Crystal Nanoarchitectonics and Characterization of the Octahedral Iron(III)–Nitrate Complexes with Isomer Dimethylurea Ligands
Abstract
:1. Introduction
2. Materials and Methods
2.1. [Hexakis(N,N’-dimethyurea-O)iron(III)] Nitrate (Compound 1)
2.2. [Diaquatetrakis(N,N-dimethyurea-O)iron(III)] Nitrate (Compound 2) and [Hexakis(N,N-dimethyurea-O)iron(III)] Nitrate Trihidrate (Compound 3)
2.3. Powder X-ray Diffractometry
2.4. Single Crystal X-ray Diffraction
2.5. Infrared Spectroscopy
2.6. Raman Spectroscopy
2.7. UV–Vis Spectroscopy
3. Results and Discussion
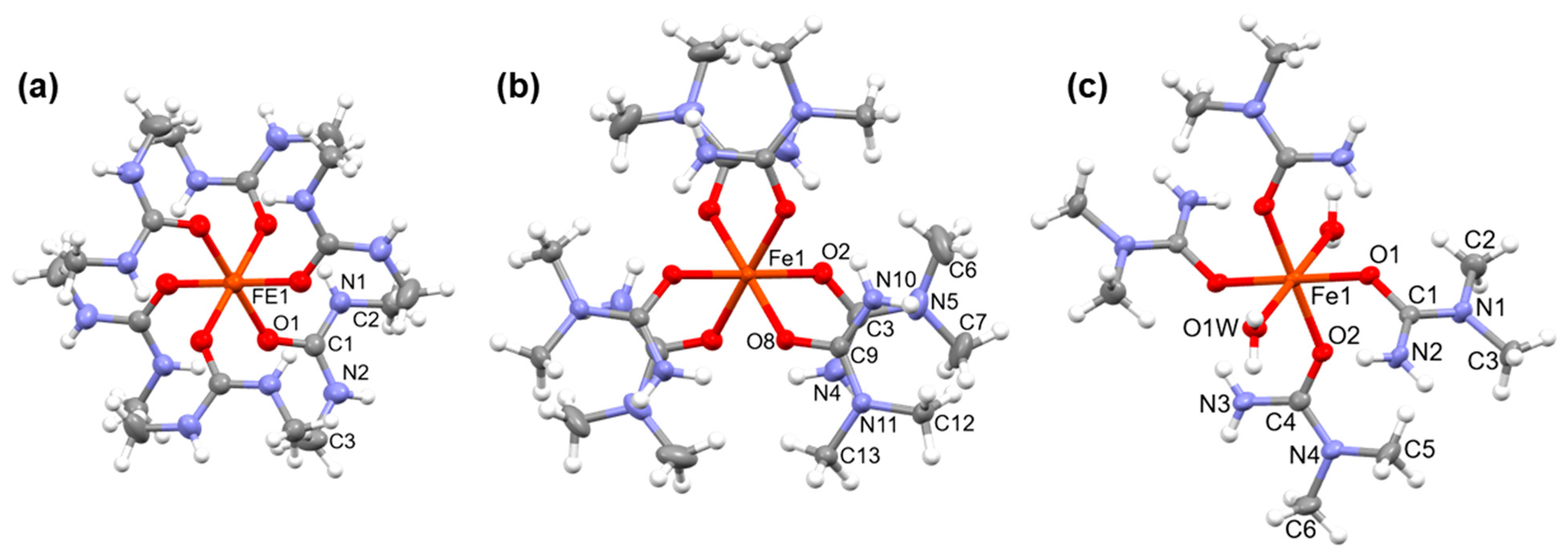
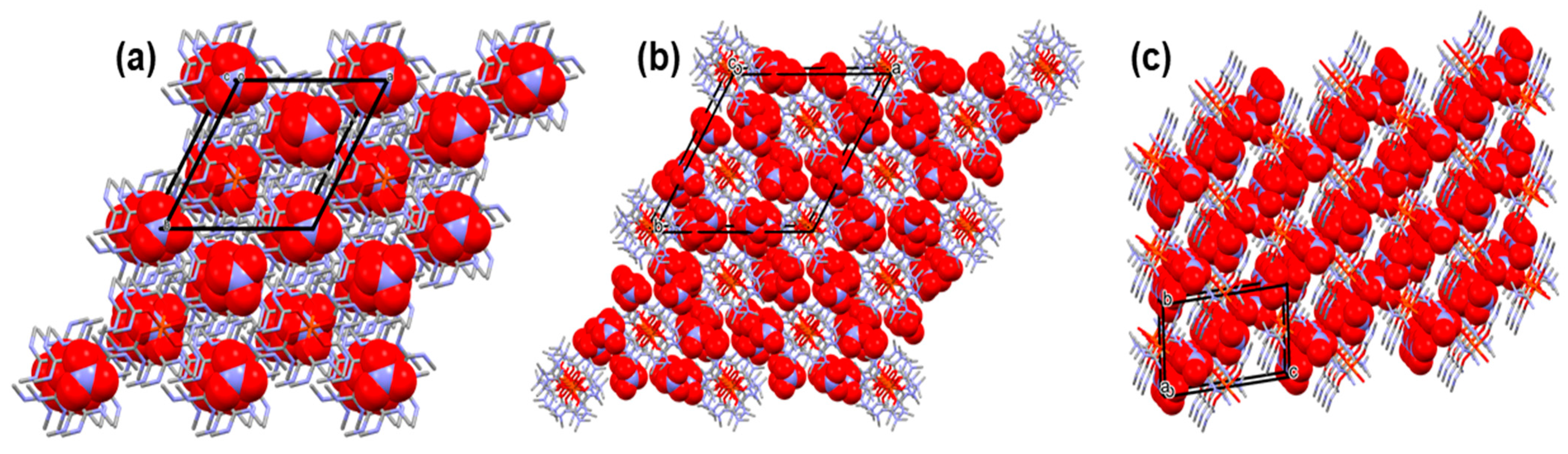
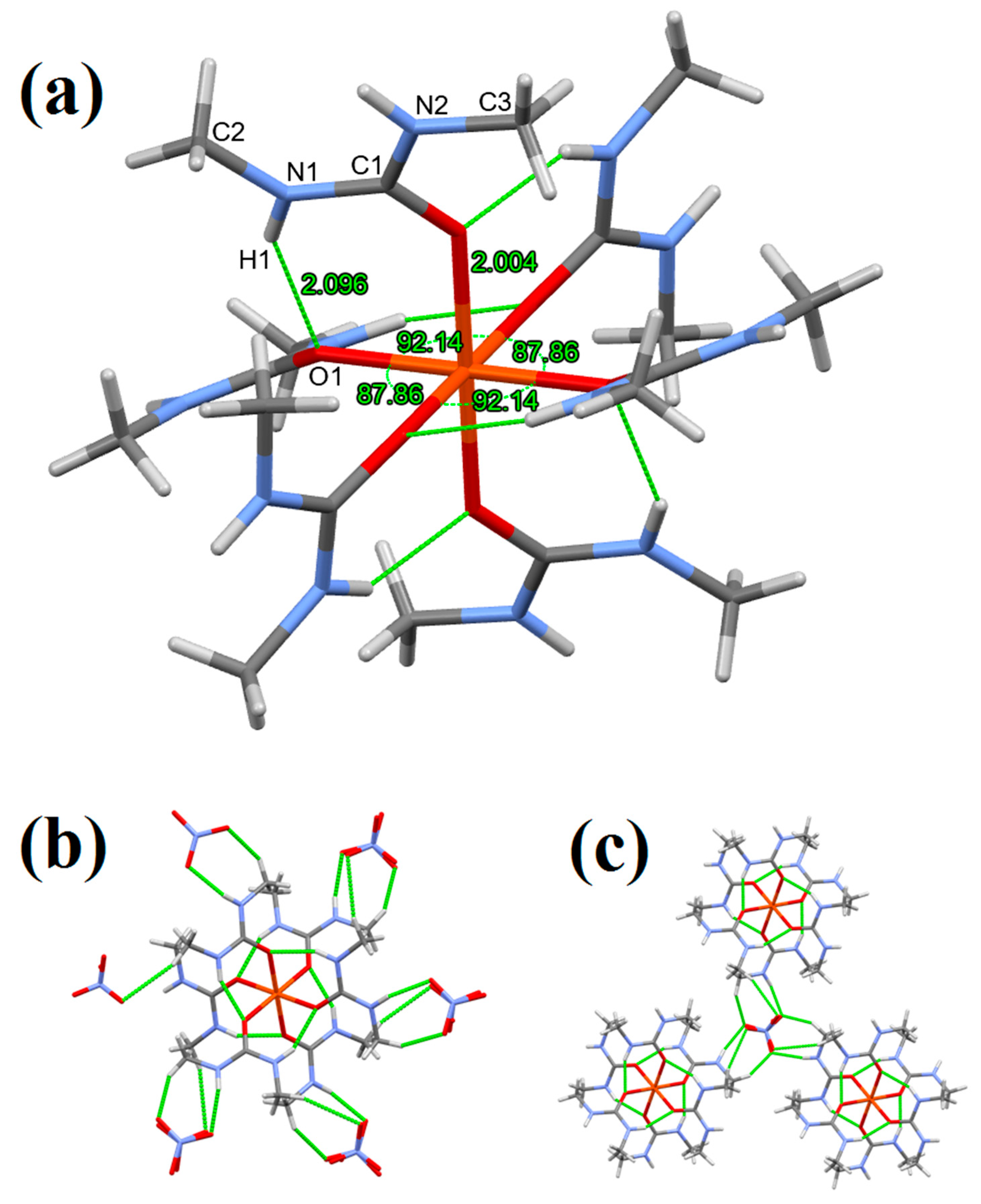
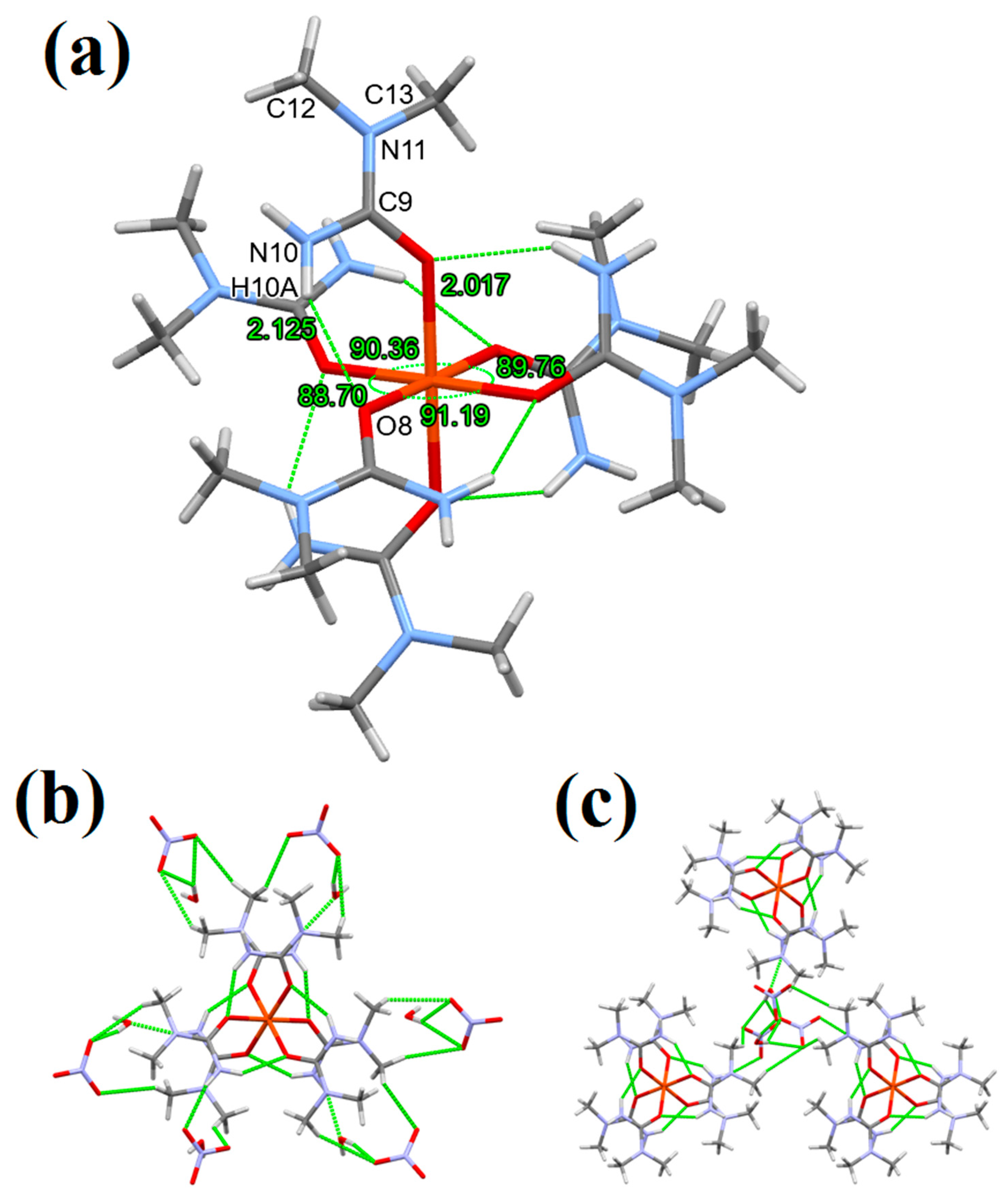
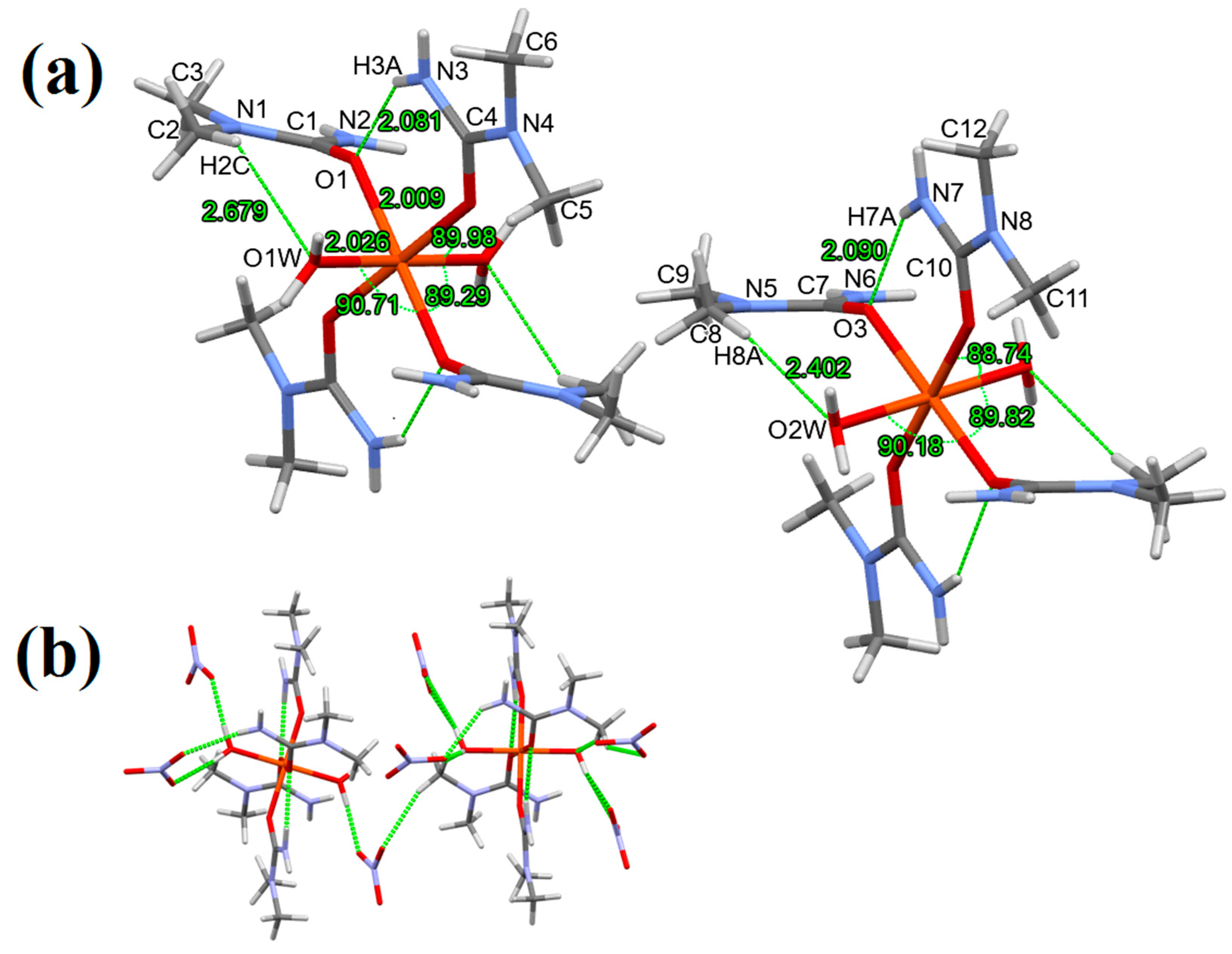
3.1. Single Crystal Structure of Compounds 1–3
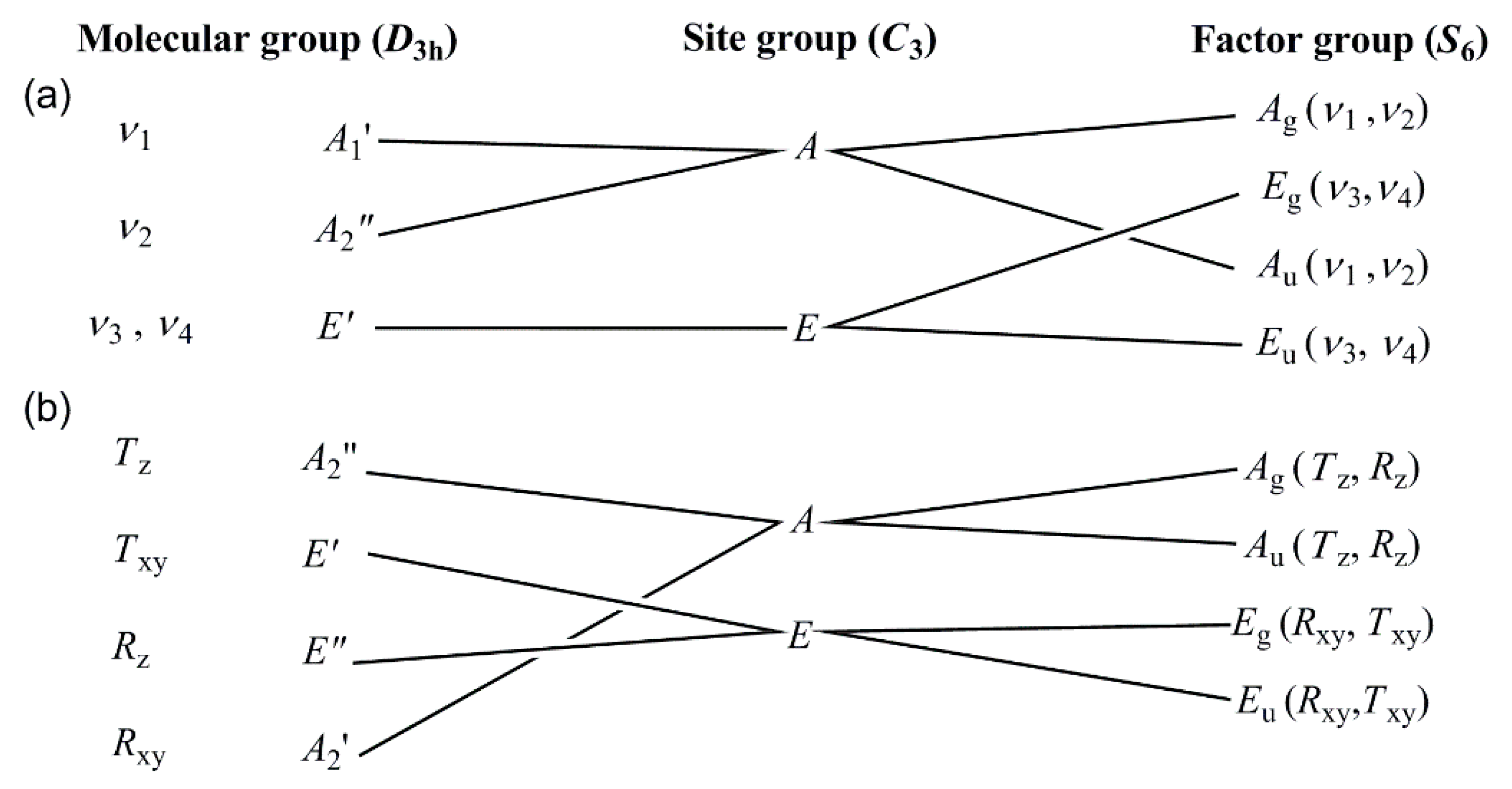
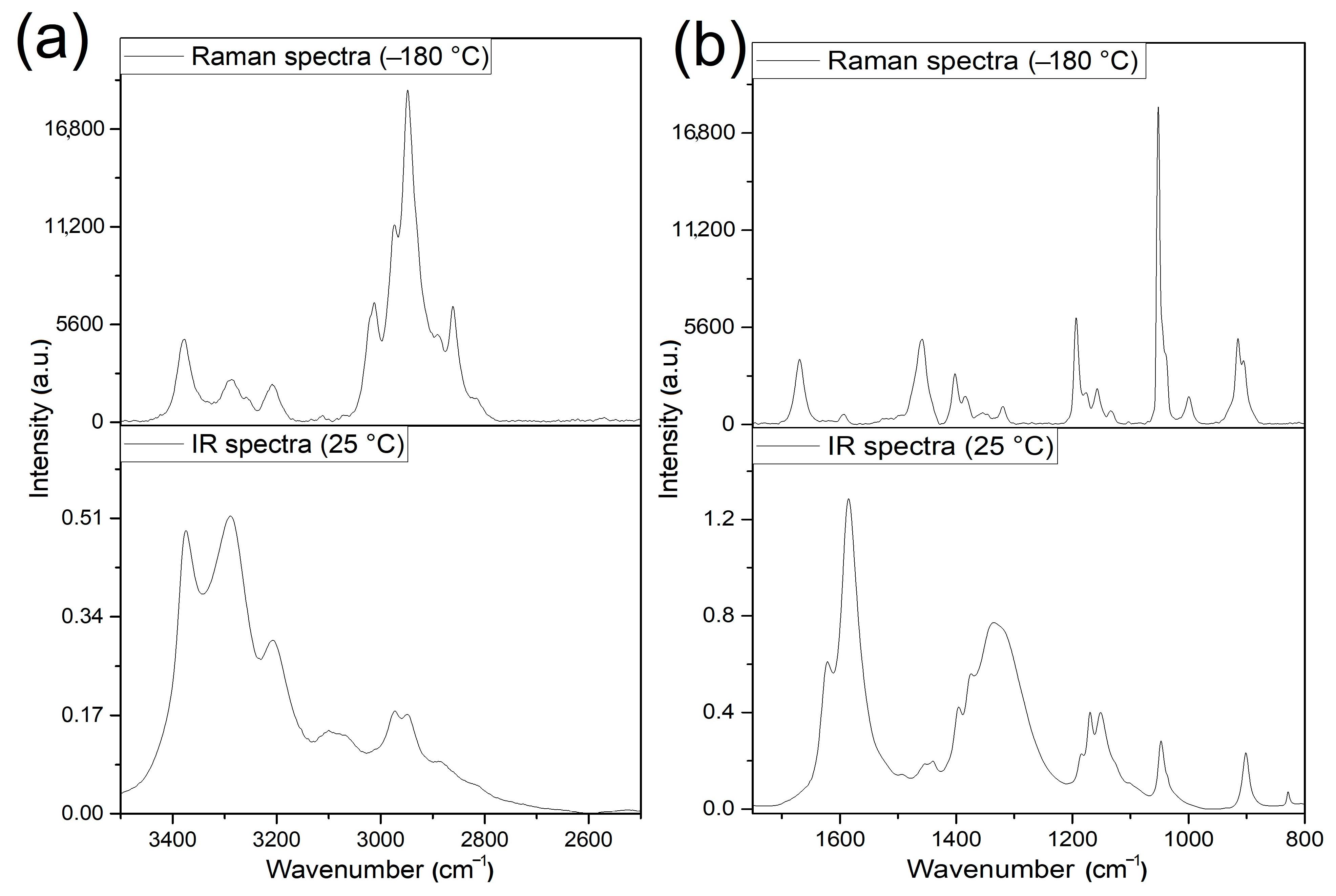
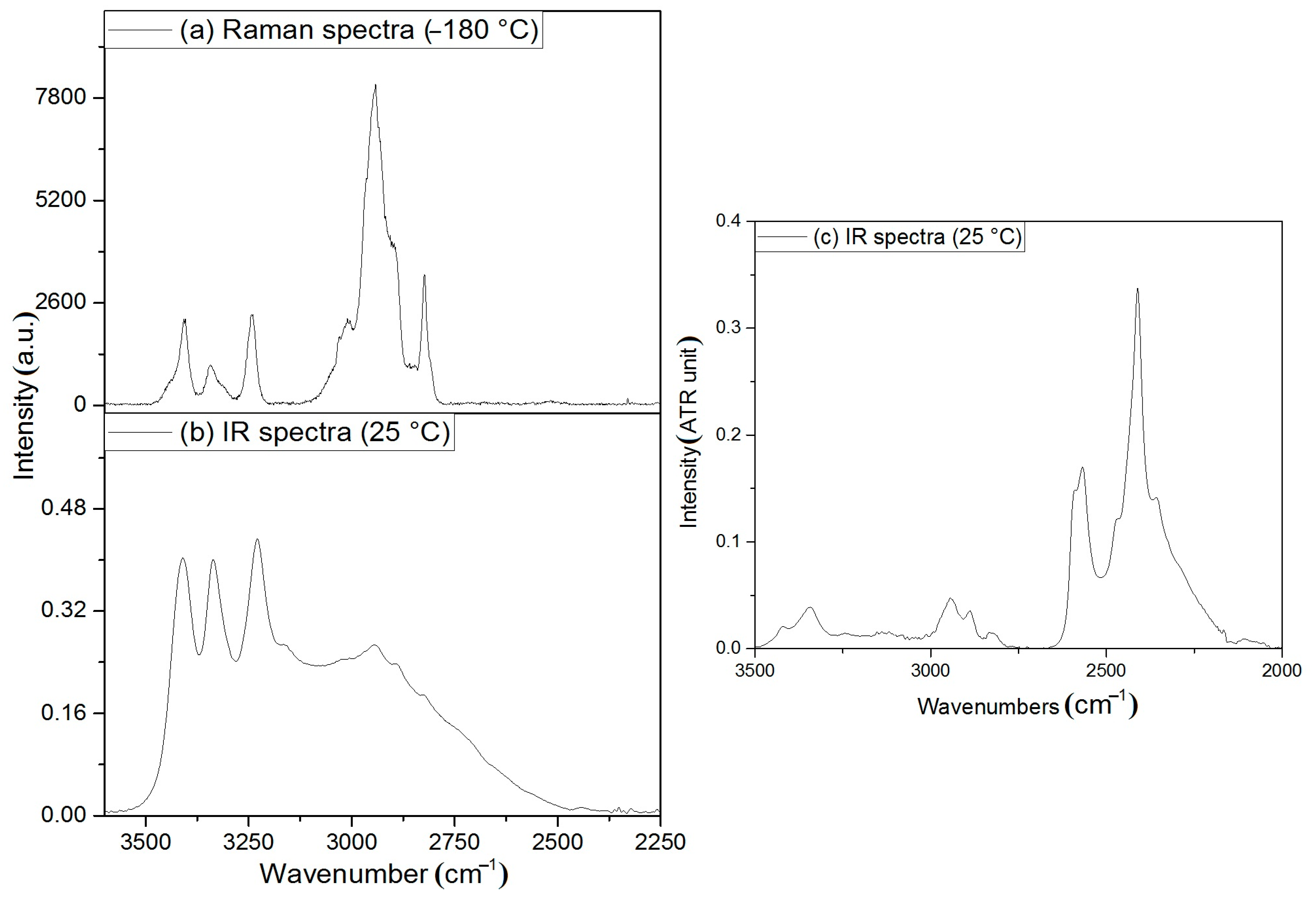
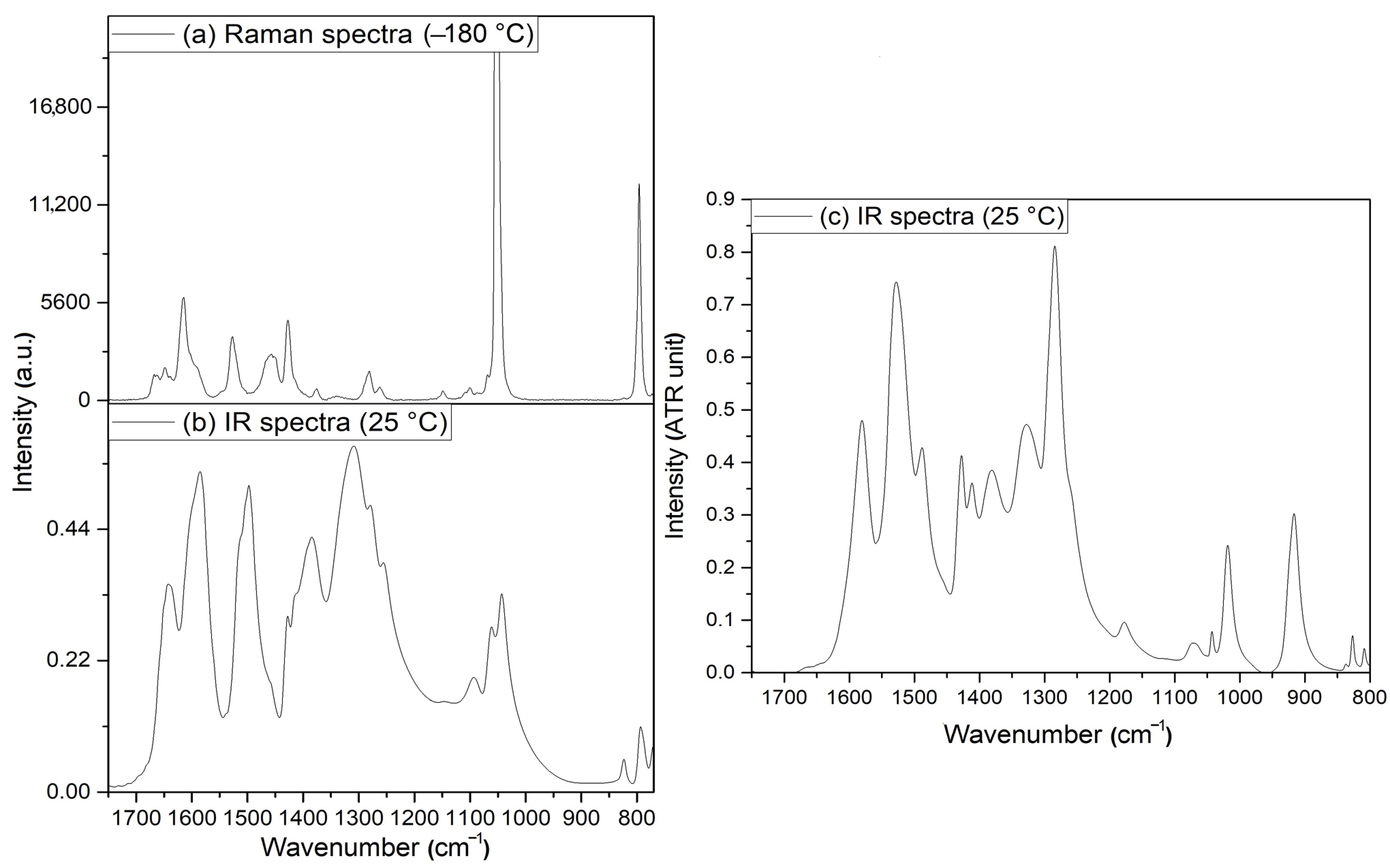
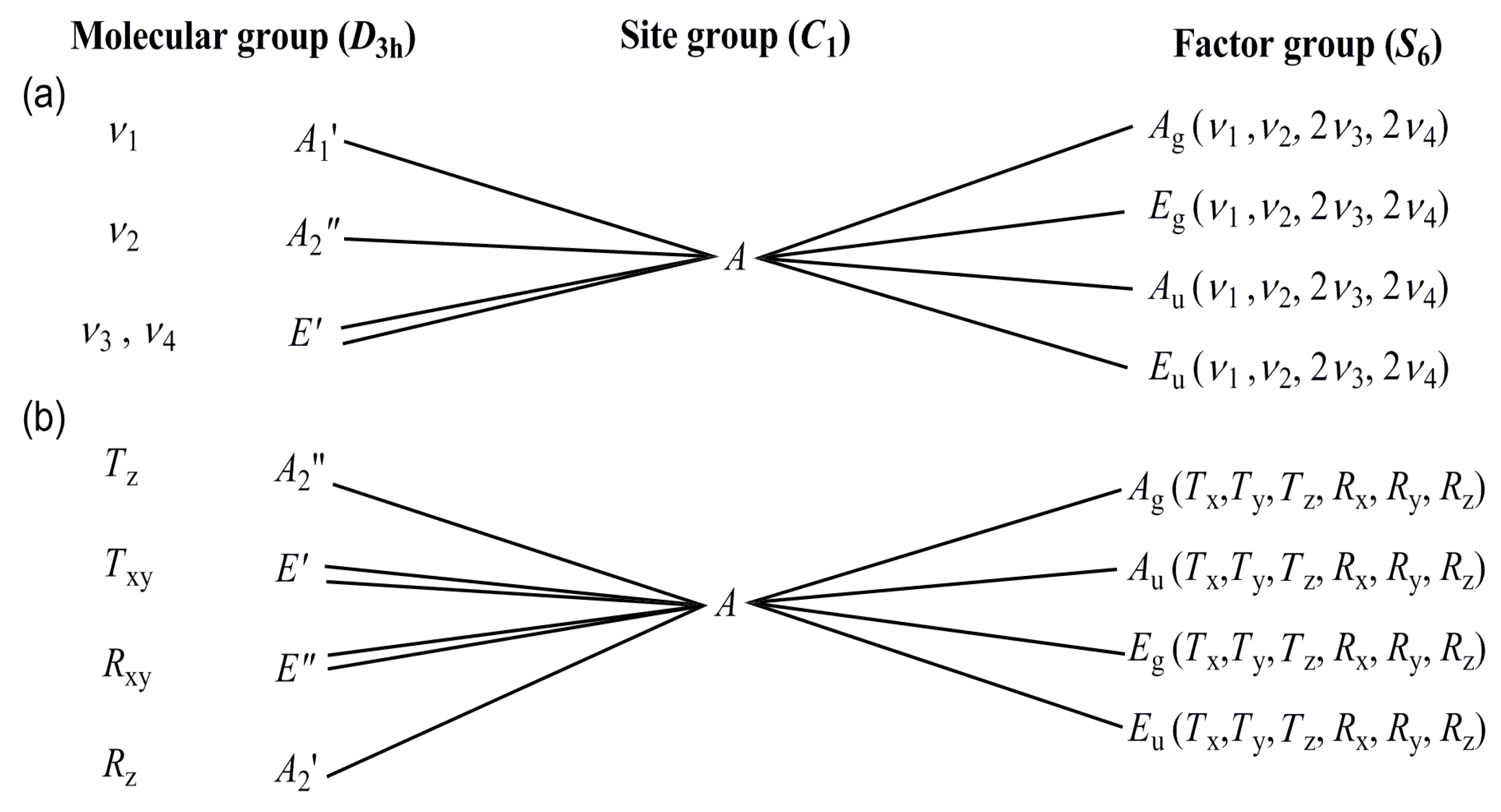
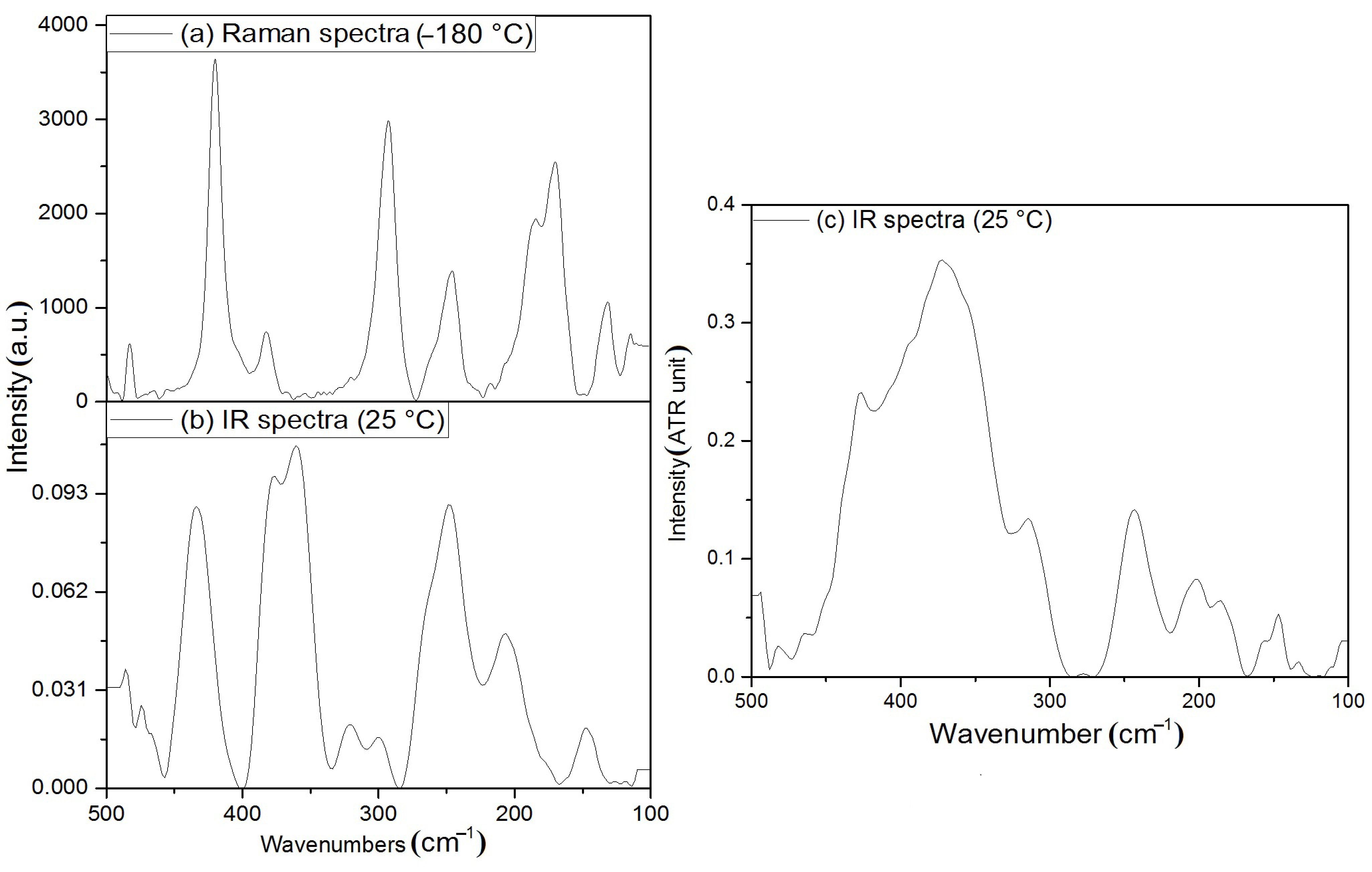
3.2. Vibrational Spectrum of Compound 1
3.3. Vibrational Spectrum of Compound 2
3.4. UV Spectra of Compounds 1 and 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Béres, K.A.; Homonnay, Z.; Kvitek, L.; Dürvanger, Z.; Kubikova, M.; Harmat, V.; Szilágyi, F.; Czégény, Z.; Németh, P.; Bereczki, L.; et al. Thermally Induced Solid-Phase Quasi-Intramolecular Redox Reactions of [Hexakis(urea-O)iron(III)] Permanganate: An Easy Reaction Route to Prepare Potential (Fe,Mn)Ox Catalysts for CO2 Hydrogenation. Inorg. Chem. 2022, 61, 14403. [Google Scholar] [CrossRef] [PubMed]
- Béres, K.A.; Homonnay, Z.; Barta Holló, B.; Gracheva, M.; Petruševski, V.M.; Farkas, A.; Dürvanger, Z.s.; Kótai, L. Synthesis, structure, and Mössbauer spectroscopic studies on the heat-induced solid-phase redox reactions of hexakis(urea-O)iron(III) peroxodisulfate. J. Mater. Res. 2023, 38, 1102. [Google Scholar] [CrossRef]
