Effect of Nano-Y2O3 on the Microstructure and Properties of Fe-Cr-C-N-Al Surfacing Alloy
Abstract
1. Introduction
2. Experimental Process
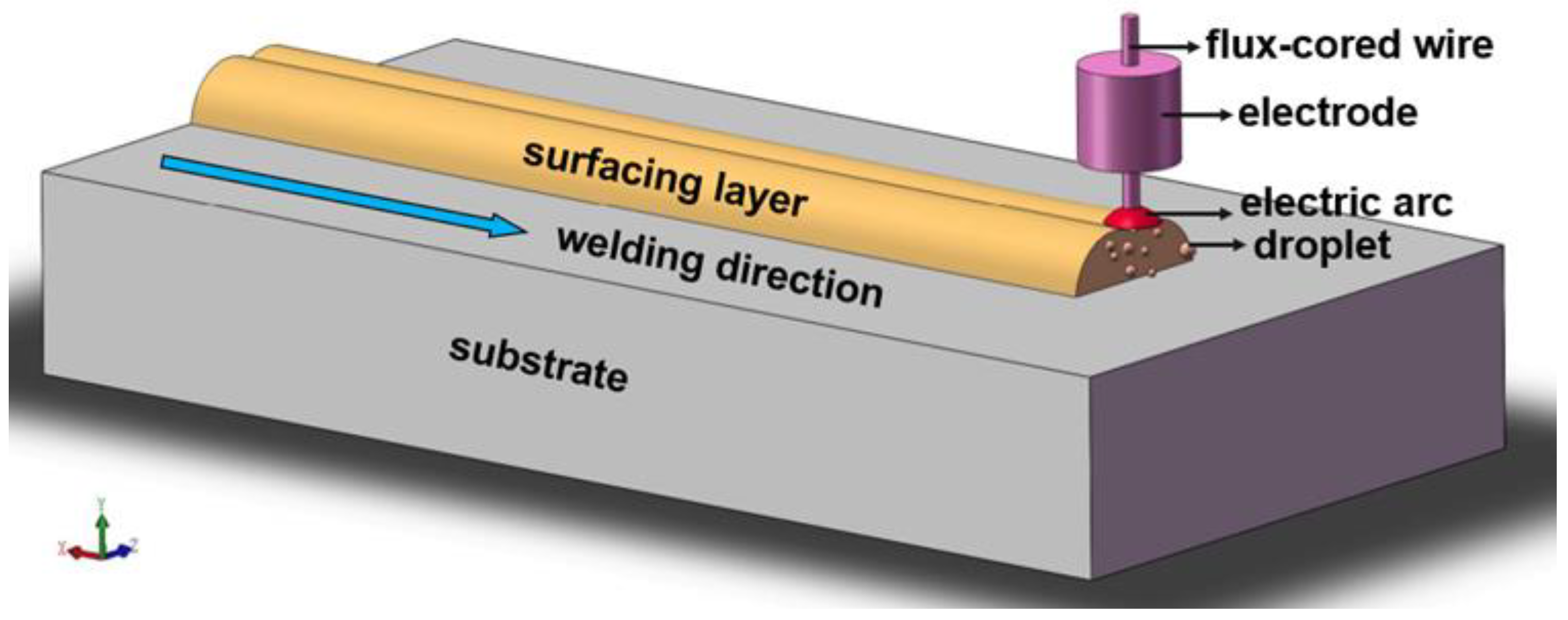
Preparation of Surfacing Alloy
3. Results and Discussion
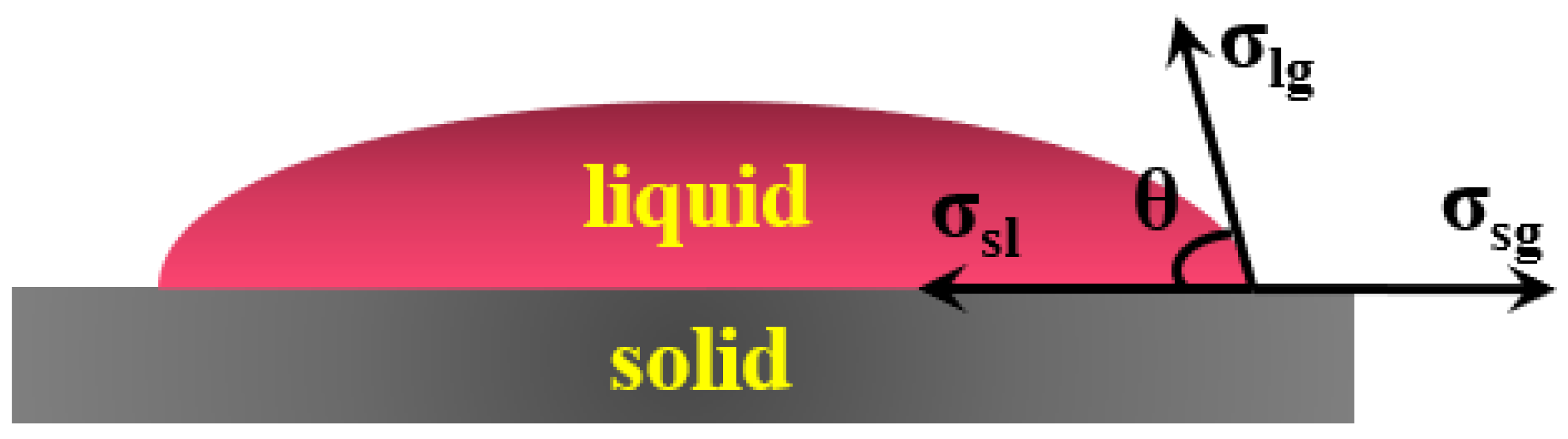
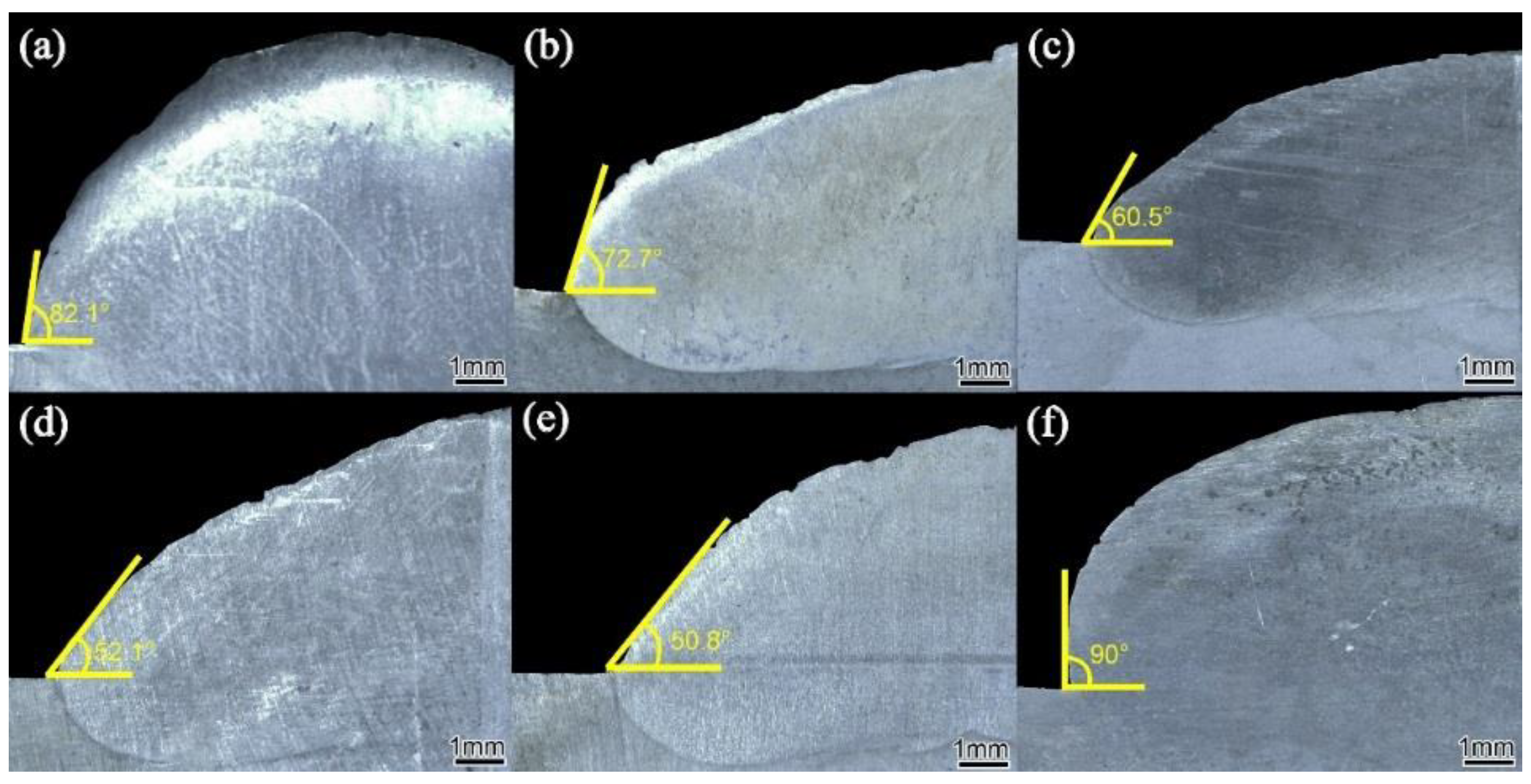
3.1. Formability Analysis
