Research on Single Crystal Preparation via Dynamic Liquid Phase Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.1.1. Copper Sulfate Powder
2.1.2. Water
2.2. Experimental Methods
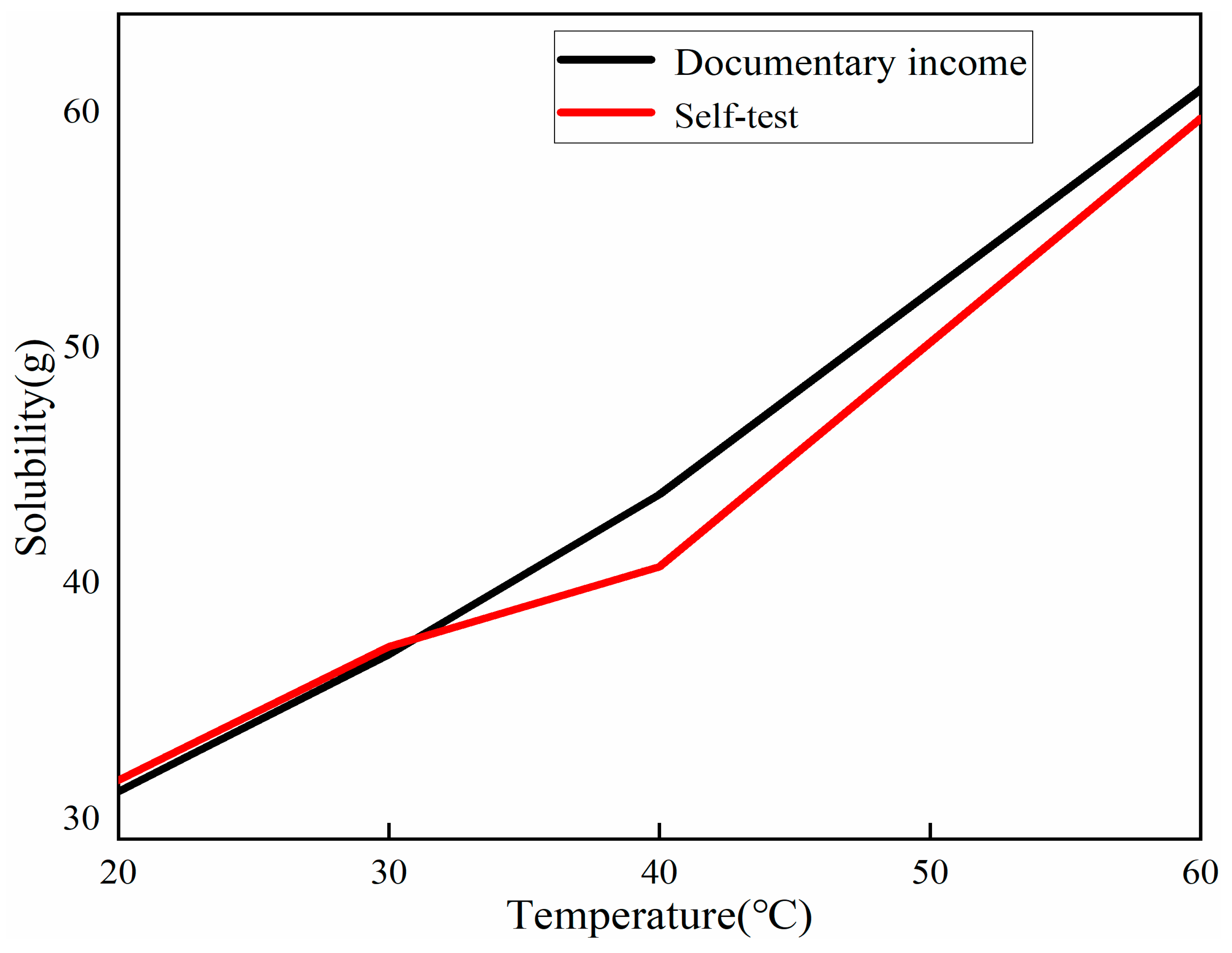
2.2.1. Preparation of Seed Crystals and Determination of Solubility
2.2.2. Traditional Solution Method
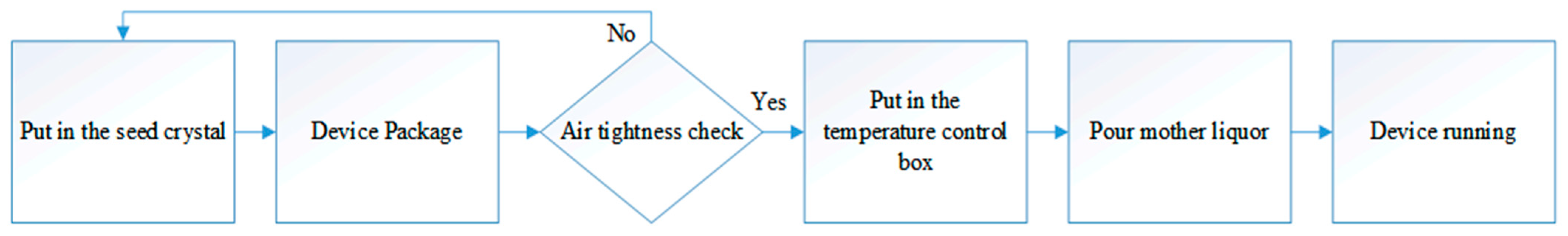
2.2.3. Dynamic Liquid Solution Growth Method
3. Results and Discussion
3.1. Crystal Structure
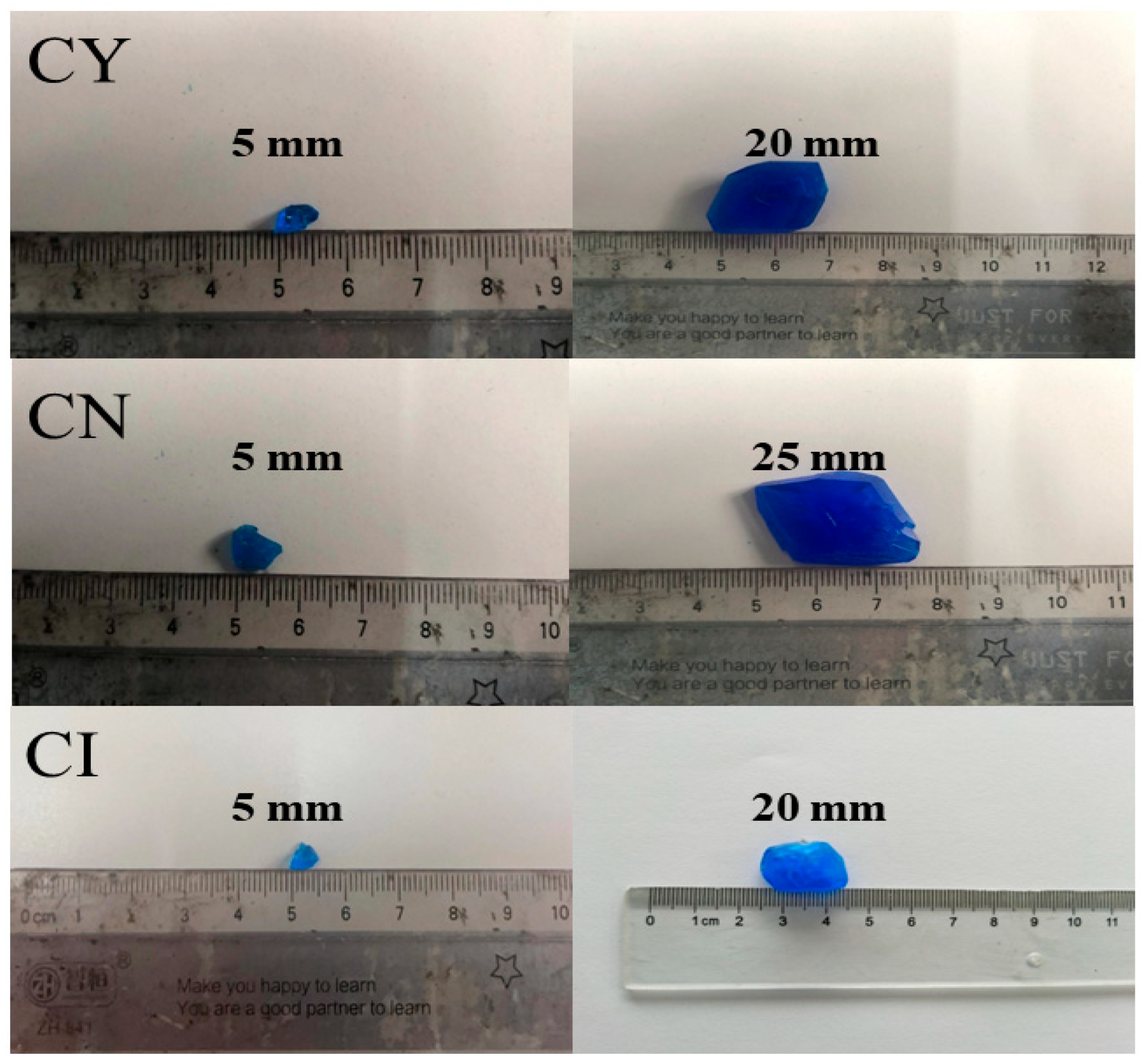
3.1.1. Crystal Shape
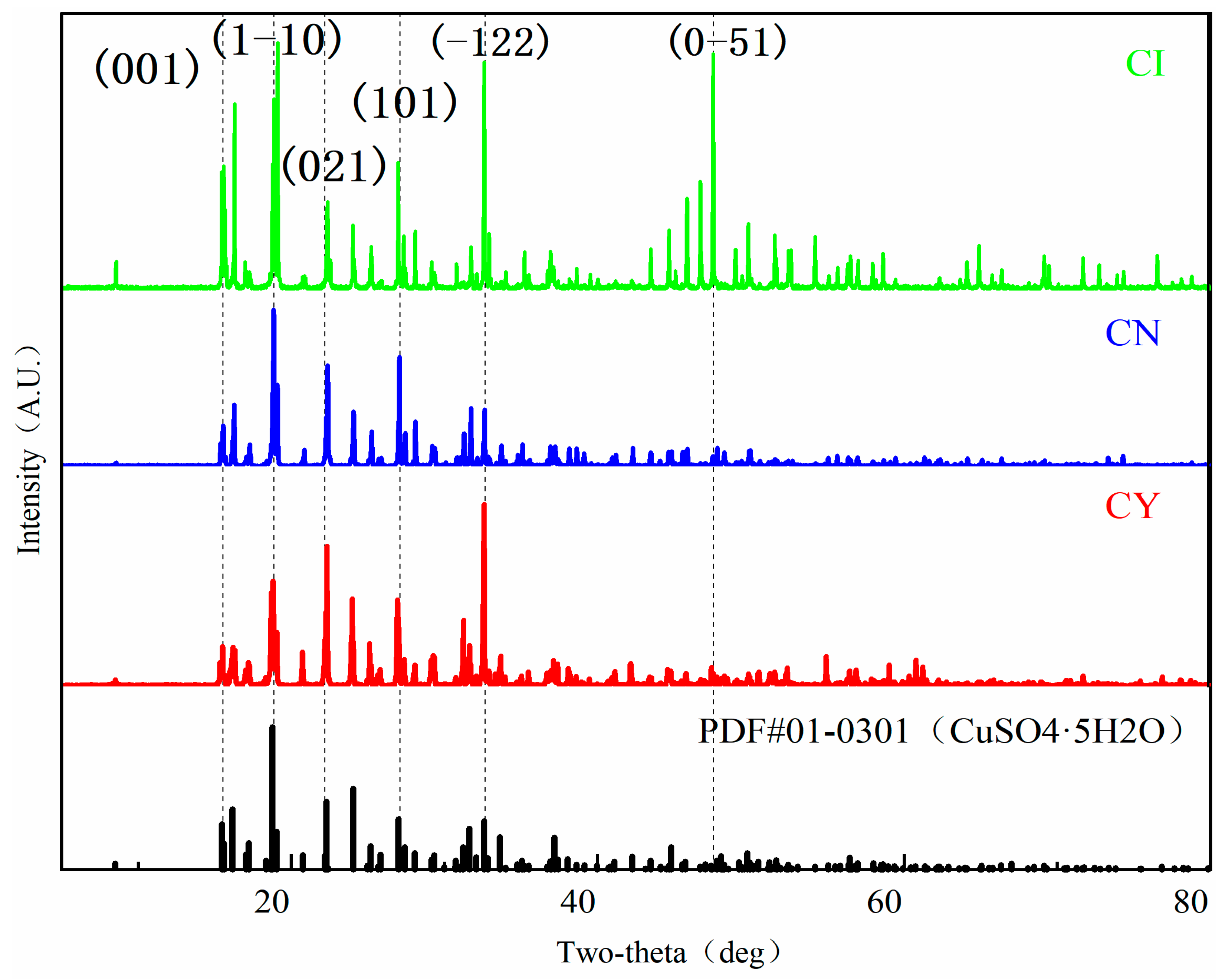
3.1.2. XRD Polycrystalline Powder Diffraction
3.1.3. XRD Single-Crystal Diffraction
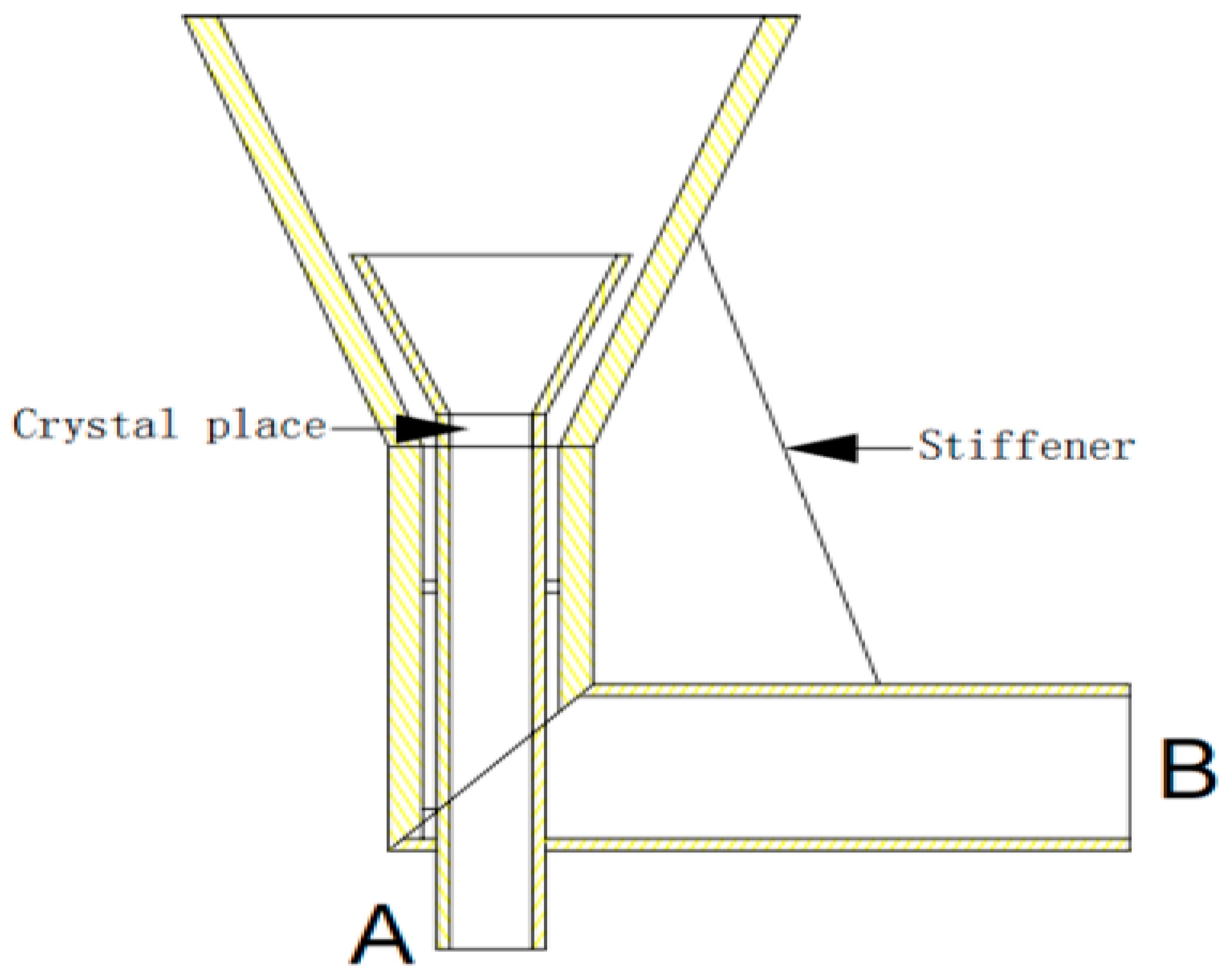
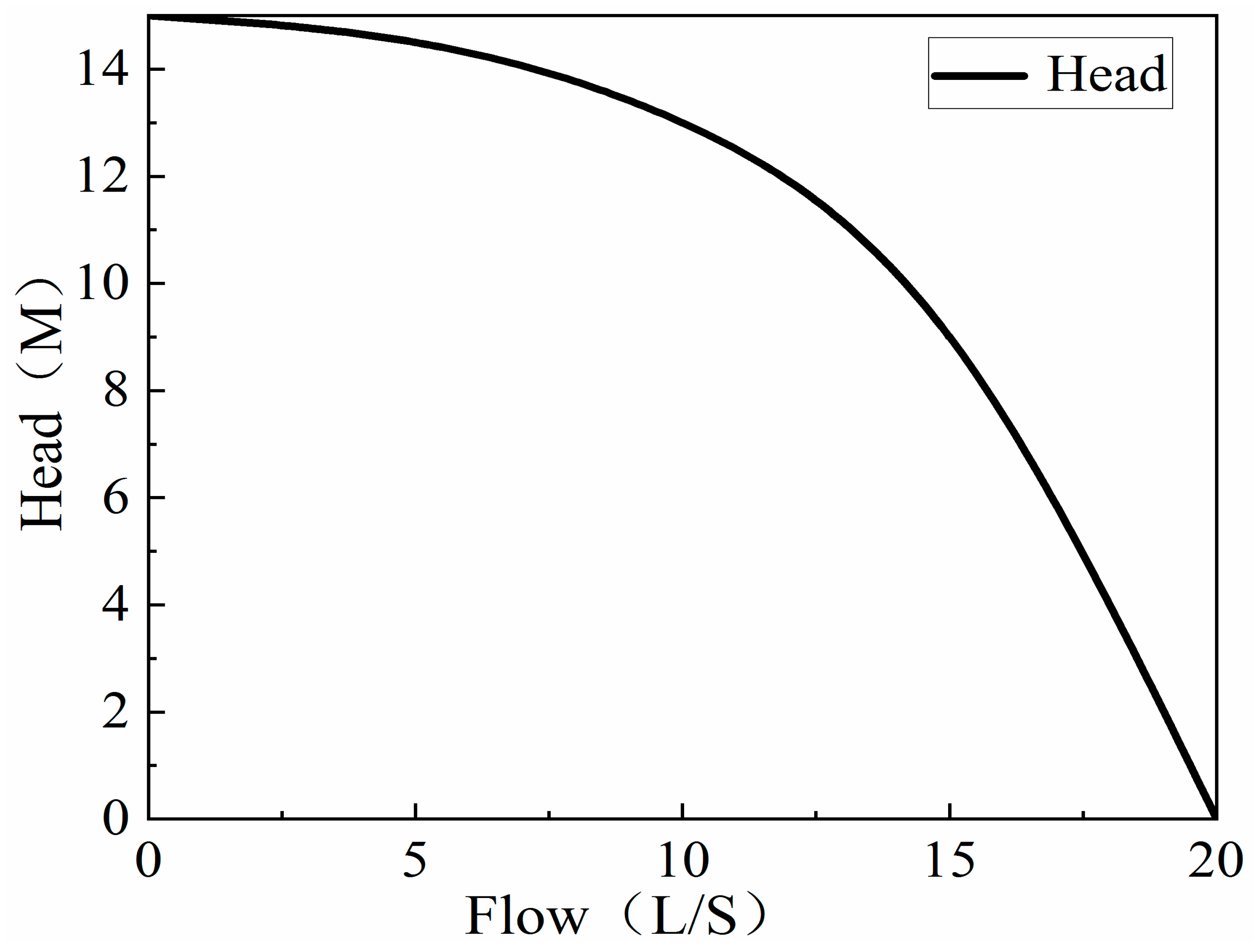
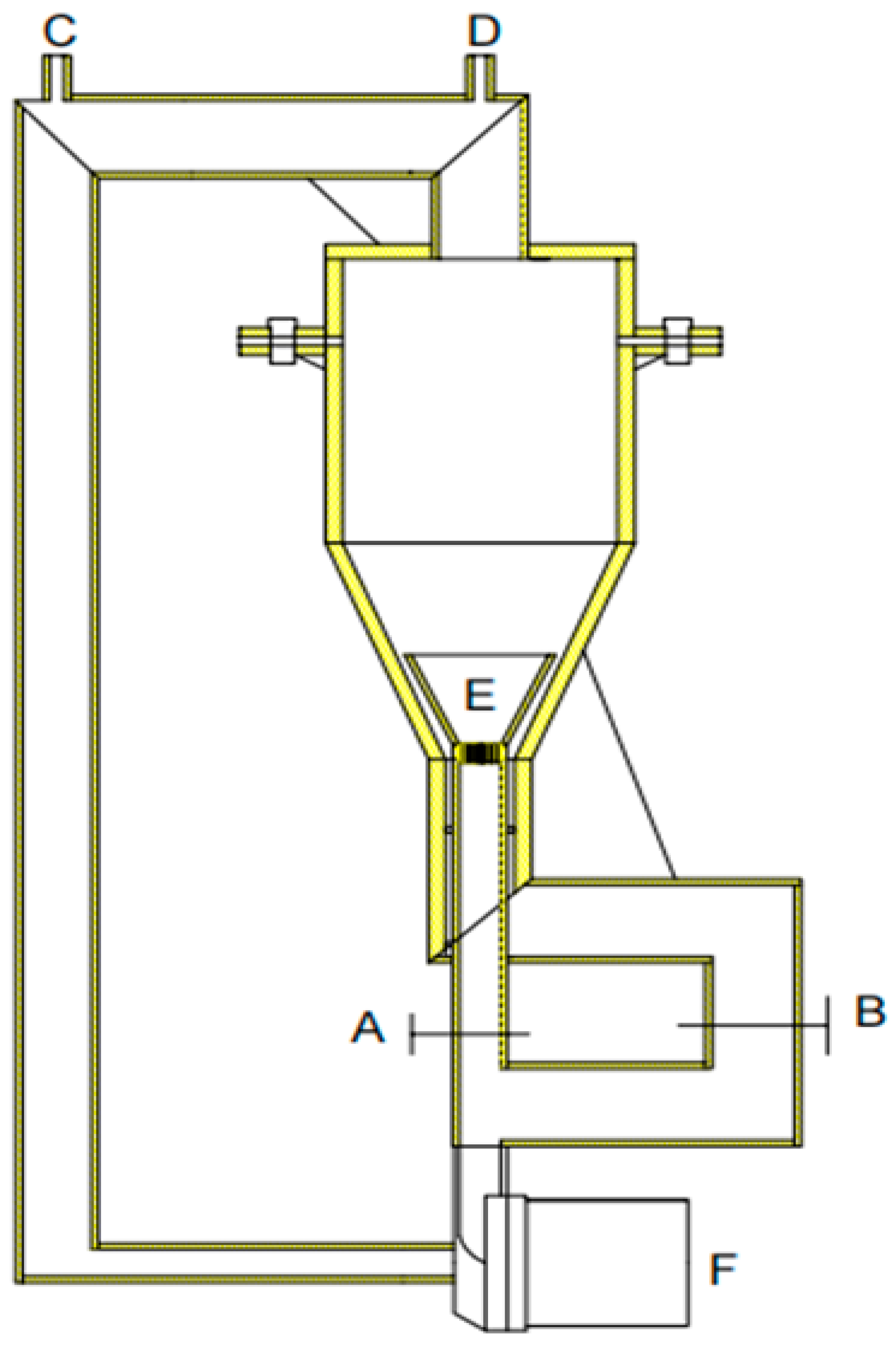
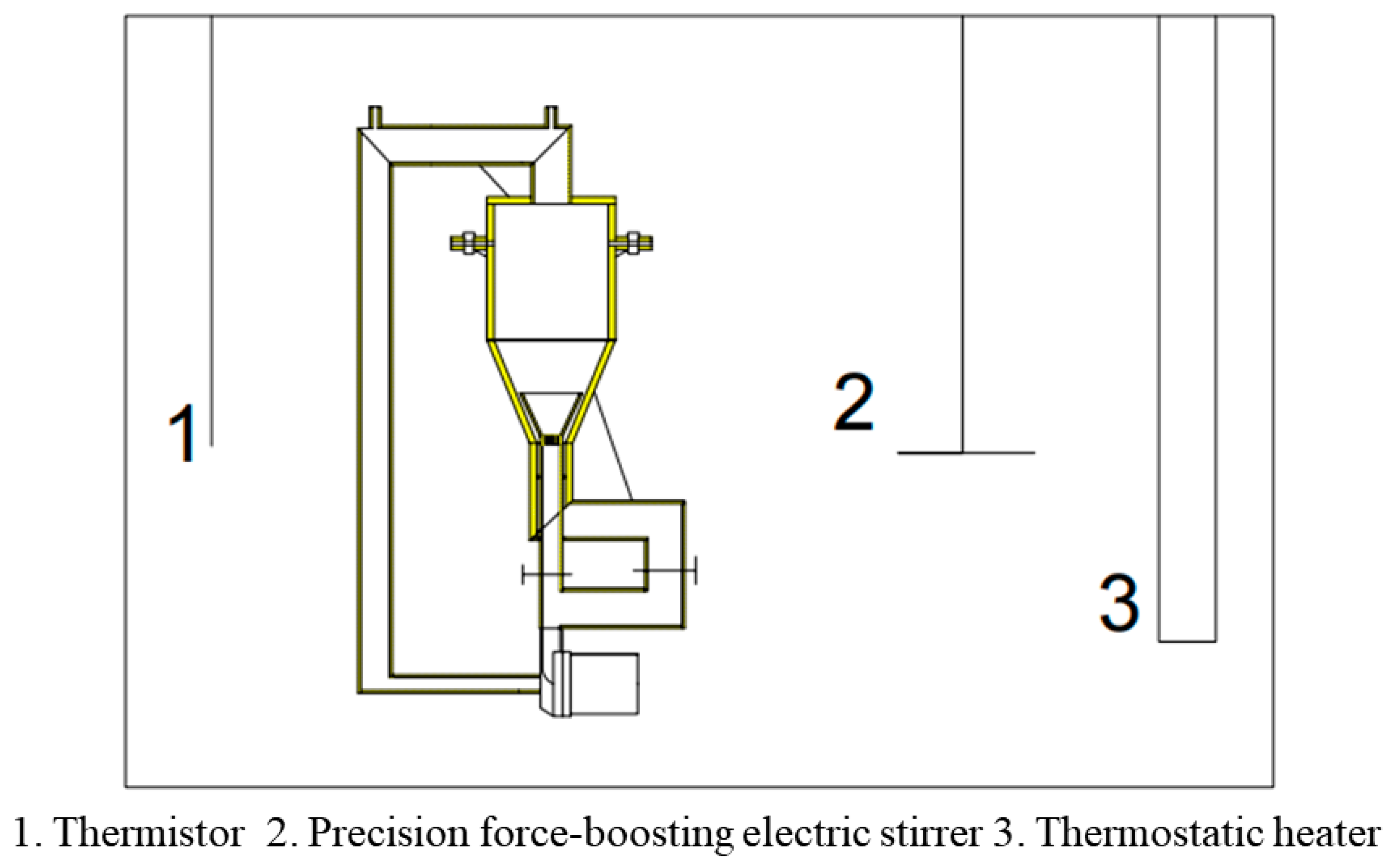
3.2. Design of Improved Solution Growth Method Apparatus
3.2.1. Design Principle
3.2.2. Design Solutions
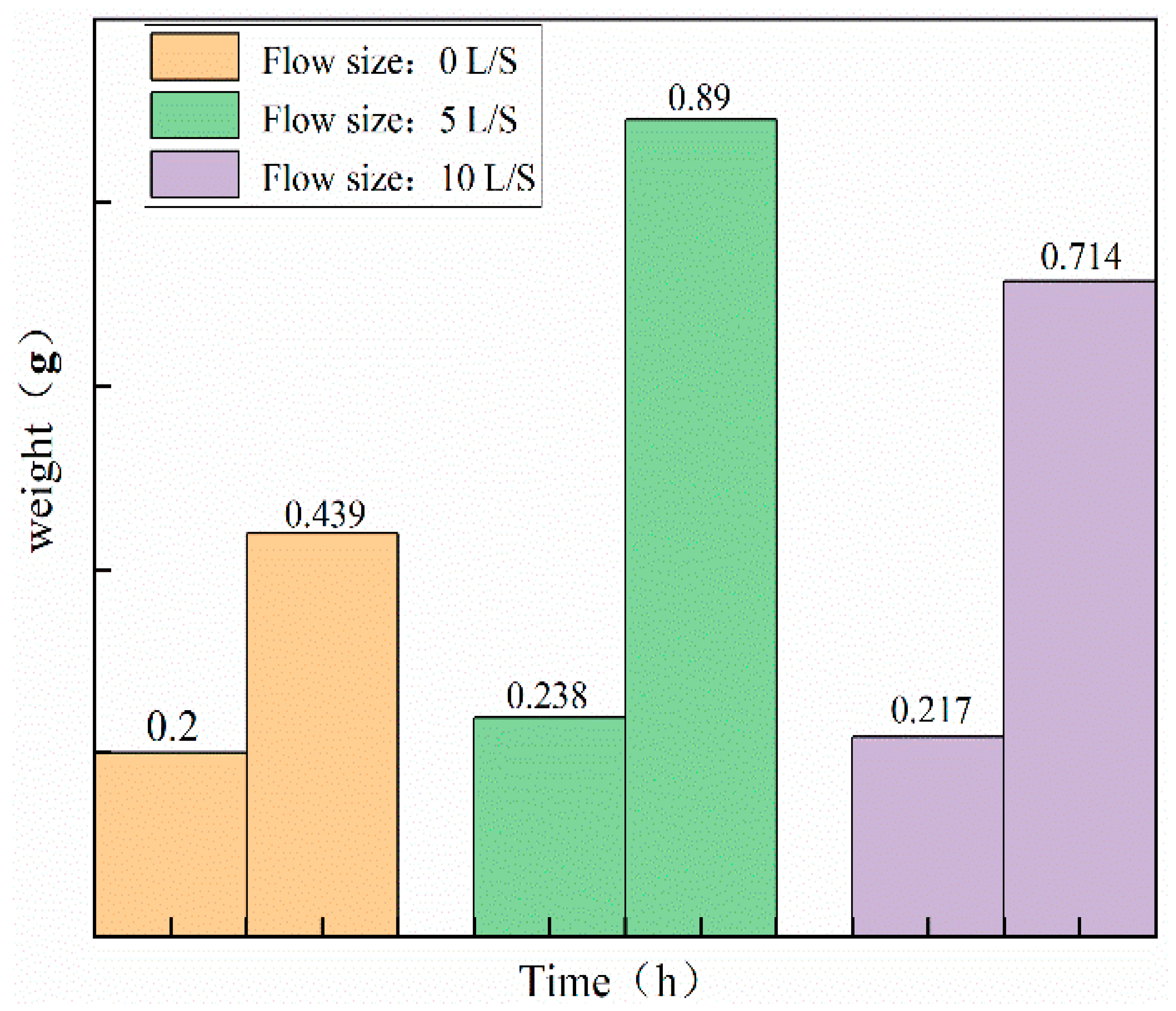
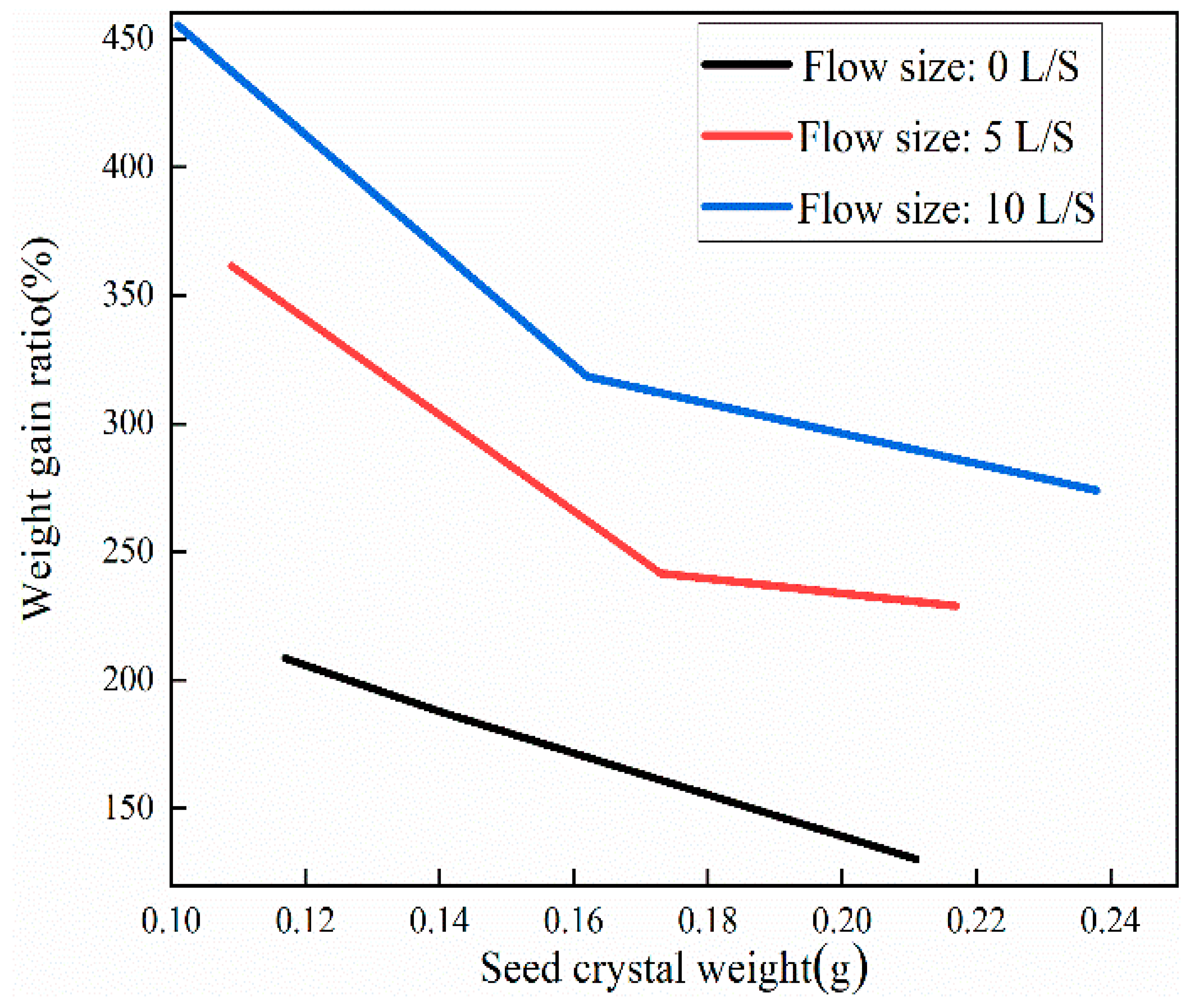
3.3. Comparative Analysis of Single Crystals Prepared using Liquid Phase Method
3.4. Discussion
3.4.1. Air Binding and Pouring Pump
3.4.2. Crystal Growth via Convection and Stirring
4. Conclusions
- (1)
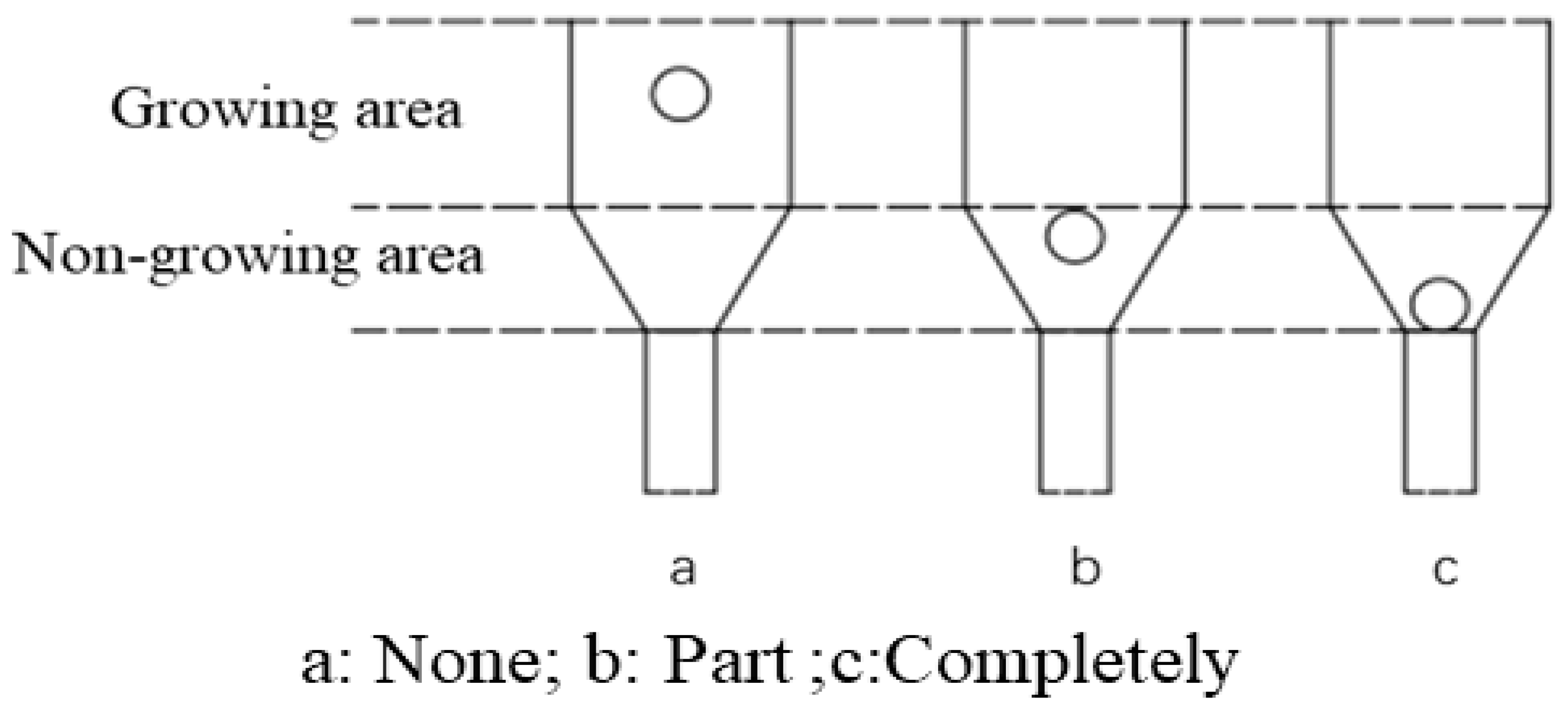
