Influence of Silicon and Chromium on the Na2SO4-Induced Hot Corrosion Behavior of Titanium Alloys
Abstract
1. Introduction
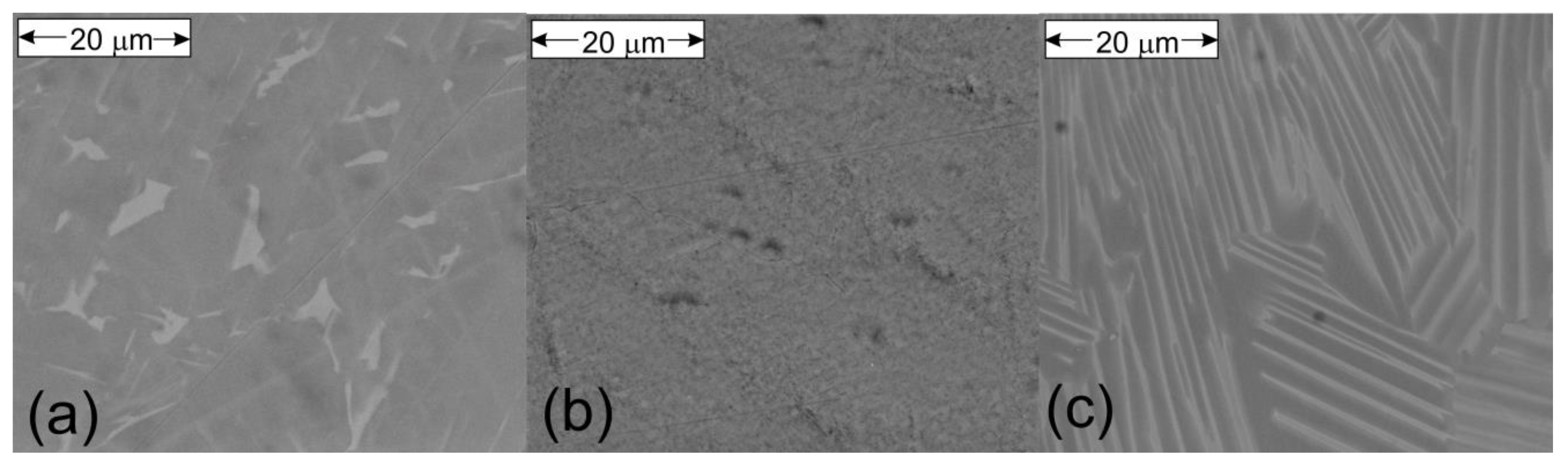
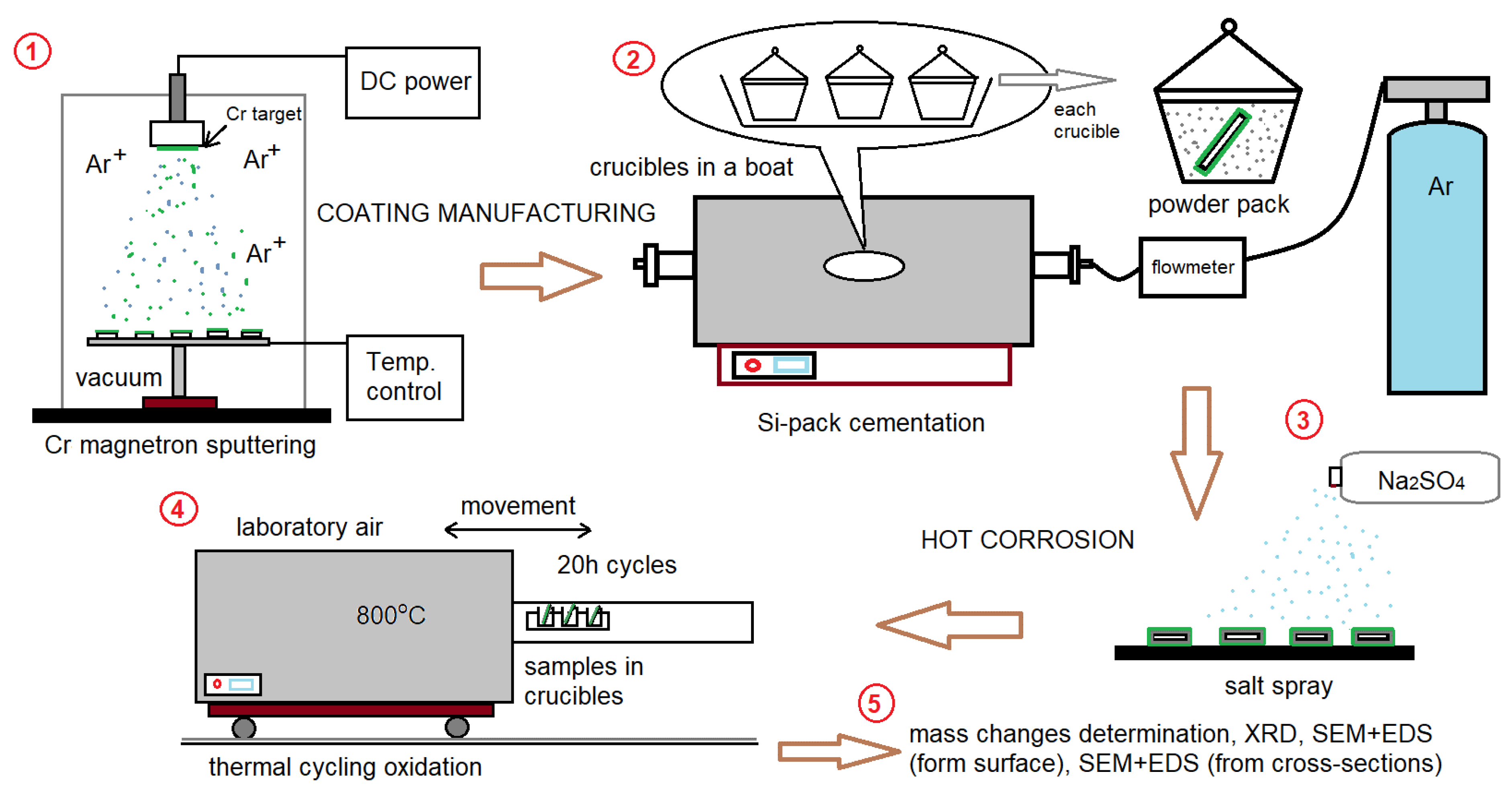