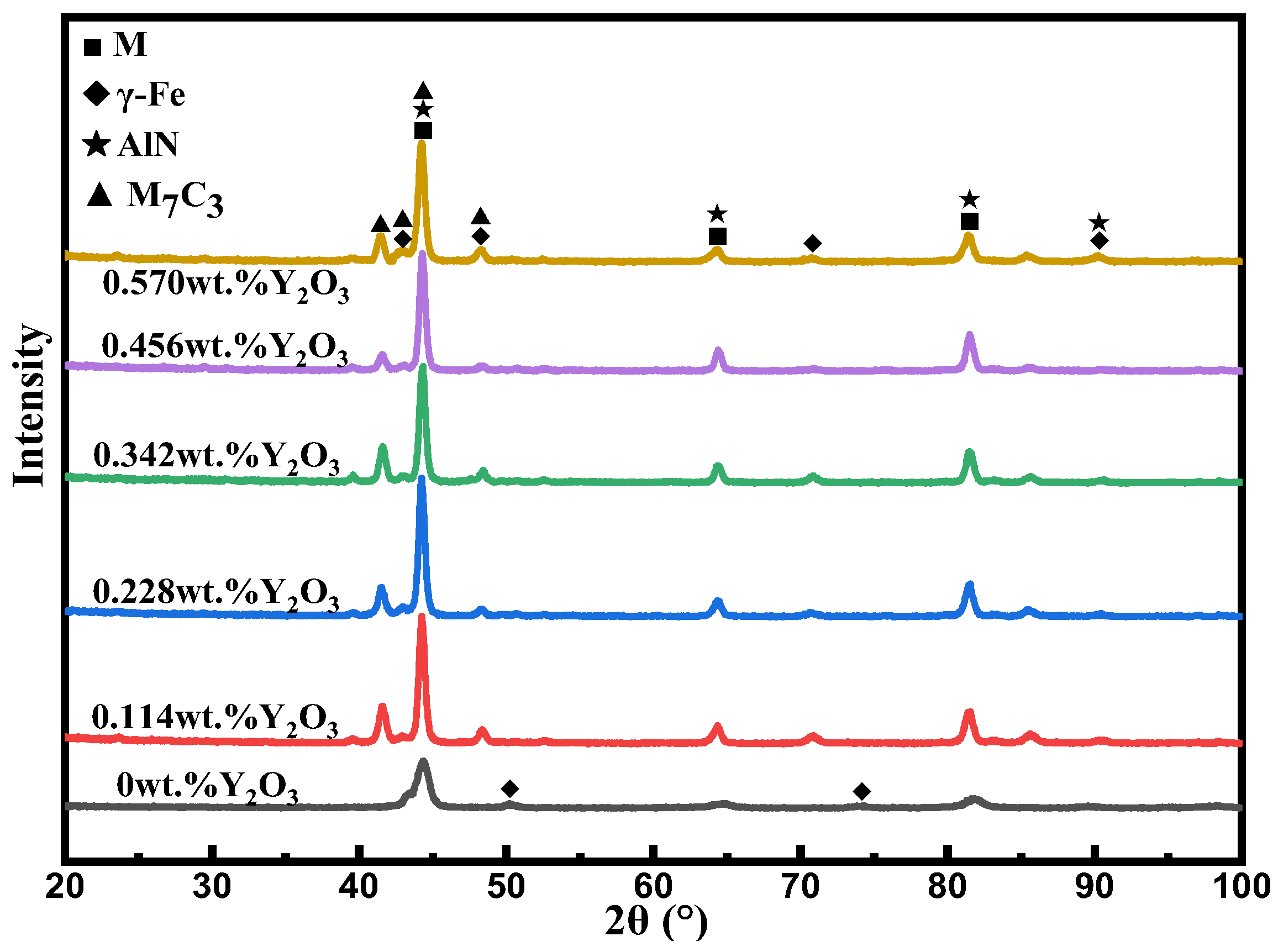
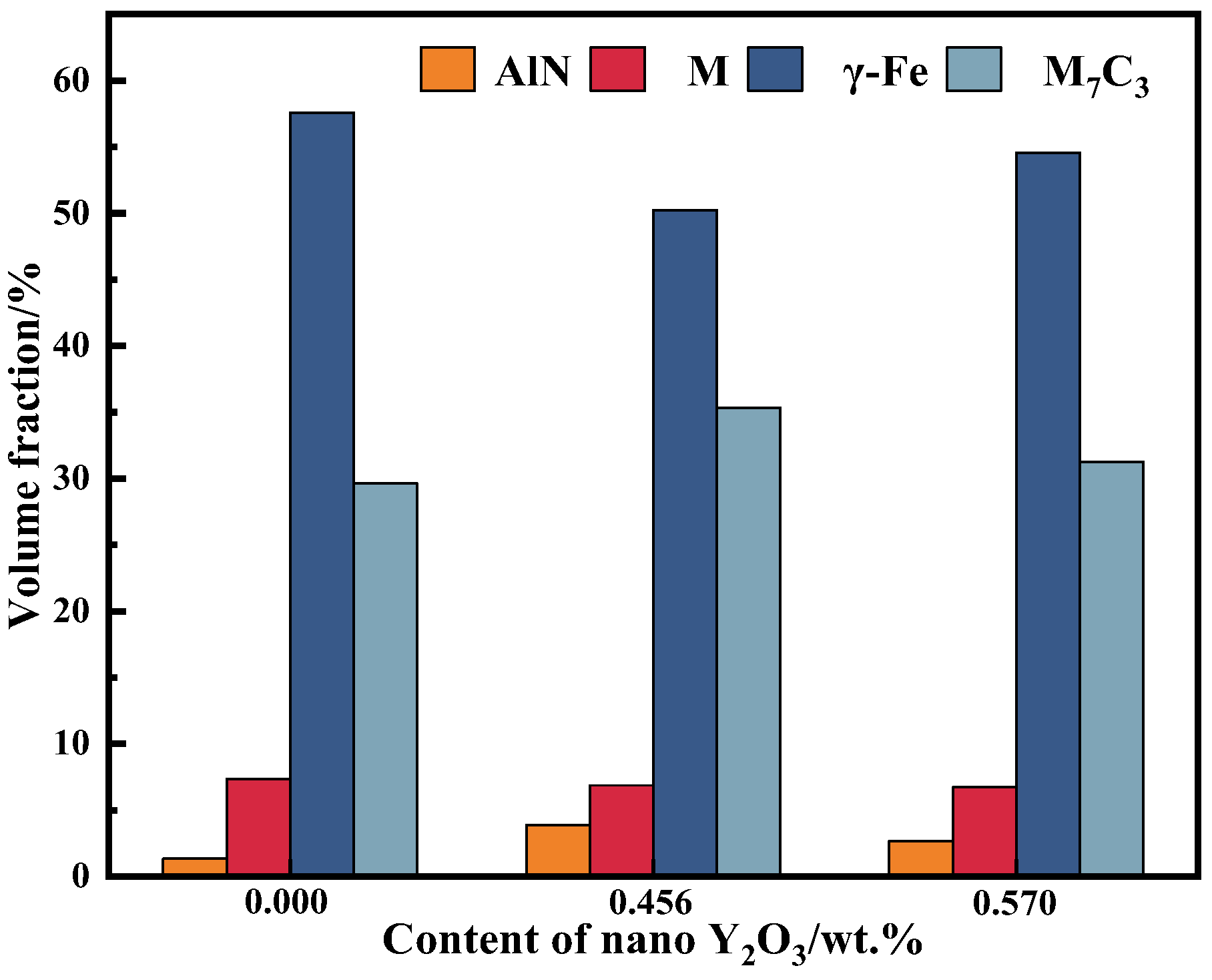
3.2. Phase Analysis
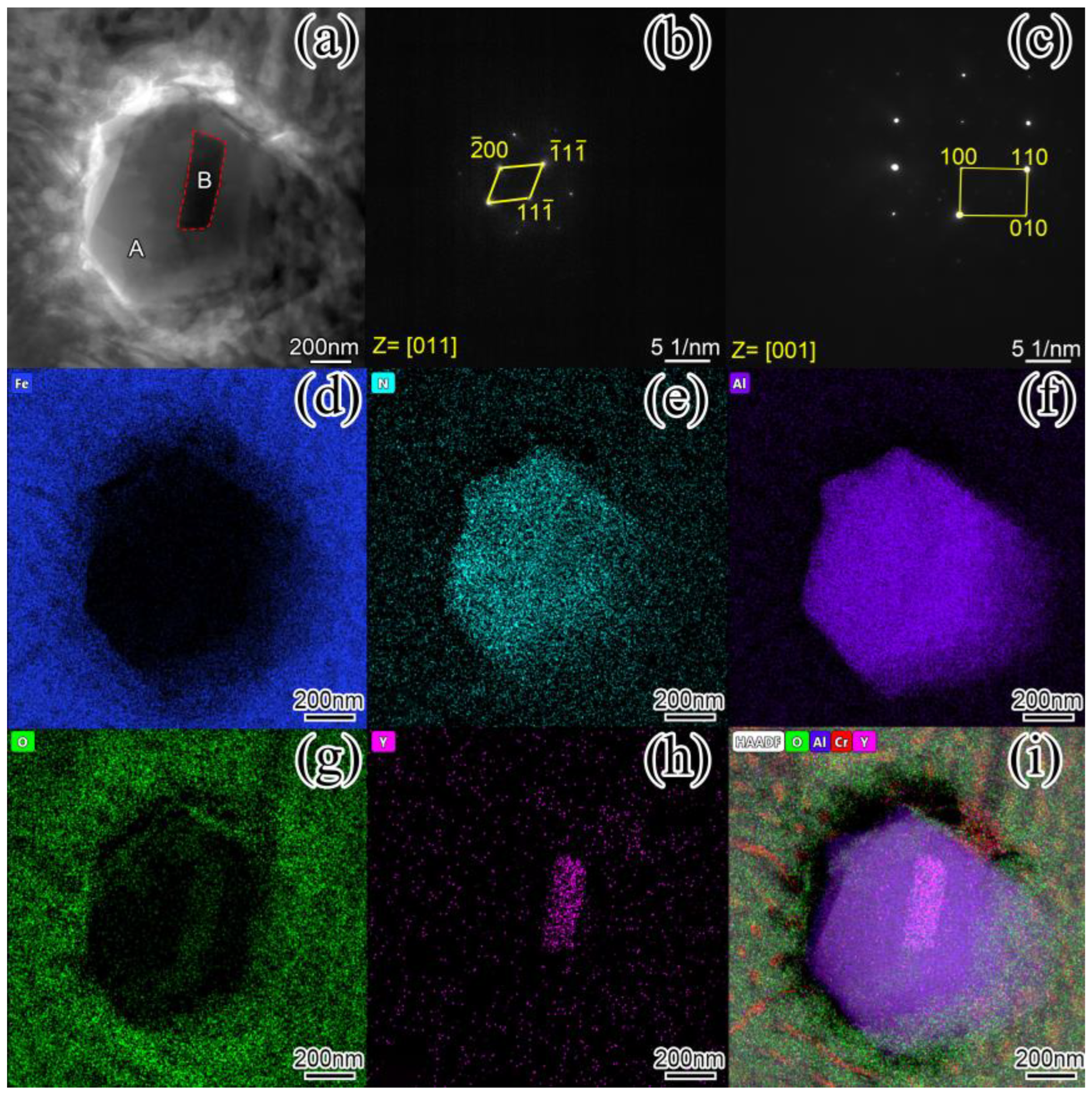
3.3. Microstructure Analysis
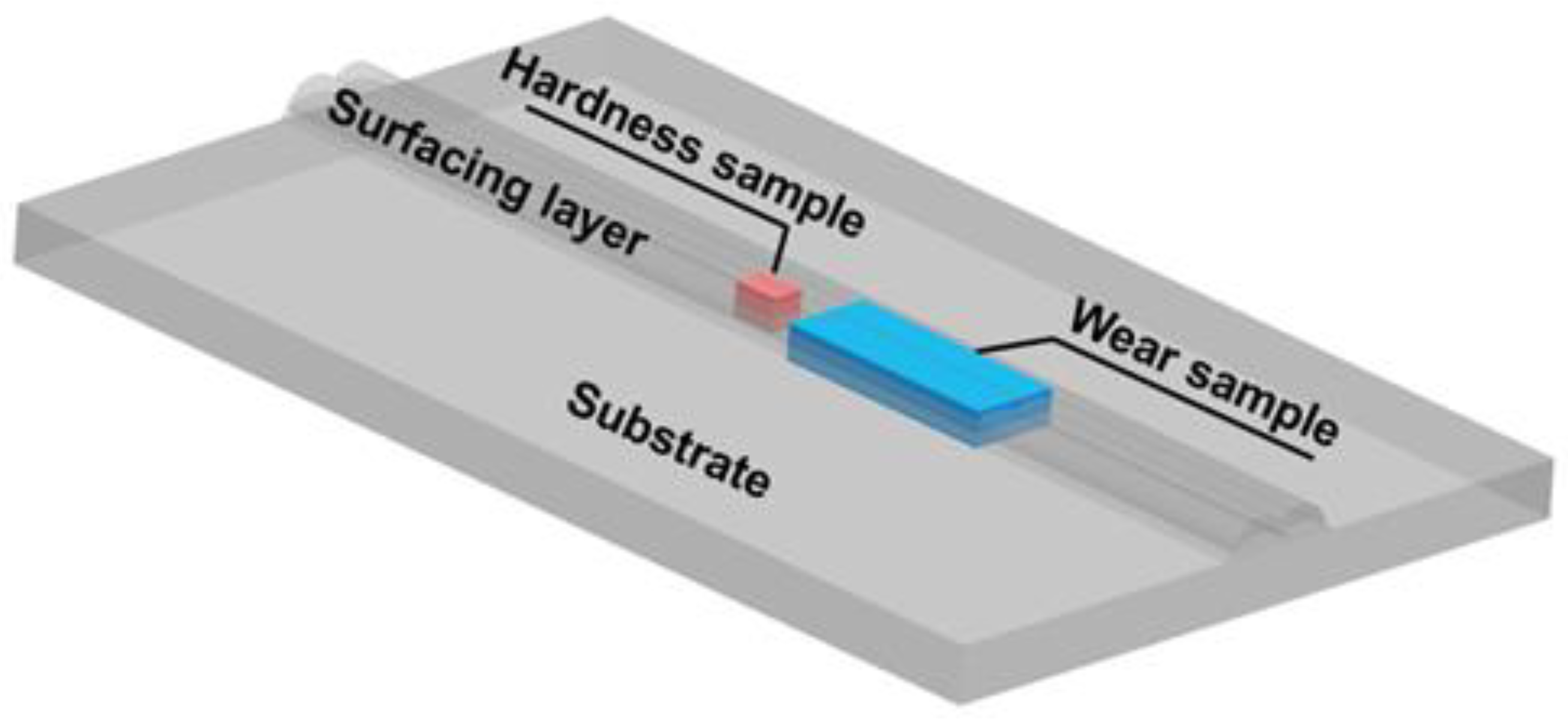
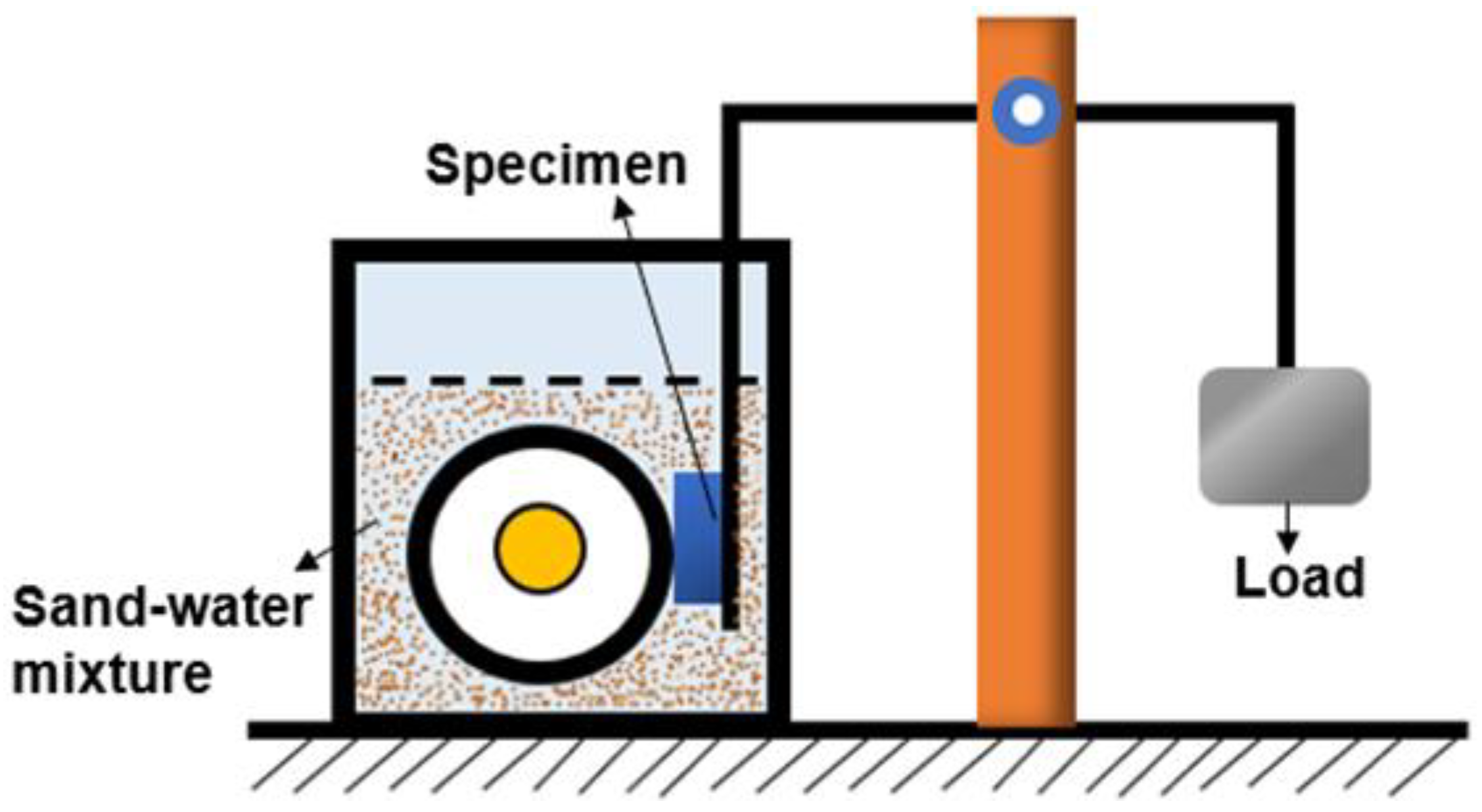
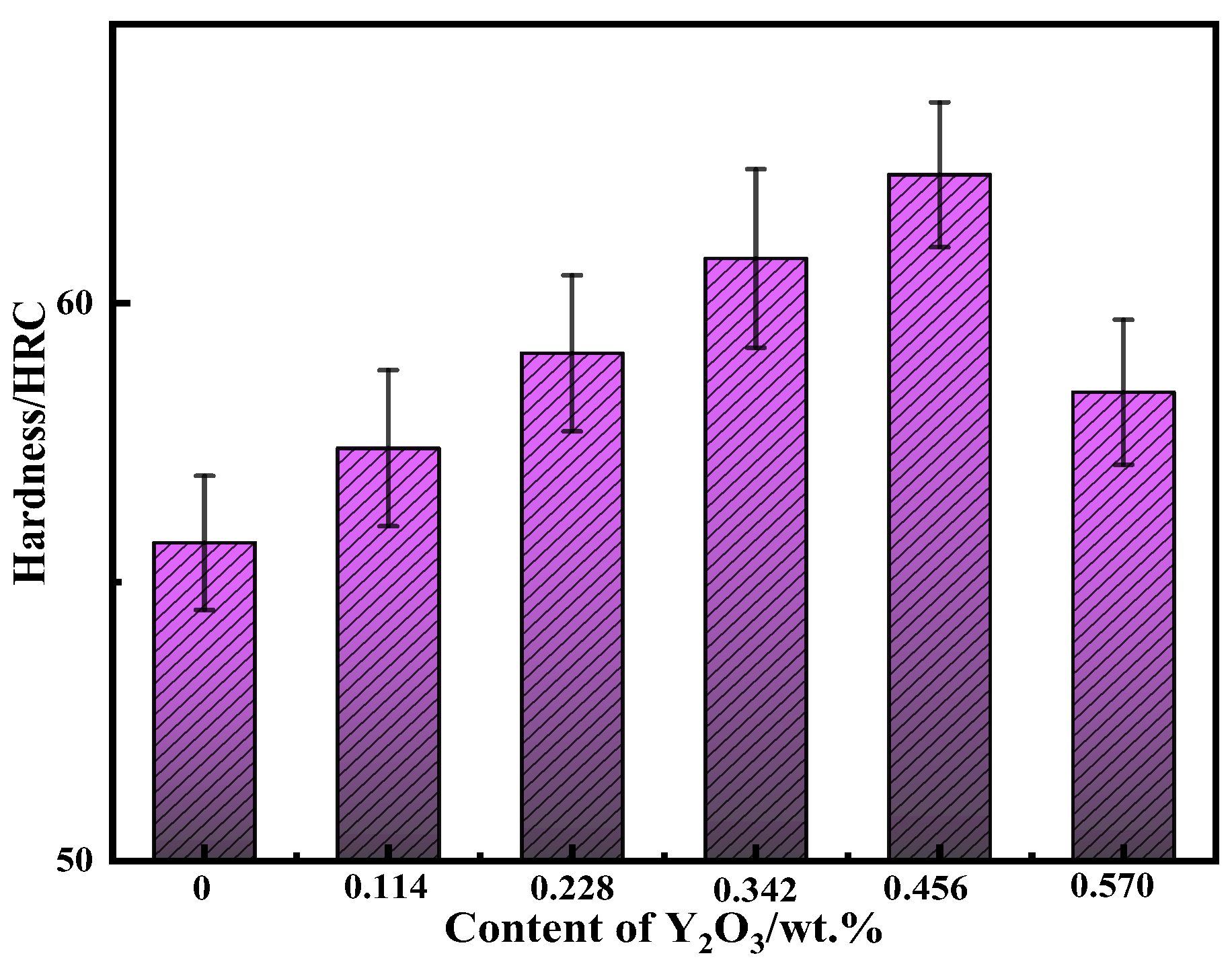
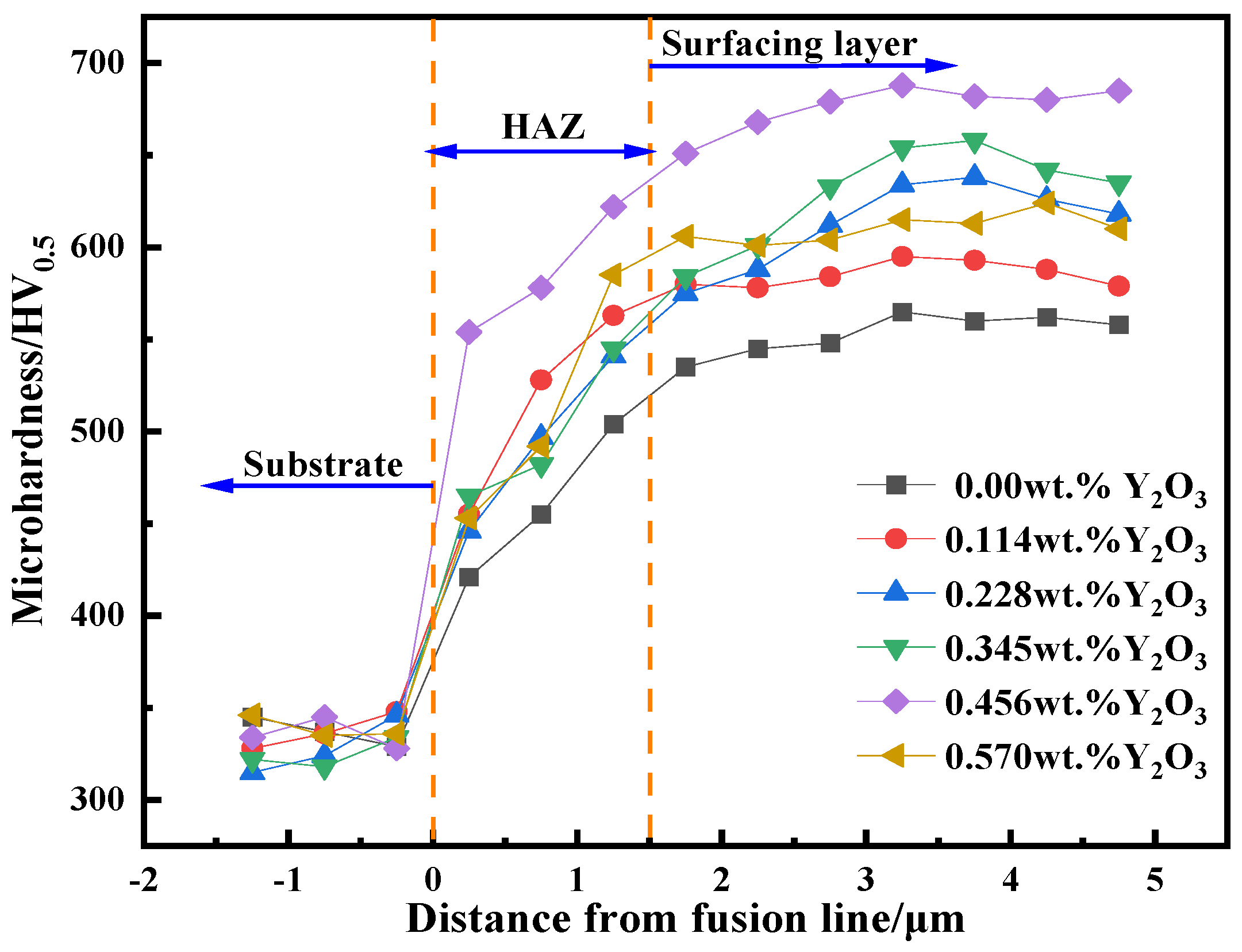