- Bereczki, L.; Petruševski, V.M.; Franguelli, F.P.; Béres, K.A.; Farkas, A.; Holló, B.B.; Czégény, Z.; Szilágyi, I.M.; Kótai, L. [Hexaamminecobalt(III)] Dichloride Permanganate—Structural Features and Heat-Induced Transformations into (CoII,MnII)(CoIII,MnIII)2O4 Spinels. Inorganics 2022, 10, 252. [Google Scholar] [CrossRef]
- Franguelli, F.P.; Kováts, É.; Czégény, Z.; Bereczki, L.; Petruševski, V.M.; Barta Holló, B.; Béres, K.A.; Farkas, A.; Szilágyi, I.M.; Kótai, L. Multi-Centered Solid-Phase Quasi-Intramolecular Redox Reactions of [(Chlorido)Pentaamminecobalt(III)] Permanganate—An Easy Route to Prepare Phase Pure CoMn2O4 Spinel. Inorganics 2022, 10, 18. [Google Scholar] [CrossRef]
- Müller, T.G.; Mogk, J.; Conrad, M.; Kraus, F. Octaammine EuII and YbII Azides and Their Thermal Decompositions to the Nitrides. Eur. J. Inorg. Chem. 2016, 26, 4162–4169. [Google Scholar] [CrossRef]
- Hetmanczyk, L.; Hetmanczyk, J. Comparison of vibrational dynamics, thermal behaviour, and phase transition in [Ni(NH3)4](ReO4)2 and [Ni(NH3)6](ReO4)2. J. Therm. Anal. Calorim. 2015, 119, 1415–1428. [Google Scholar] [CrossRef] [Green Version]
- Fogaca, L.A.; Kováts, É.; Németh, G.; Kamarás, K.; Béres, K.A.; Németh, P.; Petruševski, V.; Bereczki, L.; Holló, B.B.; Sajó, I.E.; et al. Solid-Phase Quasi-Intramolecular Redox Reaction of [Ag(NH3)2]MnO4: An Easy Way to Prepare Pure AgMnO2. Inorg. Chem. 2021, 60, 3749. [Google Scholar] [CrossRef] [PubMed]
- Fogaça, L.A.; Bereczki, L.; Petruševski, V.M.; Barta-Holló, B.; Franguelli, F.P.; Mohai, M.; Béres, K.A.; Sajó, I.E.; Szilágyi, I.M.; Kotai, L. A Quasi-Intramolecular Solid-Phase Redox Reaction of Ammonia Ligands and Perchlorate Anion in Diamminesilver(I) Perchlorate. Inorganics 2021, 9, 38. [Google Scholar] [CrossRef]
- Kovács, G.B.; May, N.v.; Bombicz, P.A.; Klébert, S.; Németh, P.; Menyhárd, A.; Novodárszki, G.; Petrusevski, V.; Franguelli, F.P.; Magyari, J.; et al. An Unknown Component of a Selective and Mild Oxidant: Structure and Oxidative Ability of a Double Salt-Type Complex Having κ 1 O-Coordinated Permanganate Anions and Three- and Four-Fold Coordinated Silver Cations. RSC Adv. 2019, 9, 28387. [Google Scholar] [CrossRef] [Green Version]
