Phase-Field Simulation of the Microstructure Evolution in the Eutectic Alloy NiAl-31Cr-3Mo
Abstract
1. Introduction
2. Materials and Methods
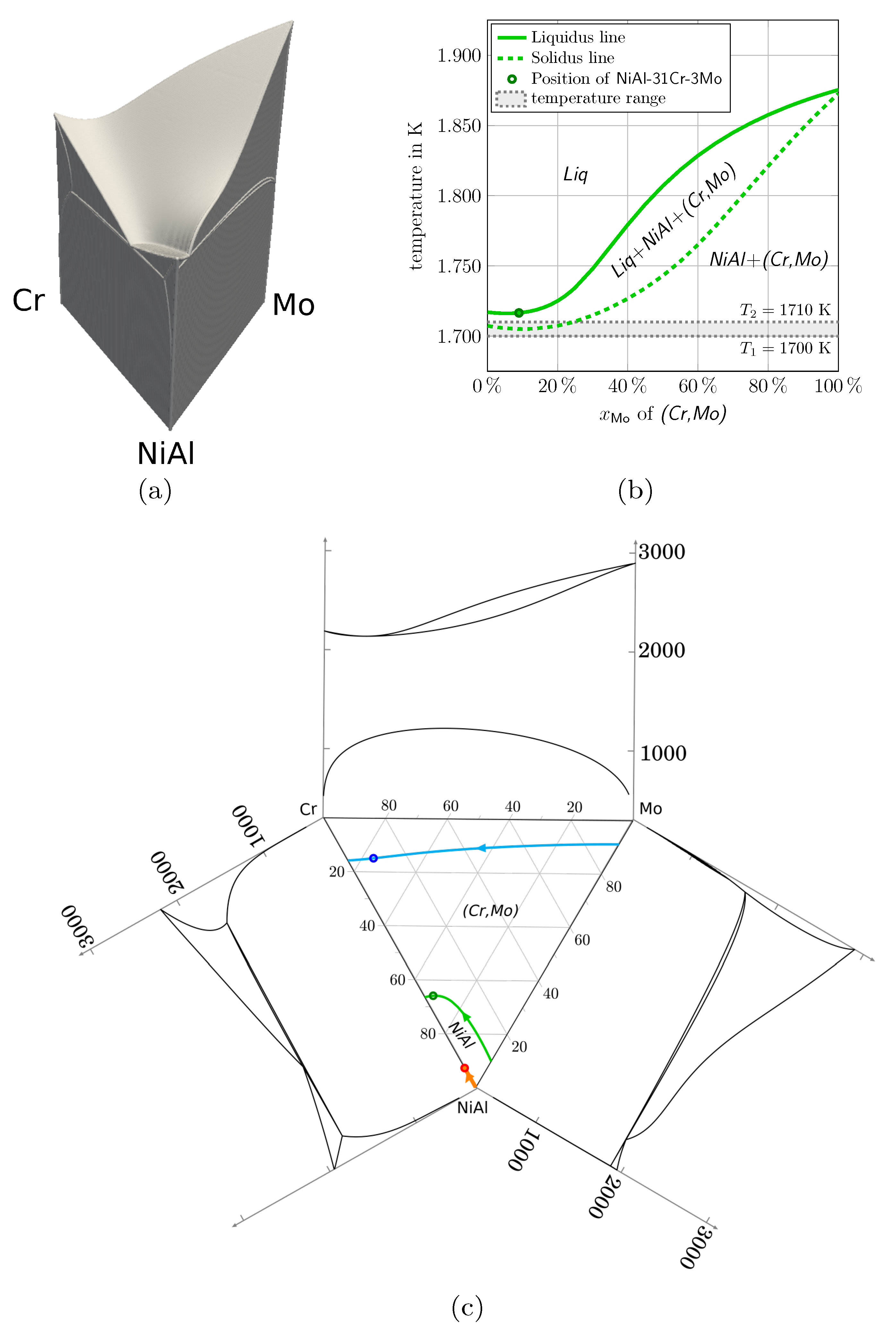
2.1. The Quaternary System NiAl-(Cr,Mo)
2.2. Phase-Field Method
2.3. Analysis Method
2.4. Model
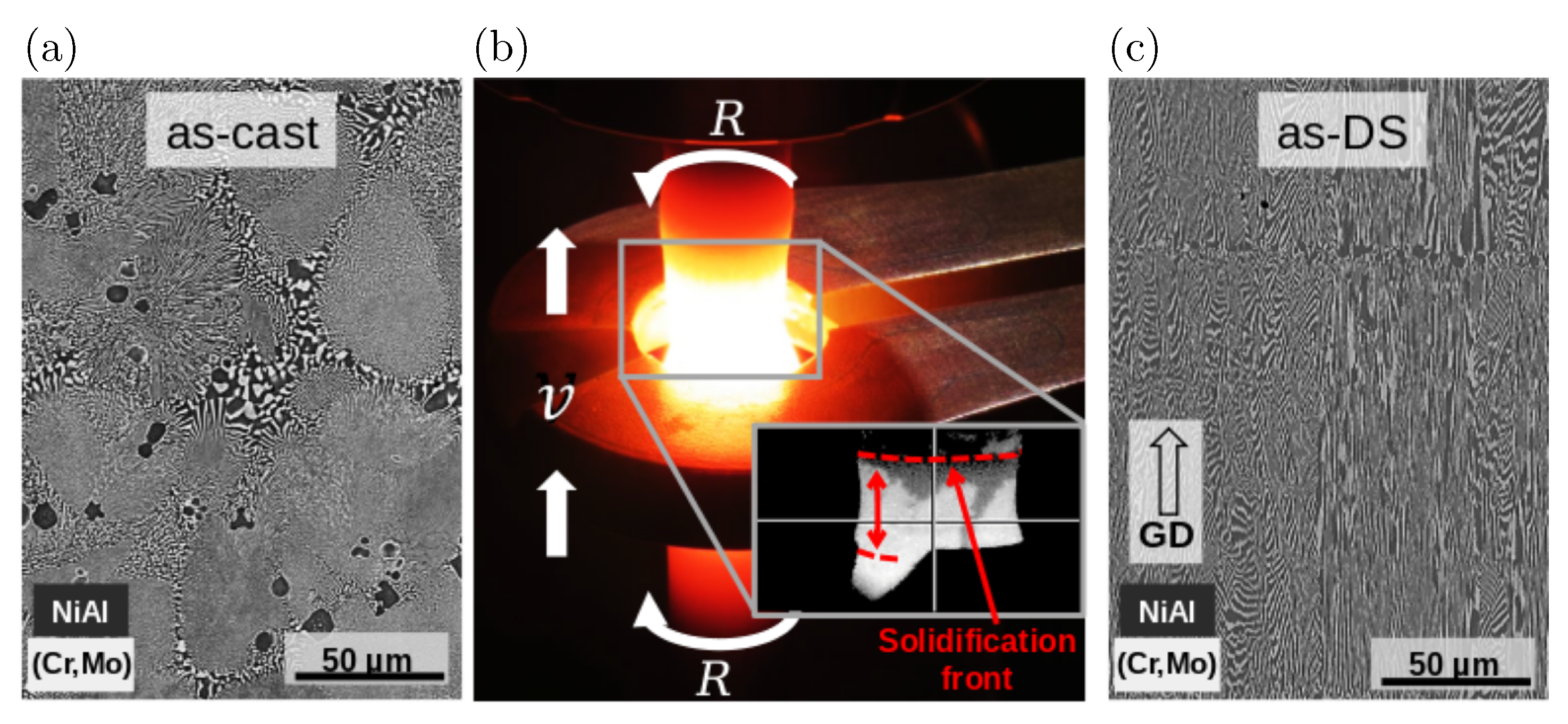
2.5. Experimental Setup
2.6. Simulation Setup
3. Results and Discussion
4. Conclusions
- (i)
- The generated Gibbs energy functions for the modeling of the quaternary system NiAl-31Cr-3Mo show an excellent behavior in reproducing the equilibrium concentrations of the solids, given in the thermodynamic Calphad database. Within the defined temperature range for the modeling, the deviation between the Calphad data and the rebuilt equilibrium concentrations is ∼0.1%. Also for temperatures up to eutectic temperature and down to below predefined temperature range a very good accordance with a maximum deviation of ∼0.9% is detected.