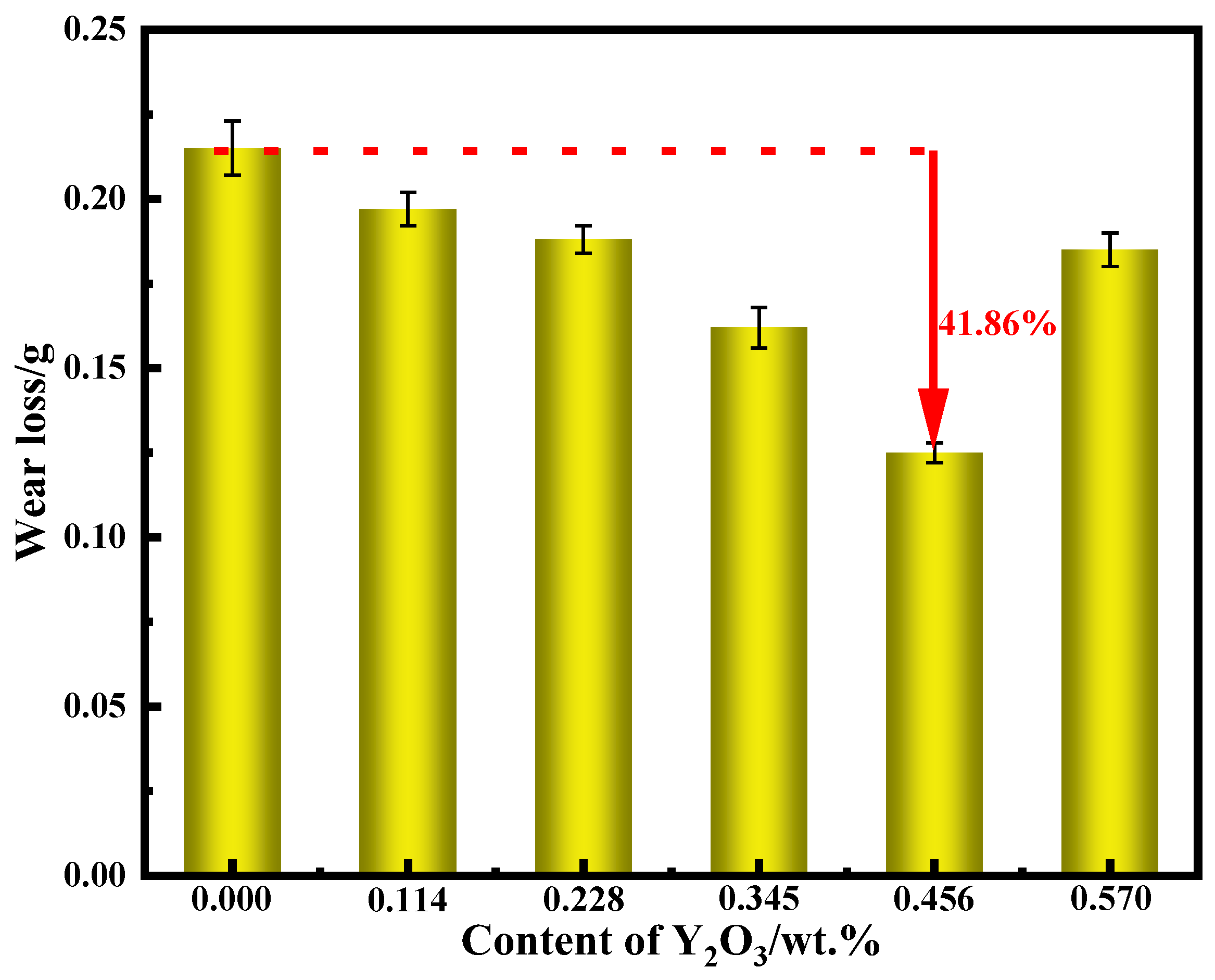
3.4. Analysis of Hardness and Wear Resistance
4. Conclusions
- (1)
- The formability of surfacing alloy with different content of nano-Y2O3 was analyzed. It was found that adding an appropriate amount of nano-Y2O3 can obtain a surfacing alloy with good forming quality. When the nano-Y2O3 content is 0.456 wt.