cl], [Mn(L42)Cl], [Mn(L43)Cl], where (L41)2− = N,N′-benzene-bis(4-aminodiethylenesalicylideneiminato) dianion, (L42)2− = N,N′-3-methylbenzene-bis(3-ethoxysalicylideneiminato) dianion, (L43)2− = N,N′-ethylene-bis(5-chlorosalicylideneiminato) dianion, and (L44)2− = N,N′-ethylene-bis(3-ethoxysalicylideneiminato) dianion, were prepared according to the literature procedures [18,19]. Complexes 1a–c and 2a–c were prepared using the same procedure as follows:
A water solution (10 mL) of K[Ag(CN)2] or K[Au(CN)2] (50 mg) was added to a methanol solution (10 mL) of [Mn(L4x)Cl] (137 mg for 1a, 127 mg for 1b, 107 mg for 1c, 95 mg for 2a, and 77 mg for 2b); the stoichiometric ratio between K[Ag/Au(CN)2] and [Mn(L4x)Cl] was 1:1. The reaction mixture was stirred at room temperature for 30 min and then kept undisturbed in dark. After 7 days, single crystals suitable for X-ray analysis formed. The resulting crystals were filtered off from the mother liquor, washed with water and diethyl ether, dried in a drying kiln, and finally stored in a desiccator.
[{Mn(L41)}{µ-Ag(CN)2}]n (1a): Yield: 78% (132 mg). Elem. anal. Calcd (%) for C30H32N6O2Mn1Ag1: C, 53.66; H, 4.80; N, 12.51. Found: C, 53.27; H, 4.86; N, 12.31. ΛM (DMF, S cm2 mol−1): 24.7. FT-IR (Nujol, cm−1): 596 m; 581 m; 541 s; 524 s; 513 m; 497 m; 465 w; 450 w; 424 w; 403 w; 358 w; 328 m; 297 m; 286 m ν(Mn−N); 230 w ν(Mn−O). FT-IR (ATRd, cm−1): 3429 w; 3027 w ν(C−H)ar; 2969 w ν(C−H)alip; 2123 m ν(C≡N); 1614 vs. ν(C=N)ar; 1559 vs. ν(C=C)ar; 1558 vs. ν(C=C)ar; 1517 m ν(C=C)ar; 1491 s ν(C=C)ar; 1429 w; 1408 w; 1366 w; 1345 m; 1316 w; 1273 w; 1247 m; 1211 m; 1174 w; 1141 w; 1078 w; 1014 w; 972 w; 823 m; 728 w; 740 w; 704 w; 654 w; 611 w; 518 w.
[{Mn(L42)(H2O)}{µ-Ag(CN)2}] (1b): Yield: 72% (117 mg). Elem. anal. Calcd (%) for C27H26N4O5Mn1Ag1: C, 49.94; H, 4.03; N, 8.62. Found: C, 49.58; H, 3.92; N, 8.75. ΛM (DMF, S cm2 mol−1): 19.1. FT-IR (Nujol, cm−1): 581 m; 537 s; 526 w; 512 m; 496 m; 481 w; 469 w; 449 w; 396 w; 329 m; 300 m; 280 w ν(Mn−N); 246 w ν(Mn−O); 230 w. FT-IR (ATRd, cm−1): 3416 m; 3057 w ν(C−H)ar; 2979 w ν(C−H)alip; 2927 w ν(C−H)alip; 2873 w; 2148 w ν(C≡N); 1638 w ν(C=N)ar; 1587 vs. ν(C=C)ar; 1540 s ν(C=C)ar; 1472 w ν(C=C)ar; 1433 s; 1382 m; 1307 m; 1246 s; 1205 m; 1180 m; 1080 w; 1023 w; 958 w; 906 w; 849 w; 779 w; 728 w; 636 w; 601 w; 540 w.
[{Mn(L43)}{µ-Ag(CN)2}]n (1c): Yield: 71% (98 mg). Elem. anal. Calcd (%) for C18H12N4O2Cl2Mn1Ag1: C, 39.30; H, 2.19; N, 10.18; Cl, 12.89. Found: C, 39.26; H, 2.21; N, 10.43; Cl, 13.21. ΛM (DMF, S cm2 mol−1): 15.2. FT-IR (Nujol, cm−1): 552 m; 491 s; 465 m; 409 w; 373 w; 362 w; 349 w; 304 w; 287 w ν(Mn−N); 259 w ν(Mn−O). FT-IR (ATRd, cm−1): 3083 w ν(C−H)ar; 3043 w ν(C−H)ar; 2966 w ν(C−H)alip; 2920 w ν(C−H)alip; 2858 w; 2642; 2159 m ν(C≡N); 1629 vs. ν(C=N)ar; 1586 m ν(C=C)ar; 1531 m ν(C=C)ar; 1452 m ν(C=C)ar; 1439 m; 1422 w; 1374 m; 1327 w; 1340; 1280 s; 1242 w; 1202 w; 1181 m; 1134 w; 1092 w; 1042 w; 996 w; 981 w; 959 w; 883 w; 862 w; 820 w; 799 m; 733 w; 704 m; 658 w; 600 w; 551 w.
[{Mn(L41)}{µ-Au(CN)2}]n (2a). Yield: 74%, Anal. Calcd. for C30H32N6O2Mn1Au1: C, 47.37; H, 4.24; N, 11.05. Found: C, 47.42; H, 4.36; N, 11.40%. ΛM (DMF, S cm2 mol−1): 16.1. FT-IR (Nujol, cm−1): 598 w; 570 s; 537 w; 514 m; 496 m; 459 m; 410 m; 383 m; 356 m; 336 w; 320 w; 301 w; 290 w ν(Mn−N); 255 w ν(Mn−O); 244 w; 225 w; 180 w; 158 w. FT-IR (ATRd, cm−1): 3069 w; 2969 w; 2932 w; 2895 w; 2165 w ν(C≡N); 2129 m ν(C≡N); 1611 m ν(C=N)ar; 1555 m; 1515 m; 1487 m ν(C=C)ar; 1429 w ν(C=C)ar; 1407 w; 1336 m; 1313 w; 1274 w; 1244 w; 1209 w; 1173 w; 1138 w; 1078 w; 1012 w; 872 w; 819 m; 780 w; 736 m; 700 m; 652 w; 609 w.
[{Mn(L44)(H2O)}{µ-Au(CN)2}] (2b). Yield: 81%, Anal. Calcd. for C22H24N4O5Mn1Au1: C, 39.07; H, 3.58; N, 8.28. Found: C, 39.51; H, 3.64; N, 8.63%. ΛM (DMF, S cm2 mol−1): 12.6. FT-IR (Nujol, cm−1): 568 m; 554 m; 540 m; 515 m; 491 w; 474 w; 460 m; 429 s; 390 w; 380 w; 363 w; 352 m; 332 w; 319 w; 306 w; 295 w; 286 m ν(Mn−N); 270 w; 260 w; 240 m ν(Mn−O); 219 w; 206 w; 198 w; 183 w; 173 w; 162 w. FT-IR (ATRd, cm−1): 3410 m; 3024 w; 3005 w; 2973 w; 2920 w; 2875 w; 2175 m ν(C≡N); 2151 m ν(C≡N); 1647 m ν(C=N)ar; 1618 vs. ν(C=N)ar; 1597 s; 1551 m; 1464 w ν(C=C)ar; 1439 m ν(C=C)ar; 1390 m; 1330 w; 1297 m; 1252 m; 1215 m; 1176 w; 1114 w; 1082 w; 1047 w; 1018 w; 977 w; 950 w; 903 w; 849 w; 763 w; 731 w; 640 w; 602 w.
2.2. Measurements
Temperature-dependent (T = 2–300 K, B = 0.2 T) and field-dependent (B = 0–5 T, T = 2 and 5 K) dc magnetization measurements were performed on a Quantum Design MPMS–XL magnetometer. The magnetic data were corrected for the diamagnetism of the constituent atoms (χdia/10−12 m3mol−1 = −5 × Mr) and the diamagnetism of the capsule.
Elemental analysis was performed by a Flash 2000 CHNS Elemental Analyzer (Thermo Scientific, Waltham, MA, USA). A Jasco FT/IR-4700 spectrometer (Jasco, Easton, MD, USA) was used for the collection of the infrared (IR) spectra of the studied ligand and complexes in the range of 400–4000 cm−1 by using the attenuated total reflection (ATR) technique on a diamond plate.
2.3. Crystallography
X-ray measurements on the selected crystals of 1a–1c and 2b were performed on an Oxford Diffraction XcaliburTM2 equipped with a Sapphire2 CCD detector using the Mo-Kα radiation at 100 K. The CrysAlis program package (version 1.171.33.52, Oxford Diffraction) was used for data collection and reduction [20]. The crystal structure of 2a was determined using an XtaLAB Synergy-I diffractometer (Rigaku) with a HyPix3000 hybrid pixel array detector and micro-focused PhotonJet-I X-ray source (Cu Kα). The multi-scan absorption corrections were applied using the program CrysAlisPro 1.171.40.82a [21].
The molecular structures were solved by SHELXT [22], and all non-hydrogen atoms were refined anisotropically on F2 using full-matrix least-square procedure SHELXL [22]. All the hydrogen atoms were found in differential Fourier maps, and their parameters were refined using a riding model with Uiso(H) = 1.2 (CH, CH2, OH) or 1.5Ueq (−CH3). Non-routine aspects of the structure refinement are as follows: In 1a, disorder of N-diethyl moiety was modelled as positional disorder over two positions with site occupation factors: 0.63:0.37. In 1b, the positional disorder of the methyl group over two positions was modelled with occupation factors: 0.54:0.46. In 2a, disorder of N-diethyl moiety was modelled as positional disorder over two positions with site occupation factors: 0.51:0.49. In 2b, the positional disorder of the ethylene bridge was modelled over two positions with site occupation factors: 0.74:0.26.
