Multi-Theory Comparisons of Molecular Simulation Approaches to TiO2/H2O Interfacial Systems
Abstract
1. Introduction
2. Methodology
2.1. Forcefield MD
2.2. DFTB-MD
2.3. DFT-MD
3. Results and Discussion
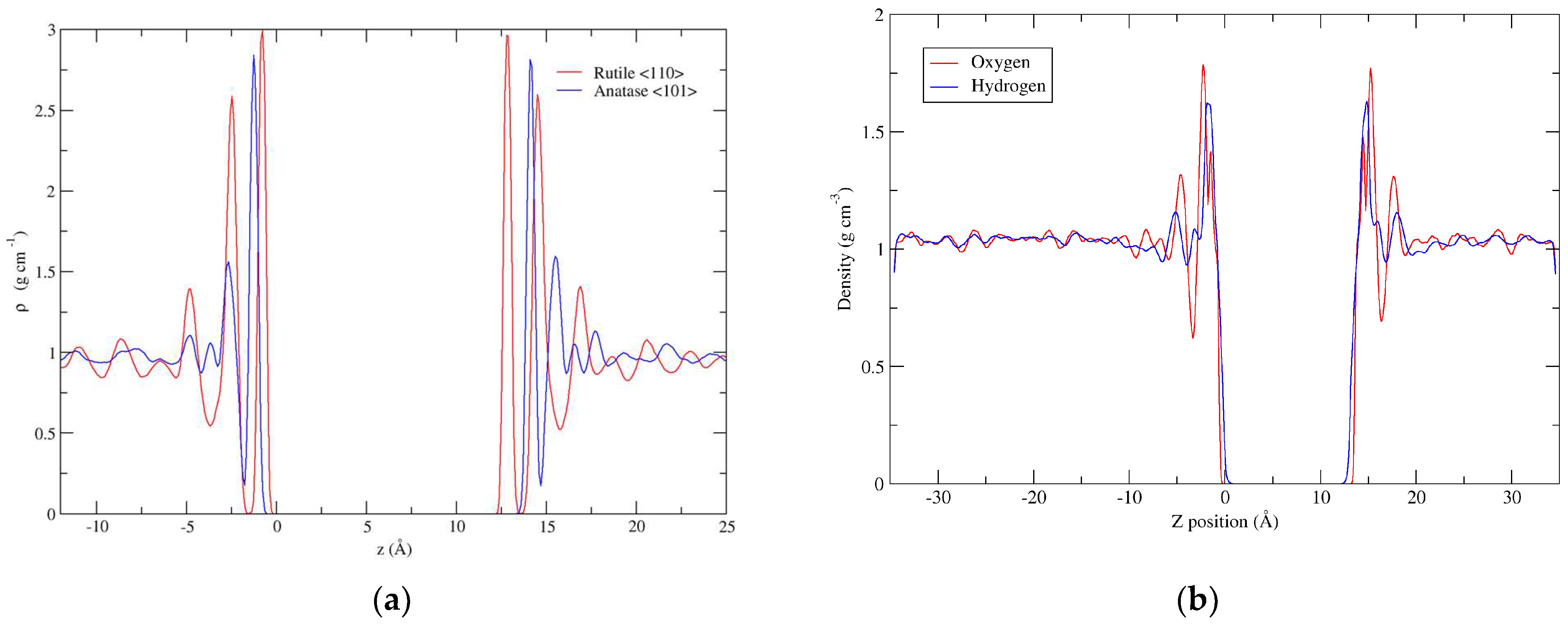
3.1. Water Layering
3.2. Forcefield MD
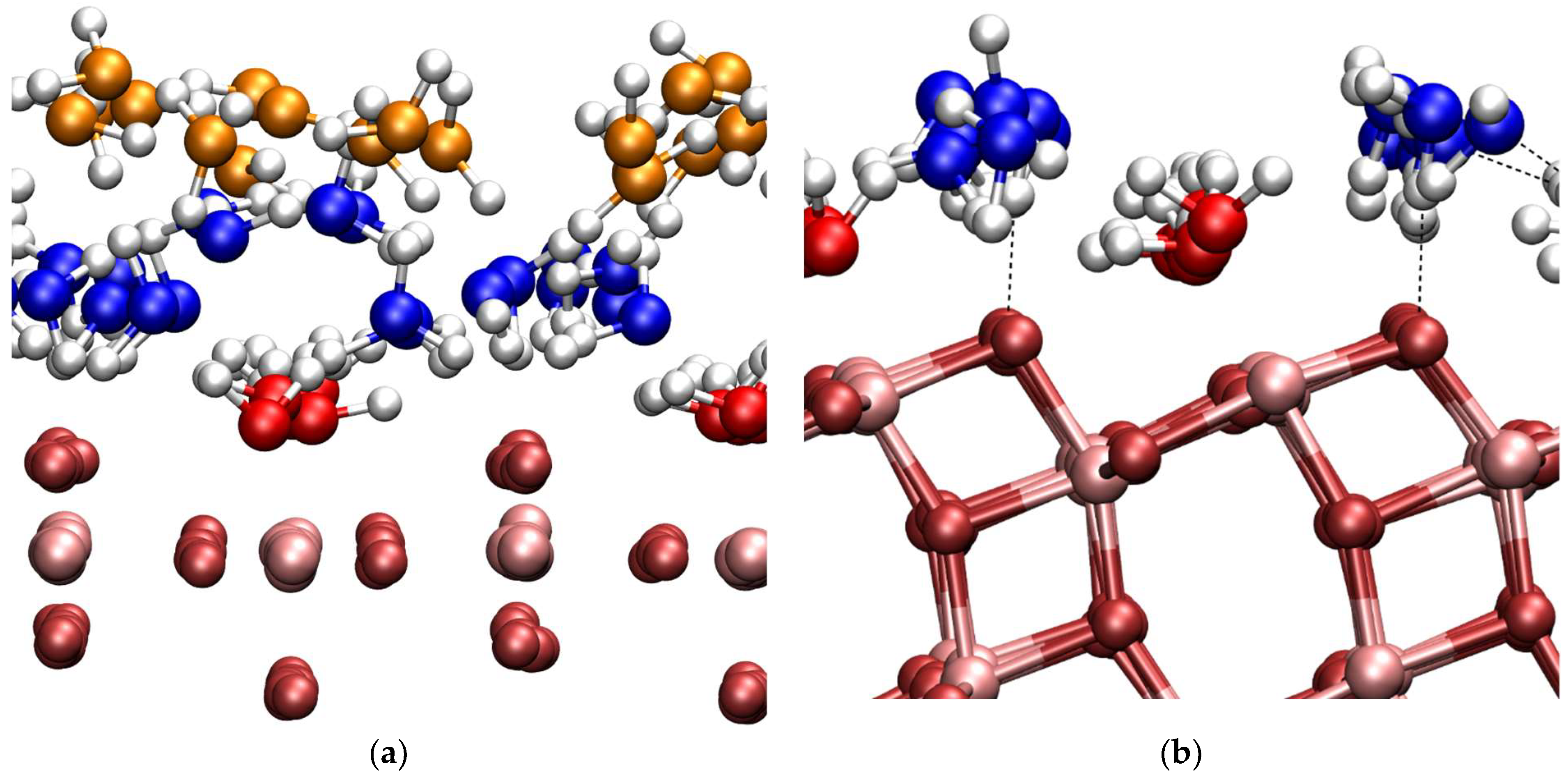
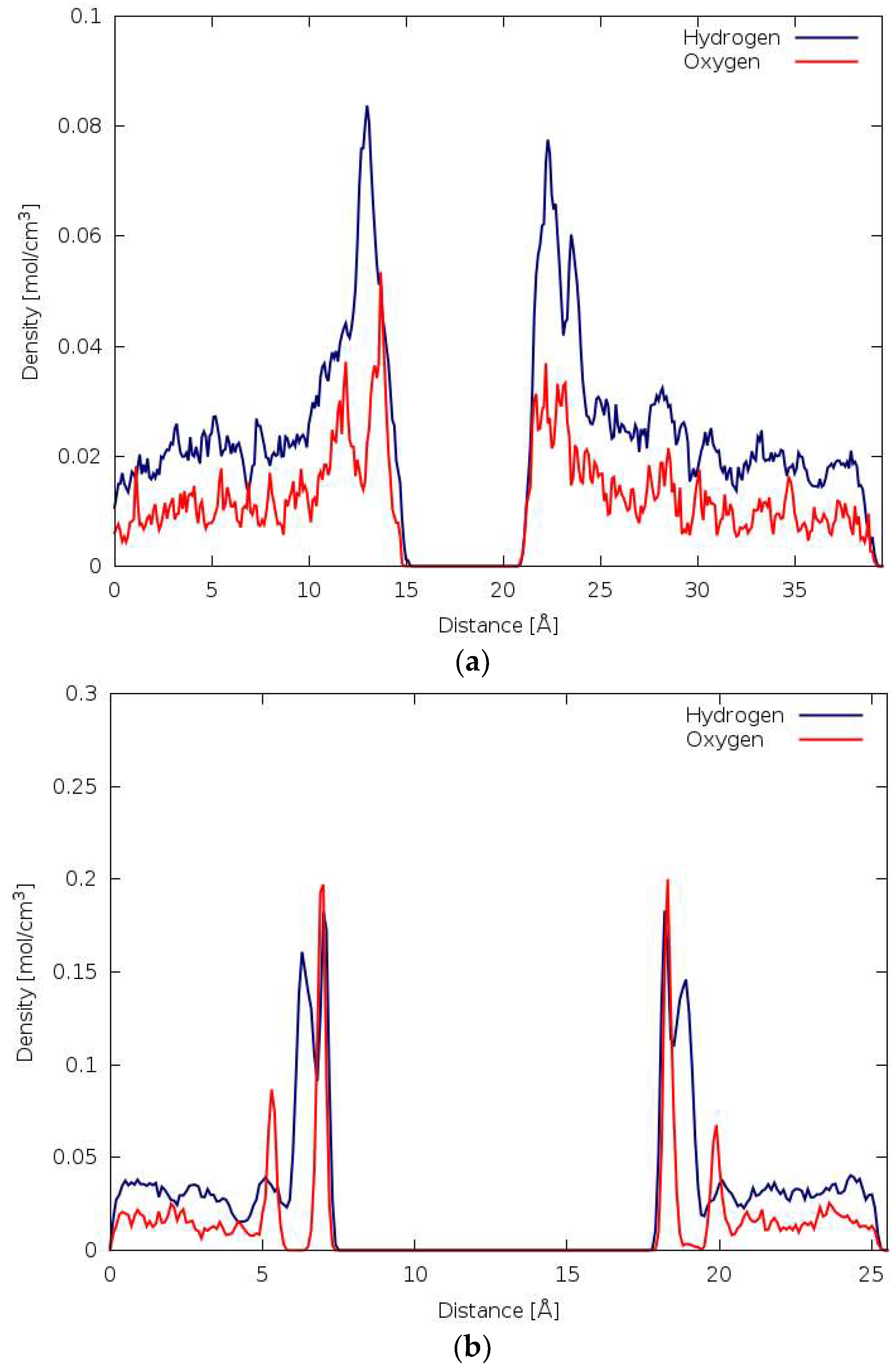
3.3. DFTB-MD
3.4. DFT
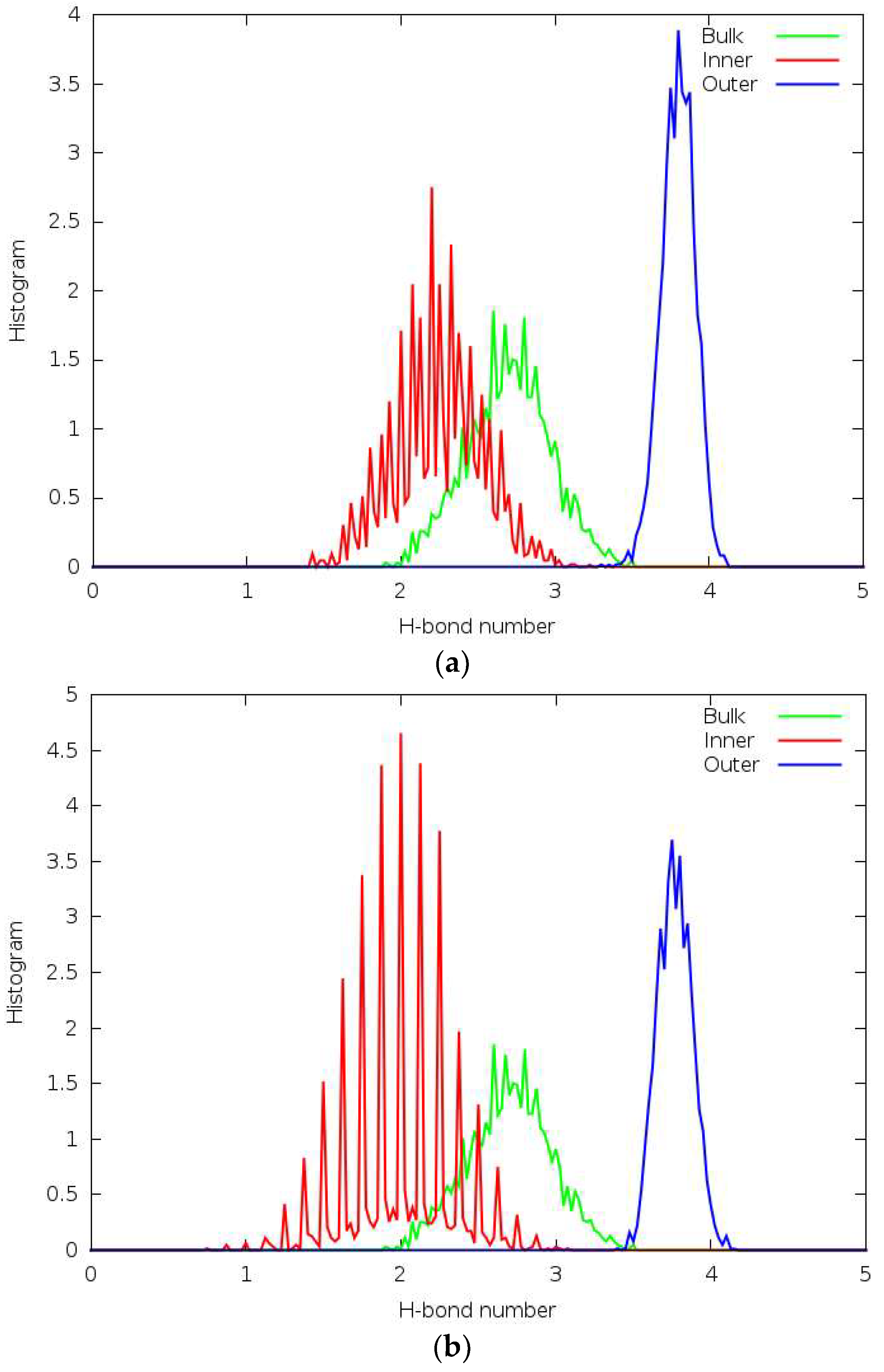
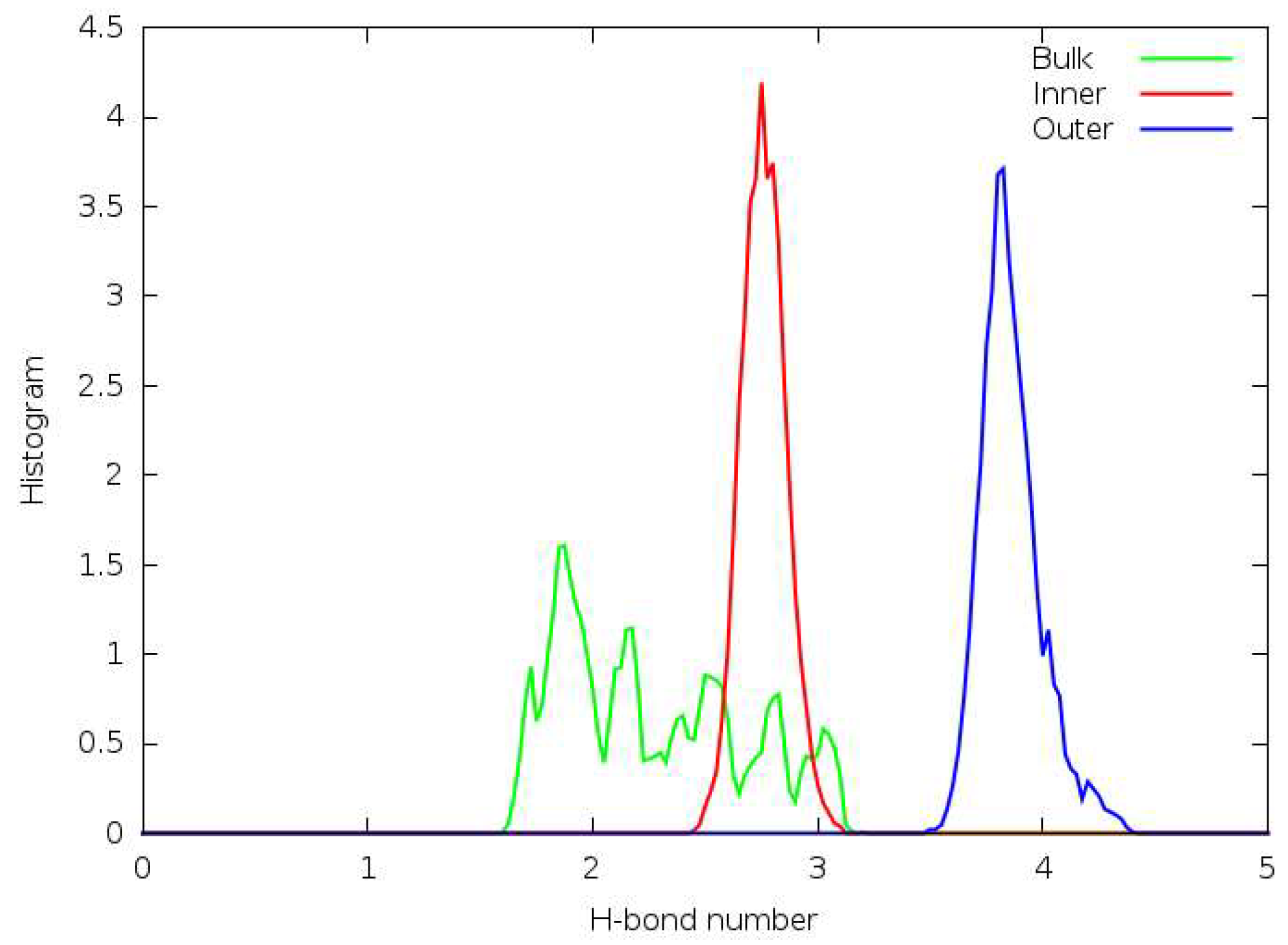
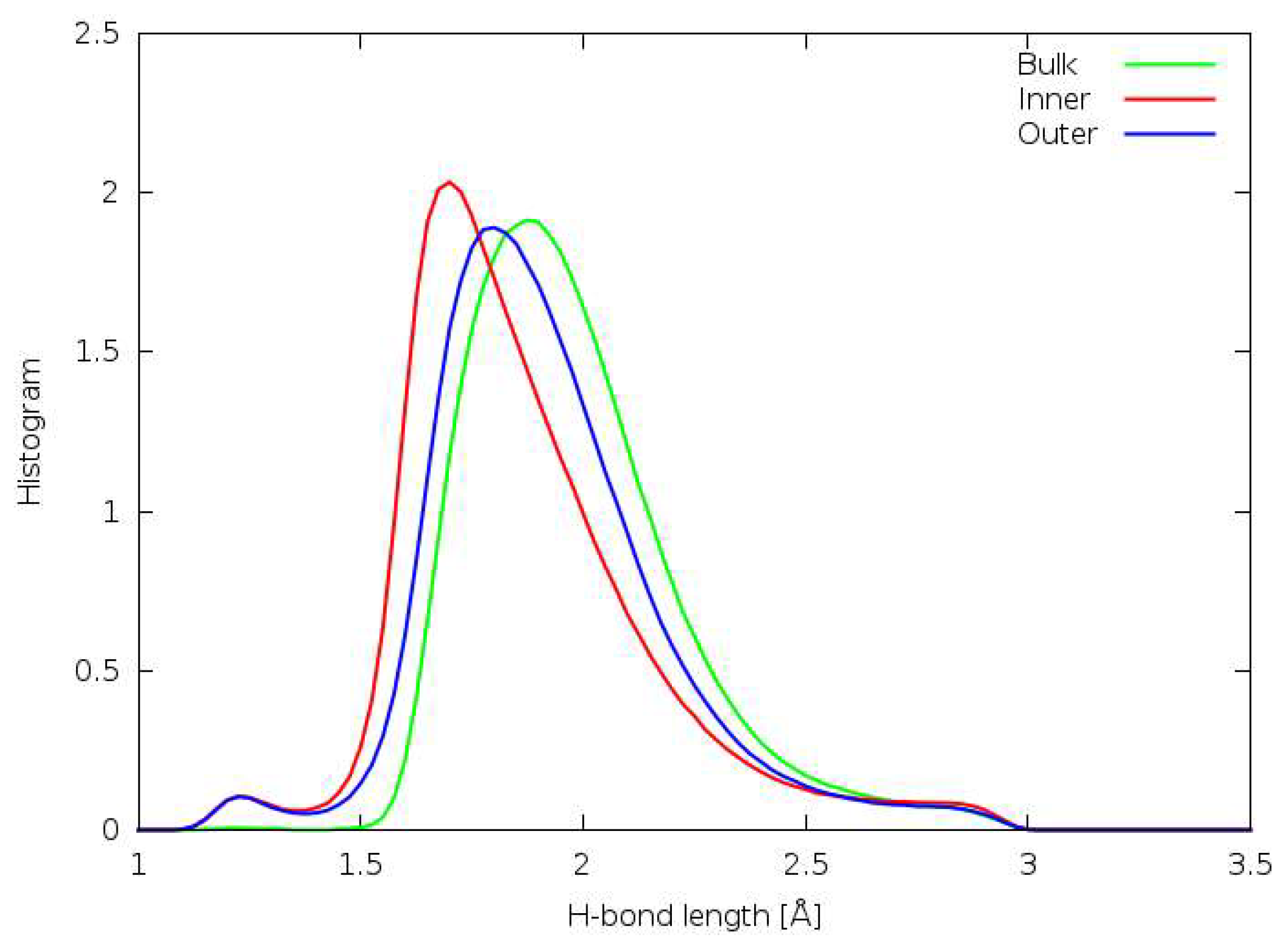
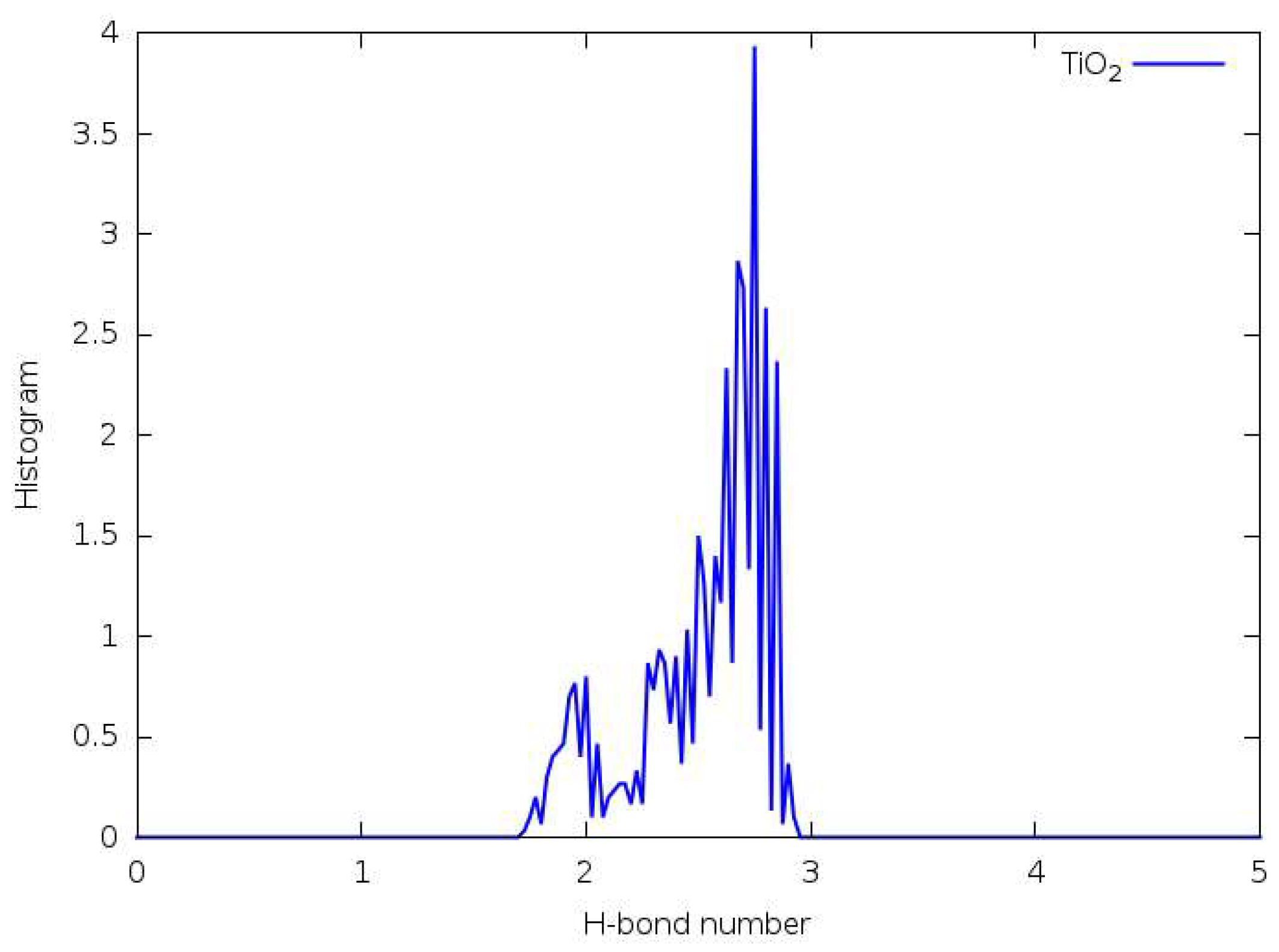
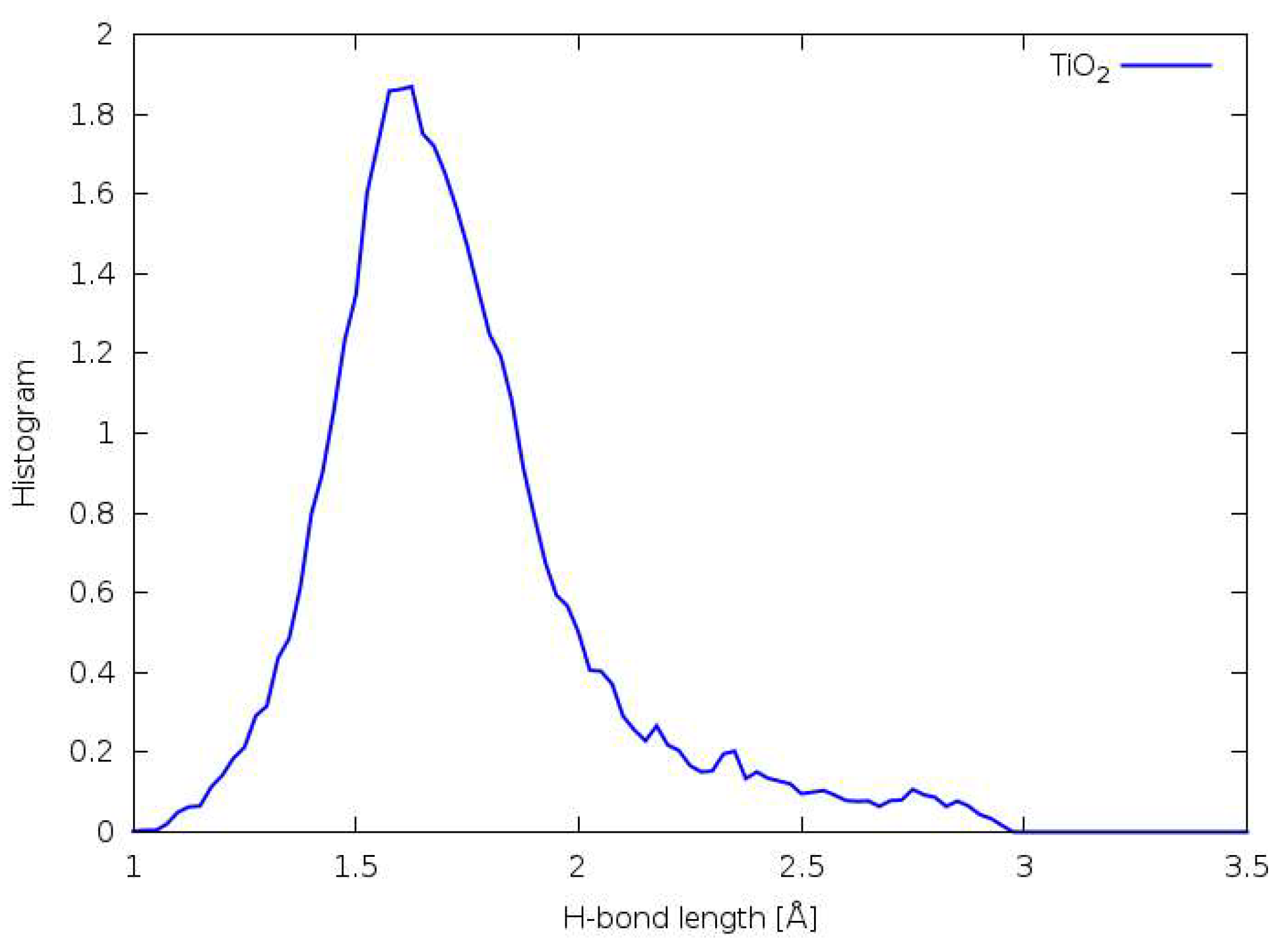
3.5. Hydrogen Bonding
3.6. Forcefield MD
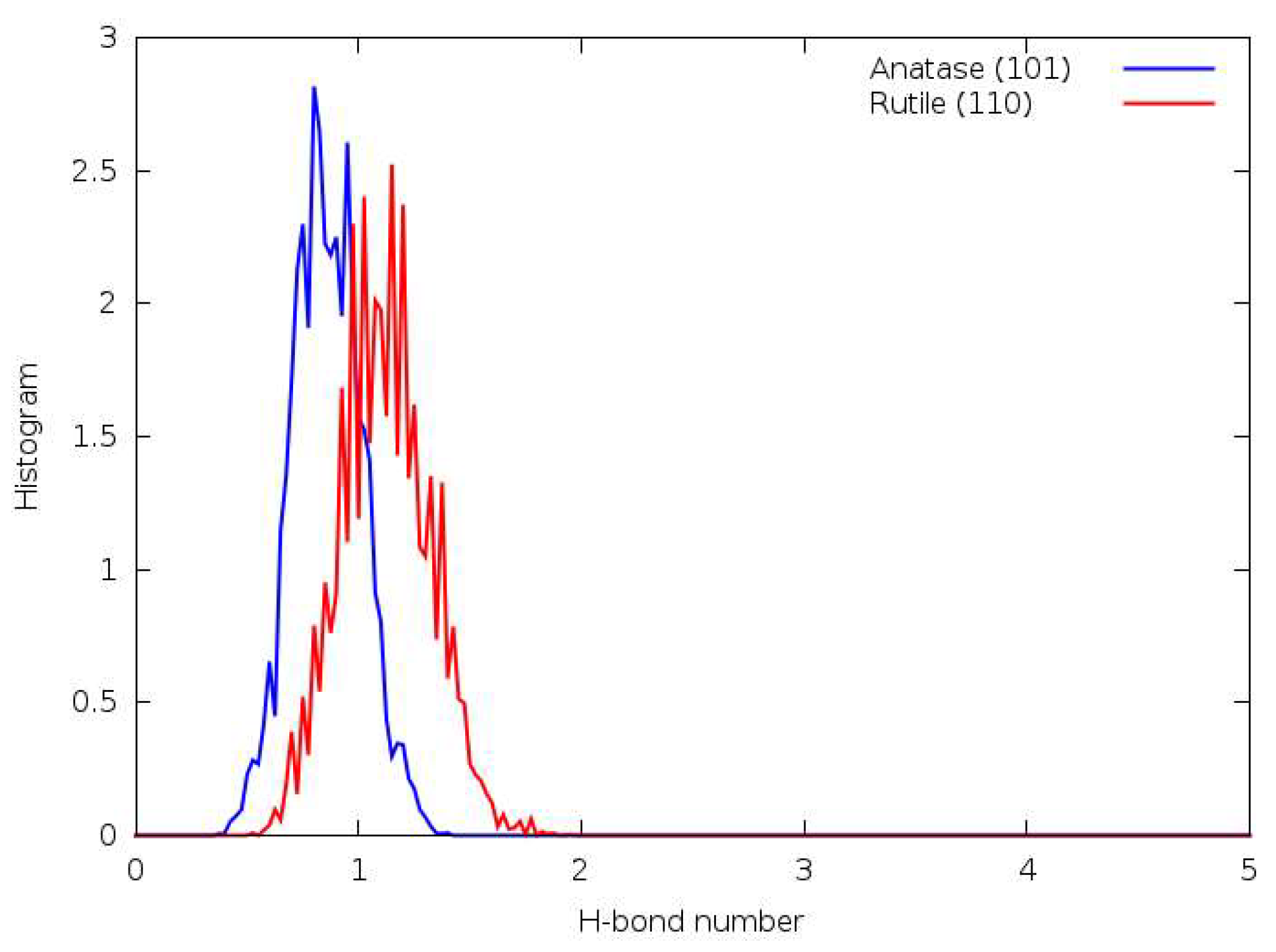
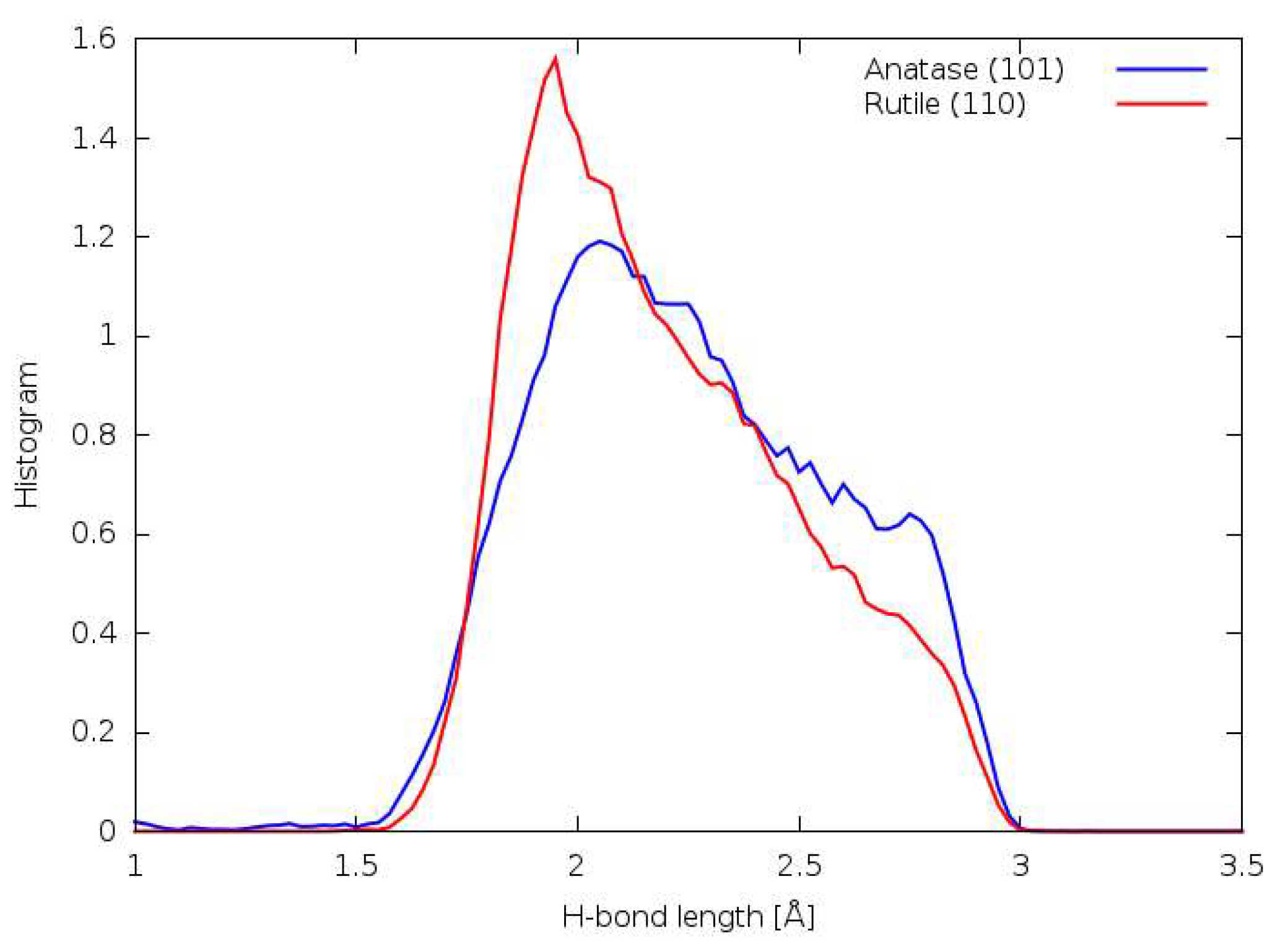
3.7. DFTB
3.8. DFT
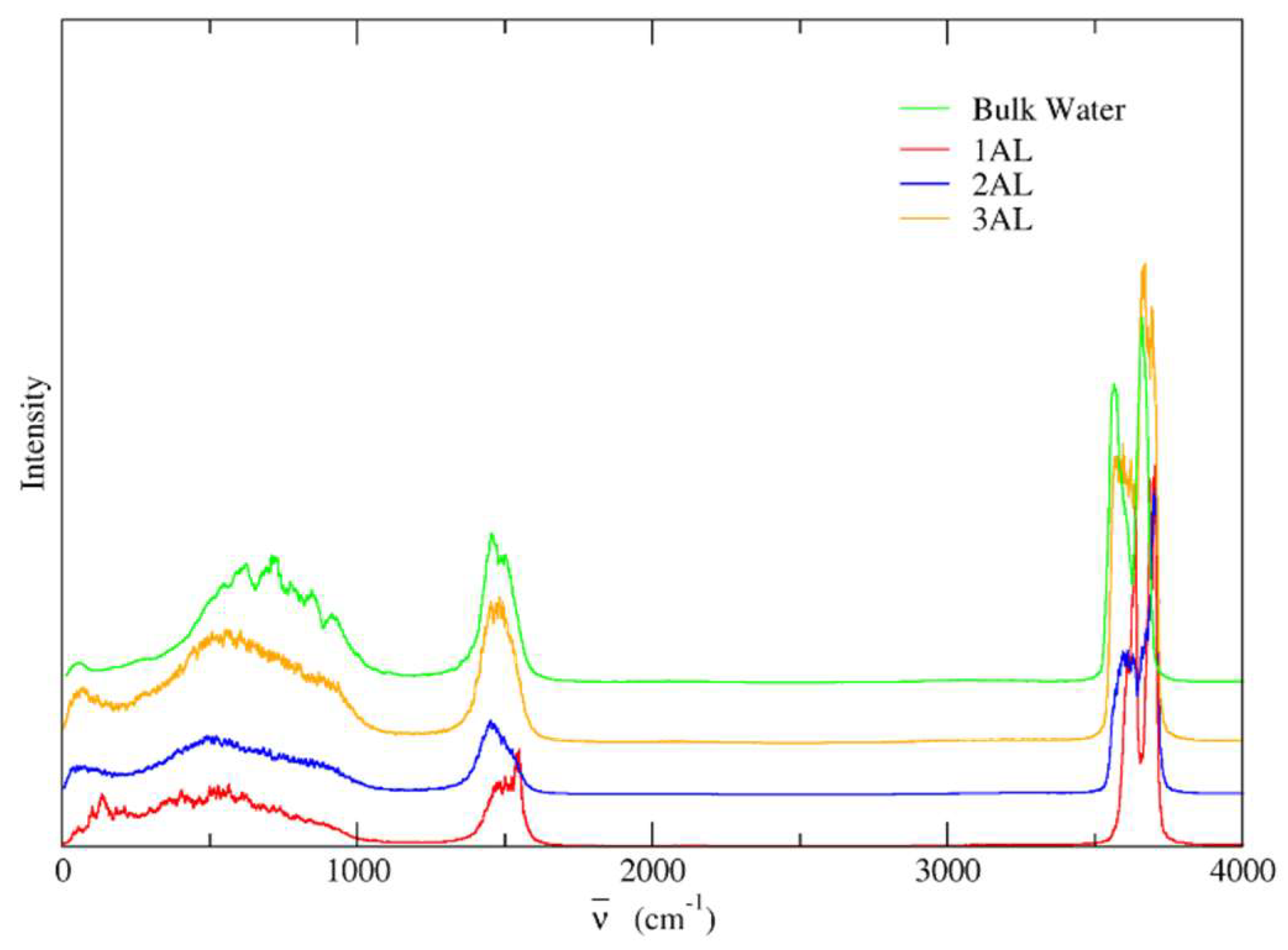
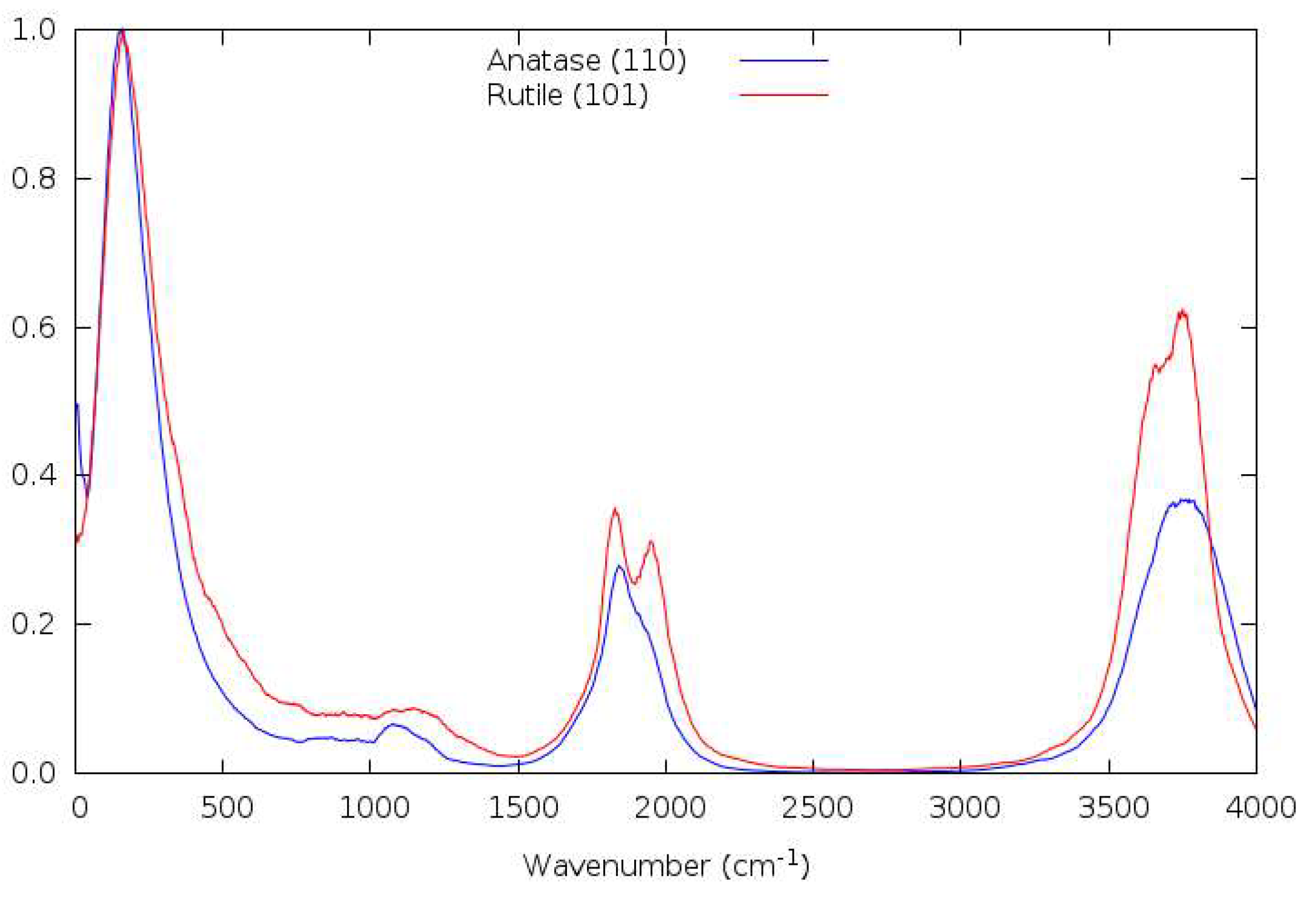
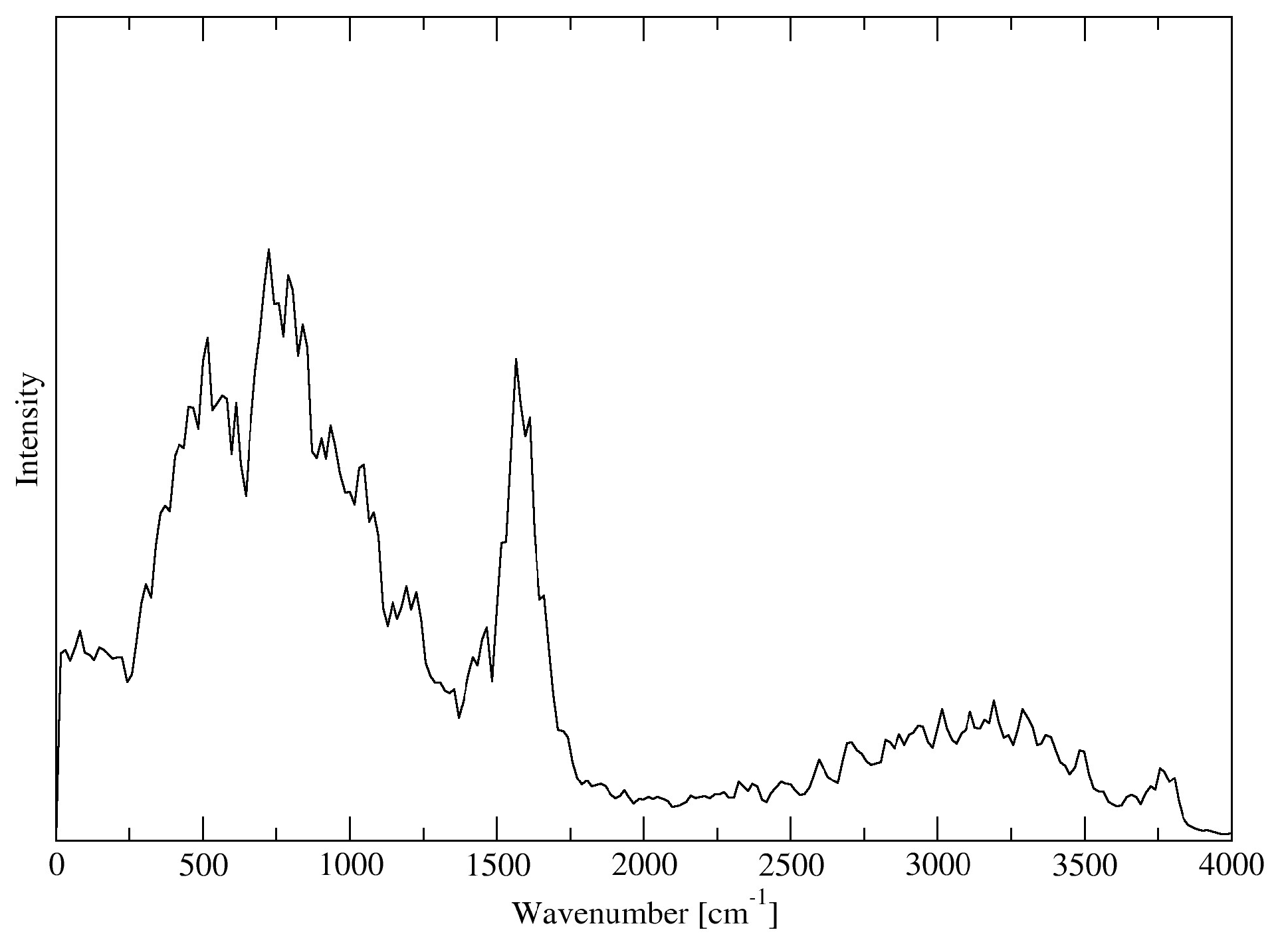
3.9. Vibrational Spectra
3.10. Forcefield MD
3.11. DFTB
3.12. DFT
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Selli, D.; Fazio, G.; Di Valentin, C. Using Density Functional Theory to Model Realistic TiO2 Nanoparticles, Their Photoactivation and Interaction with Water. Catalysts 2017, 7, 357. [Google Scholar] [CrossRef]
- Koskinen, P.; Mäkinen, V. Density-functional tight-binding for beginners. Comput. Mater. Sci. 2009, 47, 237–253. [Google Scholar] [CrossRef]
