Electronic and Magnetic Properties of Cr and V Doped CaZ (Z = S, Se)
Abstract
1. Introduction
2. Computational Details
3. Result and Discussion
3.1. Structural Properties
3.1.1. Elastic Properties
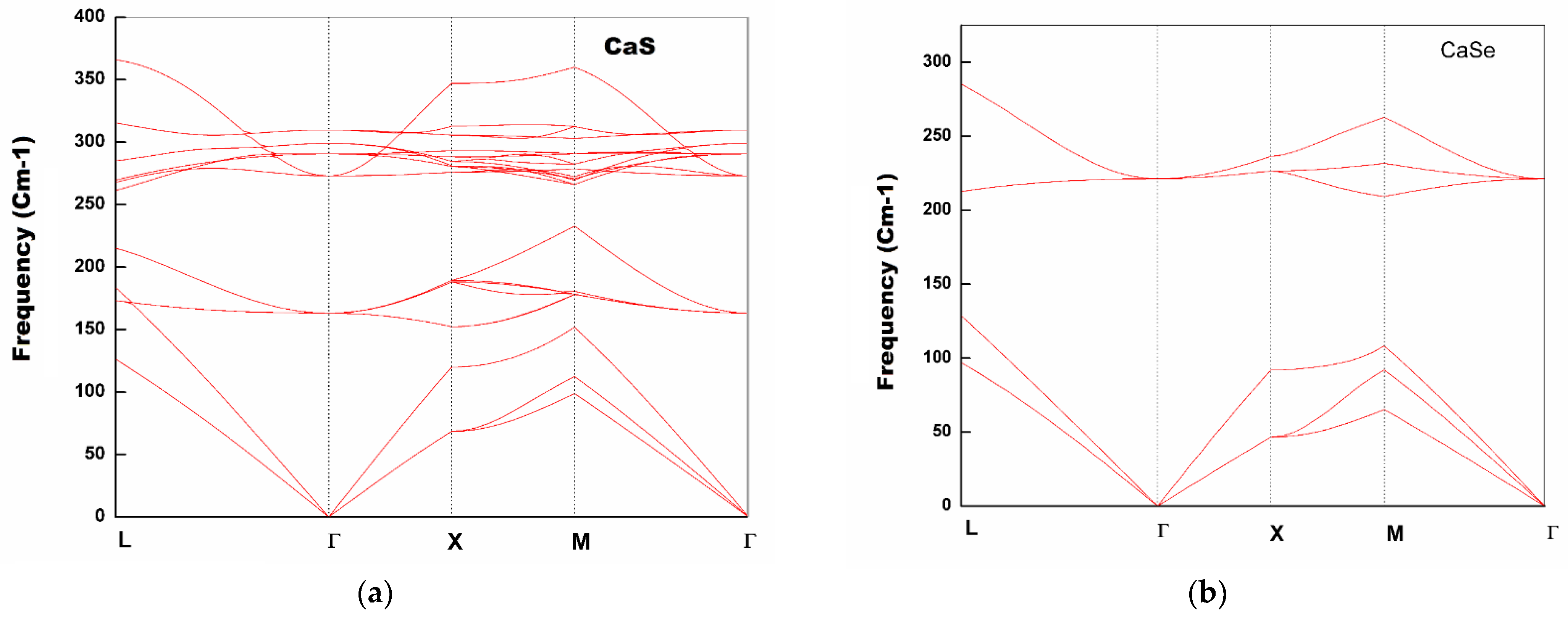
3.1.2. Phonon Dispersion Curves
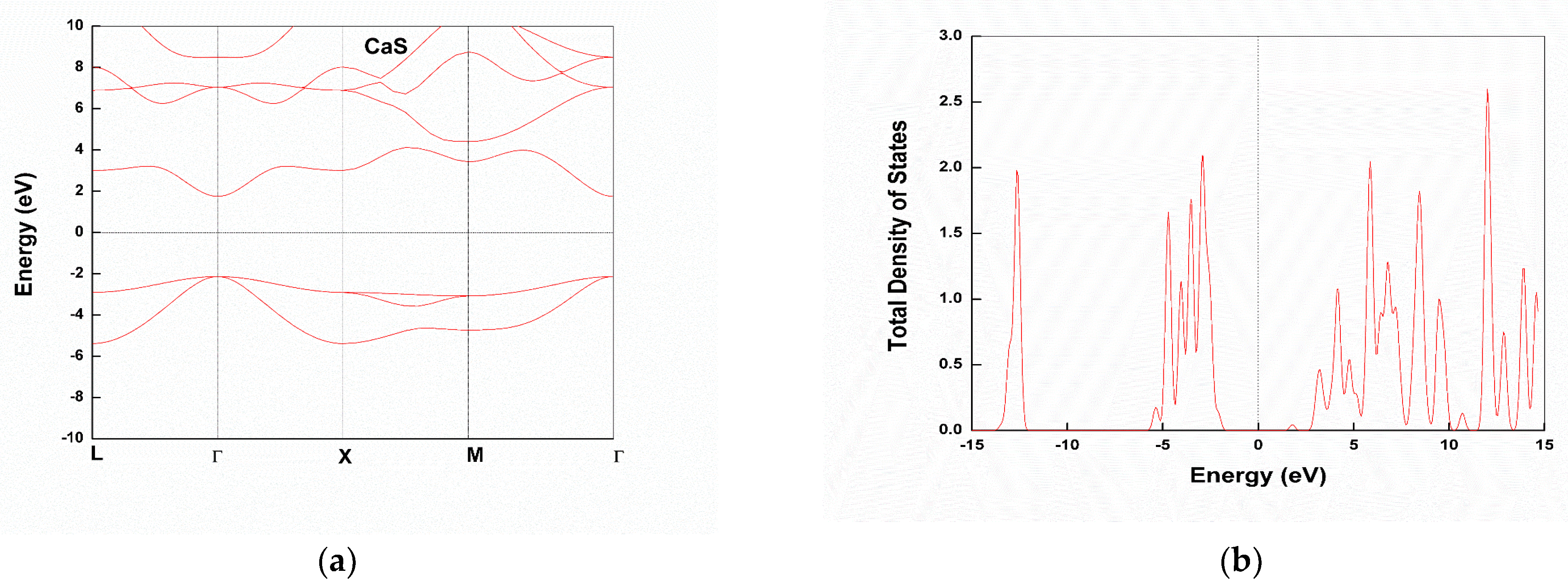
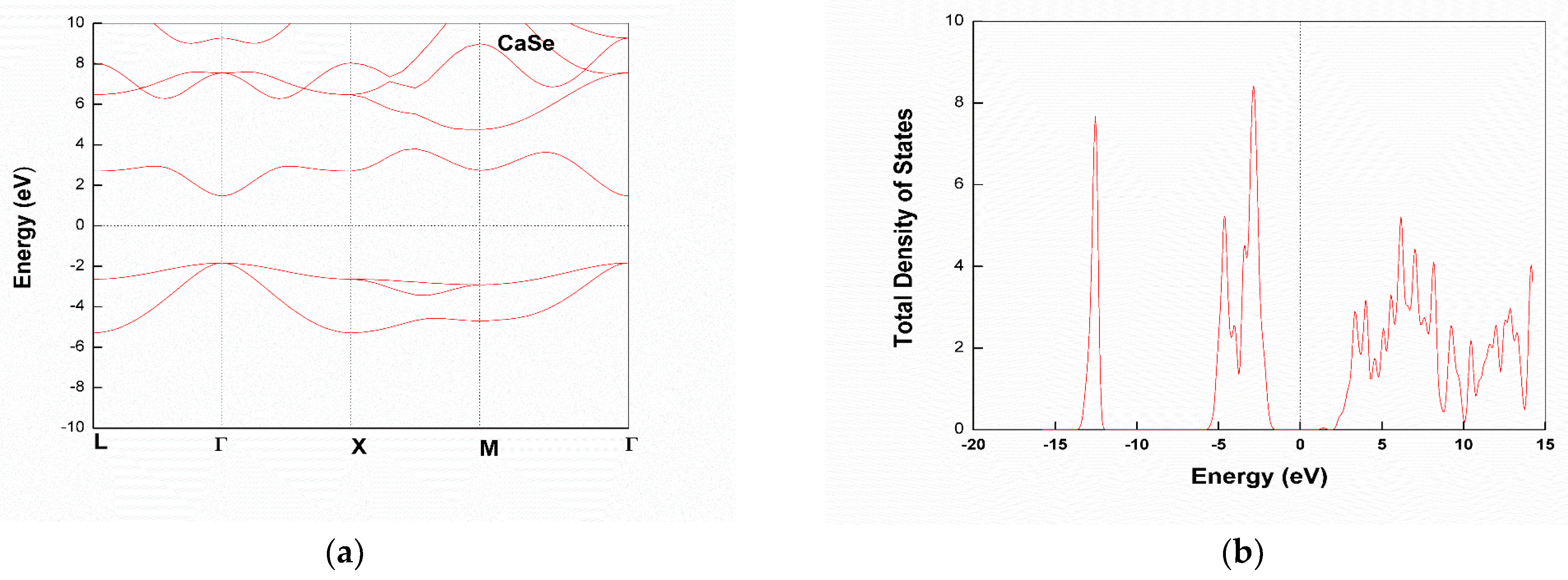
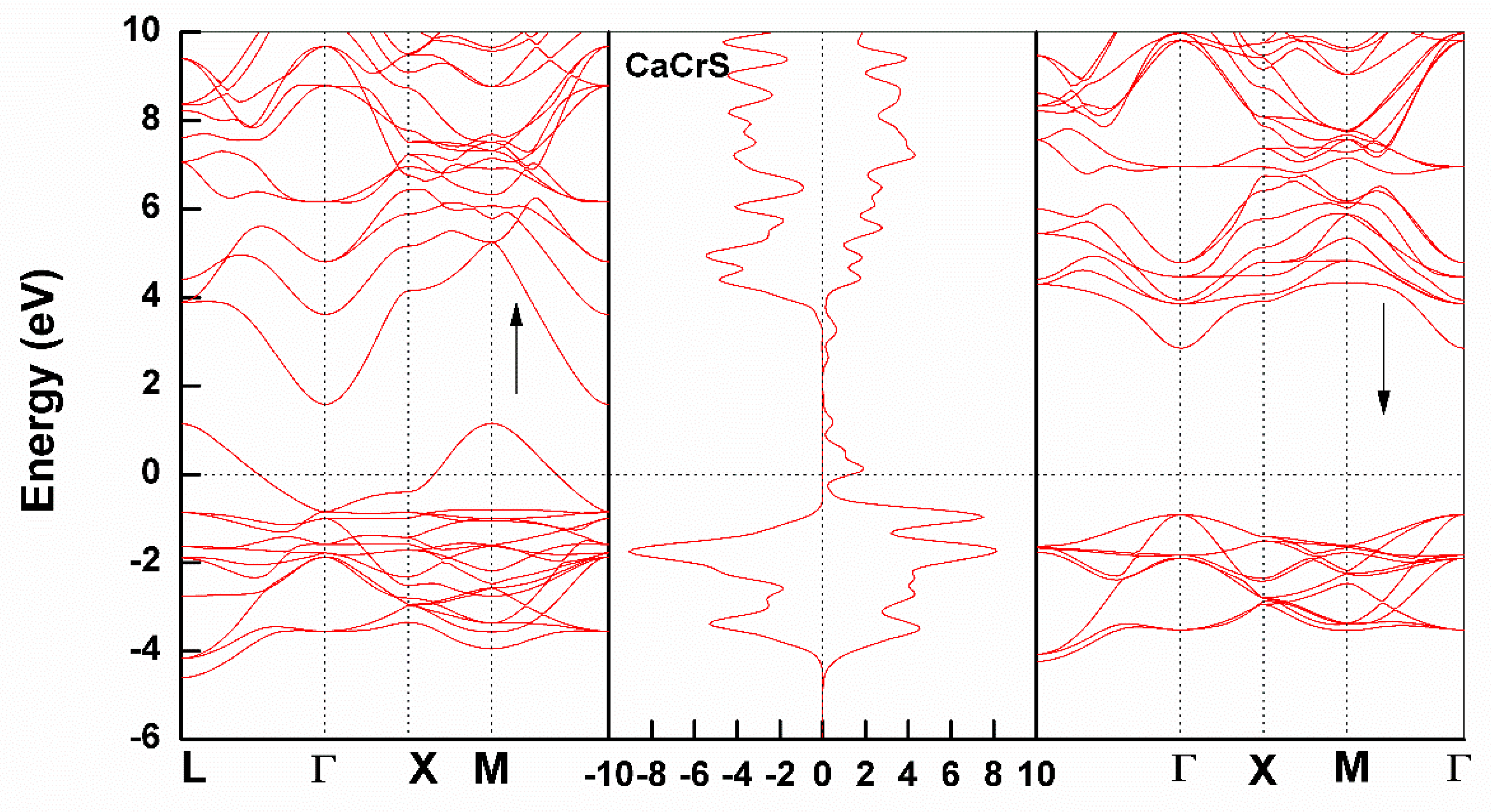
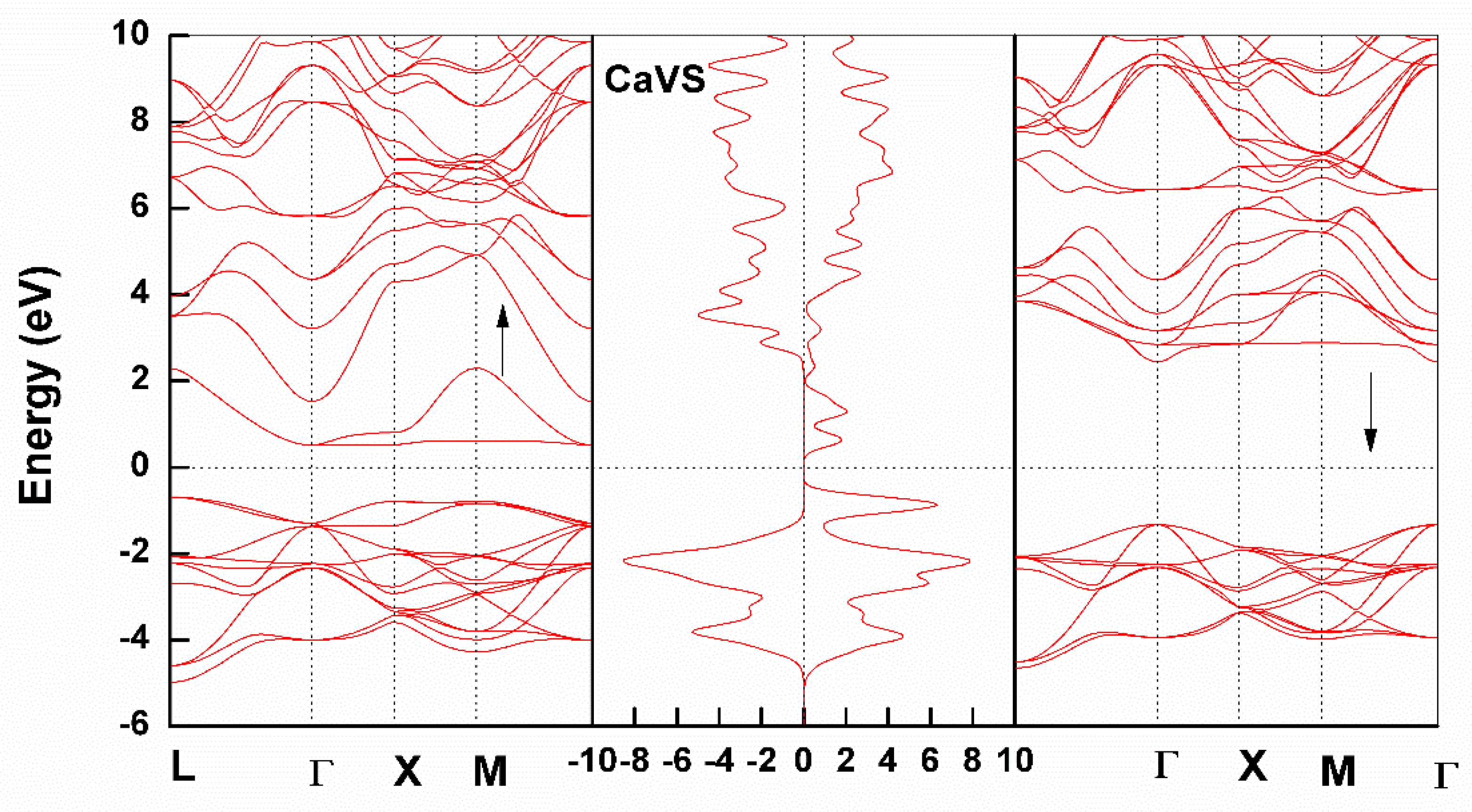
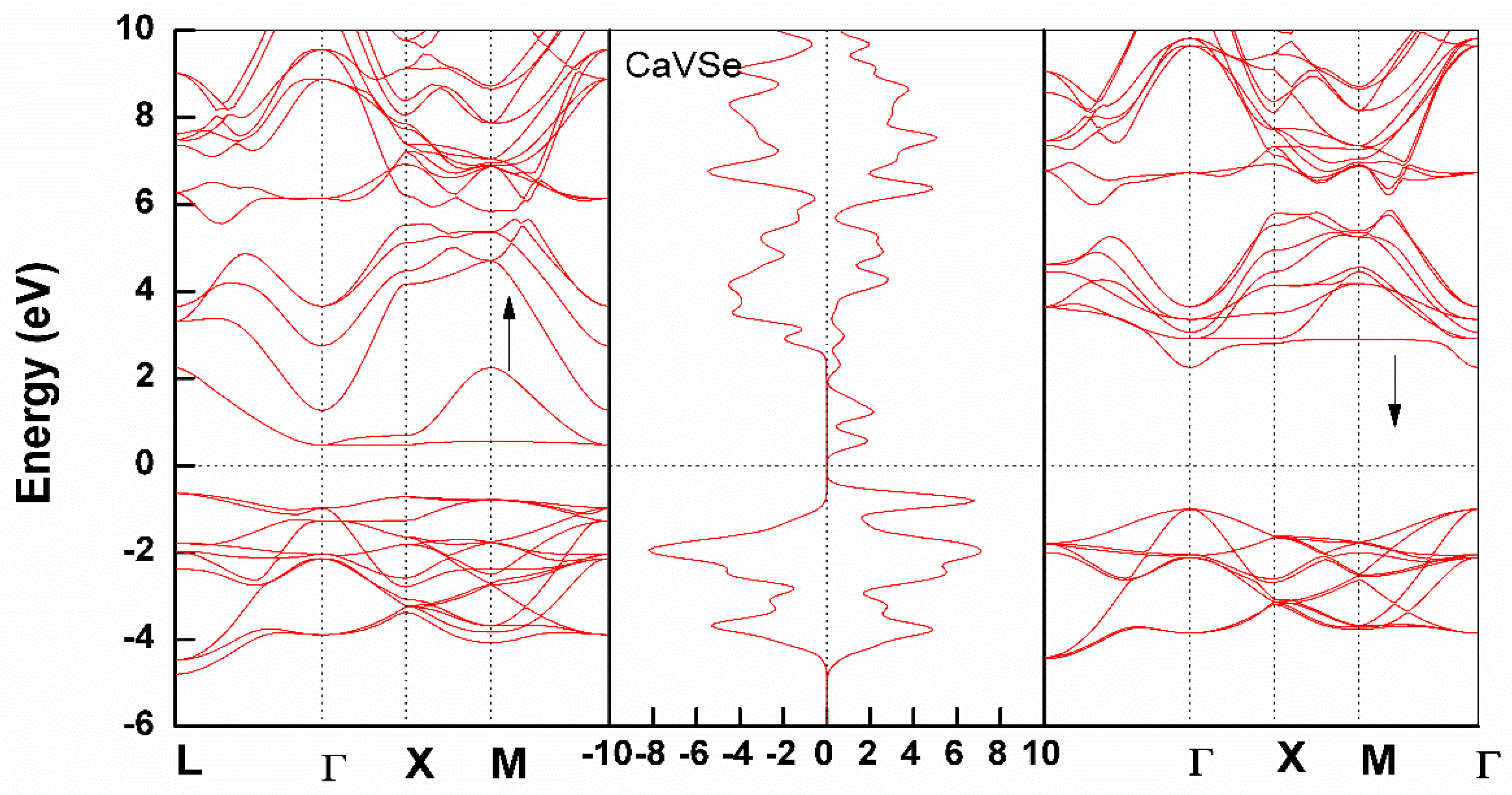
3.2. Electronic Structure
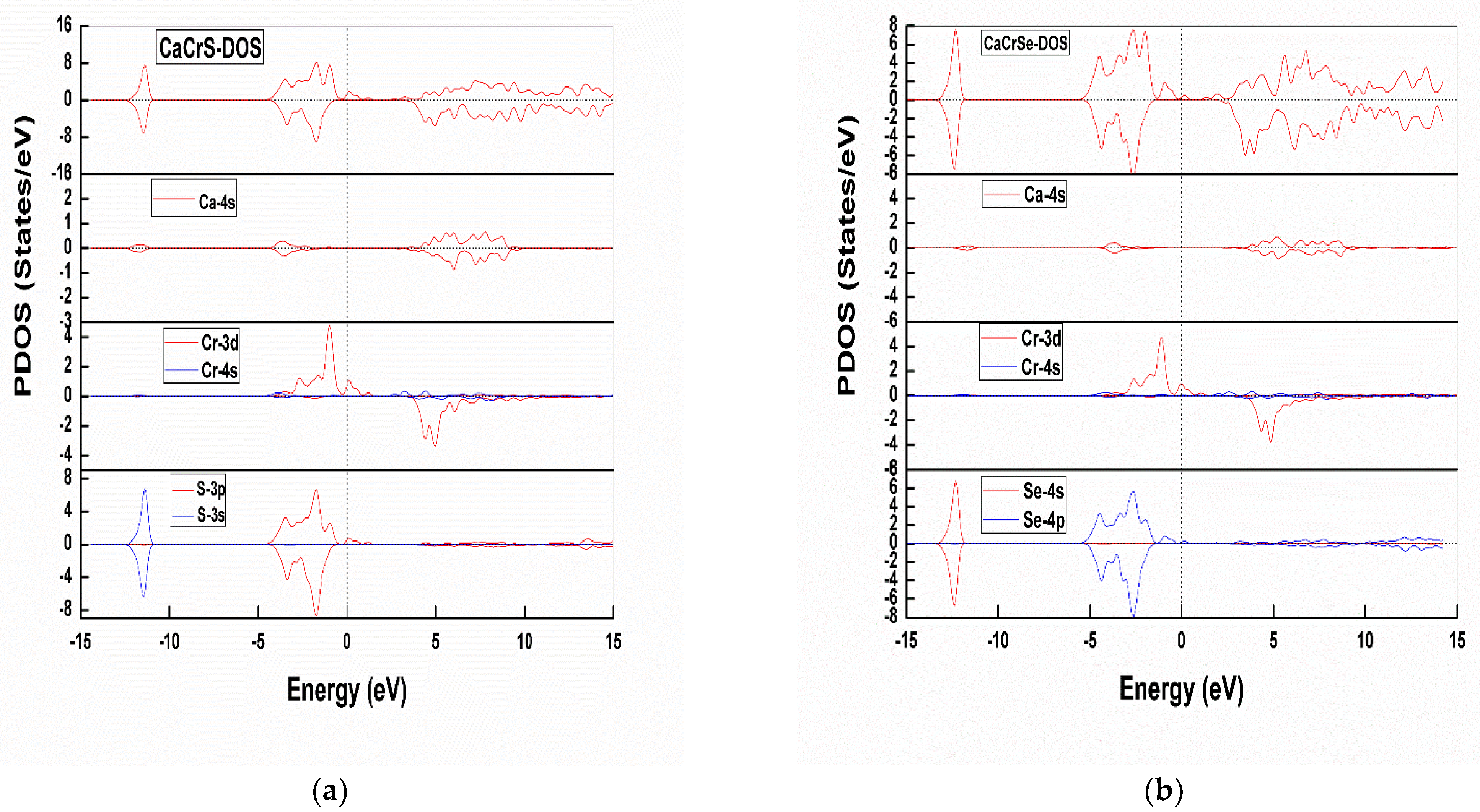
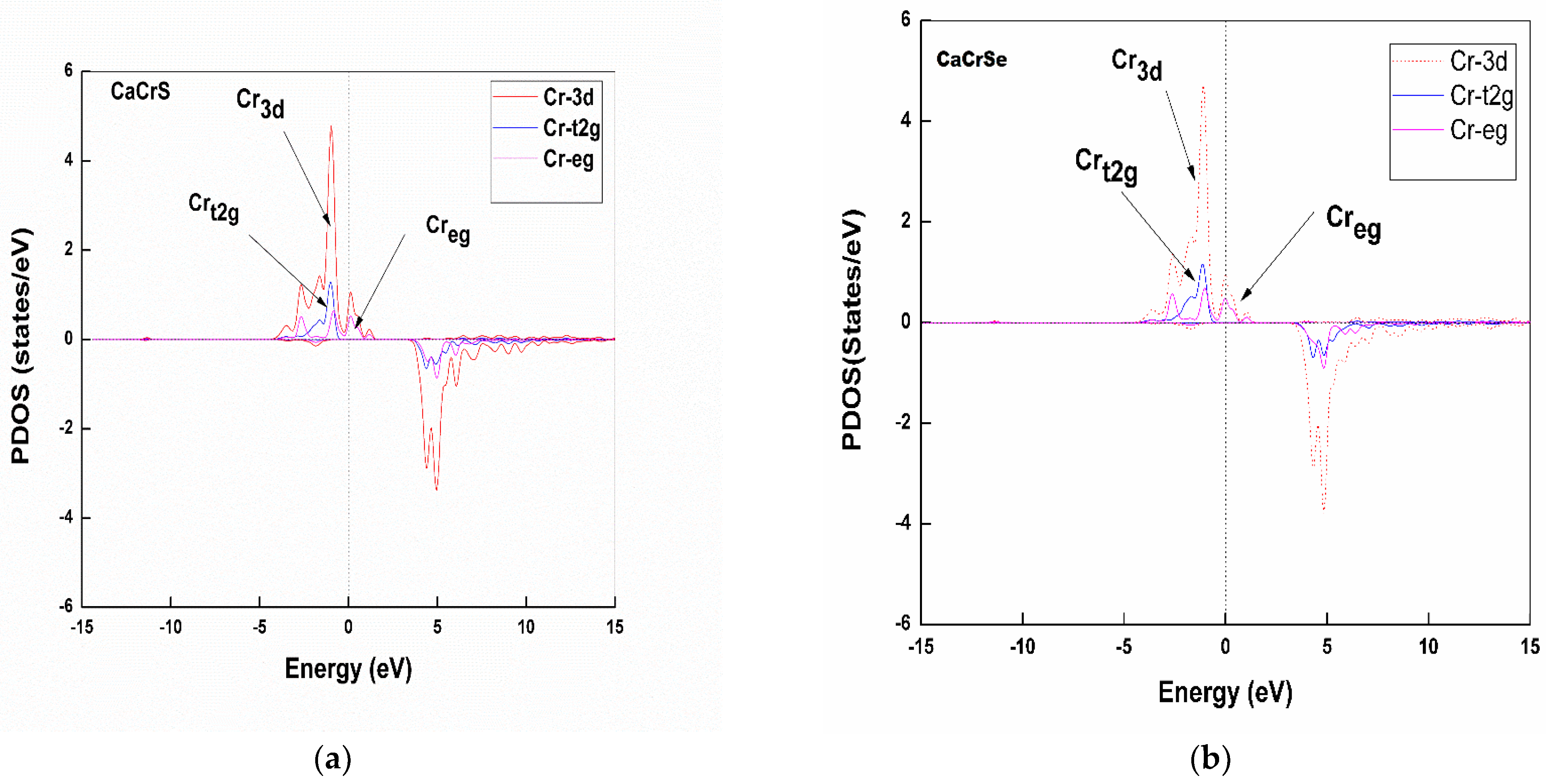
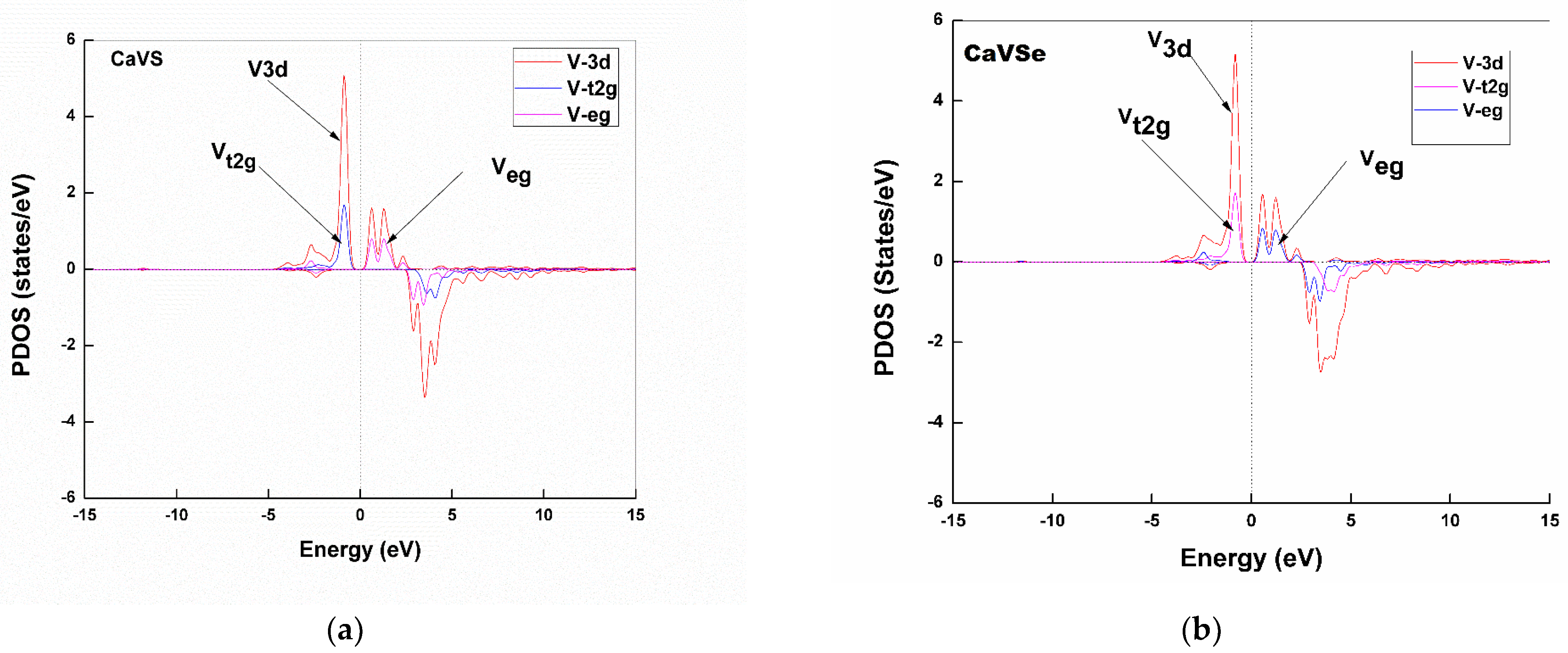
Partial Density of States
3.3. Magnetic Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chambers, S.A.; Droubay, T.C.; Wang, C.M.; Rosso, K.M.; Heald, S.M.; Schwartz, D.A.; Kittilstved, K.R.; Gamelin, D.R. Ferromagnetism in oxide semiconductors. Mater. Today 2006, 9, 28. [Google Scholar] [CrossRef]
- Furdyna, J.K. Diluted Magnetic Semiconductors. J. Appl. Phys. 1988, 64, R29. [Google Scholar] [CrossRef]
- Ramdas, A.K.; Rodriguez, S. Raman Scattering by Magnetic Excitations in Diluted Magnetic Semiconductors. MRS Online Proc. Libr. 1986, 89, 49–58. [Google Scholar] [CrossRef]
- Ohno, H.; Munekata, H.; Von Molnar, S.; Chang, L. Diluted magnetic III-V semiconductors. J. Appl. Phys. 1991, 69, 6103. [Google Scholar] [CrossRef]
- Tsai, C.; Chen, S.; Chuu, D.; Chou, W. Fabrication and physical properties of radio frequency sputtered Cd1-xMnxS thin films. Phys. Rev. B 1996, 54, 11555. [Google Scholar] [CrossRef] [PubMed]