- Solt, H.E.; Németh, P.; Mohai, M.; Sajó, I.E.; Klébert, S.; Franguelli, F.P.; Fogaca, L.A.; Pawar, R.P.; Kótai, L. Temperature-Limited Synthesis of Copper Manganites along the Borderline of the Amorphous/Crystalline State and Their Catalytic Activity in CO Oxidation. ACS Omega 2021, 6, 1523. [Google Scholar] [CrossRef]
- Franguelli, F.P.; Barta-Holló, B.; Petruševski, V.M.; Sajó, I.E.; Klébert, S.; Farkas, A.; Bódis, E.; Szilágyi, I.M.; Pawar, R.P.; Kótai, L. Thermal Decomposition and Spectral Characterization of Di[Carbonatotetraamminecobalt(III)] Sulfate Trihydrate and the Nature of Its Thermal Decomposition Products. J. Therm. Anal. Calorim. 2021, 145, 2907. [Google Scholar] [CrossRef]
- Trif, L.; Franguelli, F.P.; Lendvay, G.; Majzik, E.; Béres, K.; Bereczki, L.; Szilágyi, I.M.; Pawar, R.P.; Kótai, L. Thermal Analysis of Solvatomorphic Decakis (Dimethylammonium) Dihydrogendodecatungstate Hydrates. J. Therm. Anal. Calorim. 2021, 144, 81. [Google Scholar] [CrossRef]
- Petrushevski, V.M.; Béres, K.A.; Bombicz, P.; Farkas, A.; Kótai, L.; Bereczki, L. Structural and Raman Spectroscopic Characterization of Tetrapyridinesilver(I) Perrhenate, [Agpy4]ReO4. Maced. J. Chem. Chem. Eng. 2022, 41, 37. [Google Scholar] [CrossRef]
- May, N.V.; Bayat, N.; Béres, K.A.; Bombicz, P.; Petruševski, V.M.; Lendvay, G.; Farkas, A.; Kótai, L. Structure and Vibrational Spectra of Pyridine Solvated Solid Bis(Pyridine)Silver(I) Perchlorate, [Agpy2ClO4]·0.5py. Inorganics 2022, 10, 123. [Google Scholar] [CrossRef]
- Mehrotra, R.N. Review on the chemistry of [M(NH3)n](XO4)m (M=transition metal, X=Mn, Tc or Re, n=1–6, m=1–3) ammine complexes. Inorganics, 2023; 2023050956, accepted for publication. [Google Scholar]
- Hu, B.; Frueh, S.; Garces, H.F.; Zhang, L.; Aindow, M.; Brooks, C.; Kreidler, E.; Suib, S.L. Selective Hydrogenation of CO2 and CO to Useful Light Olefins over Octahedral Molecular Sieve Manganese Oxide Supported Iron Catalysts. Appl. Catal. B 2013, 132, 54. [Google Scholar] [CrossRef]