2. Materials and Methods
3. Results and Discussion
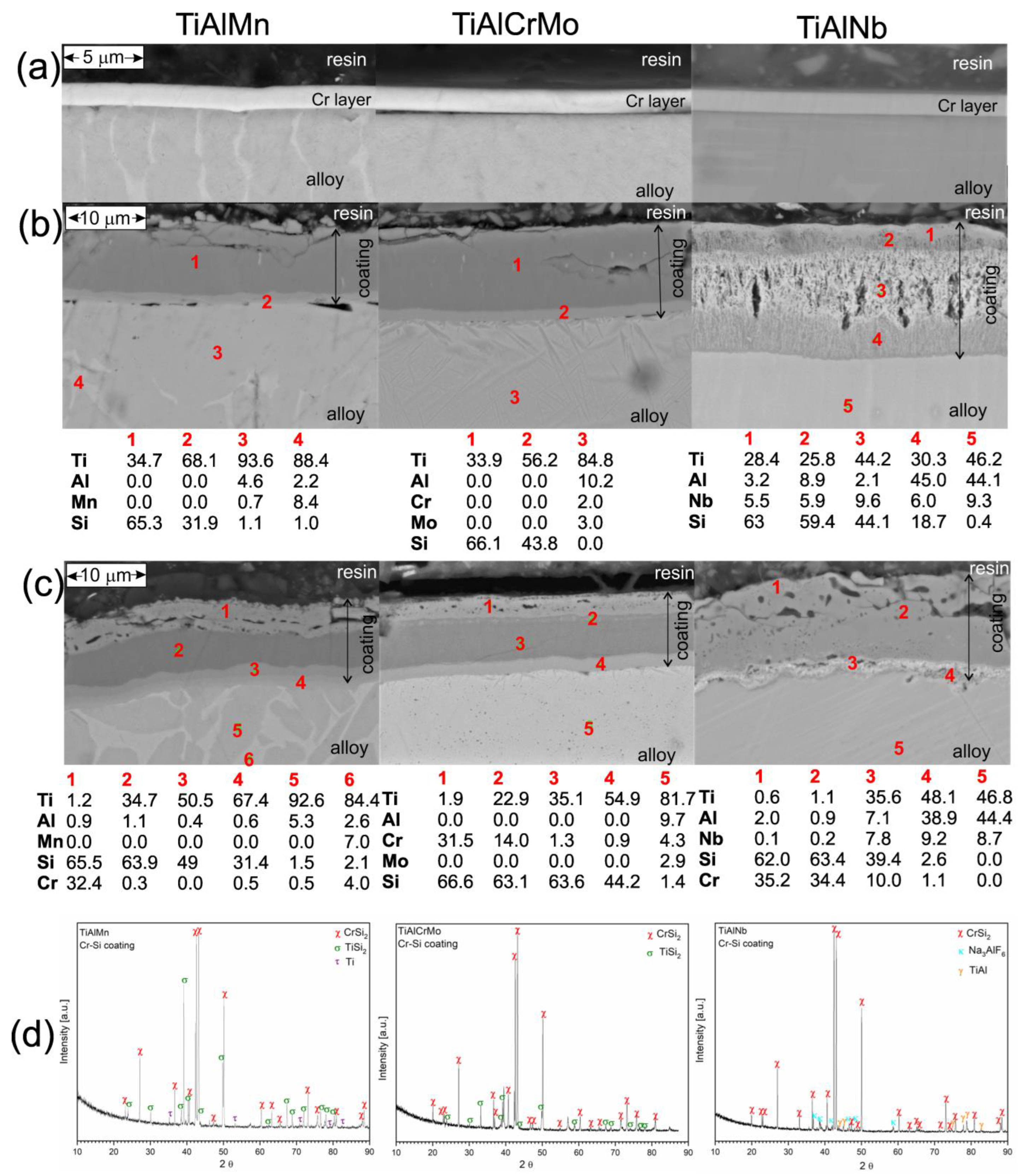
3.1. Coating Deposition