- Rodic, D.; Spasojevic, V.; Bajorek, A.; Orinerud, P. Similarity of structural properties of Hg1-xMnxS and Cd1-xMnxS. J. Magn. Magn. Mater. 1996, 152, 159. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Zhou, Y.; Zhang, M.; Luo, X. First-principles investigation on the electronic and magnetic properties of cubic Be0.75Mn0.25X (X = S, Se, Te). J. Alloys Compd. 2013, 575, 190. [Google Scholar] [CrossRef]
- Nazir, S.; Ikram, N.; Siddiqi, S.A.; Saeed, Y.; Shaukat, A.; Reshak, A.H. First principles density functional calculations of half-metallic ferromagnetism in Zn1-xCrxS and Cd1-xCrxS. Curr. Opin. Solid State Mater. Sci. 2010, 14, 6. [Google Scholar] [CrossRef]
- Aiche, A.; Tadjer, A.; Mazouz, H.M.A.; Doumi, B.; Khachai, H. Electronic Structure and Ferromagnetic Properties of Doped Calcium Sulfide Ca1−xTMxS (TM == V, Cr and Co). SPIN 2020, 10, 2. [Google Scholar] [CrossRef]
- Daoudi, Y.; Mazouz, H.M.A.; Lagoun, B.; Benghia, A. Structural, Electronic and Magnetic Properties of CaSe Doped with 3d (V, Cr and Mn). SPIN 2021, 11, 2150026. [Google Scholar] [CrossRef]
- Ziati, M.; Ez-Zahraouy, H. Theoretical Investigation of Structural, Electronic Properties and Half-Metallic Ferromagnetism in Ca1−xTixS ternary alloys. J. Supercond. Nov. Magn. 2021, 34, 1441–1452. [Google Scholar] [CrossRef]
- Hamidane, O.; Meddour, A.; Bourouis, C. Half-metallic ferromagnetism character in Cr-doped CaS diluted magnetic insulator and semiconductor: An ab initio study. J. Supercond. Nov. Magn. 2019, 32, 2155–2164. [Google Scholar] [CrossRef]
- Hamidane, N.; Baaziz, H.; Ocak, H.; Kmaml, B.U.; Sule, U.; Gokay, Z. Charifi Ab Initio Full-Potential Study of the Structural, Electronic, and Magnetic Properties of the Cubic Sr0.75Ti0.25X (X = S, Se, and Te) Ternary Alloys. J. Supercond. Nov. Magn. 2020, 33, 3263–3272. [Google Scholar] [CrossRef]