- Jiang, J.; Wen, C.; Tian, Z.; Wang, Y.; Zhai, Y.; Chen, L.; Li, Y.; Liu, Q.; Wang, C.; Ma, L. Manganese-Promoted Fe 3O4 Microsphere for Efficient Conversion of CO2 to Light Olefins. Ind. Eng. Chem. Res. 2020, 59, 2155. [Google Scholar] [CrossRef]
- Liu, B.; Geng, S.; Zheng, J.; Jia, X.; Jiang, F.; Liu, X. Unravelling the New Roles of Na and Mn Promoter in CO2 Hydrogenation over Fe3O4 -Based Catalysts for Enhanced Selectivity to Light α-Olefins. ChemCatChem 2018, 10, 4718. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.-F.; Bao, J.; Zhang, Y. Manganese-Modified Fe3O4 Microsphere Catalyst with Effective Active Phase of Forming Light Olefins from Syngas. ACS Catal. 2015, 5, 3905. [Google Scholar] [CrossRef]
- Okaya, Y.; Pepinsky, R.; Takeuchi, Y.; Kuroya, H.; Shimida, A.; Gallitelli, P.; Stemple, N.; Beevers, A. X-ray analyses of some complex-ion structures. Acta Crystallogr. 1957, 10, 798. [Google Scholar]
- Kuz’mina, N.E.; Palkina, K.K.; Savinkina, E.V.; Kozlova, I.A. Products of Reactions of Manganese(II) and Iron(II) Iodides with Urea: Comparison of Structures and Properties. Zh. Neorg. Khim. 2000, 45, 332–337. [Google Scholar]
- Galeazzi, G.; Russo, U.; Valle, G.; Calogero, S. Mössbauer study of some iron(III) complexes with urea type ligands and the crystal structure of hexakisdimethylureairon(III) perchlorate. Transit. Met. Chem. 1981, 6, 325. [Google Scholar] [CrossRef]
- Russo, U.; Calogero, S.; Pra, A.D. Characterization of some high-spin iron(III) complexes with urea derivaties. Crystal structure of diaquatetrakis(perhydropyrimidin-2-one)iron trichloride dihydrate and of perhydropyrimidin-2-one. J. Chem. Soc. Dalton Trans. 1980, 4, 646. [Google Scholar]
- Carp, O.; Patron, L.; Diamandescu, L.; Reller, A. Thermal decomposition study of the coordination compound [Fe(urea)6](NO3)3. Thermochim. Acta 2002, 390, 169. [Google Scholar] [CrossRef]
- Zhao, S.; Sin, A. Synthesis of iron(III)-urea complex in organic solvent and its thermal decomposition. Huaxue Shiji 2010, 32, 108. [Google Scholar]
- Theophanides, T.; Harvey, P.D. Structural and spectroscopic properties of metal-urea complexes. Coord. Chem. Rev. 1987, 76, 237. [Google Scholar] [CrossRef]