2.4. Theoretical Calcualtions
The broken-symmetry DFT calculations were used for calculation of the isotropic exchange coupling constants. The isotropic exchange J was calculated for H = −J(S1S2) spin Hamiltonian with the help of Ruiz [23]
and Yamaguchi [24] formulas:
The values of the J constants calculated by Yamaguchi formula reproduced magnetic exchange in 1b and 2b reasonably. Thus, only their values were used in discussion of the magnetic properties.
The Laplacian of electron density was investigated and visualized using AIMQB software package [25].
3. Results
3.1. Structural Overview
The crystal data and structure refinements for compounds reported in this article are provided in Supplementary (Tables S1 and S2). In 1a–1c, 2b, the manganese atom is hexacoordinated, with two oxygen and two nitrogen donor atoms forming the equatorial plane (a tetradentate Schiff base). The axial positions are occupied either by two nitrogen donor atoms (from silver/gold cyanide bridges) in 1a, 1c, and 2a, or by one cyanide nitrogen and by one aqua oxygen atom (1b, 2b). Therefore, the coordination sphere of the manganese atom in discussed complexes adopts either {MnN4O2} (in 1a, 1c and 2a) or {MnN3O3} (in 1b and 2b) donor sets. The coordination polyhedra can be described as strongly axially elongated octahedrons due to the Jahn–Teller effect. In general, based on herein and previously reported salen-type complexes, it can be concluded that the Mn(III) salen-based compounds tend to possess a rather long axial [5]. This was also observed for 1a–2b with the axial bonds exhibiting lengths longer than 2.3 Å (Table 1). The equatorial bond lengths are much shorter, and the lengths of M–Nim and M–Oph bonds range between 1.86 and 2.00 Å (Nim stands for imine nitrogen atom; OPh stands for phenolic oxygen atom). The Ag(I) or Au(I) atoms are coordinated by two carbon atoms resulting in C–Ag/Au–C angles measuring 180.00(2)° (for 1a), 175.36(19)° (for 1b), 177.49(8)° (for 1c), 180.0(7)° (for 2a), and 174.96(9)° or 175.56(9) (for 2b). The Ag–C or Au–C bond lengths range from 2.05 to 2.06 Å.

Table 1.
Selected bond lengths (Å) of the prepared silver and gold complexes.
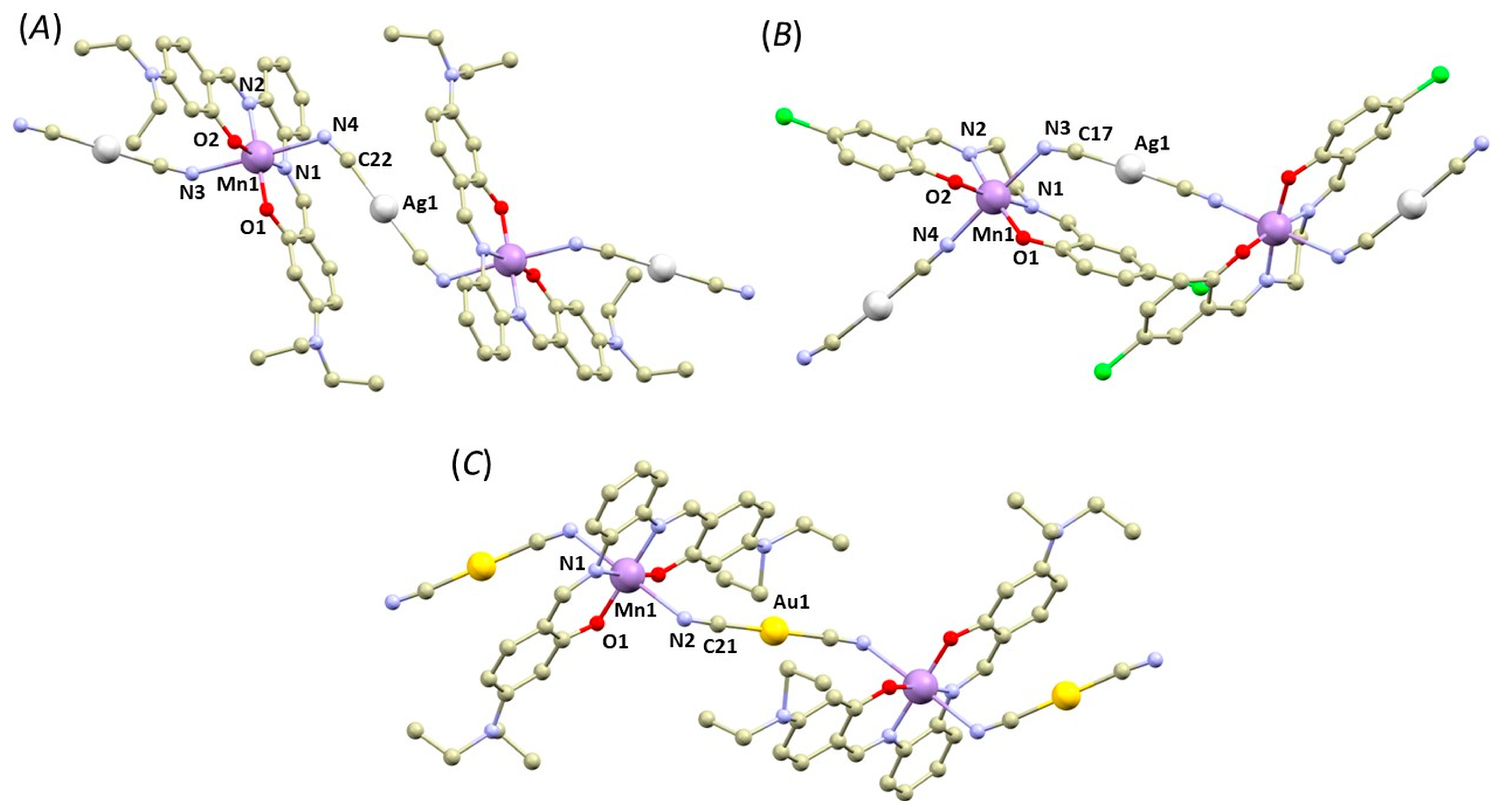
The crystal structures of 1a–2b can be categorized into two types: polymeric complexes bridged by the Au/Ag cyanido-bridging complexes (1a, 1c, and 2a, Figure 1) and dimeric complexes (in 1b and 2b, Figure 2). In the first type, the cyanide complexes ([Ag(CN)2]− or [Au(CN)2]−) act as bridging units between the [Mn(L4x)]+ units, connecting them through the Mn–N coordination bonds in the axial positions. The overall crystal structure can be then described as 1D polymeric, in which the chains interact through weak non-covalent interactions, such as π–π stacking of the aromatic rings (see Supplementary, Figure S1).
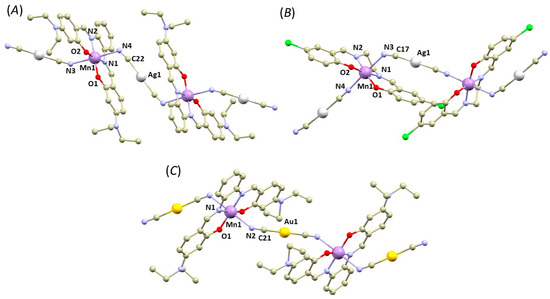
Figure 1.
A perspective views illustrating fragments of the crystal structures of 1a (A), 1c (B), 2a (C). Hydrogen atoms were omitted for clarity. Colour code: carbon (light brown), chlorine (green), gold (yellow), manganese (violet), nitrogen (light blue), oxygen (red), silver (white).
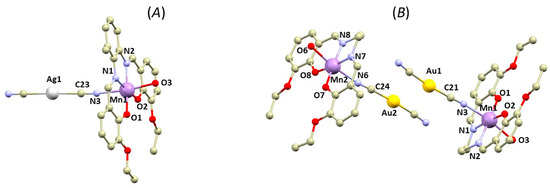
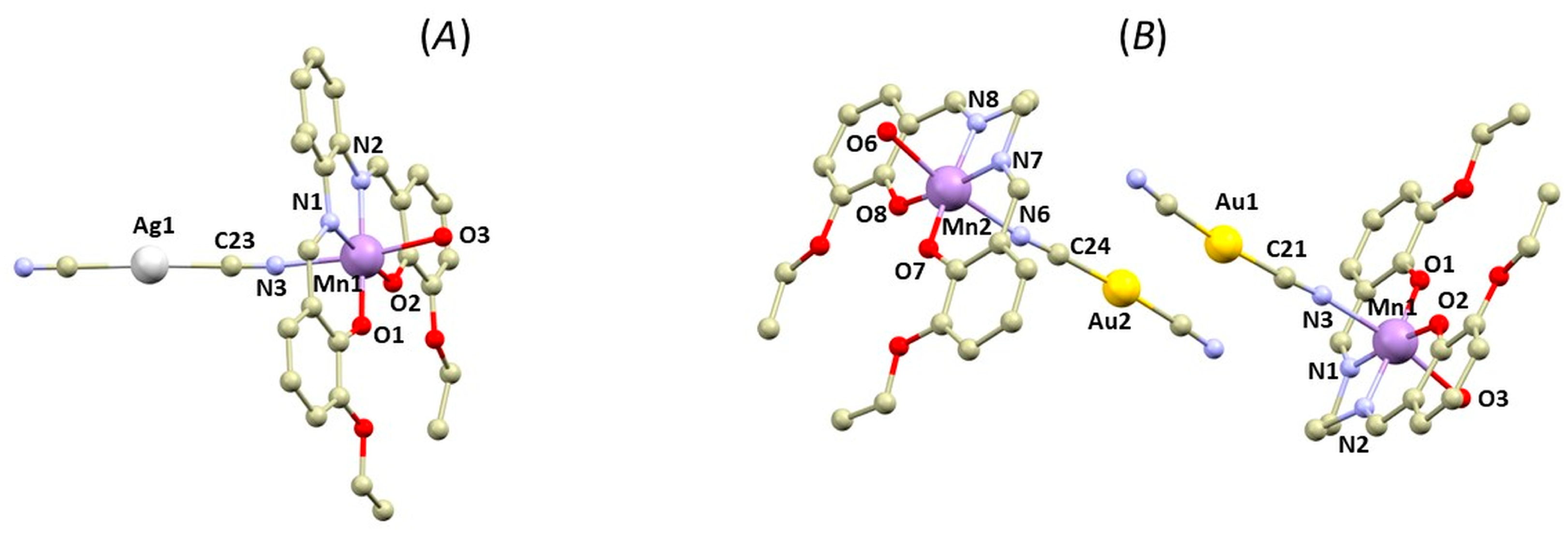
Figure 2.