- Doumi, B.; Boutaleb, M.; Mokaddem, A.A. Investigation of the structural properties and the magneto-electronic performances in new Ba1-xCrxS materials. Opt. Quant. Electron. 2022, 54, 747. [Google Scholar] [CrossRef]
- Daoudi, Y.; Mazouz, H.M.A.; Fadla, M.A.; Benghia, A. Ab intio investigation of electronic and magnetic properties of Ca TM Te (TM = V, Cr, and Mn). J. Magn. Magn. Mater. 2021, 538, 168315. [Google Scholar] [CrossRef]
- Kaneko, Y.; Koda, T.N. New Developments in IIa–VIb (alkaline-earth chalcogenide) binary semiconductor. J. Cryst. Growth 1988, 86, 72–78. [Google Scholar] [CrossRef]
- Louail, L.; Haddadi, K.; Maouche, D.; Sahraoui, F.A.; Hachemi, A. Electronic band structure of calcium selenide under pressure. Phys. B Condens. Matter. 2008, 403, 3022–3026. [Google Scholar] [CrossRef]
- Versluys, J.; Poelman, D.; Wauters, D.; Meirhaeghe, R.L.V. Photoluminescent and structural properties of CaS: Pb electron beam deposited thin films. J. Phys. Condens. Matter. 2001, 13, 5709–5716. [Google Scholar] [CrossRef]
- Debnath, B.; Sarkar, U.; Debbarma, M.; Bhattacharjee, R.; Chattopadhyaya, S. Modification of band gaps and optoelectronic properties of binary calcium chalcogenides by means of doping of magnesium atom(s) in rock-salt phase- a first principle based theoretical initiative. J. Solid State Chem. 2018, 258, 358–375. [Google Scholar] [CrossRef]
- Palomino-Rojas, L.A.; Cocoletzi, G.H.; de Coss, R.; Takeuchi, N. Structural properties and phase transformations under pressure of XTe compounds (X = Be, Mg, and Ca): The role of the exchange–correlation potential. Solid State Sci. 2009, 11, 1451–1455. [Google Scholar] [CrossRef]