- A dynamic liquid phase single-crystal growth apparatus was designed. Firstly, acrylic material was chosen for the construction of the apparatus. Copper sulfate solution was used as the mother liquor for crystal growth, and a concentric dual-channel ring fluid inlet structure was designed. Due to the drag forces, particles can move upward. However, considering that the seed crystal is not a perfectly spherical shape, it tends to disperse in all directions where there is insufficient drag force, eventually sinking to the bottom. To address this issue, the aforementioned design was implemented, allowing the seed crystal to return to its initial position where drag forces are provided, thus achieving a cyclic motion, even if the seed crystal sinks.
- (2)
- To ensure the accuracy of copper sulfate solubility in the experiments, the solubility of copper sulfate was self-tested in this study. Based on the measured solubility, a saturated solution of copper sulfate was prepared as the mother liquor for crystal growth. A comparison was made between the dynamic liquid phase method and the static liquid phase method for single-crystal growth. It was found that the single crystals prepared using the dynamic liquid phase method exhibited a complete external structural appearance. Additionally, a higher flow rate environment could accelerate the growth rate of the seed crystal. However, it should be noted that an excessively high flow rate may not be beneficial, although this upper limit was not investigated in the present study. X-ray diffraction (XRD) analysis of the polycrystalline powder confirmed that the prepared copper sulfate single crystals had high purity without impurities. Moreover, the XRD patterns indicated that the CI sample had higher crystallinity. Therefore, single crystals grown using the dynamic liquid phase method exhibited a good crystal structure and external appearance, and they could reduce the time required for crystal growth.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lespiaux, J.; Deprat, F.; Goncalves, B.R.; Souc, J.; Leverd, F.; Juhel, M.; Mattei, J.-G.; Giroud-Garampon, C.; Roman, A.; Magis, T.; et al. Trench filling with phosphorus-doped monocrystalline and polycrystalline silicon. Mater. Sci. Semicond. Process. 2022, 144, 106549. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Yin, X.; Bai, M.; Liu, W. Micro/Nanostructures for Light Trapping in Monocrystalline Silicon Solar Cells. J. Nanomater. 2022, 2022, 8139174. [Google Scholar] [CrossRef]
- Wu, L.; Cui, L.; He, W.; Guo, J.; Yu, B.; Qian, L. Toward Controllable Wet Etching of Monocrystalline Silicon: Roles of Mechanically Driven Defects. ACS Appl. Mater. Interfaces 2022, 14, 29366–29376. [Google Scholar] [CrossRef]
- Feigelson, R.S. Crystal growth History: Theory and melt growth processes. J. Cryst. Growth 2022, 594, 29366–29376. [Google Scholar] [CrossRef]