A perspective view illustrating fragments of the crystal structures of 1b (A) and 2b (B). Hydrogen atoms were omitted for clarity. Colour code: carbon (light brown), chlorine (green), gold (yellow), manganese (violet), nitrogen (light blue), oxygen (red), silver (white).
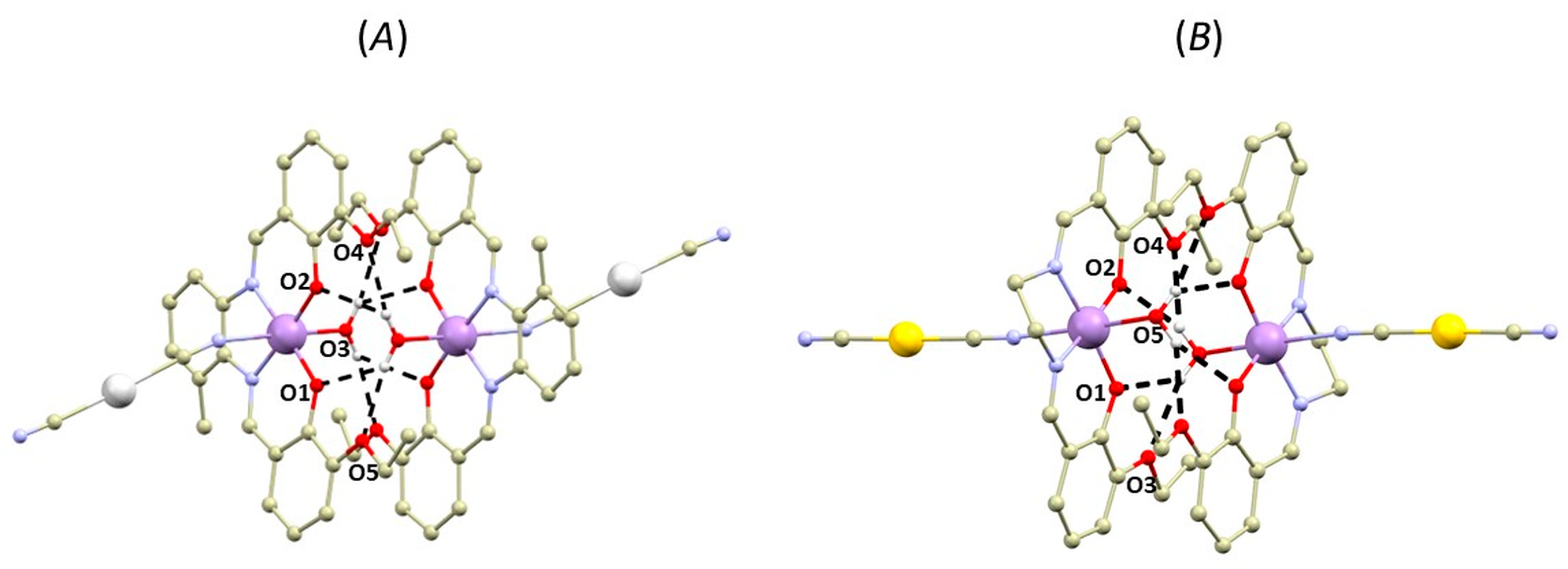
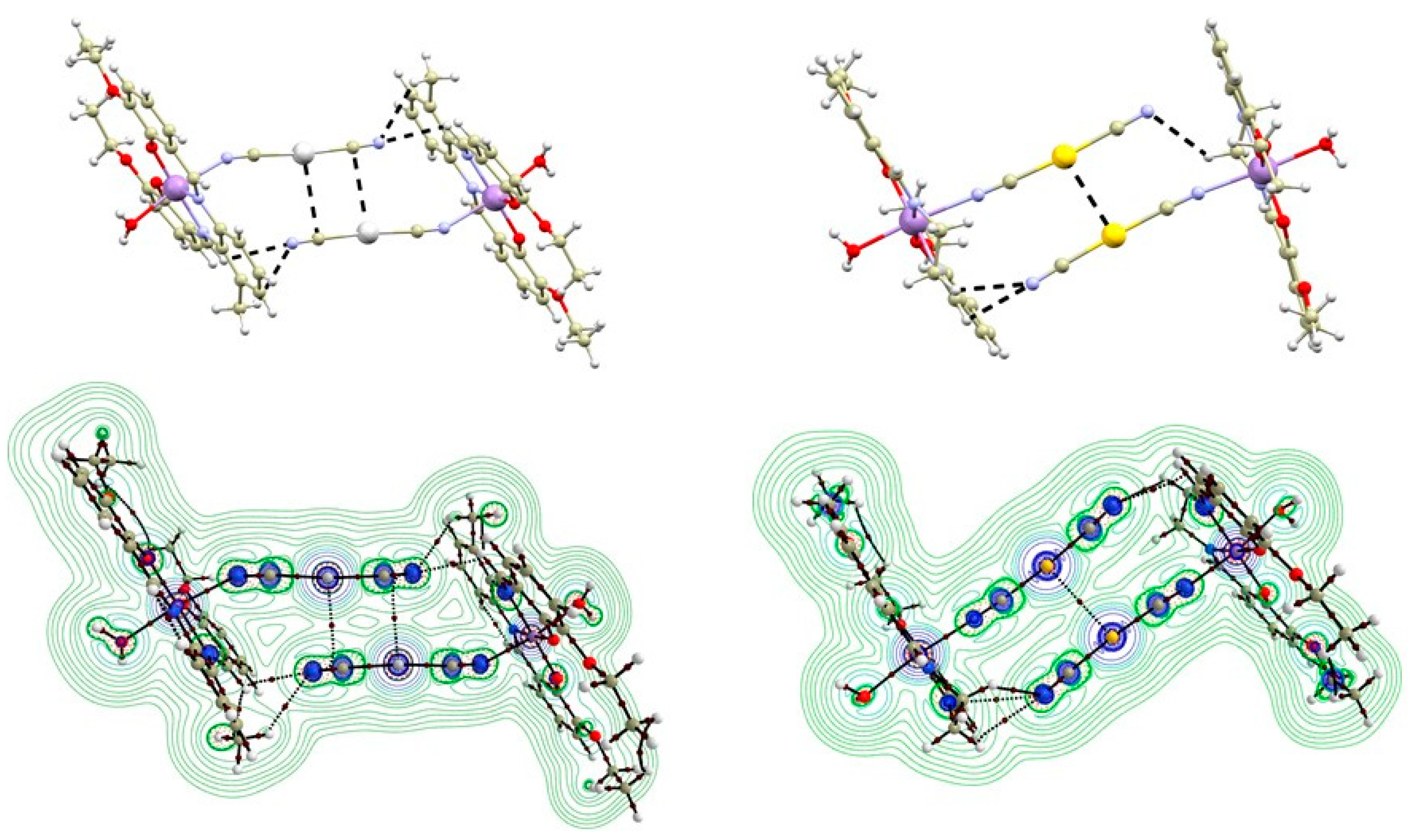
In the second type of complex, one of the axial positions of the [Mn(L4x)]+ moiety is occupied by the aqua ligand, which forms O-H∙∙∙O hydrogen bonding with the phenolic and ethoxy (OEtO) oxygen atoms from the adjacent complex molecule forming well-known hydrogen-bonded supramolecular dimeric unit topology (Figure 3) [5]. The supramolecular building unit exhibits the following lengths of bifurcated hydrogen bonds: (OH2O….OPh) = 2.89–2.91 Å and (OH2O….OEtO) = 2.83–2.96 Å. The Mn….Mn distances within the supramolecular unit are relatively short (4.7667(7) Å (in 1b) and 4.6719(5) Å, or 4.7211(5) Å (in 2b)).
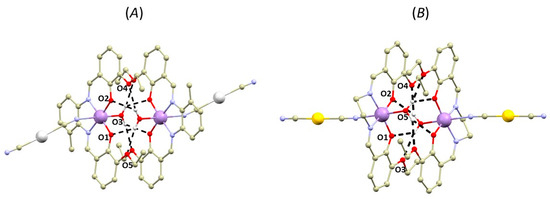
Figure 3.
A perspective views illustrating supramolecular O-H···O hydrogen-bonded dimers of the crystal structures of 1b (A) and 2b (B). Hydrogen atoms were omitted for clarity except for those involved in hydrogen bonding (black dashed lines). Colour code: carbon (light brown), chlorine (green), gold (yellow), manganese (violet), nitrogen (light blue), oxygen (red), silver (white). Selected donor···acceptor distances of hydrogen bonding (in Å): 1b, d(O3···O1) = 2.890(4), d(O3···O2) = 2.916(3), d(O3···O4) = 2.959(4), d(O3···O5) = 2.935(4), 2b, d(O5···O1) = 2.864(2), d(O5···O2) = 2.890(2), d(O5···O3) = 2.946(2), d(O5···O4) = 3.040(3), d(O6···O7) = 2.942(2), d(O6···O8) = 2.877(2), d(O6···O9) = 3.041(3), d(O6···O10) = 2.990(2).