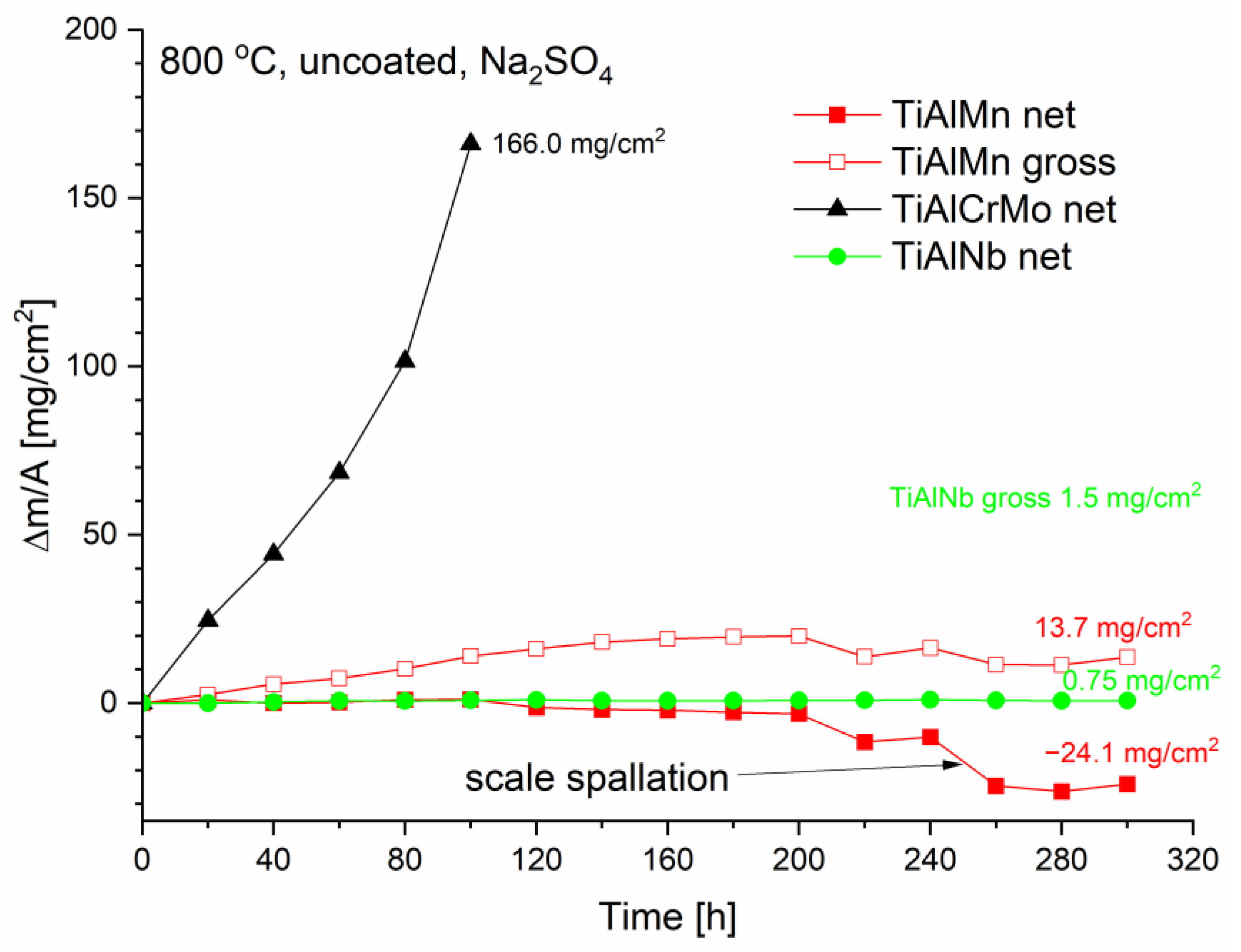
3.2. Hot Corrosion Behavior of Uncoated Samples