- Zaitseva, N.; Carman, L.; Klapper, H. Growth mechanisms of large, faceted crystals grown from solutions. J. Cryst. Growth 2022, 597, 126841. [Google Scholar] [CrossRef]
- Sciacca, B.; Mann, S.A.; Tichelaar, F.D.; Zandbergen, H.W.; Van Huis, M.A.; Garnett, E.C. Solution-Phase Epitaxial Growth of Quasi-Monocrystalline Cuprous Oxide on Met-al Nanowires. Nano Lett. 2014, 14, 5891–5898. [Google Scholar] [CrossRef]
- Sharma, A.; Khangarot, R.K.; Kumar, N.; Chattopadhyay, S.; Misra, K.P. Rise in UV and blue emission and reduction of surface roughness due to the presence of Ag and Al in monocrystalline ZnO films grown by sol-gel spin coating. Mater. Technol. 2021, 36, 541–551. [Google Scholar] [CrossRef]
- Meng, F.; Gong, J.; Fan, Z.; Li, H.; Yuan, J. Hydrothermal synthesis and mechanism of triangular prism-like monocrystalline CeO2 nanotubes via a facile template-free hydrothermal route. Ceram. Int. 2016, 42, 4700–4708. [Google Scholar] [CrossRef]
- Wang, S.; Yang, F.; Zhu, J.; Cao, Q.; Zhong, Y.; Wang, A.; Du, W.; Liu, X. Growth of metal halide perovskite materials. Sci. China Mater. 2020, 63, 1438–1463. [Google Scholar] [CrossRef]
- Chen, M.; Yuan, Y.; Liu, Y.; Cao, D.; Xu, C. High-quality all-inorganic CsPbBr3 single crystals prepared by a facile one-step solution growth method. RSC Adv. 2022, 12, 14838–14843. [Google Scholar] [CrossRef]