In some of the studied complexes 1a–2b, the silver and gold atoms are involved in the formation of non-covalent interactions. In 1a and 2a, the Ag/Au atoms are surrounded by aromatic rings and ethylamine groups originating from the [Mn(L4x)]+ moieties of neighbouring polymeric chains (see Supplementary, Figure S2). Such crystal packing arrangement limits the occurrence of significant non-covalent interactions for the Ag/Au atoms. In 1b, the Ag atom forms close contact with the cyanide ligand of the neighboring complex molecule related through the inversion center (Figure 3 left). The distance between the carbon and silver atoms (3.294(9) Å) is shorter than the sum of their van der Waals radii (ΣRvdw(C, Ag) = 3.42 Å). Additionally, a relatively short C-H∙∙∙N interaction (3.190(5) Å) is observed between the C–H group of the imino moiety and the cyanide nitrogen atom (Figure 3 left), supporting the formation of this interaction. The positions of the Ag atoms in the crystal packing of 1c resemble those in 1a and 2a (see Supplementary, Figure S3). Similarly, they do not form any significant contacts with donor-acceptor distances shorter than the sum of the van der Waals radii. The crystal packing in 2b is very similar to that in 1b, exhibiting dimers interacting via C-H∙∙∙N hydrogen bonds between the imino and methylene groups of the ligand and cyanido nitrogen atom (3.045(5) and 3.298(4) Å (Figure 3 right), respectively). However, an important distinction is that in 2b, the Au atoms form a non-covalent interaction with a distance of d(Au∙∙∙Au) = 3.3938(3) Å, slightly longer than the sum of the Au van der Waals radii (ΣRvdw(Au, Au) = 3.32 Å). A closer inspection of the non-covalent interactions using Quantum Theory Atoms in Molecules (QT-AIM) is provided in the following text.
3.2. QT–AIM Analysis of Non-Covalent Interactions
The numismophilic (metallophilic) interactions [26] manifest themselves as weak electrostatic attractive forces between low-valent closed-shell (n-1)d10ns0 and metal atoms [9]. These interactions are commonly known as argentophilic [27] and aurophilic [28,29] interactions when referring to the Ag(I) and Au(I) atoms, respectively. In the 1a–2b compounds, X-ray diffraction experiments confirmed the existence of non-covalent interactions involving Ag (1b) and Au (2b) atoms as was discussed above. To study the nature of these interactions, we performed DFT and QT–AIM calculations using ORCA 4.2.1 and Multiwfn calculation packages on the structural fragments chosen from experimentally determined crystal structures. The positions of the hydrogen atoms were normalized to more accurate the distances based on distances obtained by precise neutron diffraction experiments [30].
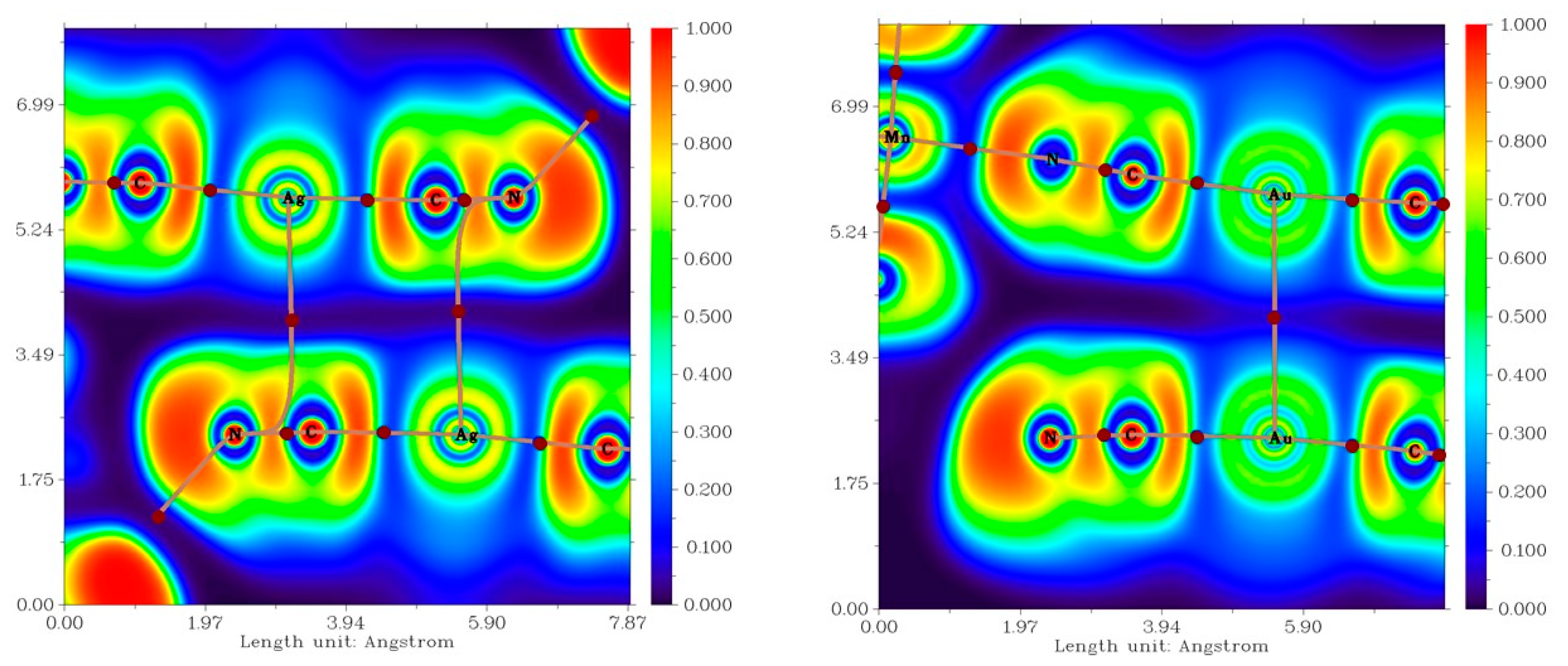
In 1b, the Ag atoms were shifted away from the ideal alignment, resulting in a deviation from the shortest Ag∙∙∙Ag distance. This allowed for the rise of an interaction between the silver atom and cyanido ligand of the neighbouring molecule (Figure 4 left). The distance between the silver and carbon atoms was relatively short (3.294(4) Å), even shorter than the sum of their van der Waals radii (ΣRvdw(C, Ag) = 3.42 Å). The topological analysis of the calculated electron density revealed the presence of a (3,−1) bond critical point (BCP) between Au and C atoms. It is important to mention that the bond path did not directly connect with the carbon atom but merged with the bond pathway between the C and N atoms of the cyanide ligand (Figure 4 left below). This indicated that the interaction is due to interaction of the π electrons of the cyanido ligand with the Ag atom [31]. To further investigate this possibility, we also calculated electron localization function (ELF), which maps the probability of the electron localization (Figure 5 below) [32,33,34,35,36]. The highest likelihood of electron occurrence was found in areas corresponding to the covalent bonds (C-N) and lone pairs, while the bond pathway of the interaction intersected an area with low electron localization, thus supporting the previous assumption of an Ag···π type of the contact. A non-covalent nature of the contact was also confirmed by the calculation of the topological and energetic properties at (3,−1) BCP: he(r) > 0, ρ(r) > 0, (|V(r)|/G(r) < 1 [37]. he(r) stands for energy density, G(r) stands for Lagrangian kinetic energy, and V(r) stands for potential energy density (see Supplementary, Table S3). Based on the calculation of the interaction energy Eint, the contact can be considered to be very weak (Eint = 1.47 kcal/mol).
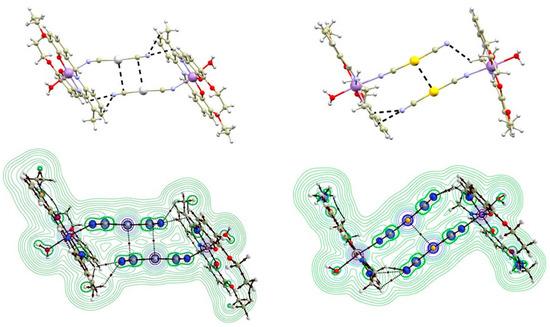
Figure 4.
A perspective view illustrating the fragments of the crystal structures of 1b (top left) and 2b (top right) exhibiting C-H∙∙∙N hydrogen bonding and non-covalent interactions (black dashed lines) involving Ag or Au atoms. A relief section through the mean plane of the metal cyanide groups is shown in the contour plot of the Laplacian of electron density (bottom left for 1b, bottom right for 2b). The (3,−1) bond critical points are depicted as brown circles; bond paths are depicted as dashed brown lines.
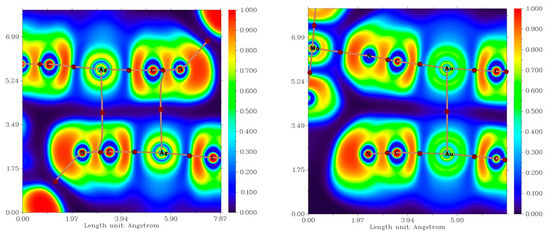
Figure 5.
The calculated Electron localization function (ELF) for the fragments of the crystal structures of 1b (left) and 2b (right), depicting non-covalent interactions involving Ag (1b) and Au (2b) atoms. The (3,−1) bond critical points are depicted as brown circles; bond paths are depicted as brown lines.