- Solar, J.M.; Artacho, E.; Gale, J.D.; Garcia, A.; Junquera, J.; Ordejon, P.; Sanchez-Portal, D. The SIESTA method for ab initio order-N materials simulation. J. Phys. Condens. Matter 2002, 14, 2745. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Ahmad, I.; Amin, B. Robust half metallicity in Ga1-xMnxP and Ga1-xMnxAs. Comput. Mater. Sci. 2013, 68, 55–60. [Google Scholar] [CrossRef]
- Murnaghan, F. The compressibility of media under extreme pressures. Proc. Natl. Acad. Sci. USA 1944, 30, 244. [Google Scholar] [CrossRef]
- Mohammad, R.; Katircioglu, S. A comparative study for structural and electronic properties of single-crystal ScN. Condens. Matter Phys. 2011, 14, 23701. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, R. Study of half-metallic ferromagnetism and elastic properties of Cd1−x CrxZ (Z = S, Se). Appl. Phys. A 2016, 122, 11. [Google Scholar] [CrossRef]
- Charifi, Z.; Baaziz, H.; Hassan, F.E.H.; Bouarissa, N. High pressure study of structural and electronic properties of calcium chalcogenides. J. Phys. Condens. Matter 2003, 17, 4083. [Google Scholar] [CrossRef]
- Cortona, P.; Masri, P. Cohesive properties and behaviour under pressure of CaS, CaSe, and CaTe: Results of ab initio calculations. J. Phys. Condens. Matter 1998, 10, 8947. [Google Scholar] [CrossRef]
- Marinelli, F.; Lichanot, A. Elastic constants and electronic structure of alkaline-earth chalcogenides. Performances of various hamiltonians. Chem. Phys. Lett. 2003, 367, 430–438. [Google Scholar] [CrossRef]
- Dieter, S. CaS: Elastic constants. In New Data and Updates for Several IIa-VI Compounds (Structural Properties, Thermal and Thermodynamic Properties, and Lattice Properties); Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Straub, G.K.; Harrison, W.A. Self-consistent tight-binding theory of elasticity in ionic solids. Phys. Rev. B 1989, 39, 10325. [Google Scholar] [CrossRef] [PubMed]