- de Juan, D.; Meseguer, V.F.; Lozano, L.J. A contribution to the study of CuSO4 center dot 5H2O solubility in aqueous media. Rev. De Metal. 1999, 35, 47–52. [Google Scholar]
- Aslandukov, A.; Aslandukov, M.; Dubrovinskaia, N.; Dubrovinsky, L. Domain Auto Finder (DAFi) program: The analysis of single-crystal X-ray diffraction data from polycrystalline samples. J. Appl. Crystallogr. 2022, 55, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Cross, R. Measurements of the drag force on balls in water. Eur. J. Phys. 2020, 41, 055003. [Google Scholar] [CrossRef]
- Singh, N.; Kroells, M.; Li, C.; Ching, E.J.; Ihme, M.; Hogan, C.J.; Schwartzentruber, T.E. General Drag Coefficient for Flow over Spherical Particles. AIAA J. 2022, 60, 587–597. [Google Scholar] [CrossRef]
- Kalman, H.; Matana, E. Terminal velocity and drag coefficient for spherical particles. Powder Technol. 2022, 396, 181–190. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, T.; Yan, Z.; Duan, W.; Deng, J.; Luo, G. Liquid-liquid dispersion and flow characteristics in a miniaturized annular rotating device. Chem. Eng. J. 2023, 454, 140374. [Google Scholar] [CrossRef]
- Lin, S.; Liu, J.; Xia, H.; Zhang, Z.; Ao, X. A numerical study of particle-laden flow around an obstacle: Flow evolution and Stokes number effects. Appl. Math. Model. 2022, 103, 287–307. [Google Scholar] [CrossRef]
- Akhshik, S.; Rajabi, M. Computer simulation of the effect of particle stiffness coefficient on the particle-fluid flows. Part. Sci. Technol. 2022, 40, 233–242. [Google Scholar] [CrossRef]