In the crystal structure of 2b, the neighbouring Au atoms exhibited better aligned than Ag atoms in 1b, leading to a shorter contact distance of 3.3938(3) Å (Figure 4). The QTAIM analysis of electron density in 2b confirmed the presence of a bond pathway and (3,−1) BCP between the Au atoms (Figure 4, right below, and Figure 5). At this BCP, he(r) adopted a small and positive value, as well as ρ(r) and |V(r)|/G(r) < 1 (0.92, see ESI). These parameters collectively indicated the non-covalent nature of the Au∙∙∙Au interaction in 2b.
3.3. Experimental and Theoretical Investigation of Magnetic Properties
As can be anticipated from the previous works on Mn(III) salen-based complexes, the magnetic properties of the studied complexes will be significantly influenced by Jahn–Teller axial elongation and, thus, by deviation from the ideal 5E ligand field ground term. This situation inevitably brings non-negligible magnetic anisotropy, and, in the case of axial elongation, the magnetic anisotropy of axial type can be expected to be observed [8]. Therefore, to properly analyze the magnetic data, we had to introduce spin Hamiltonian involving the Zeeman term, as well as the zero-filed splitting (ZFS) term, with axial (D) and rhombic (E) parameters of magnetic anisotropy. The polymeric Mn(III) complexes bridged by the diamagnetic cyanometallates (1a, 1c, and 2a) tend to exhibit weak magnetic-exchange interactions. Such exchanges may become significant in the case of the complexes with hydrogen-bonded supramolecular dimeric unit topology in which the antiferromagnetic exchange interaction is mediated via O-H…O hydrogen bonding (1b and 2b). Therefore, the first group of the compounds (1a, 1c, and 2a) were treated with spin Hamiltonian containing ZFS parameters (D and E) and the molecular-field correction parameter (zj)
and Zeeman term was defined for the a-direction of the magnetic field as Ba = B(sin(θ)cos(φ), sin(θ)sin(φ), and cos(θ)), exploiting the polar coordinates. Then, the molar magnetization for a-direction of the magnetic field was computed as
where Za is the matrix element of the Zeeman term for the a-direction of the magnetic field and C comprises the eigenvectors resulting from the diagonalization of the complete spin Hamiltonian matrix. The incorporation of the zj-parameter means that an iterative procedure must be applied [38]. Finally, the integral average of the molar magnetization was calculated as
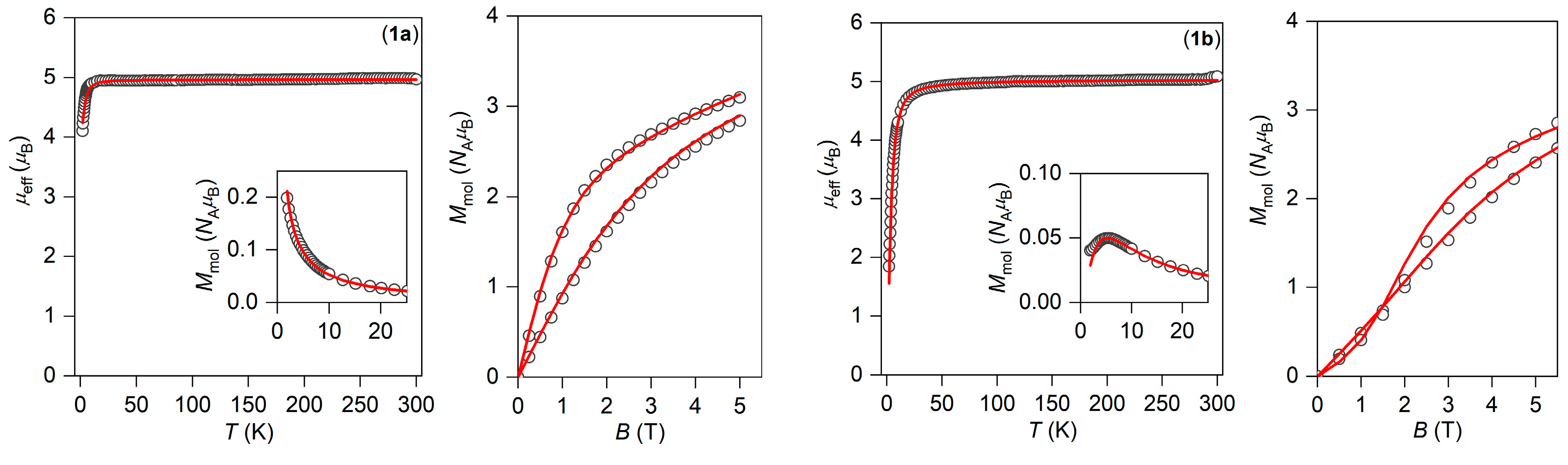
to properly evaluate experimental powder magnetization data. The analysis was performed with the program POLYMAGNET, and both temperature and field-dependent magnetic data were fitted simultaneously. For all fitted parameters, the standard deviations were also calculated with 95% probability. The preliminary calculations showed that the analysis is not sensitive to rhombic ZFS-parameter E. Thus, for the final analysis, E was fixed to zero. The best fit for 1a is showed on Figure 6, as well as for 1c and 2a in Figure S4 (Supplementary). The axial ZFS parameter D was found to be close to −3 cm−1 (Table 2).
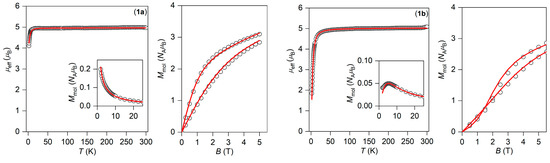
Figure 6.
Temperature dependence of the effective magnetic moment, the molar magnetization (inset), and the isothermal magnetizations measured at T = 2 and 5 K for 1a (left) and 1b (right). Empty circles—experimental data, full lines—calculated data with parameters in the text. All data are scaled per one Mn(III) ion.

Table 2.
Summary of calculated (CASSCF/NEVPT2) and experimentally derived (magnetometry) spin Hamiltonian parameters.
The above-mentioned procedure was not successful for 1b and 2b due to a much stronger antiferromagnetic exchange mediated by the hydrogen bonds within the supramolecular dimers. This is evidenced by a much more pronounced decrease of μeff/μB having values of ca. 1.8 (1b) and 1.3 (2b) at 2 K. Furthermore, if the temperature dependence of magnetization is plotted, we clearly see a maximum at low temperatures for both complexes (Figure 6 and Figure S4 in the insets). Thus, a spin Hamiltonian was then applied
where an isotropic magnetic exchange term was also added. The fitting of magnetic data resulted in J = −0.58(2) cm−1 for 1b and J = −0.73(7) cm−1 for 2b, thus confirming a slightly stronger antiferromagnetic exchange in 2b. However, the magnetic anisotropy parameter D was found larger for 1b (Table 2).
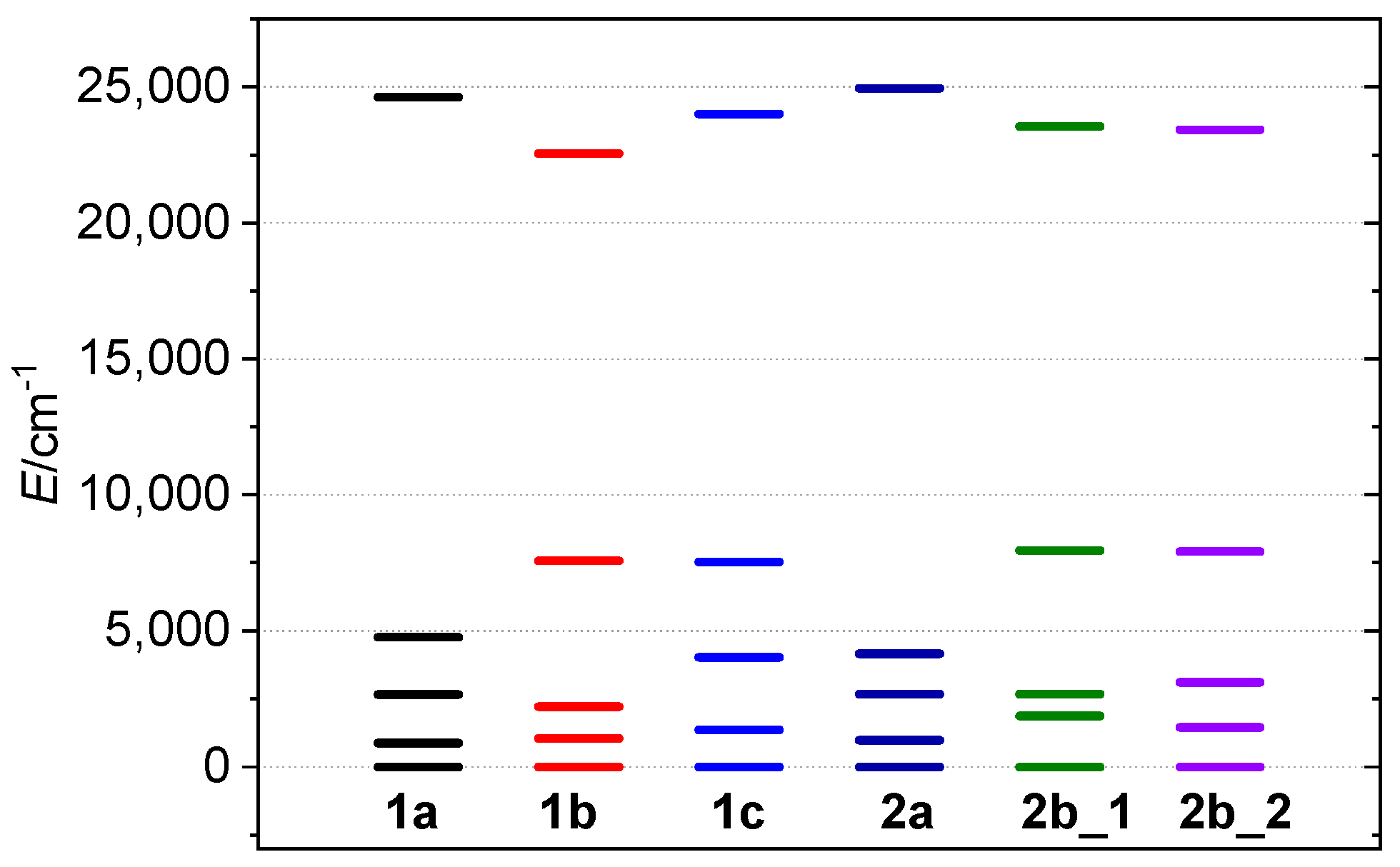
The calculations of the ZFS parameters were performed utilizing a multi-reference method based on the Spin-Averaged Complete Active Space Self Consistent Field (SA–CASSCF). To determine the energy levels of the central Mn(III) atom with 3d4 configuration, we utilized an ORCA 4.2 computational package. The active space was defined as consisting of four electrons in five d-orbitals (CAS(4,5) and considering five quintets and 45 triplets) and dynamic electronic configuration, which was handled using the NEVPT2 method. In addition, the ab initio ligand field theory (AILFT), refs. [37,39] was employed to calculate the splitting of the d-orbitals, as illustrated in Figure 7. To improve efficiency and reduce computational costs, we conducted all calculations performed on selected fragments from the crystal structures in which the cyanometallate bridging complexes were substituted by cyanide ligands. The basis set was used for the calculation consisted of def2-SVP basis for hydrogen and carbon atoms, while def2-TZVP was used for the remaining atoms. The costs of calculations were decreased by the use of the def2/J and def2-TZVP/C auxiliary basis sets [40,41] together with the chain-of-spheres (RIJCOSX) [42,43] approximation to exact exchange, as is implemented in ORCA. Increased integration grids (Grid6) and tight SCF convergence criteria were used.