- Drakopoulou, L.; Papatriantafyllopoulou, C.; Terzis, A.; Perlepes, S.P.; Manessi-Zoupa, E.; Papaefstathiou, G.S. Synthesis, X-Ray Structure, and Characterization of a Complex Containing the Hexakis(urea)cobalt(II) Cation and Lattice Urea Molecule. Bioinorg. Chem. Appl. 2007, 2007, 51567. [Google Scholar] [CrossRef] [Green Version]
- Bala, R.; Sachdeva, D.; Kumar, M.; Parakash, V. Advances in coordination chemistry of hexaurea complexes of chromium(III). J. Coord. Chem. 2020, 73, 2801. [Google Scholar] [CrossRef]
- Aghabozorg, H.; Palenik, G.J.; Stoufer, R.C.; Summers, J. Dynamic Jahn-Teller effect in a manganese(III) complex. Synthesis and structure of hexakis(urea)manganese(III) perchlorate. Inorg. Chem. 1982, 21, 3903. [Google Scholar] [CrossRef]
- Figgis, B.N.; Wadley, L.G.B.; Graham, J. Crystal structure of hexaurea salts of trivalent metals. I. Ti(urea)6(ClO4)3 at room temperature. Acta Cryst. 1972, 28, 187. [Google Scholar] [CrossRef]
- Russo, U.; Calogero, S.; Burriesci, N.; Petrera, M. Mössbauer characterization of some new high-spin iron complexes with urea and thiourea derivatives. J. Inorg. Nucl. Chem. 1979, 41, 25. [Google Scholar] [CrossRef]
- Yamauchi, S.; Sakai, Y.; Tominaga, T. Paramagnetic relaxation effects on Mössbauer spectra of hexakis/alkylurea/iron/III/complexes. J. Radioanal. Nucl. Chem. 1987, 119, 283. [Google Scholar] [CrossRef]
- Yamauchi, S.; Sakai, Y.; Nishioji, H.; Tominaga, T. Mössbauer spectroscopic study of the magnetic relaxation in tris(β-diketonato)iron(III) complexes. Int. J. Appl. Radiat. Isot. 1983, 34, 977. [Google Scholar] [CrossRef]
- Martiz, A.; Károly, Z.; Domján, A.; Mohai, M.; Bereczki, L.; Trif, L.; Farkas, A.; László, K.; Menyhárd, A.; Kótai, L. Nano-ZrO2@C, Nano-(ZrC, ZrO2)@C and Nano-ZrC@C Composites Prepared by Plasma-Assisted Carbonization of Zr-Loaded Iminodiacetate-Functionalized Styrene-Divinylbenzene Copolymers. Inorganics 2022, 10, 77. [Google Scholar] [CrossRef]