- Guan, D.; Zhong, J.; Xu, H.; Huang, Y.-C.; Hu, Z.; Chen, B.; Zhang, Y.; Ni, M.; Xu, X.; Zhou, W.; et al. A universal chemical-induced tensile strain tuning strategy to boost oxygen-evolving electrocatalysis on perovskite oxides. Appl. Phys. Rev. 2022, 9, 011422. [Google Scholar] [CrossRef]
- Daqin, G.; Chenliang, S.; Hengyue, X.; Yuxing, G.; Jian, Z.; Yuchen, S.; Zhiwei, H.; Meng, N.; Zongping, S. Simultaneously mastering operando strain and reconstruction effects via phase-segregation strategy for enhanced oxygen-evolving electrocatalysis. J. Energy Chem. 2023, 82, 572–580. [Google Scholar]
- Huang, B.; Xu, H.; Jiang, N.; Wang, M.; Huang, J.; Guan, L. Tensile-Strained RuO2 Loaded on Antimony-Tin Oxide by Fast Quenching for Proton-Exchange Membrane Water Electrolyzer. Adv. Sci. 2022, 9, 2201654. [Google Scholar] [CrossRef]
- Xu, H.; Guan, D.; Ma, L. The bio-inspired heterogeneous single-cluster catalyst Ni100–Fe4S4 for enhanced electrochemical CO2 reduction to CH4. Nanoscale 2023, 15, 2756–2766. [Google Scholar] [CrossRef]
- Futera, Z.; English, N.J. Exploring Rutile (110) and Anatase (101) TiO2 Water Interfaces by Reactive Force-Field Simulations. J. Phys. Chem. C 2017, 121, 6701–6711. [Google Scholar] [CrossRef]
- Bredow, T.; Jug, K. Theoretical investigation of water adsorption at rutile and anatase surfaces. Surf. Sci. 1995, 327, 398–408. [Google Scholar] [CrossRef]
- Lindan, P.J.D.; Zhang, C. Exothermic water dissociation on the rutile TiO2 (110) surface. Phys. Rev. B 2005, 72, 075439. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, J.; Luo, Y.; Monti, S.; Carravetta, V. Quantum molecular dynamics study of water on TiO2(110) surface. J. Chem. Phys. 2008, 129, 064703. [Google Scholar] [CrossRef]