- Jing, H.X.; Zhang, D.H.; Li, G.D. Pressure variations of fluid transients in a pressurized pipeline. Fluid Dyn. Res. 2018, 50, 045514. [Google Scholar] [CrossRef]
- Ouchiha, Z.; Loraud, J.C.; Ghezal, A.; Kessal, M.; Benzaoui, A.; Ghiaasiaan, S.M. An investigation of highly pressurized transient fluid flow in pipelines. Int. J. Press. Vessel. Pip. 2012, 92, 106–114. [Google Scholar] [CrossRef]
- Kobylkin, M.; Rikker, Y.; Batukhtin, A.; Akimov, I. Measurement of fluid pressure through the pipeline wall in heat and power pro-cesses. Iop Conference Series. Earth Environ. Sci. 2022, 1070, 12041. [Google Scholar]
- Razvarz, S.; Vargas-Jarillo, C.; Jafari, R.; Gegov, A. Flow Control of Fluid in Pipelines Using PID Controller. IEEE Access 2019, 7, 25673–25680. [Google Scholar] [CrossRef]
- Zuo, Z.; Liu, S.; Sun, Y.; Wu, Y. Pressure fluctuations in the vaneless space of High-head pump-turbines—A review. Renew. Sustain. Energy Rev. 2015, 41, 965–974. [Google Scholar] [CrossRef]
- Han, Y.; Li, H.; Tiganik, T.; Wang, Y.; Zhou, L. Influence Mechanism of Trimming Impeller Diameter in a Centrifugal Pump by Computa-tional Fluid Dynamics Investigation. J. Fluids Eng. Trans. Asme 2023, 145, 021205. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Zeng, Y.; Zhu, B.; Hu, B.; Hua, H. Investigation of the Noise Induced by Unstable Flow in a Centrifugal Pump. Energies 2020, 13, 589. [Google Scholar] [CrossRef]