- Khachai, H.; Khenata, R.; Haddou, A.; Bouhemadou, A.; Boukortt, A.; Soudini, B.; Boukabrine, F.; Abid, H. First-principles study of structural, electronic and elastic properties under pressure of calcium chalcogenides. Phys. Procedia 2009, 2, 5. [Google Scholar] [CrossRef]
- Bayrakci, M.; Colakoglu, K.; Deligoz, E.; Ciftci, Y. A first-principle study of the structural and lattice dynamical properties of CaX (X = S, Se, and Te). High Press. Res. 2009, 29, 187–203. [Google Scholar] [CrossRef]
- Hill, R. Elastic properties of reinforced solids: Some theoretical principles. Mech. Phys. Solids 1963, 11, 357–372. [Google Scholar] [CrossRef]
- Born, M.; Huang, K. Dynamical Theory of Crystal Lattices; Clarendon: Oxford, UK, 1954. [Google Scholar]
- Golesorkhtabar, R.; Pavone, P.; Spitaler, J.; Puschnig, P.; Draxl, C. Elastic: A tool for calculating second-order elastic constants from first principles. Comput. Phys. Commun. 2013, 184, 1861. [Google Scholar] [CrossRef]
- Haines, J.; Leger, J.; Bocquillon, G. Synthesis and Design of Superhard Materials. Ann. Rev. Mater. Res. 2001, 31, 23. [Google Scholar] [CrossRef]
- Zener, C. Elasticity and Anelasticity of Metals; University of Chicago Press: Chicago, IL, USA, 1948. [Google Scholar]
- Rajput, K.; Roy, D.R. h-CaS and h-CaSe nanosheets in CaX (X = O, S, Se and Te) series: Promising thermoelectric materials under DFT investigation. Appl. Nanosci. 2019, 9, 1845–1856. [Google Scholar] [CrossRef]
- Boucenna, S.; Medkour, Y.; Louail, L.; Boucenna, M.; Hachemi, A.; Roumili, A. High pressure induced structural, elastic and electronic properties of Calcium Chalcogenides CaX (X = S, Se and Te) via first-principles calculations. Comput. Mater. Sci. 2013, 68, 325–334. [Google Scholar] [CrossRef]