- Allegretti, F.; O’Brien, S.; Polcik, M.; Sayago, D.I.; Woodruff, D.P. Adsorption Bond Length for H2O on TiO2(110): A Key Parameter for Theoretical Understanding. Phys. Rev. Lett. 2005, 95, 226104. [Google Scholar] [CrossRef]
- Walle, L.E.; Borg, A.; Uvdal, P.; Sandell, A. Experimental evidence for mixed dissociative and molecular adsorption of water on a rutile TiO2 (110) surface without oxygen vacancies. Phys. Rev. B 2009, 80, 235436. [Google Scholar] [CrossRef]
- Wu, L.; Fu, C.; Huang, W. Surface chemistry of TiO2 connecting thermal catalysis and photocatalysis. Phys. Chem. Chem. Phys. 2020, 22, 9875–9909. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; de Coss, R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Xu, C.B.; Ren, Z.F.; Yang, W.S.; Ma, Z.B.; Dai, D.X.; Fan, H.J.; Minton, T.K.; Yang, X.M. Stepwise Photocatalytic Dissociation of Methanol and Water on TiO2(110). J. Am. Chem. Soc. 2012, 134, 13366–13373. [Google Scholar] [CrossRef]
- Panayotov, D.A.; Burrows, S.P.; Morris, J.R. Photooxidation Mecanism of Methanol on Rutile TiO2 Nanoparticles. J. Phys. Chem. C 2012, 116, 6623–6635. [Google Scholar] [CrossRef]
- Santo, V.D.; Naldoni, A. Titanium Dioxide Photocatalysis. Catalysts 2018, 8, 591. [Google Scholar] [CrossRef]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes based on TiO2 and α-Fe2O3 for solar water splitting—Superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef]