- (ii)
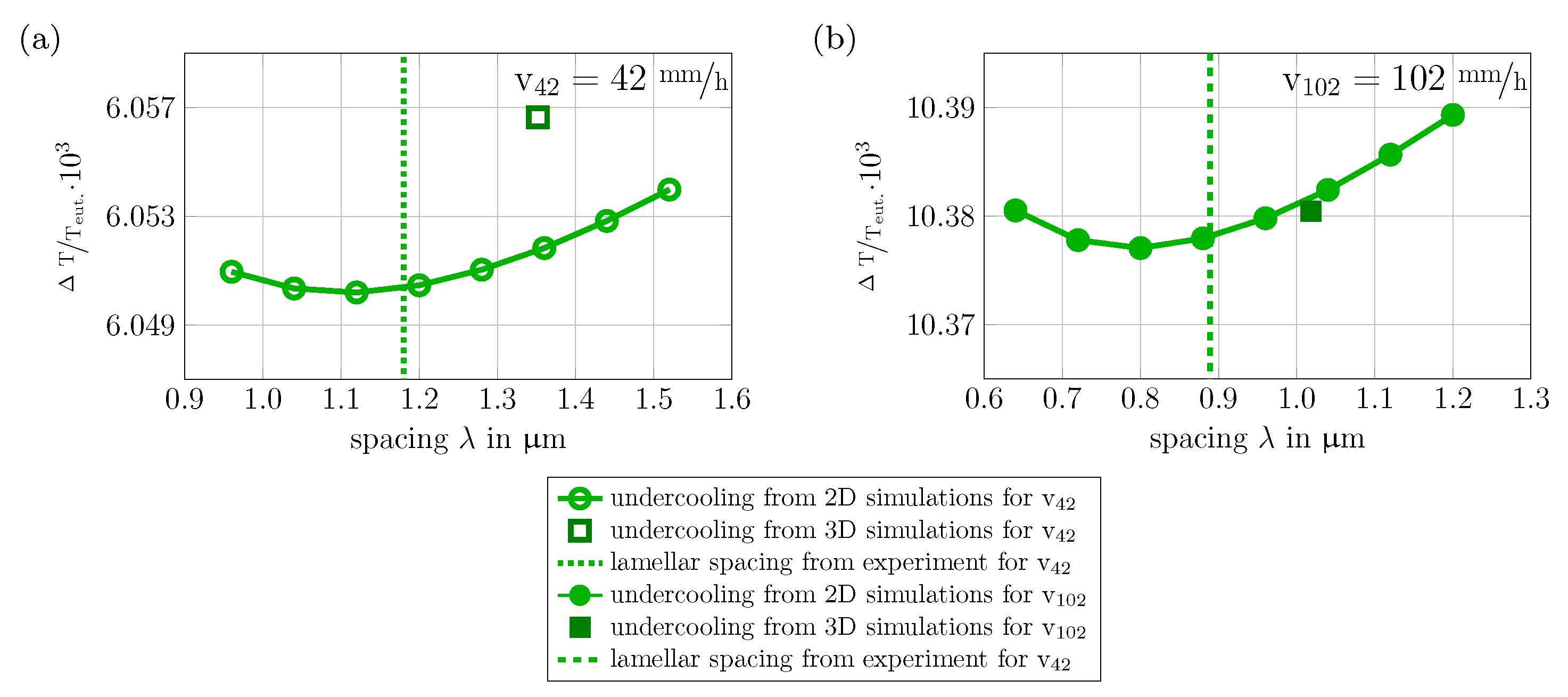
- The undercooling-spacing relationships of the two-dimensional phase-field simulations follow the expected curve for temperature dependent solidification described in the theory of Jackson and Hunt. For both velocities a defined minimum is found.
- (iii)
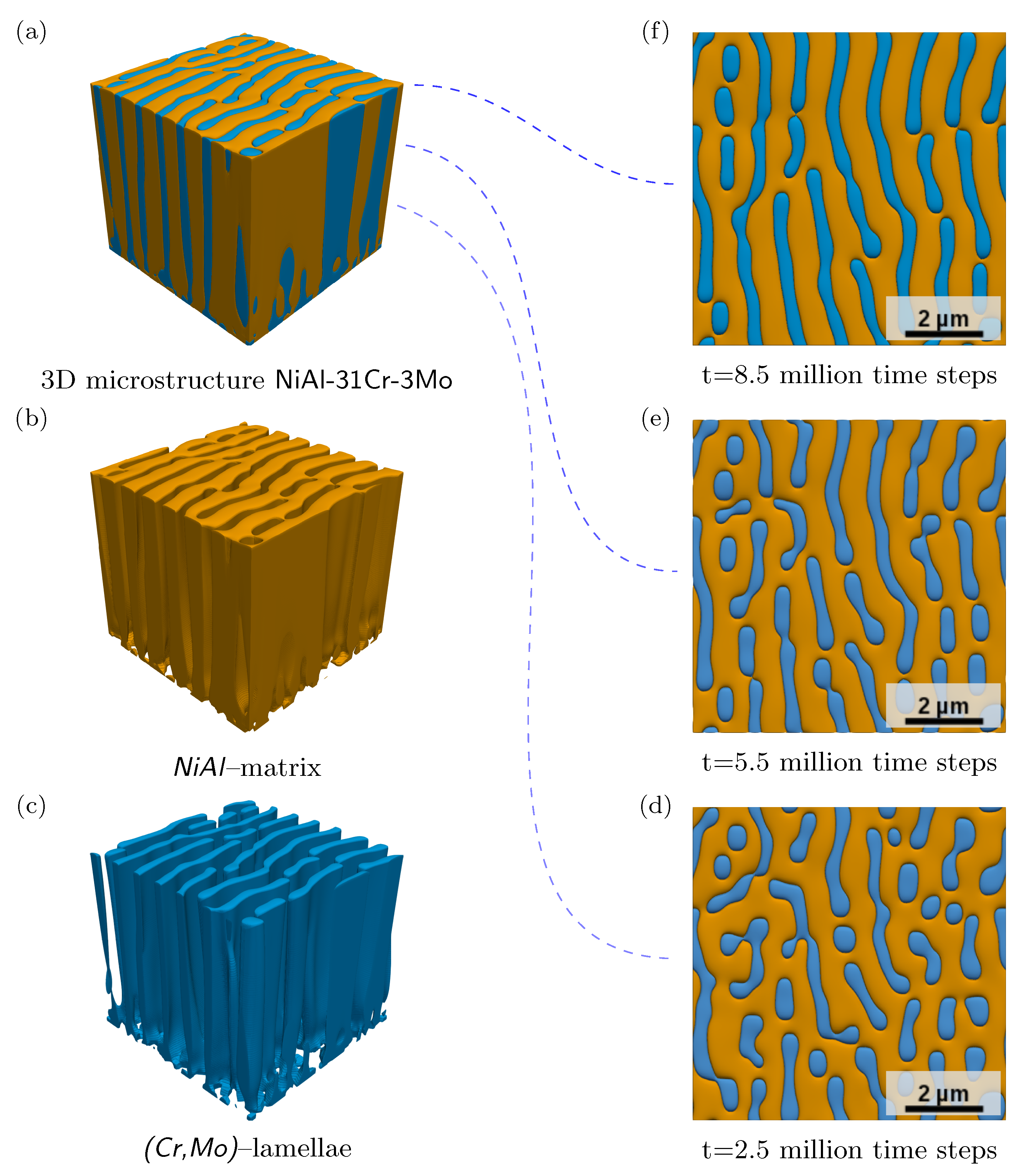
- The establishment of a lamellar microstructure in 3D can be ascribed to the used two-fold anisotropy formulation, as simulations without anisotropy lead to the growth of a merely fibrous structure. Although this anisotropy formulation is not motivated based on the investigated material system, the results demonstrate the capability of the approach to validate the derived model of NiAl-31Cr-3Mo to describe the microstructure evolution during directional solidification. To increase the variety within the microstructure with the formation of eutectic two-phase cell boundaries, a more general interfacial anisotropy formulation can be used including crystallographic orientations in 3D to enable the description of eutectic colony boundaries.
- (iv)
- The evolved microstructures of the 3D large-scale phase-field simulations for the quaternary system NiAl-31Cr-3Mo are in qualitative and quantitative accordance with experimental micrographs. The deviations in the lamellar spacings are ∼9% for v42 and ∼2% for v102, respectively. To reduce the deviations and the impact of a single missing lamellae pair, larger simulation domains are necessary. Furthermore disturbances need to be implemented into the solidification process together with the activation of a nucleation mechanism.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Parameter Sets for Simulations
| General Parameter Set | ||
|---|---|---|
| Parameter | Simulation Value | Physical Value |
| 1.0 | ||
| 4.0 | ||
| , | ||
| , | ||
| , | ||
| - | ||
| - | ||
| For 2D Simulations | ||
| 1.0 | ||
| v42 | ||
| v102 | ||
| 0 | ||
| , | 49 | - |
| , | 28 | - |
| , | 25 | - |
| For 3D Simulations | ||
| 1.0 | ||
| v42 | ||