- Zhou, P.J.; Wang, F.J.; Mou, J.G. Investigation of rotating stall characteristics in a centrifugal pump impeller at low flow rates. Eng. Comput. 2017, 34, 1989–2000. [Google Scholar] [CrossRef]
- Jin, X.; Wang, B. Numerical investigation of the effects of axial temperature gradient and cooling rate on InGaSb crystal growth under micro-gravity. J. Cryst. Growth 2023, 607, 127110. [Google Scholar] [CrossRef]
- Murakami, N.; Arafune, K.; Koyama, T.; Momose, Y.; Ozawa, T.; Okano, Y.; Dost, S.; Dao, L.H.; Kumagawa, M.; Hayakawa, Y. Effect of gravity on InGaSb crystal growth. Microgravity Sci. Technol. 2005, 16, 79–83. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Kumar, V.N.; Arivanandhan, M.; Rajesh, G.; Koyama, T.; Momose, Y.; Sakata, K.; Ozawa, T.; Okano, Y.; Inatomi, Y. Effects of Gravity and Crystal Orientation on the Growth of InGaSb Ternary Alloy Semiconductors: Experiments at the International Space Station and on Earth. Int. J. Microgravity Sci. Appl. 2017, 34, 340111. [Google Scholar]
- Sun, J.-K.; Sobolev, Y.I.; Zhang, W.; Zhuang, Q.; Grzybowski, B.A. Enhancing crystal growth using polyelectrolyte solutions and shear flow. Nature 2020, 579, 73–79. [Google Scholar] [CrossRef]








| Impurity Name | Content (%) |
|---|---|
| Water insoluble substance | 0.010 |