%, the formability of the surfacing alloy is the best, and the wetting angle is the smallest, which is 50.8°.
- (2)
- The addition of nano-Y2O3 does not change the phase composition of the surfacing alloy. The microstructure of the alloy is still M + γ-Fe + M7C3 + AlN. When the content of nano-Y2O3 is 0.456 wt.%, the precipitation of AlN and M7C3 is the most, and the grain size is the smallest.
- (3)
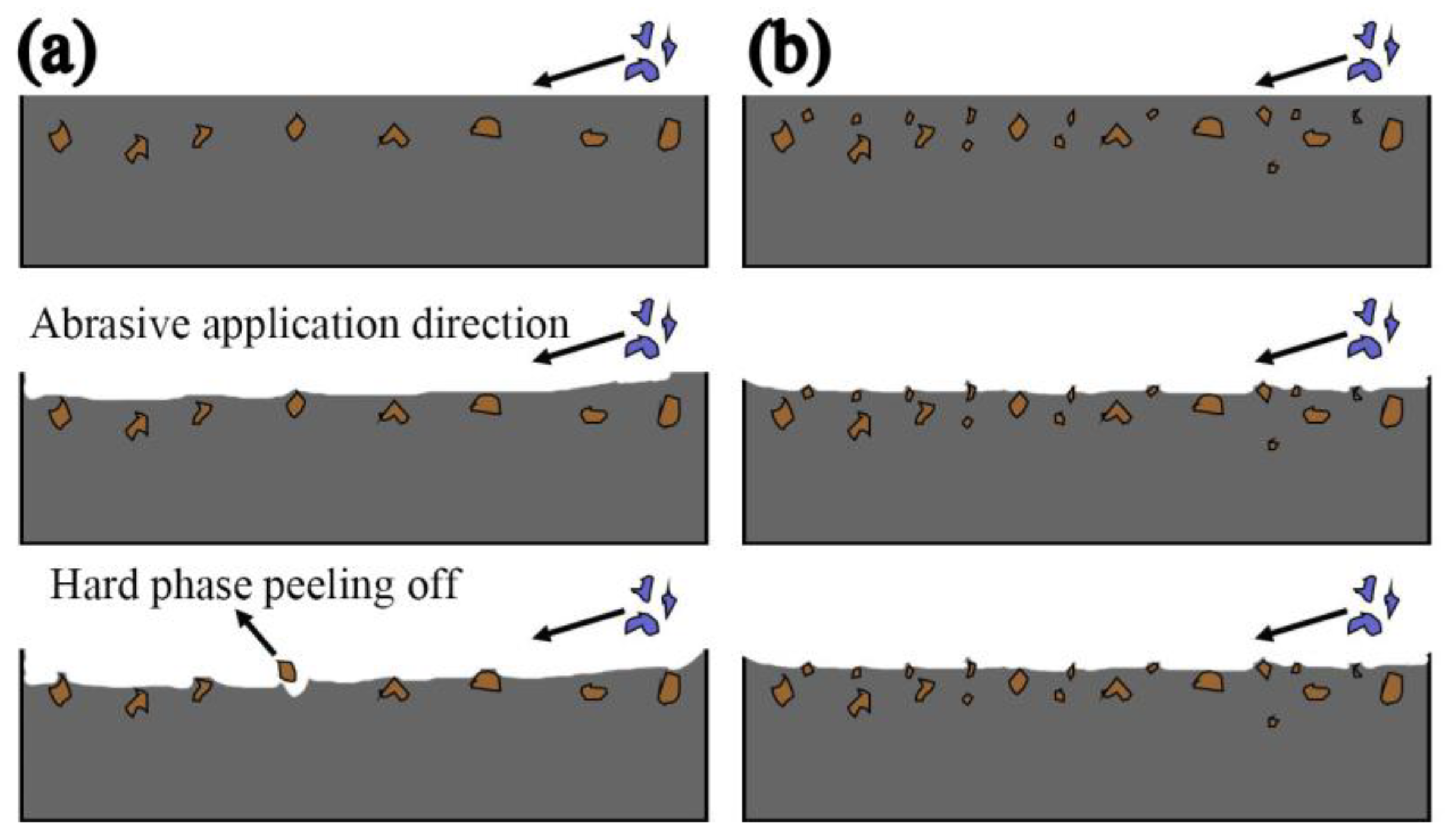
- Compared with no nano-Y2O3, the hardness and wear resistance of the surfacing alloy modified by nano-Y2O3 were significantly improved. When the nano-Y2O3 content is 0.456 wt.%, the hardness of the surfacing alloy is 62.3 HRC, and the Rockwell hardness is increased by 11.8%. The minimum wear weight loss is 0.125 g, and the wear resistance is increased by 41.86%. The wear mechanism of surfacing alloy is mainly a plastic deformation mechanism, and the material removal process comprises microcutting and furrow wear.