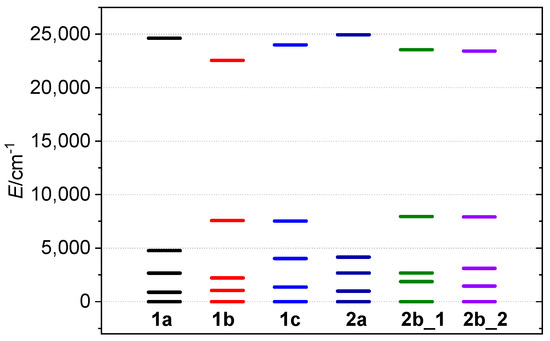
Figure 7.
The splitting of the d-orbitals as calculated for 1a–2b by AILFT method.
The calculated splitting pattern matches the expected pattern for an axially elongated octahedron, with the and orbitals having the lowest energy, while has the highest energy. The overall configuration of d orbitals in 1a–2b is . The calculated ZFS parameters are in agreement with the experimentally derived parameters, with D parameters ranging from −2.80 cm−1 (1a) to −3.16 cm−1 (2b). The variations in the D values reflect different levels of axial elongation, with the complexes exhibiting longer axial bonds having the smallest |D| values. The rhombicity was calculated to be low in all compounds, with E/D values being very small (≤0.03, Table 2).
We utilized broken-symmetry DFT calculations at the B3LYP and ZORA-def2-TZVP levels of theory to investigate the magnetic-exchange interactions in 1a–2b. Again, the fragments selected from the crystal structures were used as input coordinates, and the positions of the hydrogen atoms were optimized using DFT calculations (B3LYP def2-SVP level of theory). We investigated possible magnetic-exchange interactions mediated by diamagnetic bridging ligands (1a, 1c, 2a), as well as the assemblies involving interactions of Ag (1b) and Au (2b, Figure 3) atoms, which were investigated for the possible mediation of magnetic exchange. However, none of these systems were found to mediate even weak magnetic-exchange interactions. Subsequently, we performed calculations to assess the magnetic-exchange interactions within the O-H∙∙∙O hydrogen-bonded dimers present in 1b and 2b. These calculations confirmed the mediation of weak antiferromagnetic-exchange interactions in complexes (J = −0.46 (1b) and −0.60/−0.56 (2b) cm−1). These results are consistent with previous observations and also align with the experimental data (Table 2, Table S4) [5].
4. Discussion
Our investigations have revealed two examples of interesting interactions involving Ag and Au atoms in Compounds 1a–2b. In 1b, we observed Ag∙∙∙π interactions in 1b, while 2b exhibited Au∙∙∙Au interactions. These interactions occur in compounds with 0D structure composed of Mn–Ag or Mn–Au molecules. Although these molecules are, in terms of covalent bonding, isolated, they assemble into O-H∙∙∙O hydrogen-bonded supramolecular dimers. However, a search in the CSD database (Cambridge Structural Database) shows that this is not a general rule, and that the topology of the salen-based (and related) Mn(III) complexes with [Ag/Au(CN)2]− bridging complexes exhibiting metallophilic interactions is much more diverse than that.
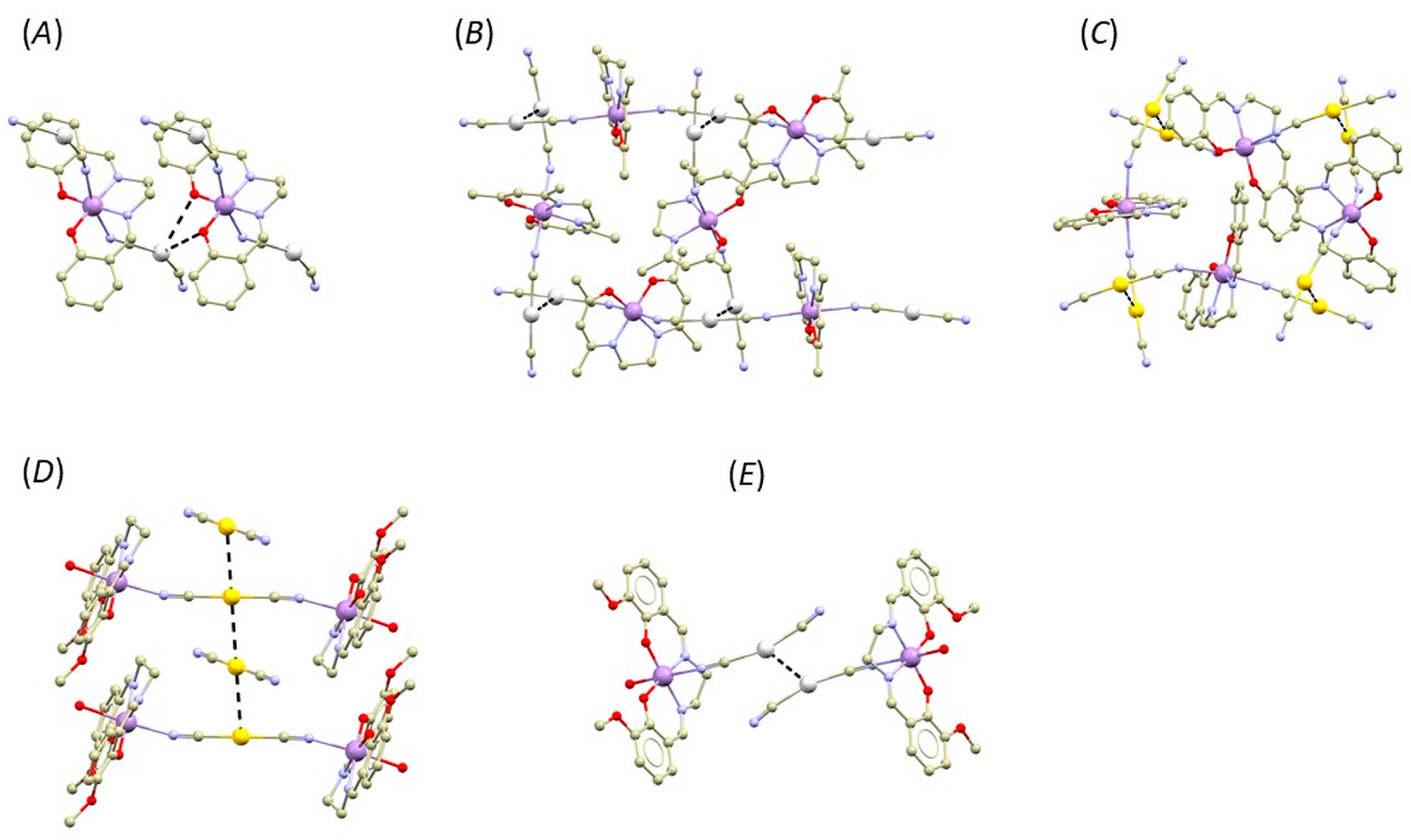
The reactions between the [Ag/Au(CN)2]− bridging complexes and precursors containing a basic [Mn(salen)]+ complex result in structurally different outcomes (Figure 8).
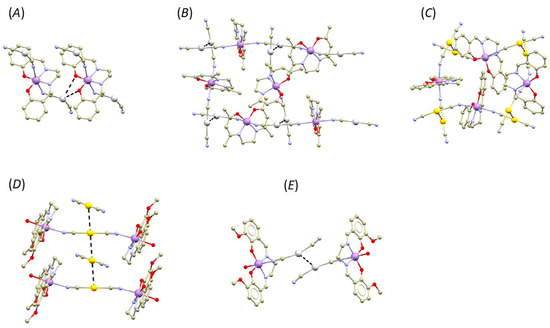
Figure 8.
A perspective illustrating selected non-covalent interactions (black dashed lines) in the fragments of crystal structures of [Mn(salen){Ag(CN)2}]n, (A), [Mn(salen){Au(CN)2}]n, (B), [Mn(acacen){Ag(CN)2}]n, (C), [Mn(valen)(H2O)]2[Au(CN)2][Au(CN)2], (D), and [Mn(valen)(H2O)]2[Ag(CN)2][Ag(CN)2] (E). Hydrogen atoms were omitted for clarity. Colour code: carbon (light brown), chlorine (green), gold (yellow), manganese (violet), nitrogen (light blue), oxygen (red), and silver (white).