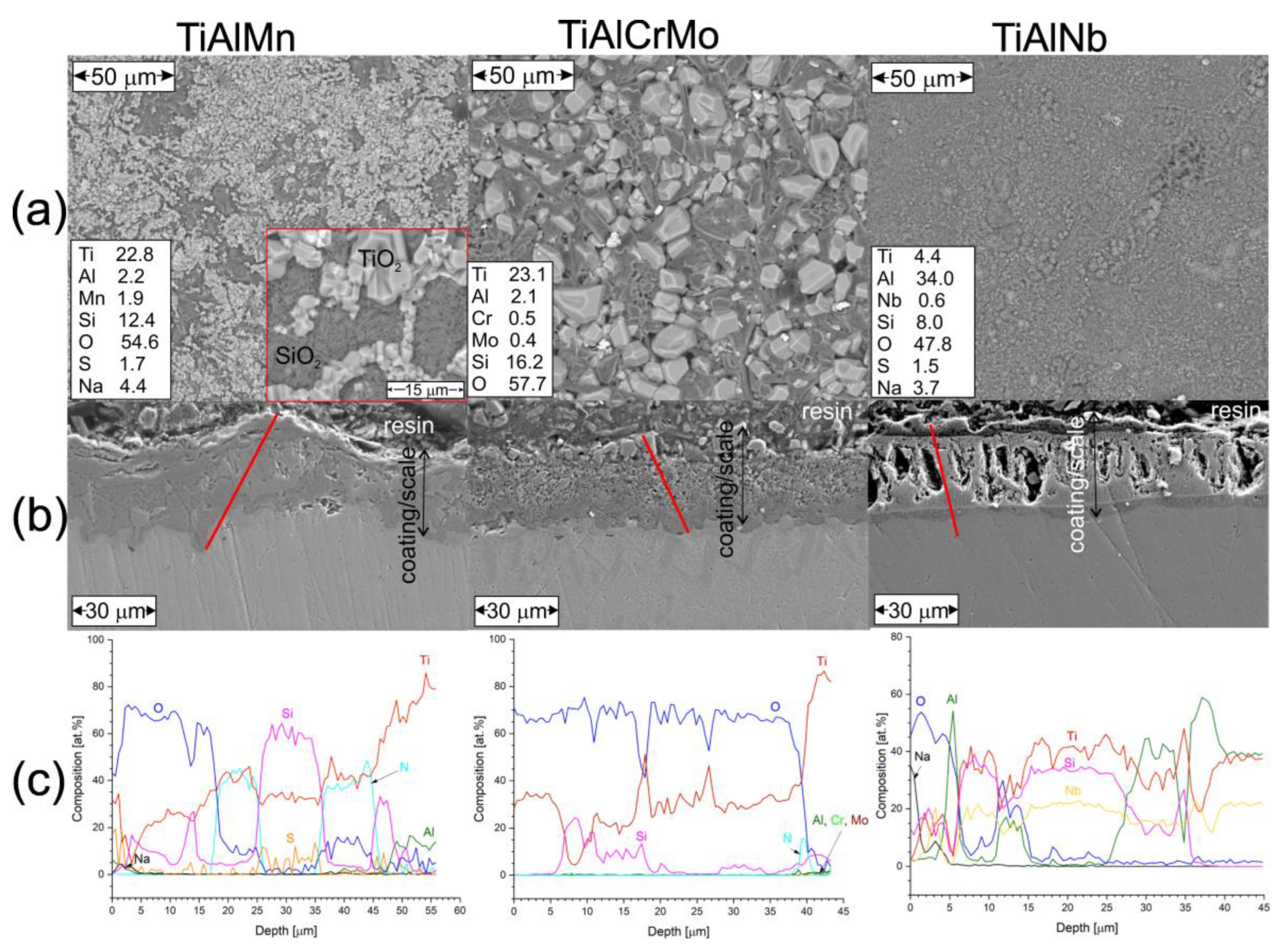
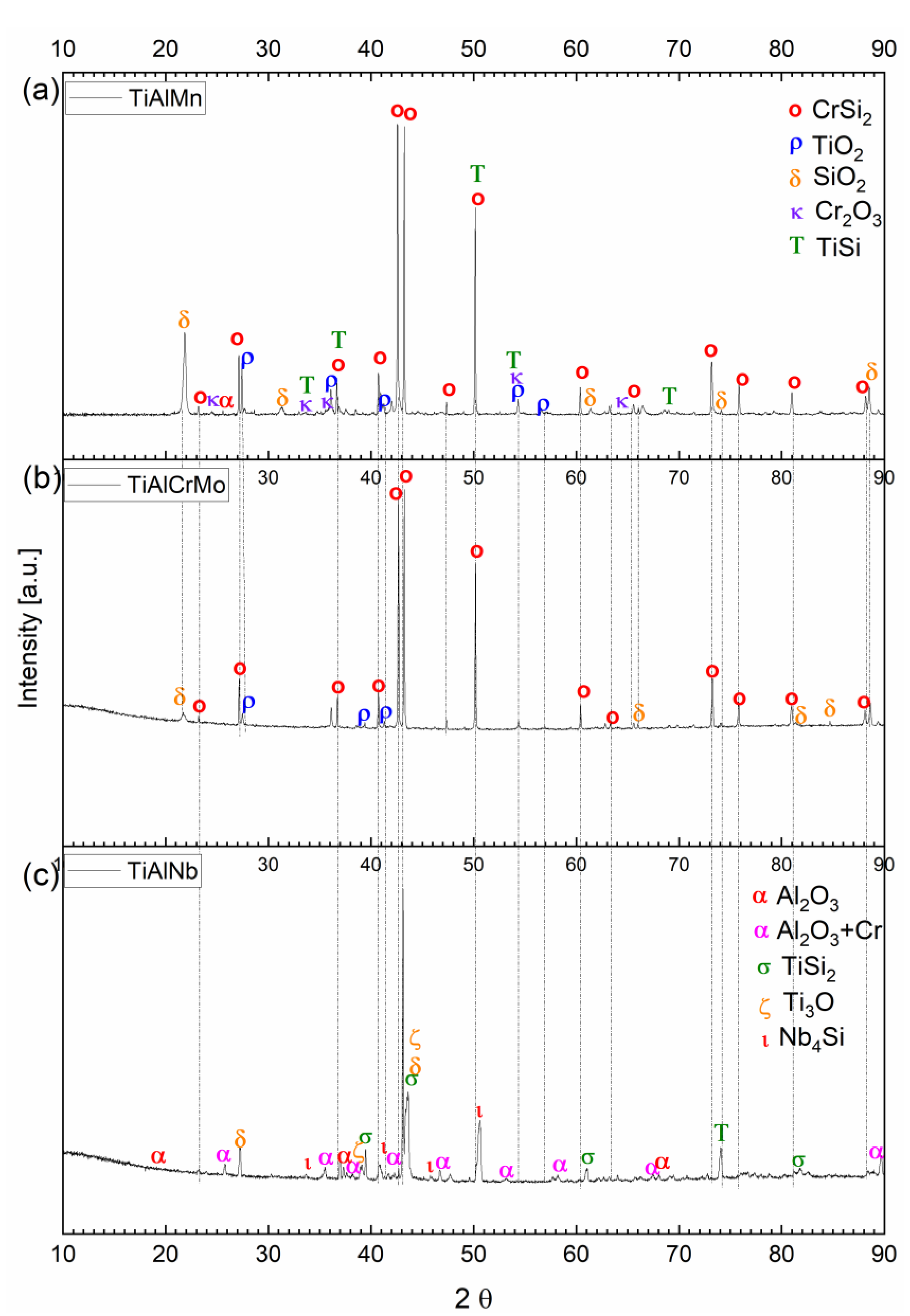
3.3. Hot Corrosion Behavior of Si-Coated Samples