| Chloride (Cl) | 0.002 |
| Iron (Fe) | 0.005 |
| Hydrogen sulfide non-precipitates (as silicate) | 0.150 |
| Temperature (°C) | 10 | 20 | 30 | 40 | 60 |
|---|---|---|---|---|---|
| Amount of solute (g) | 10.541 | 12.989 | 15.258 | 16.614 | 24.225 |
| Solubility (g) | 26.353 | 32.473 | 38.145 | 41.535 | 60.563 |
| Time Period | Start Time | Termination Time | Start-Up Temperature (°C) | Termination Temperature (°C) |
|---|---|---|---|---|
| 1 | 00:00 | 04:00 | 29.8 | 30.0 |
| 2 | 04:00 | 08:00 | 29.6 | 29.8 |
| 3 | 08:00 | 12:00 | 29.4 | 29.6 |
| 4 | 12:00 | 16:00 | 29.2 | 29.4 |
| 5 | 16:00 | 20:00 | 29.0 | 29.2 |
| 6 | 20:00 | 00:00 | 28.8 | 29.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhou, Y. Research on Single Crystal Preparation via Dynamic Liquid Phase Method. Crystals 2023, 13, 1150. https://doi.org/10.3390/cryst13071150
Wang X, Zhou Y. Research on Single Crystal Preparation via Dynamic Liquid Phase Method. Crystals. 2023; 13(7):1150. https://doi.org/10.3390/cryst13071150
Chicago/Turabian StyleWang, Xu, and Yongmin Zhou. 2023. "Research on Single Crystal Preparation via Dynamic Liquid Phase Method" Crystals 13, no. 7: 1150. https://doi.org/10.3390/cryst13071150
APA StyleWang, X., & Zhou, Y. (2023). Research on Single Crystal Preparation via Dynamic Liquid Phase Method. Crystals, 13(7), 1150. https://doi.org/10.3390/cryst13071150