- Yang, X.; Liu, R.; Du, C.; Dai, P.; Zheng, Z.; Wang, D. Improving Hematite-based Photoelectrochemical Water Splitting with Ultrathin TiO2 by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2014, 6, 12005–12011. [Google Scholar] [CrossRef]
- Deng, A.; Zhu, Y.; Guo, X.; Zhou, L.; Jiang, Q. Synthesis of Various TiO2 Micro-/Nano-Structures and Their Photocatalytic Performance. Materials 2018, 11, 995. [Google Scholar] [CrossRef]
- Futera, Z.; English, N.J. Oscillating electric-field effects on adsorbed-water at rutileand anatase-TiO2 surfaces. J. Chem. Phys. 2016, 145, 204706. [Google Scholar] [CrossRef] [PubMed]
- Futera, Z.; English, N.J. Electric-Field Effects on Adsorbed-Water Structural and Dynamical Properties at Rutile- and Anatase-TiO2 Surfaces. J. Phys. Chem. C 2016, 120, 19603–19612. [Google Scholar] [CrossRef]
- Schaefer, A.; Lanzilotto, V.; Cappel, U.; Uvdal, P.; Borg, A.; Sandell, A. First layer water phases on anatase TiO2(101). Surf. Sci. 2018, 674, 25–31. [Google Scholar] [CrossRef]
- Smith, W.; Forester, T.R. DL_POLY_2.0: A general-purpose parallel molecular dynamics simulation package. J. Mol. Graph. 1996, 14, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Akaogi, M. Molecular Dynamics Simulation of the Structural and Physical Properties of the Four Polymorphs of TiO2. Mol. Simul. 1991, 6, 239–244. [Google Scholar] [CrossRef]