- (4)
- After studying the abrasive wear mechanism, it is found that the wear resistance of the surfacing layer is not only affected by the hardness when the external conditions are constant. The size, quantity and distribution of the second phase precipitated in the surfacing alloy play a decisive role in the wear resistance of the surfacing alloy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bembenek, M.; Prysyazhnyuk, P.; Shihab, T.; Machnik, R.; Ivanov, O.; Ropyak, L. Microstructure and Wear Characterization of the Fe-Mo-B-C—Based Hardfacing Alloys Deposited by Flux-Cored Arc Welding. Materials 2022, 15, 5074. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Sousa, J.; Lobato, M.; Garcia, D.; Machado, P. Abrasion resistance of Fe-Cr-C coating deposited by FCAW welding process. Wear 2021, 476, 203688. [Google Scholar] [CrossRef]
- Venkatesh, B.; Sriker, K.; Prabhakar, V. Wear characteristics of hardfacing alloys: State-of-the-art. Procedia Mater. Sci. 2015, 10, 527–532. [Google Scholar] [CrossRef]
- Rojacz, H.; Katsich, C.; Kirchgassner, M.; Kirchmayer, R. Badisch EImpact-abrasive wear of martensitic steels and complex iron-based hardfacing alloys. Wear 2022, 492–493, 204183. [Google Scholar]
- Garbade, R.; Dhokey, N. Effect of mechanical alloying of Ti and B in pre alloyed gas atomized powder on carbide dispersed austenitic matrix of Iron based hardfacing alloy. Mater. Charact. 2022, 191, 112134. [Google Scholar] [CrossRef]
- Günen, A.; Gürol, U.; Koçak, M.; Çam, G. A new approach to improve some properties of wire arc additively manufactured stainless steel components: Simultaneous homogenization and boriding. Surf. Coat. Technol. 2023, 460, 129395. [Google Scholar] [CrossRef]
- Günen, A.; Gürol, U.; Koçak, M.; Çam, G. Investigation into the influence of boronizing on the wear behavior of additively manufactured Inconel 625 alloy at elevated temperature. Prog. Addit. Manuf. 2023. [Google Scholar] [CrossRef]
- Buytoz, S. Microstructural properties of M7C3 eutectic carbides in a Fe-Cr-C Alloy. Mater. Lett. 2006, 60, 605–608. [Google Scholar] [CrossRef]
- Fan, C.; Chen, M.; Chia Chang, M.; Wu, W. Microstructure Change Caused by (Cr, Fe)23C6 Carbides in High Chromium Fe-Cr-C Hardfacing Alloys. Surf. Coat. Technol. 2006, 201, 908–912. [Google Scholar] [CrossRef]
- Yang, K.; Xie, X.; Bao, Y. Effect of niobium and titanium carbonitride precipitates on the high-temperature wear behavior of hardfacing alloy. Tribology 2010, 30, 15–19. [Google Scholar]
- Ke, Y.; Qin, Y.; Bao, Y. Effect of carbonitride precipitates on the solid/liquid erosion behaviour of hardfacing alloy. Appl. Surf. Sci. 2013, 284, 540–544. [Google Scholar]
- Wei, S.; Liu, Y.; Zhang, G.; Xu, L.; Li, J.; Ren, Y. Microstructure and Wear Resistance of Fe-Cr-C Hardfacing Alloy Reinforced by Titanium Carbonitride. Tribol. Trans. 2015, 58, 745–749. [Google Scholar] [CrossRef]
- Amuda, M.; Akinlabi, E.T.; Moolla, M. Microstructure evolution in Ti6Al4V alloy laser cladded with Premix Ti + TiB2 powders. Mater. Today Proc. 2017, 4, 763–7736. [Google Scholar]
- Li, M.; Huang, J.; Zhu, Y. Effect of heat input on the microstructure of in-situ synthesized TiN-TiB/Ti based composite coating by laser cladding. Surf. Coat. Technol. 2012, 206, 4021–4026. [Google Scholar] [CrossRef]
- Qi, C.; Zhan, X.; Gao, Q. The influence of the pre-placed powder layers on the morphology, microscopic characteristics and microhardness of Ti-6Al-4V/WC MMC coatings during laser cladding. Opt. Laser Technol. 2019, 119, 1055721–1055729. [Google Scholar] [CrossRef]
- Li, Q.; Lei, Y.; Fu, H. Growth Characteristics and Reinforcing Behavior of In-situ NbCp in Laser Cladded Fe-based Composite Coating. J. Mater. Sci. Technol. 2015, 31, 766–772. [Google Scholar] [CrossRef]
- Li, J.; Luo, X.; Li, G. Effect of Y2O3 on the sliding wear resistance of TiB/TiC-reinforced composite coatings fabricated by laser cladding. Wear 2014, 310, 72–82. [Google Scholar] [CrossRef]
- Debta, M.K.; Masanta, M. Effect of nano-Y2O3 on the microstructure and wear behaviour of TIG cladded TiC-Co-nY2O3 coating. Int. J. Refract. Met. H. 2023, 111, 106078. [Google Scholar] [CrossRef]
- Shu, D.; Cui, X.; Li, Z. Effect of the Rare Earth Oxide CeO2 on the Microstructure and Properties of the Nano-WC-Reinforced Ni-Based Composite Coating. Metals 2020, 10, 383. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, Y.; Zou, Z.D.; Wu, D.T. Microstructure and properties of Fe-based composite coating by laser cladding Fe-Ti-V-Cr-C-CeO2 powder. Opt. Laser Technol. 2015, 65, 119–125. [Google Scholar] [CrossRef]
- Zhang, T.; Xiao, H.; Zhang, Z. Effect of Y2O3 Addition on Microstructural Characteristics and Microhardness of Laser-Cladded Ti-6Al-4V Alloy Coating. J. Mater. Eng. Perform. 2020, 29, 8221–8235. [Google Scholar] [CrossRef]
- Li, M.; Zhang, S.; Li, H. Effect of nano-CeO2 on cobalt-based alloy laser coatings. J. Mater. Process. Technol. 2008, 202, 107–111. [Google Scholar] [CrossRef]
- Zhang, K.; Zou, J.; Jun, L. Synthesis of Y2O3 particle enhanced Ni/TiC composite on TC4 Ti alloy by laser cladding. Trans. Nonferrous Met. Soc. China 2012, 22, 1817–1823. [Google Scholar] [CrossRef]