When [Ag(CN)2]− reacts with [Mn(salen)(CH3COO)]·H2O, the resulting compound [Mn(salen){Ag(CN)2}]n is 1D polymeric without exhibiting any metallophilic interactions (CSD Reference code: WUJGIR) [12]. Instead, the Ag atoms form Ag···O interactions with the phenolic oxygen atoms of the salen ligand of the adjacent polymeric chain. We performed QT–AIM analysis of electron density, and we revealed that both Ag···O interactions are of a non-covalent nature (he(r) and ρ(r) > 0, |V(r)|/G(r) < 0) and of relatively weak strength as can be judged based on their low values of interaction energies at particular (3,−1) BCP (Eint = 1.61 and 1.03 kcal/mol, see Table S3). The magnetic properties were not studied in the original report. BS–DFT calculations indicate that the magnetic behavior of this compound will not be dominated by magnetic-exchange interactions because no significant exchange pathway was found (see Supplementary, Table S5).
When [Au(CN)2]− reacts with [Mn(salen)(H2O)]ClO4, the resulting compound is again a polymer composed of [Mn(salen){Au(CN)2}]n chains and co-crystalized H2O molecules (CSD: TIJDUM) [14]. In this case, the distance between the Au atoms of neighbouring chains is shorter than the sum of van der Waals radii (3.150(1) Å, ΣRvdw(Au, Au) = 3.32 Å). The QT–AIM analysis of electron density revealed that at (3,−1) BCP, the values of he(r) and ρ(r) are small and positive, while |V(r)|/G(r) is slightly larger than 1 (1.04, see Table S3). In the original report, the authors also analyzed magnetic data measured for this compound. In addition, they revealed that the axial magnetic anisotropy dominates the magnetic behaviour (D = −5.5 cm−1), and that the molecular field parameter zj (+0.12 cm−1) was necessary for successful fitting of the magnetic data. Therefore, we also conducted BS–DFT calculations to investigate if magnetic exchanges could mediate via the metallo-cyanide bridge but with the negative outcome (see Supplementary, Table S5). In the same paper, the authors also report on the structure of the polymeric complex with the salen analogue acacen (H2acacen = N,N′-ethylenebis(acetylacetonylideneiminate)) and formula [Mn(acacen){Ag(CN)2}]n, (CSD: TIJDOG). In the crystal structure, the Ag atoms from neighbouring chains form Ag···Ag interactions, with the distance shorter than the sum of their van der Waals radii: d(Ag···Ag) = 3.0967(7) Å, ΣRvdw(Ag, Ag) = 3.44 Å. The results of the QT–AIM analysis of electron density reflects a relatively short contact distance. At (3,−1) BCP of this contact, the value of he(r) is negative, ρ(r) is of positive value, and the |V(r)|/G(r) ratio is significantly larger than 1 (1.09, see Table S3). This indicates that the interaction is stabilized by local charge concentration and, thus, it exhibits some degree of covalency [44]. The interaction energy is relatively large (Eint = 4.27 kcal/mol). The magnetic properties of this compound were analyzed using a one-dimensional chain formula and a weak antiferromagnetic-exchange interaction (J = −0.1 cm−1). Again, we investigated magnetism of this compound by BS–DFT calculations, but we did not reveal any significant magnetic-exchange pathway (see Supplementary, Table S5).
The last two examples are related to Compounds 1b and 2b since they are 0D compound assemblies into supramolecular dimers by O-H···O hydrogen bonding. The reaction between [Mn(valen)(H2O)(CH3CN)](ClO4)·CH3CN and K[Ag(CN)2] led to the isolation of the compound with the formula [Mn(valen)(H2O)]2[Ag(CN)2][Ag(CN)2], CSD: GIMGAL [16]. In its crystal structure, the Ag atoms form short intermolecular contact with d(Ag···Ag) = 3.0922(7) Å, which is shorter than sum of the van der Waals radii. The QT–AIM analysis of the electron density again revealed that, at corresponding BCP, the value of he(r) is negative, ρ(r) is of positive value, and the |V(r)|/G(r) ratio is significantly larger than 1 (1.09, see Table S3), indicating covalency contribution. Furthermore, this interaction possesses a relatively large value of Eint (4.34 kcal/mol). Magnetic properties of this compound were not studied in the original report. The BS–DFT calculations indicate that the magnetic behavior of this compound will be dominated by exchange interactions mediated within the supramolecular dimer by O-H···O hydrogen bonding and magnetic anisotropy of the Mn(III) centers (J = −0.59 cm−1, see Supplementary, Table S5).
By reacting [Mn(valen)(H2O)(CH3CN)](ClO4)·CH3CN with K[Au(CN)2], the coordination compound with formula [Mn(valen)(H2O)]2[Au(CN)2][Au(CN)2]·H2O was obtained (H2valen = N,N′-bis(3-methoxysalicylidene)ethylenediamine, CSD: DOKDOY) [45]. In the crystal structure, the Au···Au interaction is present with d(Au···Au) = 3.3946(8) Å, which is longer than the sum of their van der Waals radii (ΣRvdw(Au, Au) = 3.32 Å). The QT–AIM analysis of electron density revealed (3,−1) BCP between the Au atoms, and the calculated topological and energetic properties confirmed the non-covalent nature of the interaction (he(r) > 0, ρ(r) > 0, |V(r)|/G(r) < 1.0, see ESI) and its weak strength (Eint = 2.21 kcal/mol). The magnetic properties were not studied in the original report. The insertion of co-crystalized water molecules between neighboring [Mn(valen)(H2O)]+ moieties disrupted the formation of the standard O-H···O hydrogen-bonded supramolecular dimer. As a result, no effective magnetic-exchange pathways were predicted by BS–DFT (see Supplementary, Table S5).
In summary, we investigated topological and energetic properties of five new (1a–2b) and previously reported complexes of the Mn(III) salen-based family exhibiting metallophilic interactions of the Ag/Au atoms. We found that the strength of the Ag···Ag and Au···Au interactions varies between 2.2 and 5.2 kcal/mol in this group of compounds. In addition, by utilizing BS–DFT calculations, we determined that these interactions do not have any significant influence over their magnetic properties. This is due to very long distances between the paramagnetic metal centres when [Ag/Au(CN)2]− bridging complexes are considered as primary mediators of the magnetic exchange. The long distance, along with the diamagnetic nature of the Ag(I)/Au(I) centers, makes this super-exchange pathway very ineffective. Therefore, the magnetic properties of the compounds are dominated by magnetic anisotropy of the Mn(III) centers, and also by exchange interactions mediated by O-H···O hydrogen bonding in the compounds exhibiting supramolecular dimer topology.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst13081217/s1. Table S1: Crystal data and details of structure determination of complexes 1a–1c; Table S2: Crystal data and details of structure determination of complexes 2a and 2b; Table S3: Topological and energetic properties of ρ(r) calculated for interactions involving Ag/Au atoms; Table S4: The results of BS-DFT calculations for 1a–2b; Table S5: The results of BS-DFT calculations for Mn(III) salen-based complexes with [Ag/Au(CN)2]− bridging complexes; Figure S1: Projection along the c-axis for the complex 1a (above) and complex 1c (below), showing a 2D network structure, in which are shown π-π interaction between two 1D polymeric networks; Figure S2: A perspective view illustrating localization of the Ag/Au atoms in 1a and 2a; Figure S3: A perspective view illustrating localization of the Ag atoms in 1c; Figure S4: Temperature dependence of the effective magnetic moment and the molar magnetization (inset), and the isothermal magnetizations measured at T = 2 and 5 K for 1c, 2a, and 2b.
Author Contributions
Conceptualization, T.Š. and I.N.; methodology, I.N. and R.H.; formal analysis, T.Š., R.H. and I.N.; investigation, T.Š., R.H. and I.N.; writing—original draft preparation, T.Š., R.H. and I.N; writing—review and editing, T.Š., R.H. and I.N.; visualization, I.N.; supervision, I.N. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge financial support from institutional sources of the Department of Inorganic Chemistry at Palacký University Olomouc, Czech Republic.