- Martiz, A.; Károly, Z.; Trif, L.; Mohai, M.; Bereczki, L.; Németh, P.; Molnár, Z.; Menyhárd, A.; Pawar, R.P.; Tekale, S.; et al. Plasma-assisted preparation of nano-(ZrC, ZrO2)@ carbon composites from Zr-loaded sulfonated styrene–divinylbenzene copolymers. J. Therm. Anal. Calorim. 2022, 147, 9353–9365. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT– Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226. [Google Scholar] [CrossRef] [Green Version]
- Kocsis, T.; Magyari, J.; Sajó, I.E.; Pasinszki, T.; Homonnay, Z.; Szilágyi, I.M.; Farkas, A.; May, Z.; Effenberger, H.; Szakáll, S.; et al. Evidence of quasi-intramolecular redox reactions during thermal decomposition of ammonium hydroxodisulfitoferriate(III), (NH4)2[Fe(OH)(SO3)2]·H2O. J. Therm. Anal. Calorim. 2018, 132, 493. [Google Scholar] [CrossRef] [Green Version]
- Smeets, S.; Lutz, M. Hexakis(urea-κO)zinc(II) dinitrate at 110 and 250 K: Uniaxial negative thermal expansion. Acta Crystallogr. C Struct. Chem. 2011, 67, 50. [Google Scholar] [CrossRef] [PubMed]
- Kuz’mina, N.E.; Palkina, K.K.; Savinkina, E.V.; Kuznetsova, N.T.; Kozlova, I.A. Syntheses and Crystal Structures of Nickel(II) and Cobalt(II) Urea Diiodoiodates [Ni(CON2H4)6][I3]2·2(CON2H4) and [Co(CON2H4)6][I3]2 · 2(CON2H4). Zh. Neorg. Khim. 2000, 45, 780. [Google Scholar]
- Palkina, K.K.; Kuz’mina, N.E.; Orlova, V.; Kondokova, I. Coordination Compounds of Some Transition Metal, Magnesium, and Calcium Nitrates with Dimethylurea. Zh. Neorg. Khim. 1999, 44, 963. [Google Scholar]
- Figgis, B.N.; Skelton, B.W.; White, A.H. Crystal structures of hexa(N-methylurea)cobalt(II) sulfate and thiosulfate. Aust. J. Chem. 1980, 33, 425. [Google Scholar] [CrossRef]
- Masłowska, J.; Cedzyńska, K. The structure of iron(III) complexes with carbamide derivatives. J. Mol. Struct. 1973, 19, 521. [Google Scholar] [CrossRef]
- Mido, Y.; Murata, H. Infrared Absorption Spectra of Urea-formaldehyde Initial Condensation Products. II. Dimethylurea. Bull. Chem. Soc. JPN 1969, 42, 3372. [Google Scholar] [CrossRef] [Green Version]
- Penland, R.B.; Lane, T.J.; Quagliano, J.V. Infrared Absorption Spectra of Inorganic Coordination Complexes. VII. Structural Isomerism of Nitro- and Nitritopentamminecobalt(III) Chlorides. J. Am. Chem. Soc. 1956, 78, 887. [Google Scholar] [CrossRef]