- Szczytko, J.; Mac, W.; Twardowski, A.; Matsukara, F.; Ohno, H. Antiferromagnetic p−d exchange in ferromagnetic Ga1−xMnxAs epilayers. Phys. Rev. B 1999, 59, 12935. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, R. Ab-initio study of (Ga,Cr)N and (Ga,Mn)N DMSs: Under hydrostatic pressure. J. Mater. Res. Express 2018, 5, 036104. [Google Scholar] [CrossRef]
- Kaur, K.; Rani, A. Study of structural, electronic, magnetic, and elastic properties of GaP and Ga0.75X0.25P (where X = Cr, Mn, and Fe) using DFT studies. Appl. Phys. A 2017, 123, 791. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, R. Hydrostatic Pressure Effect on Ga0.75Cr0.25As DMS: DFT Study. J. Supercond. Nov. Magn. 2017, 30, 3079–3084. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, R. DFT Study of Hydrostatic Pressure Effect on Cd1 – xZ x X (Z = Cr, Mn; X = S, Se) DMSs. J. Supercond. Nov. Magn. 2017, 30, 2175–2185. [Google Scholar] [CrossRef]
- Gutowski, J.; Sebald, K.; Voss, T. Landolt-Bornstein, New Series Group III B; Springer: Berlin/Heidelberg, Germany, 2009; Volume 44. [Google Scholar]


| Systems | Configuration | dd1 | dd2 | EAFM (eV) | EFM (eV) | ΔE = EAFM − EFM (eV) | Stable State | Tc (Curie Temperature) (K) |
|---|---|---|---|---|---|---|---|---|
| Ca0.75V0.25S | (0,1) | 0.74 | 0.71 | −3677.831 | −3677.827 | −0.031 | AFM | 959.3 K |
| (0,2) | 1.05 | 1.02 | −3677.850 | −3677.853 | −0.022 | AFM | 680.8 K | |
| (0,3) | 1.15 | 1.14 | −3677.869 | −3677.868 | −0.019 | AFM | 587.9 K | |
| Ca0.75Cr0.25S | (0,1) | 0.74 | 0.71 | −3860.632 | −3860.662 | 0.030 | FM | 928.39 K |
| (0,2) | 1.05 | 1.02 | −3860.455 | −3860.482 | 0.027 | FM | 835.55 K | |
| (0,3) | 1.15 | 1.13 | −3860.701 | −3860.718 | 0.017 | FM | 526.09 K | |