- Chen, J. Effects of CeO2 on Microstructural Evolution, Corrosion and Tribology Behavior of Laser Cladded TiC Reinforced Co-based Coatings. Int. J. Electrochem. Sci. 2021, 16, 210511. [Google Scholar] [CrossRef]
- Yun, X.; Zhou, Y.; Zhao, B. Influence of Nano-Y2O3 on Wear Resistance of Hypereutectic Fe-Cr-C Hardfacing Coating. Tribol. Lett. 2015, 58, 23. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, K.S.; Inoue, M. Effects of Ag and Cu addition on microstructure properties and oxidation resistance of Sn-Zn eutectic alloy. J. Alloys Compd. 2008, 454, 310–320. [Google Scholar] [CrossRef]
- Chen, C.M.; Chen, C.H. Interfacial Reactions between Eutectic SnZn Solder and Bulk or Thin-Film Cu Substrates. J. Electron. Mater. 2007, 36, 1363–1371. [Google Scholar] [CrossRef]
- Sharif, A.; Chan, Y.C. Effect of substrate metallization on interfacial reactions and reliability of Sn-Zn-Bi solder joints. Microelectron. Eng. 2007, 84, 328–335. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wang, T.H. Effects of Rare Earth Y2O3 on the Microstructure and Wear Behaviors of Ti-6Al-4V Cladding with SiC. Mater. Sci. Forum 2018, 934, 89–94. [Google Scholar] [CrossRef]
- Liu, W.; Tong, W.; Lu, X. Effects of different types of rare earth oxide additives on the properties of silicon nitride ceramic substrates. Ceram. Int. 2019, 45, 12436–12442. [Google Scholar] [CrossRef]
- Attinger, D.; Zhao, Z.; Poulikakos, D. An Experimental Study of Molten Microdroplet Surface Deposition and Solidification: Transient Behavior and Wetting Angle Dynamics. J. Heat Transf. 2000, 122, 544–556. [Google Scholar] [CrossRef]
- Zhu, R.D.; Li, Z.Y.; Li, X.X. Microstructure and properties of the low-power-laser clad coatings on magnesium alloy with different amount of rare earth addition. Appl. Surf. Sci. 2015, 353, 405–413. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.H.; Qu, K.L. Effect of Rare Earth Oxide on Microstructure and High Temperature Oxidation Properties of Laser Cladding Coatings on 5CrNiMo Die Steel Substrate. Opt. Laser Technol. 2019, 119, 105597. [Google Scholar] [CrossRef]
- Li, X.F.; Feng, Y.H.; Liu, B. Influence of NbC Particles on Microstructure and Mechanical Properties of AlCoCrFeNi High-Entropy Alloy Coatings Prepared by Laser Cladding. J. Alloys Compd. 2019, 788, 485–494. [Google Scholar] [CrossRef]
- Sun, S.T.; Fu, H.G.; Ping, X.L. Effect of CeO2 Addition on Microstructure and Mechanical Properties of in-situ (Ti, Nb)C/Ni Coating. Surf. Coat. Technol. 2019, 359, 300–313. [Google Scholar] [CrossRef]
- Wang, C.L.; Gao, Y.; Wang, R. Microstructure of Laser-Clad Ni60 Cladding Layers Added with Different Amounts of Rare-Earth Oxides on 6063 Al Alloys. J. Alloys Compd. 2018, 740, 1099–1107. [Google Scholar] [CrossRef]
- Ma, S.Q.; Xing, J.D.; He, Y.L.; Li, Y.F.; Huang, Z.F.; Liu, G.Z.; Geng, Q.J. Microstructure and crystallography of M7C3 carbide in chromium cast iron. Mater. Chem. Phys. 2015, 161, 65–73. [Google Scholar] [CrossRef]
- Wen, Y.; Guan, B.; Xin, Y. Solute atom mediated Hall-Petch relations for magnesium binary alloys. Scr. Mater. 2022, 210, 114451. [Google Scholar] [CrossRef]
- Hou, Q.Y.; Huang, Z.Y.; Gao, J.S. Effects of Y2O3 on the microstructure and wear resistance of cobalt-based alloy coatings deposited by plasma transferred arc process. Rare Earth 2007, 26, 103–109. [Google Scholar] [CrossRef]
- Yang, J.; Hou, X.R.; Zhang, P.; Zhou, Y.F.; Yang, Y.L.; Ren, X.J.; Yang, Q.X. Mechanical properties of the hypereutectoid Fe-Cr-C hardfacing coatings with different nano-Y2O3 additives and the mechanism analysis. Mater. Sci. Eng. A 2016, 655, 346–354. [Google Scholar] [CrossRef]
- Chang, C.M.; Chen, Y.C.; Wu, W. Microstructural and abrasive characteristics of high carbon Fe-Cr-C hardfacing alloy. Tribol. Int. 2010, 43, 929–934. [Google Scholar] [CrossRef]






| C | Si | Mn | S | p | Fe | |
|---|---|---|---|---|---|---|
| Q235 | 0.14–0.22 | ≤0.30 | 0.30–0.65 | <0.045 | <0.045 | Bal |
| H08A | <0.01 | ≤0.03 | 0.30–0.55 | ≤0.03 | ≤0.03 | Bal |
| CaF2 | SiO2 | CaO | MgO | Al2O3 | |
|---|---|---|---|---|---|
| Content | 1.2–1.5 | 0.8–1.0 | 0.3–0.5 | 0.2–0.5 | 1.0–1.5 |
| Number | C | Cr | N | Al | Y | Fe |
|---|---|---|---|---|---|---|
| 0 | 2.67 | 15.32 | 0.39 | 3.24 | — | Bal. |
| 1 | 2.43 | 13.85 | 0.39 | 3.63 | <0.01 | Bal. |
| 2 | 2.66 | 13.61 | 0.41 | 3.69 | <0.01 | Bal. |
| 3 | 2.58 | 15.31 | 0.45 | 3.84 | 0.02 | Bal. |
| 4 | 2.71 | 16.21 | 0.51 | 3.81 | 0.03 | Bal. |
| 5 | 2.57 | 14.87 | 0.48 | 3.79 | 0.04 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, X.; Liu, Z.; Zou, Z. Effect of Nano-Y2O3 on the Microstructure and Properties of Fe-Cr-C-N-Al Surfacing Alloy. Crystals 2023, 13, 1023. https://doi.org/10.3390/cryst13071023
Ai X, Liu Z, Zou Z. Effect of Nano-Y2O3 on the Microstructure and Properties of Fe-Cr-C-N-Al Surfacing Alloy. Crystals. 2023; 13(7):1023. https://doi.org/10.3390/cryst13071023
Chicago/Turabian StyleAi, Xingyu, Zhengjun Liu, and Zongxuan Zou. 2023. "Effect of Nano-Y2O3 on the Microstructure and Properties of Fe-Cr-C-N-Al Surfacing Alloy" Crystals 13, no. 7: 1023. https://doi.org/10.3390/cryst13071023
APA StyleAi, X., Liu, Z., & Zou, Z. (2023). Effect of Nano-Y2O3 on the Microstructure and Properties of Fe-Cr-C-N-Al Surfacing Alloy. Crystals, 13(7), 1023. https://doi.org/10.3390/cryst13071023