- Cotton, S.A.; Gibson, J.F. Electron paramagnetic resonance and vibrational spectra of some iron(III) complexes with oxygen-containing ligands. J. Chem. Soc. A 1971, 1690. [Google Scholar] [CrossRef]
- Butorc, V.; Simeon, V.; Tomisic, V. Effect of temperature on the UV spectra of concentrated NaNO3 aqueous solutions. Croat. Chem. Acta 2007, 80, 533. [Google Scholar]
| Label | Compound 1 | Compound 2 | Compound 3 |
|---|---|---|---|
| Compound | [Fe(N,N’-DMU)6](NO3)3 | [Fe(N,N-DMU)4(H2O)2](NO3)3 | [Fe(N,N-DMU)6](NO3)3·3H2O |
| Empirical formula | C18H48FeN15O15 | C12H36FeN11O15 | C18H54FeN15O18 |
| Formula weight | 770.56 | 630.37 | 824.61 |
| Temperature | 100.00(10) | 113(2) | 105(2) K |
| Radiation and wavelength | Cu-Kα, λ = 1.54184Å | Mo-Kα, λ = 0.71073Å | Cu-Kα, λ = 1.54184 Å |
| Crystal system | Trigonal | Triclinic | Trigonal |
| Space group | R-3 | P-1 | R-3 |
| Unit cell dimensions | a = 12.1232(2)Å | a = 9.8655(6)Å | a = 22.0051(3) Å |
| b = 12.1232(2)Å | b = 10.5568(7)Å | b = 22.0051(3) Å | |
| c = 22.5665(4)Å | c = 15.5569(10)Å | c = 13.9132(2) Å | |
| α = 90° | α = 77.658(5)° | α = 90° | |
| β = 90° | β = 76.538(5)° | β = 90° | |
| γ = 120° | γ = 62.239(4)° | γ = 120° | |
| Volume | 2872.30(11)Å3 | 1383.86(16)Å3 | 5834.51(18) Å3 |
| Z | 3 | 2 | 6 |
| Density (calculated) | 1.337 Mg/m3 | 1.513 Mg/m3 | 1.408 Mg/m3 |
| Absorption coefficient, μ | 3.844 mm−1 | 0.629 mm−1 | 3.887 mm−1 |
| F(000) | 1221 | 662 | 2622 |
| Crystal color | orange | orange | orange |
| Crystal description | block | prism | rod |
| Crystal size | 0.39 × 0.37 × 0.29 mm | 0.37 × 0.33 × 0.32 mm3 | 0.197 × 0.066 × 0.049 mm3 |
| Absorption correction | Gaussian | numerical | Gaussian |
| Max. and min. transmission | 0.2790.328 | 0.9070.962 | 1.0000.569 |
| Θ-range for data collection | 4.645 ≤ Θ ≤ 75.409° | 5.116 ≤ Θ ≤ 25.349° | 3.934 ≤ Θ ≤ 75.583° |
| Index ranges | −15 ≤ h ≤ 15; −15 ≤ k ≤ 14; −28 ≤ l ≤ 28 | −11 ≤ h ≤ 11; −12 ≤ k ≤ 12; −18 ≤ l ≤ 18 | −27 ≤ h ≤ 27; −27 ≤ k ≤ 27; −17 ≤ l ≤ 17 |