| Ca0.75V0.25Se | (0,1) | 4.39 | 4.35 | −3366.987 | −3366.954 | −0.028 | AFM | 866.5 K |
| (0,2) | 6.21 | 6.19 | −3366.782 | −3366.761 | −0.021 | AFM | 649.8 K | |
| (0,3) | 7.60 | 7.58 | −3366.685 | −3366.670 | −0.015 | AFM | 464.19 K | |
| Ca0.75Cr0.25Se | (0,1) | 4.39 | 4.35 | −3550.696 | −3550.725 | 0.029 | FM | 902.3 K |
| (0,2) | 6.21 | 6.19 | −3550.489 | −3550.507 | 0.018 | FM | 557.03 K | |
| (0,3) | 7.60 | 7.58 | −3550.790 | −3550.805 | 0.015 | FM | 464.19 K |
| Parameters | CaS | Ca0.75V0.25S | Ca0.75Cr0.25S | ||
|---|---|---|---|---|---|
| Present | Exp. | Other Theoretical | Present | Present | |
| a (Å) | 5.72 | 5.689 [16] | 5.717 [25] 5.598 [28] 5.721 [29] | 4.778 | 4.463 |
| Beq (GPa) | 86.24 | 64 [16] | 57.42 [24] 115.67 [30] | 64.56 | 63.29 |
| B’eq | 4.05 | 4.2 [16] | 3.8 [24] 4.1 [30] | 3.32 | 3.19 |
| C11 (GPa) | 227.04 | - | 122.87 [24] 135 [29] 202.35 [30] 123.3 [31] | 181.30 | 180.34 |
| C12 (GPa) | 17.24 | - | 32.01 [24] 31.45 [32] 23.6 [33] | 19.65 | 18.85 |
| C44 (GPa) | 32.46 | 36.08 [24] 38 [29] 67.45 [30] 33.5 [32] | 30.99 | 29.32 | |
| Parameters | CaSe | Ca0.75V0.25Se | Ca0.75Cr0.25Se | ||
|---|---|---|---|---|---|
| Present | Exp. | Other. Theoretical | Present | Present | |
| a (Å) | 6.02 | 5.916 [16] | 5.968 [24] 6.087 [29] 5.91 [31] | 4.961 | 4.792 |
| Beq (GPa) | 75.24 | 51 [16] | 56.2 [28] 88.92 [30] 63.94 [32] 60.88 [31] | 67.27 | 66.81 |
| B’eq | 5.3 | 4.2 [16] | 4.1 [28] 4.39 [32] | 3.81 | 3.75 |
| C11 (GPa) | 199.55 | - | 155.25 [30] 135.0 [32] 120.32 [31] 104.6 [34] | 156.14 | 160.73 |
| C12 (GPa) | 16.89 | - | 23.12 [28] 18 [29] 28.38 [32] 20.6 [34] | 18.42 | 18.84 |
| C44 (GPa) | 28.58 | - | 29.90 [24] 31 [29] 28.5 [34] | 22.70 | 23.52 |