| v102 | ||
| 0 | ||
| , | 76 | - |
| , | 43 | - |
| , | 38 | - |
References
- Moore, G.E. Cramming more components onto integrated circuits. IEEE Solid-State Circuits Soc. Newsl. 2006, 11, 33–35, reprinted in Electronics 1965, 38, 114. [Google Scholar] [CrossRef]
- Steinmetz, P.; Hötzer, J.; Kellner, M.; Dennstedt, A.; Nestler, B. Large-scale phase-field simulations of ternary eutectic microstructure evolution. Comput. Mater. Sci. 2016, 117, 205–214. [Google Scholar] [CrossRef]
- Lewis, D.; Warren, J.; Boettinger, W.; Pusztai, T.; Gránásy, L. Phase-field models for eutectic solidification. JOM 2004, 56, 34–39. [Google Scholar] [CrossRef]
- Nestler, B.; Choudhury, A. Phase-field modeling of multi-component systems. Curr. Opin. Solid State Mater. Sci. 2011, 15, 93–105. [Google Scholar] [CrossRef]
- Akamatsu, S.; Plapp, M. Eutectic and peritectic solidification patterns. Curr. Opin. Solid State Mater. Sci. 2016, 20, 46–54. [Google Scholar] [CrossRef]
- Hötzer, J.; Kellner, M.; Steinmetz, P.; Nestler, B. Phase-field modeling of solidification processes. J. Indian Inst. Sci. 2016, 96, 235–256. [Google Scholar]
- Choudhury, A.; Kellner, M.; Nestler, B. A method for coupling the phase-field model based on a grand-potential formalism to thermodynamic databases. Curr. Opin. Solid State Mater. Sci. 2015, 19, 287–300. [Google Scholar] [CrossRef]
- Noubary, K.; Kellner, M.; Hötzer, J.; Seiz, M.; Seifert, H.J.; Nestler, B. Data workflow to incorporate thermodynamic energies from Calphad databases into grand-potential-based phase-field models. J. Mater. Sci. 2021, 56, 11932–11952. [Google Scholar] [CrossRef]
- Hötzer, J.; Jainta, M.; Steinmetz, P.; Nestler, B.; Dennstedt, A.; Genau, A.; Bauer, M.; Köstler, H.; Rüde, U. Large scale phase-field simulations of directional ternary eutectic solidification. Acta Mater. 2015, 93, 194–204. [Google Scholar] [CrossRef]
- Steinmetz, P.; Kellner, M.; Hötzer, J.; Dennstedt, A.; Nestler, B. Phase-field study of the pattern formation in Al-Ag-Cu under the influence of the melt concentration. Comput. Mater. Sci. 2016, 121, 6–13. [Google Scholar] [CrossRef]