Data Availability Statement
All data contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gao, S. Molecular Nanomagnets and Related Phenomena. In Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Kachi-Terajima, C.; Ishii, R.; Tojo, Y.; Fukuda, M.; Kitagawa, Y.; Asaoka, M.; Miyasaka, H. Ferromagnetic Exchange Coupling in a Family of MnIII Salen-Type Schiff-Base Out-of-Plane Dimers. J. Phys. Chem. C 2017, 121, 12454–12468. [Google Scholar] [CrossRef]
- Bogani, L.; Vindigni, A.; Sessoli, R.; Gatteschi, D. Single chain magnets: Where to from here? J. Mater. Chem. 2008, 18, 4750–4758. [Google Scholar] [CrossRef]
- Ferbinteanu, M.; Miyasaka, H.; Wernsdorfer, W.; Nakata, K.; Sugiura, K.; Yamashita, M.; Coulon, C.; Clérac, R. Single-Chain Magnet (NEt4)[Mn2(5-MeOsalen)2Fe(CN)6] Made of MnIII−FeIII−MnIII Trinuclear Single-Molecule Magnet with an ST = 9/2 Spin Ground State. J. Am. Chem. Soc. 2005, 127, 3090–3099. [Google Scholar] [CrossRef]
- Nemec, I.; Herchel, R.; Šilha, T.; Trávníček, Z. Towards a better understanding of magnetic exchange mediated by hydrogen bonds in Mn(III)/Fe(III) salen-type supramolecular dimers. Dalton Trans. 2014, 43, 15602–15616. [Google Scholar] [CrossRef]
- Nemec, I.; Šilha, T.; Herchel, R.; Trávníček, Z. Investigation of Magnetic Exchange Pathways in Heterotrinuclear Manganese(III) Schiff Base Complexes Involving Tetrathiocyanidoplatinate(II) Bridges. Eur. J. Inorg. Chem. 2013, 2013, 5781–5789. [Google Scholar] [CrossRef]
- Šilha, T.; Nemec, I.; Herchel, R.; Trávníček, Z. Structural and Magnetic Characterizations of the First Manganese(III) Schiff Base Complexes Involving Hexathiocyanidoplatinate(IV) Bridges. CrystEngComm 2013, 15, 5351–5358. [Google Scholar] [CrossRef]
- Nemec, I.; Herchel, R.; Trávníček, Z. Pentacoordinate and Hexacoordinate Mn(III) Complexes of Tetradentate Schiff-Base Ligands Containing Tetracyanidoplatinate(II) Bridges and Revealing Uniaxial Magnetic Anisotropy. Molecules 2016, 21, 1681. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Elguero, J.; Frontera, A. Not Only Hydrogen Bonds: Other Noncovalent Interactions. Crystals 2020, 10, 180. [Google Scholar] [CrossRef]
- Raju, S.; Singh, H.B.; Butcher, R.J. Metallophilic Interactions: Observations of the Shortest Metallophilicinteractions between Closed Shell (d10⋯d10, d10⋯d8, d8⋯d8) Metal Ions [M⋯M′ M = Hg(ii) and Pd(ii) and M′ = Cu(i), Ag(i), Au(i), and Pd(ii)]. Dalton Trans. 2020, 49, 9099–9117. [Google Scholar] [CrossRef]
- Zheng, Q.; Borsley, S.; Nichol, G.S.; Duarte, F.; Cockroft, S.L. The Energetic Significance of Metallophilic Interactions. Angew. Chem. Int. Ed. 2019, 58, 12617–12623. [Google Scholar] [CrossRef]
- Panja, A.; Shaikh, N.; Vojtíšek, P.; Gaoc, S.; Banerjee, P. Synthesis, crystal structures and magnetic properties of 1D polymeric [MnIII(salen)N3] and [MnIII(salen)Ag(CN)2] complexes. New J. Chem. 2002, 26, 1025–1028. [Google Scholar] [CrossRef]
- Kosone, Y.; Suzuki, Y.; Kanadani, C.; Saito, T.; Kitazawa, T. Structural isomers of {MnII(L)2[AgI(CN)2]2} (L = 3-methylpyridine or 4-methylpyridine), bilayer structure with binuclear argentophilic interaction and interpenetrated structure with 1D chain argentophilic interaction; synthesis, crystal structure, and magnetic properties. Bull. Chem. Soc. Jpn. 2009, 82, 347–351. [Google Scholar]
- Feng, Y.; Guo, Y.; Yang, Y.O.; Liu, Z.; Liao, D.; Cheng, P.; Yan, S.; Jiang, Z. 2D warp-and-woof interwoven networks constructed by helical chains with different chirality. Chem. Commun. 2007, 35, 3643–3645. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, H.; Tian, L.; Jiang, J.; Ni, Z.H. Rational design of cyanide-bridged heterometallic M(I)–Mn(II) (M = Ag, Au) one-dimensional chain complexes: Synthesis, crystal structures and magnetic properties. CrystEngComm 2009, 11, 2447–2451. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, Y.; Zhou, N.; Liu, Z.Q.; Wang, Q.L.; Yan, S.P.; Liao, D.Z. Three dicyanidometallate(I)-based complexes incorporating hydrogenbonding, π–π packing and d10d10 interactions with auxiliary 2,2′-bipyridyl like ligands. Z. Anorg. Allg. Chem. 2010, 636, 865–871. [Google Scholar] [CrossRef]
- Nastase, S.; Tuna, F.; Maxim, C.; Muryn, C.A.; Avarvari, N.; Winpenny, R.E.P.; Andruh, M. Supramolecular dimers and chains resulting from second coordination sphere interactions. Cryst. Growth Des. 2007, 7, 1825–1831. [Google Scholar] [CrossRef]
- Jayaseeli, A.M.I.; Ramdass, A.; Rajagopal, S. Selective H2O2 oxidation of organic sulfides to sulfoxides catalyzed by cobalt (III)–salen ionw. Polyhedron 2015, 100, 59–66. [Google Scholar] [CrossRef]
- Gravert, D.J.; Griffin, J.H. Steric and electronic effects, enantiospecificity, and reactive orientation in DNA binding/cleaving by substituted derivatives of [SalenMnIII]+. Inorg. Chem. 1996, 35, 4837–4847. [Google Scholar] [CrossRef]
- CrysAlis CCD and CrysAlis RED, version 1.171.33.52; Oxford Diffraction Ltd.: Oxford, UK, 2009.
- CrysAlisPro, version 1.171.40.82a; Rigaku Oxford Diffraction: Oxford, UK, 2020.
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. In Acta Crystallographica Section A Foundations and Advances; International Union of Crystallography (IUCr): Chester, UK, 2015; Volume 71, pp. 3–8. [Google Scholar]
- Ruiz, E.; Cano, J.; Alvarez, S.; Alemany, P. Broken symmetry approach to calculation of exchange coupling constants for homobinuclear and heterobinuclear transition metal complexes. J. Comput. Chem. 1999, 20, 1391–1400. [Google Scholar] [CrossRef]
- Soda, T.; Kitagawa, Y.; Onishi, T.; Takano, Y.; Shigeta, Y.; Nagao, H.; Yoshioka, Y.; Yamaguchi, K. Ab initio computations of effective exchange integrals for H-H, H-He-H and Mn2O2 complex: Comparison of broken-symmetry approaches. Chem. Phys. Lett. 2000, 319, 223–230. [Google Scholar] [CrossRef]
- Todd, A.; Keith, T.K. AIMAll, version 19.10.12; Gristmill Software: Overland Park, KS, USA, 2019.
- Pyykkö, P. Strong Closed-Shell Interactions. Rev. Inorg. Chem. 1997, 97, 597–636. [Google Scholar]
- Schmidbaur, H.; Schier, A. Argentophilic interactions. Angew. Chem. Int. Ed. 2015, 54, 746–784. [Google Scholar] [CrossRef]
- Guevara-Vela, J.M.; Hess, K.; Rocha-Rinza, T.; Martín Pendás, Á.; Flores-Álamo, M.; Moreno-Alcántar, G. Stronger-Together: The Cooperativity of Aurophilic Interactions. Chem. Comm. 2022, 58, 1398–1401. [Google Scholar] [CrossRef]
- Eryazici, I.; Moorefield, C.N.; Newkome, G.R. Square-planar Pd(II), Pt(II), and Au(III) terpyridine complexes: Their syntheses, physical properties, supramolecular constructs, and biomedical activities. Chem. Rev. 2008, 108, 1834–1895. [Google Scholar] [CrossRef]
- Shedrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Frontera, A.; Bauzá, A. Regium-π bonds: An Unexplored Link between Noble Metal Nanoparticles and Aromatic Surfaces. Chem. Eur. J. 2018, 24, 7228–7234. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Meaning and Functional Form of the Electron Localization Function. Acta Phys. Chim. Sin. 2011, 27, 2786–2792. [Google Scholar]
- Xiao, W.; Kiran, G.K.; Yoo, K.; Kim, J.; Xu, H. The Dual-Site Adsorption and High Redox Activity Enabled by Hybrid Organic-Inorganic Vanadyl Ethylene Glycolate for High-Rate and Long-Durability Lithium–Sulfur Batteries. Small 2023, 19, e2206750. [Google Scholar] [CrossRef]
- Xu, H.; Guan, D. Exceptional Anisotropic Noncovalent Interactions in Ultrathin Nanorods: The Terminal σ-Hole. ACS Appl. Mater. Interfaces 2022, 14, 51190–51199. [Google Scholar] [CrossRef]
- Lv, Z.; Xu, H.; Xu, W.; Peng, B.; Zhao, C.; Xie, M.; Lv, X.; Gao, Y.; Hu, K.; Fang, Y.; et al. Quasi-Topological Intercalation Mechanism of Bi0.67NbS2 Enabling 100 C Fast-Charging for Sodium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2300790. [Google Scholar] [CrossRef]
- Singh, S.K.; Eng, J.; Atanasov, M.; Neese, F. Covalency and chemical bonding in transition metal complexes: An ab initio based ligand field perspective. Coord. Chem. Rev. 2017, 344, 2–25. [Google Scholar] [CrossRef]
- Boča, R. Theoretical Foundations of Molecular Magnetism. Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Mingos, D.M.P.; Day, P.; Dahl, J.P. Molecular Electronic Structures of Transition Metal Complexes II; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Hellweg, A.; Hättig, C.; Höfener, S.; Klopper, W. Optimized accurate auxiliary basis sets for RI-MP2 and RI-CC2 calculations for the atoms Rb to Rn. Theor. Chem. Acc. 2007, 117, 587–597. [Google Scholar] [CrossRef]
- Izsák, R.; Neese, F. An overlap fitted chain of spheres exchange method. J. Chem. Phys. 2011, 135, 144105. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations A ‘chain-of-spheres’ algorithm for the Hartree–Fock Exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From Weak to Strong Interactions: A Comprehensive Analysis of the Topological and Energetic Properties of the Electron Density Distribution Involving X–H⋯F–Y Systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Silviu, N.; Catalin, M.; Carine, D.; Jean-Pascal, S.; Marius, A. Synthesis and crystal structures of two new cyanido-bridged [MnIII5MoIV] and [MnIII2AuI] heterometallic complexes. Rev. Roum. Chem. 2013, 58, 355–363. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).