| Compound | Spin-Up Band Gap Eg(eV) | Spin-Down Band Gap Eg (eV) | Other’s Calculations | Nature | Half-Metallic Band Gap GHM (eV) |
|---|---|---|---|---|---|
| CaS | 3.84 | - | 3.969 [31], 4.086 [27] | Semiconductor | - |
| CaSe | 3.26 | - | 3.491 [27], 3.451 [31] | Semiconductor | - |
| Ca0.75Cr0.25S | 0.439 | 3.75 | - | Half-Metallic | 2.811 |
| Ca0.75Cr0.25Se | 0.081 | 3.28 | - | Half-Metallic | 2.577 |
| Ca0.75V0.25S | 1.374 | 3.76 | - | Semiconductor | - |
| Ca0.75V0.25Se | 1.44 | 3.19 | - | Semiconductor | - |
| Parameter | Ca0.75Cr0.25S | Ca0.75Cr0.25Se | Ca0.75V0.25S | Ca0.75V0.25Se |
|---|---|---|---|---|
| ΔEC (eV) | 1.25 | 1.33 | 2.00 | 1.75 |
| ΔEv (eV) | −2.06 | −1.87 | −0.38 | −0.25 |
| N0α | 2.50 | 2.65 | 5.34 | 4.66 |
| N0β | 4.12 | 3.74 | 1.01 | 0.66 |
| M (µb) | 4.0 | 4.0 | 3.00 | 3.00 |
| mCa (µb) | −0.054 | −0.045 | −0.03 | −0.027 |
| mS (µb) | −0.567 | - | −0.265 | − |
| mSe (µb) | - | −0.638 | - | −0.301 |
| mCr (µb) | 4.61 | 4.70 | - | - |
| mV (µb) | - | - | 3.294 | 3.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Rani, A.; Alshaikhi, A.A. Electronic and Magnetic Properties of Cr and V Doped CaZ (Z = S, Se). Crystals 2023, 13, 1069. https://doi.org/10.3390/cryst13071069
Kumar R, Rani A, Alshaikhi AA. Electronic and Magnetic Properties of Cr and V Doped CaZ (Z = S, Se). Crystals. 2023; 13(7):1069. https://doi.org/10.3390/cryst13071069
Chicago/Turabian StyleKumar, Ranjan, Anita Rani, and Abdullah A. Alshaikhi. 2023. "Electronic and Magnetic Properties of Cr and V Doped CaZ (Z = S, Se)" Crystals 13, no. 7: 1069. https://doi.org/10.3390/cryst13071069
APA StyleKumar, R., Rani, A., & Alshaikhi, A. A. (2023). Electronic and Magnetic Properties of Cr and V Doped CaZ (Z = S, Se). Crystals, 13(7), 1069. https://doi.org/10.3390/cryst13071069