- Kazemi, O.; Hasemann, G.; Krüger, M.; Halle, T. Phase field simulation of a directional solidification of a ternary eutectic Mo-Si-B Alloy. In Proceedings of the IOP Conference Series: Materials Science and Engineering, 18th Chemnitz Seminar on Materials Engineering—18, Werkstofftechnisches Kolloquium, Chemnitz, Germany, 10–11 March 2016; IOP Publishing: Bristol, UK, 2016; Volume 118, p. 012028. [Google Scholar]
- Lu, Y.L.; Zheng, C.; Li, Y.S.; Wang, Y.X. Microscopic phase-field simulation coupled with elastic strain energy for precipitation process of Ni-Cr-Al alloys with low Al content. Trans. Nonferrous Met. Soc. China 2007, 17, 64–71. [Google Scholar] [CrossRef]
- Wu, K.; Zhou, N.; Pan, X.; Morral, J.; Wang, Y. Multiphase Ni–Cr–Al diffusion couples: A comparison of phase field simulations with experimental data. Acta Mater. 2008, 56, 3854–3861. [Google Scholar] [CrossRef]
- Kellner, M.; Enugala, S.N.; Nestler, B. Modeling of stoichiometric phases in off-eutectic compositions of directional solidifying NbSi-10Ti for phase-field simulations. Comput. Mater. Sci. 2022, 203, 111046. [Google Scholar] [CrossRef]
- Dargahi Noubary, K.; Kellner, M.; Nestler, B. Rotating Directional Solidification of Ternary Eutectic Microstructures in Bi-In-Sn: A Phase-Field Study. Materials 2022, 15, 1160. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.; Hunt, J. Lamellar and Rod Eutectic Growth. Trans. Met. Soc. AIME 1966, 236, 1129–1142. [Google Scholar] [CrossRef]
- Miracle, D. Overview No. 104 The physical and mechanical properties of NiAl. Acta Metall. Mater. 1993, 41, 649–684. [Google Scholar] [CrossRef]
- Darolia, R. Structural applications of NiAl. J. Mater. Sci. Technol. 1994, 10, 157–169. [Google Scholar]
- Noebe, R.D.; Bowman, R.R.; Nathal, M.V. Review of the Physical and Mechanical Properties and Potential Applications of the B2 Compound NiAl; NASA Technical Memorandum 105598, Doc ID: 19930002447; NASA: Washington, DC, USA, 1992.