- Wu, Y.; Tepper, H.L.; Voth, G.A. Flexible simple point-charge water model with improved liquid-state properties. J. Chem. Phys. 2006, 124, 024503. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Van Duin, A.C.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A Reactive Force Field for Hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Raju, M.; Kim, S.-Y.; van Duin, A.C.T.; Fichthorn, K.A. ReaxFF Reactive Force Field Study of the Dissociation of Water on Titania Surfaces. J. Phys. Chem. C 2013, 117, 10558–10572. [Google Scholar] [CrossRef]
- Aktulga, H.M.; Fogarty, J.C.; Pandit, S.A.; Grama, A.Y. Parallel Reactive Molecular Dynamics: Numerical Methods and Algorithmic Techniques. Parallel Comput. 2012, 38, 245–259. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Hjorth Larsen, A.; Jørgen Mortensen, J.; Blomqvist, J.; Castelli, I.E.; Christensen, R.; Dułak, M.; Friis, J.; Groves, M.N.; Hammer, B.; Hargus, C.; et al. The atomic simulation environment—A Python library for working with atoms. J. Phys. Condens. Matter 2017, 29, 273002. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.P.; Richards, W.D.; Jain, A.; Hautier, G.; Kocher, M.; Cholia, S.; Gunter, D.; Chevrier, V.L.; Persson, K.A.; Ceder, G. Python Materials Genomics (pymatgen): A robust, open-source python library for materials analysis. Comput. Mater. Sci. 2013, 68, 314–319. [Google Scholar] [CrossRef]