| Reflections collected | 13,522 | 22,946 | 27,109 |
| Completeness to 2Θ | 1.000 | 0.990 | 100.0% |
| Independent reflections | 1324 [R(int) = 0.0395] | 5030 [R(int) = 0.0547] | 2672 [R(int) = 0.0466] |
| Refinement method | full-matrix least-squares on F2 | full-matrix least-squares on F2 | full-matrix least-squares on F2 |
| Data/restraints/parameters | 1321/20/96 | 5030/1/363 | 2672/0/164 |
| Goodness-of-fit on F2 | 1.113 | 1.114 | 1.088 |
| Final R indices [I > 2σ(I)] | R1 = 0.0577, wR2 = 0.1672 | R1 = 0.0504, wR2 = 0.1032 | R1 = 0.0417, wR2 = 0.1233 |
| R indices (all data) | R1 = 0.0577, wR2 = 0.1673 | R1 = 0.0660, wR2 = 0.1089 | R1 = 0.0427, wR2 = 0.1242 |
| Largest diff. peak and hole | 0.978; −0.803 e.Å−3 | 0.445; −0.326 e.Å−3 | 0.522; −0.376 e.Å−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Béres, K.A.; Homonnay, Z.; Bereczki, L.; Dürvanger, Z.; Petruševski, V.M.; Farkas, A.; Kótai, L. Crystal Nanoarchitectonics and Characterization of the Octahedral Iron(III)–Nitrate Complexes with Isomer Dimethylurea Ligands. Crystals 2023, 13, 1019. https://doi.org/10.3390/cryst13071019
Béres KA, Homonnay Z, Bereczki L, Dürvanger Z, Petruševski VM, Farkas A, Kótai L. Crystal Nanoarchitectonics and Characterization of the Octahedral Iron(III)–Nitrate Complexes with Isomer Dimethylurea Ligands. Crystals. 2023; 13(7):1019. https://doi.org/10.3390/cryst13071019
Chicago/Turabian StyleBéres, Kende Attila, Zoltán Homonnay, Laura Bereczki, Zsolt Dürvanger, Vladimir M. Petruševski, Attila Farkas, and László Kótai. 2023. "Crystal Nanoarchitectonics and Characterization of the Octahedral Iron(III)–Nitrate Complexes with Isomer Dimethylurea Ligands" Crystals 13, no. 7: 1019. https://doi.org/10.3390/cryst13071019
APA StyleBéres, K. A., Homonnay, Z., Bereczki, L., Dürvanger, Z., Petruševski, V. M., Farkas, A., & Kótai, L. (2023). Crystal Nanoarchitectonics and Characterization of the Octahedral Iron(III)–Nitrate Complexes with Isomer Dimethylurea Ligands. Crystals, 13(7), 1019. https://doi.org/10.3390/cryst13071019