- Frommeyer, G.; Rablbauer, R.; Schäfer, H. Elastic properties of B2-ordered NiAl and NiAl–X (Cr, Mo, W) alloys. Intermetallics 2010, 18, 299–305. [Google Scholar] [CrossRef]
- Schulz, C.; Kauffmann, A.; Laube, S.; Kellner, M.; Nestler, B.; Heilmaier, M. Role of orientation relationship for the formation of morphology and preferred orientation in NiAl-(Cr, Mo) during directional solidification. Acta Mater. 2022, 231, 117857. [Google Scholar] [CrossRef]
- Milenkovic, S.; Caram, R. Mechanical properties and fracture behavior of directionally solidified NiAl-V eutectic composites. Metall. Mater. Trans. A 2015, 46, 557–565. [Google Scholar] [CrossRef]
- Seemüller, C.; Heilmaier, M.; Haenschke, T.; Bei, H.; Dlouhy, A.; George, E. Influence of fiber alignment on creep in directionally solidified NiAl–10Mo in-situ composites. Intermetallics 2013, 35, 110–115. [Google Scholar] [CrossRef]
- Zhang, J.F.; Jun, S.; Shang, Z.; Lei, W.; Fu, H.Z. Directional solidification and characterization of NiAl-9Mo eutectic alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 3499–3507. [Google Scholar] [CrossRef]
- Bei, H.; George, E. Microstructures and mechanical properties of a directionally solidified NiAl–Mo eutectic alloy. Acta Mater. 2005, 53, 69–77. [Google Scholar] [CrossRef]
- Albiez, J.; Sprenger, I.; Seemüller, C.; Weygand, D.; Heilmaier, M.; Böhlke, T. Physically motivated model for creep of directionally solidified eutectics evaluated for the intermetallic NiAl–9Mo. Acta Mater. 2016, 110, 377–385. [Google Scholar] [CrossRef]
- Cline, H.; Walter, J.; Lifshin, E.; Russell, R. Structures, faults, and the rod-plate transition in eutectics. Metall. Trans. 1971, 2, 189–194. [Google Scholar] [CrossRef]
- Haenschke, T.; Gali, A.; Heilmaier, M.; Krüger, M.; Bei, H.; George, E. Synthesis and characterization of lamellar and fibre-reinforced NiAl-Mo and NiAl-Cr. In Proceedings of the Journal of Physics: Conference Series, 15th International Conference on the Strength of Materials (ICSMA-15), Dresden, Germany, 16–21 August 2009; IOP Publishing: Bristol, UK, 2010; Volume 240, p. 012063. [Google Scholar]
- Cline, H.E.; Walter, J.L. The effect of alloy additions on the rod-plate transition in the eutectic NiAl-Cr. Metall. Trans. 1970, 1, 2907–2917. [Google Scholar] [CrossRef]
- Kumar, A.; Ensslen, C.; Krüger, A.; Klimenkov, M.; Kraft, O.; Schwaiger, R. Micromechanical study on the deformation behavior of directionally solidified NiAl-Cr eutectic composites. J. Mater. Res. 2017, 32, 2127–2134. [Google Scholar] [CrossRef]
- Johnson, D.; Chen, X.; Oliver, B.; Noebe, R.D.; Whittenberger, J. Processing and mechanical properties of in-situ composites from the NiAlCr and the NiAl (Cr, Mo) eutectic systems. Intermetallics 1995, 3, 99–113. [Google Scholar] [CrossRef]
- Wang, L.; Gao, L.; Shen, J.; Zhang, Y.; Wang, T.; Wang, Z.; Qu, P.; Zhang, J.; Zhang, G. Microstructure evolution of NiAl-Cr (Mo) planar eutectic lamellar structure during high temperature treatment. J. Mater. Res. 2018, 33, 3689–3699. [Google Scholar] [CrossRef]
- Peng, J.; Franke, P.; Seifert, H.J. Experimental Investigation and CALPHAD Assessment of the Eutectic Trough in the System NiAl-Cr-Mo. J. Phase Equilibria Diffus. 2016, 37, 592–600. [Google Scholar] [CrossRef]
- Kellner, M.; Sprenger, I.; Steinmetz, P.; Hötzer, J.; Nestler, B.; Heilmaier, M. Phase-field simulation of the microstructure evolution in the eutectic NiAl-34Cr system. Comput. Mater. Sci. 2017, 128, 379–387. [Google Scholar] [CrossRef]
- Gombola, C.; Kauffmann, A.; Geramifard, G.; Blankenburg, M.; Heilmaier, M. Microstructural investigations of novel high temperature alloys based on NiAl-(Cr, Mo). Metals 2020, 10, 961. [Google Scholar] [CrossRef]
- Whittenberger, J.D.; Raj, S.; Locci, I.; Salem, J. Effect of growth rate on elevated temperature plastic flow androom temperature fracture toughness of directionally solidified NiAl-31Cr-3Mo. Intermetallics 1999, 7, 1159–1168. [Google Scholar] [CrossRef]
- Whittenberger, J.D.; Raj, S.; Locci, I.E.; Salem, J.A. Elevated temperature strength and room-temperature toughness of directionally solidified Ni-33Al-33Cr-1Mo. Metall. Mater. Trans. A 2002, 33, 1385–1397. [Google Scholar] [CrossRef]
- Chen, X.; Johnson, D.; Noebe, R.; Oliver, B. Deformation and fracture of a directionally solidified NiAl-28Cr-6Mo eutectic alloy. J. Mater. Res. 1995, 10, 1159–1170. [Google Scholar] [CrossRef]