- Hourahine, B.; Aradi, B.; Blum, V.; Bonafé, F.; Buccheri, A.; Camacho, C.; Cevallos, C.; Deshaye, M.Y.; Dumitrică, T.; Dominguez, A.; et al. DFTB+, a software package for efficient approximate density functional theory based atomistic simulations. J. Chem. Phys. 2020, 152, 124101. [Google Scholar] [CrossRef] [PubMed]
- Luschtinetz, R.; Frenzel, J.; Milek, T.; Seifert, G. Adsorption of Phosphonic Acid at the TiO2 Anatase (101) and Rutile (110) Surfaces. J. Phys. Chem. C 2009, 113, 5730–5740. [Google Scholar] [CrossRef]
- Eyert, V. A Comparative Study on Methods for Convergence Acceleration of Iterative Vector Sequences. J. Comput. Phys. 1996, 124, 271–285. [Google Scholar] [CrossRef]
- Yu, V.W.-Z.; Campos, C.; Dawson, W.; García, A.; Havu, V.; Hourahine, B.; Huhn, W.P.; Jacquelin, M.; Jia, W.; Keçeli, M.; et al. ELSI—An open infrastructure for electronic structure solvers. Comput. Phys. Commun. 2020, 256, 107459. [Google Scholar] [CrossRef]
- Selli, D.; Fazio, G.; Seifert, G.; Di Valentin, C. Water Multilayers on TiO2 (101) Anatase Surface: Assessment of a DFTB-Based Method. J. Chem. Theory Comput. 2017, 13, 3862–3873. [Google Scholar] [CrossRef]
- Aradi, B.; Niklasson, A.M.N.; Frauenheim, T. Extended Lagrangian Density Functional Tight-Binding Molecular Dynamics for Molecules and Solids. J. Chem. Theory Comput. 2015, 11, 3357–3363. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll; English, N. J. A DFTB-Based Molecular Dynamics Investigation of an Explicitly Solvated Anatase Nanoparticle. Appl. Sci. 2022, 12, 78. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Tilocca, A.; Selloni, A. Structure and Reactivity of Water Layers on Defect-Free and Defective Anatase TiO2(101) Surfaces. J. Phys. Chem. B 2004, 108, 4743–4751. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, C.; Huang, L. Molecular Dynamics Simulation of First-Adsorbed Water Layer at Titanium Dioxide Surfaces. J. Chem. Eng. Data 2018, 63, 2420–2429. [Google Scholar] [CrossRef]
- O’carroll, D.; Martinez-Gonzalez, J.A.; English, N.J. Coherency spectral analysis of interfacial water at TiO2 surfaces. Mol. Simul. 2020, 1–10. [Google Scholar] [CrossRef]
- O’carroll, D.; English, N.J. Self-ordering water molecules at TiO2 interfaces: Advances in structural classification. J. Chem. Phys. 2020, 153, 064502. [Google Scholar] [CrossRef]
- Kavathekar, R.S.; English, N.J.; MacElroy, J.M.D. Spatial distribution of adsorbed water layers at the TiO2 rutile and anatase interfaces. Chem. Phys. Lett. 2012, 554, 102–106. [Google Scholar] [CrossRef]
- Sumita, M.; Hu, C.; Tateyama, Y. Interface Water on TiO2 Anatase (101) and (001) Surfaces: First-Principles Study with TiO2 Slabs Dipped in Bulk Water. J. Phys. Chem. C 2010, 114, 18529–18537. [Google Scholar] [CrossRef]
- Luzar, A.; Chandler, D. Structure and hydrogen bond dynamics of water–dimethyl sulfoxide mixtures by computer simulations. J. Chem. Phys. 1993, 98, 8160–8173. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Van den Bossche, M. DFTB-Assisted Global Structure Optimization of 13- and 55-27-Atom Late Transition Metal Clusters. J. Phys. Chem. A 2019, 123, 3038–3045. [Google Scholar] [CrossRef] [PubMed]