- Lu, Y.L.; Jia, D.; Hu, T.; Chen, Z.; Zhang, L. Phase-field study the effects of elastic strain energy on the occupation probability of Cr atom in Ni-Al-Cr alloy. Superlattices Microstruct. 2014, 66, 105–111. [Google Scholar] [CrossRef]
- Lu, Y.L.; Chen, Z.; Wang, Y.X.; Zhang, J.; Yang, K. Phase-field study the effect of elastic strain energy on the incubation period of Ni–Cr–Al alloys. Comput. Mater. Sci. 2011, 50, 1925–1931. [Google Scholar] [CrossRef]
- Kellner, M.; Kunz, W.; Steinmetz, P.; Hötzer, J.; Nestler, B. Phase-field study of dynamic velocity variations during directional solidification of eutectic NiAl-34Cr. Comput. Mater. Sci. 2018, 145, 291–305. [Google Scholar] [CrossRef]
- Parisi, A.; Plapp, M. Defects and multistability in eutectic solidification patterns. EPL (Europhys. Lett.) 2010, 90, 26010. [Google Scholar] [CrossRef]
- Ghosh, S.; Plapp, M. Influence of interphase boundary anisotropy on bulk eutectic solidification microstructures. Acta Mater. 2017, 140, 140–148. [Google Scholar] [CrossRef]
- Khanna, S.; Choudhury, A. Three-Phase Eutectic Microstructures: Influence of Interfacial Energy Anisotropy and Solute Diffusivities. Metall. Mater. Trans. A 2021, 52, 4246–4263. [Google Scholar] [CrossRef]
- Plapp, M. Unified derivation of phase-field models for alloy solidification from a grand-potential functional. Phys. Rev. E 2011, 84, 031601. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Nestler, B. Grand-potential formulation for multicomponent phase transformations combined with thin-interface asymptotics of the double-obstacle potential. Phys. Rev. E 2012, 85, 021602. [Google Scholar] [CrossRef] [PubMed]
- Nestler, B.; Garcke, H.; Stinner, B. Multicomponent alloy solidification: Phase-field modeling and simulations. Phys. Rev. E 2005, 71, 041609. [Google Scholar] [CrossRef]
- Karma, A. Phase-field formulation for quantitative modeling of alloy solidification. Phys. Rev. Lett. 2001, 87, 115701. [Google Scholar] [CrossRef]
- Echebarria, B.; Folch, R.; Karma, A.; Plapp, M. Quantitative phase-field model of alloy solidification. Phys. Rev. E 2004, 70, 061604. [Google Scholar] [CrossRef]
- Karma, A. Phase-field model of eutectic growth. Phys. Rev. E 1994, 49, 2245. [Google Scholar] [CrossRef]
- Apel, M.; Boettger, B.; Diepers, H.J.; Steinbach, I. 2D and 3D phase-field simulations of lamella and fibrous eutectic growth. J. Cryst. Growth 2002, 237, 154–158. [Google Scholar] [CrossRef]
- Zhang, A.; Du, J.; Guo, Z.; Xiong, S. Lamellar eutectic growth under forced convection: A phase-field lattice-Boltzmann study based on a modified Jackson-Hunt theory. Phys. Rev. E 2018, 98, 043301. [Google Scholar] [CrossRef]
- Steinmetz, P.; Kellner, M.; Hötzer, J.; Nestler, B. Quantitative comparison of ternary eutectic phase-field simulations with analytical 3D Jackson–Hunt approaches. Metall. Mater. Trans. B 2018, 49, 213–224. [Google Scholar] [CrossRef]
- Steinmetz, P.; Hötzer, J.; Kellner, M.; Genau, A.; Nestler, B. Study of pattern selection in 3D phase-field simulations during the directional solidification of ternary eutectic Al-Ag-Cu. Comput. Mater. Sci. 2018, 148, 131–140. [Google Scholar] [CrossRef]
- Enugala, S.N.; Kellner, M.; Kobold, R.; Hötzer, J.; Kolbe, M.; Nestler, B.; Herlach, D. Theoretical and numerical investigations of rod growth of an Ni–Zr eutectic alloy. J. Mater. Sci. 2019, 54, 12605–12622. [Google Scholar] [CrossRef]
- McCartney, D.; Hunt, J.; Jordan, R. The structures expected in a simple ternary eutectic system: Part 1. Theory. Metall. Trans. A 1980, 11, 1243–1249. [Google Scholar] [CrossRef]
- Choudhury, A.; Plapp, M.; Nestler, B. Theoretical and numerical study of lamellar eutectic three-phase growth in ternary alloys. Phys. Rev. E 2011, 83, 051608. [Google Scholar] [CrossRef]
- Hu, S.; Murray, J.; Weiland, H.; Liu, Z.; Chen, L. Thermodynamic description and growth kinetics of stoichiometric precipitates in the phase-field approach. Calphad 2007, 31, 303–312. [Google Scholar] [CrossRef]
- Hötzer, J.; Reiter, A.; Hierl, H.; Steinmetz, P.; Selzer, M.; Nestler, B. The parallel multi-physics phase-field framework Pace3D. J. Comput. Sci. 2018, 26, 1–12. [Google Scholar] [CrossRef]
- IDM—Institute for Digital Materials Research. Software Package Parallel Algorithms for Crystal Evolution in 3D—Pace3D. 2022. Available online: https://www.h-ka.de/en/idm/profile/pace3d-software (accessed on 20 June 2023).