| System | NTiO2 | NH2O | a (Å) | b (Å) | c (Å) |
|---|---|---|---|---|---|
| Non-reactive | |||||
| Rutile | 448 | 2000 | 26.50 | 40.96 | 69.87 |
| Anatase | 448 | 2000 | 23.62 | 45.38 | 69.45 |
| Reactive | |||||
| Rutile | 448 | 2000 | 26.50 | 40.96 | 69.87 |
| System | NTiO2 | NH2O | a (Å) | b (Å) | c (Å) |
|---|---|---|---|---|---|
| Anatase | 28 | 65 | 11.41 | 10.47 | 39.26 |
| Rutile | 32 | 45 | 9.307 | 11.04 | 25.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Carroll, D.; English, N.J. Multi-Theory Comparisons of Molecular Simulation Approaches to TiO2/H2O Interfacial Systems. Crystals 2023, 13, 1122. https://doi.org/10.3390/cryst13071122
O’Carroll D, English NJ. Multi-Theory Comparisons of Molecular Simulation Approaches to TiO2/H2O Interfacial Systems. Crystals. 2023; 13(7):1122. https://doi.org/10.3390/cryst13071122
Chicago/Turabian StyleO’Carroll, Dáire, and Niall J. English. 2023. "Multi-Theory Comparisons of Molecular Simulation Approaches to TiO2/H2O Interfacial Systems" Crystals 13, no. 7: 1122. https://doi.org/10.3390/cryst13071122
APA StyleO’Carroll, D., & English, N. J. (2023). Multi-Theory Comparisons of Molecular Simulation Approaches to TiO2/H2O Interfacial Systems. Crystals, 13(7), 1122. https://doi.org/10.3390/cryst13071122