- Gombola, C.; Hasemann, G.; Kauffmann, A.; Sprenger, I.; Laube, S.; Schmitt, A.; Gang, F.; Bolbut, V.; Oehring, M.; Blankenburg, M.; et al. A zone melting device for the in situ observation of directional solidification using high-energy synchrotron x rays. Rev. Sci. Instrum. 2020, 91, 093901. [Google Scholar] [CrossRef]
- Hinrichs, F.; Kellner, M.; Hötzer, J.; Nestler, B. Calibration of a concentration-driven nucleation mechanism for phase-field simulations of eutectic and off-eutectic compositions in AlCu-5Ag. Scr. Mater. 2020, 186, 89–94. [Google Scholar] [CrossRef]
- Kellner, M.; Hötzer, J.; Schoof, E.; Nestler, B. Phase-field study of eutectic colony formation in NiAl-34Cr. Acta Mater. 2020, 182, 267–277. [Google Scholar] [CrossRef]
- LRZ—Leibniz Supercomputing Centre. SuperMUC-NG Petascale System. 2022. Available online: https://doku.lrz.de/display/PUBLIC/SuperMUC-NG (accessed on 20 June 2023).
- Kuznetsov, V.; Kadyrov, R.; Rudenskii, G.E. Calculation of surface energy of metals and alloys by the electron density functional method. J. Mater. Sci. Technol. 1998, 14, 320–322. [Google Scholar]
- Kang, M.; Lui, S.C.; Mele, E.; Plummer, E.; Zehner, D. Atomic and electronic structure of the NiAl (111) surface. Phys. Rev. B 1990, 41, 4920. [Google Scholar] [CrossRef] [PubMed]


| Temp. in K | Alc in at.% | Crc in at.% | Moc in at.% | Nic in at.% |
|---|---|---|---|---|
| NiAl | ||||
| 1700 | 44.92 | 7.18 | 0.03 | 47.87 |
| 1710 | 44.83 | 7.36 | 0.03 | 47.78 |
| (Cr,Mo) | ||||
| 1700 | 10.18 | 76.41 | 8.81 | 4.59 |
| 1710 | 10.39 | 76.01 | 8.81 | 4.79 |
| Liq | ||||
| 32.94 | 31.07 | 3.06 | 32.94 | |
| Lamellar spacing in nm: | |||
| Simulation | |||
| Experiment | |||
| Deviation | ∼9% | ∼2% | |
| Widths w of NiAl–matrix in nm: | |||
| Simulation | |||
| Experiment | |||
| Deviation | ∼3% | ∼14% | |
| Widths w of (Cr,Mo)–lamellae in nm: | |||
| Simulation | |||
| Experiment | |||
| Deviation | ∼9% | ∼7% | |
| Volume fraction of (Cr,Mo)–lamellae in vol.%: | |||
| Simulation | |||
| Experiment | |||
| Deviation | ∼9% | ∼10% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kellner, M.; Schulz, C.; Kauffmann, A.; Heilmaier, M.; Nestler, B.
Phase-Field Simulation of the Microstructure Evolution in the Eutectic Alloy
Kellner M, Schulz C, Kauffmann A, Heilmaier M, Nestler B.
Phase-Field Simulation of the Microstructure Evolution in the Eutectic Alloy
Kellner, Michael, Camelia Schulz, Alexander Kauffmann, Martin Heilmaier, and Britta Nestler.
2023. "Phase-Field Simulation of the Microstructure Evolution in the Eutectic Alloy
Kellner, M., Schulz, C., Kauffmann, A., Heilmaier, M., & Nestler, B.
(2023). Phase-Field Simulation of the Microstructure Evolution in the Eutectic Alloy