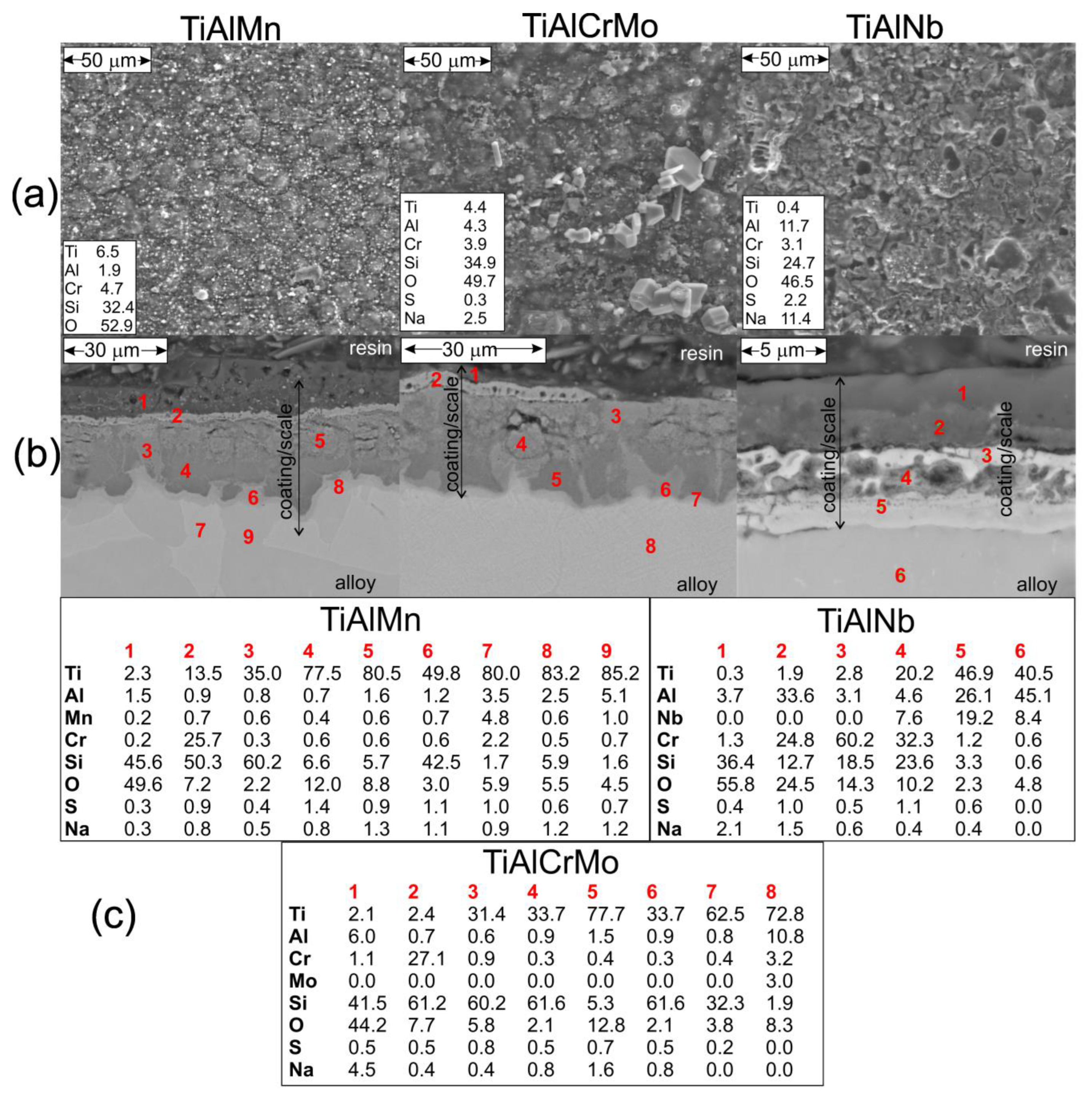
3.4. Hot Corrosion Behavior of (Cr, Si)-Coated Samples
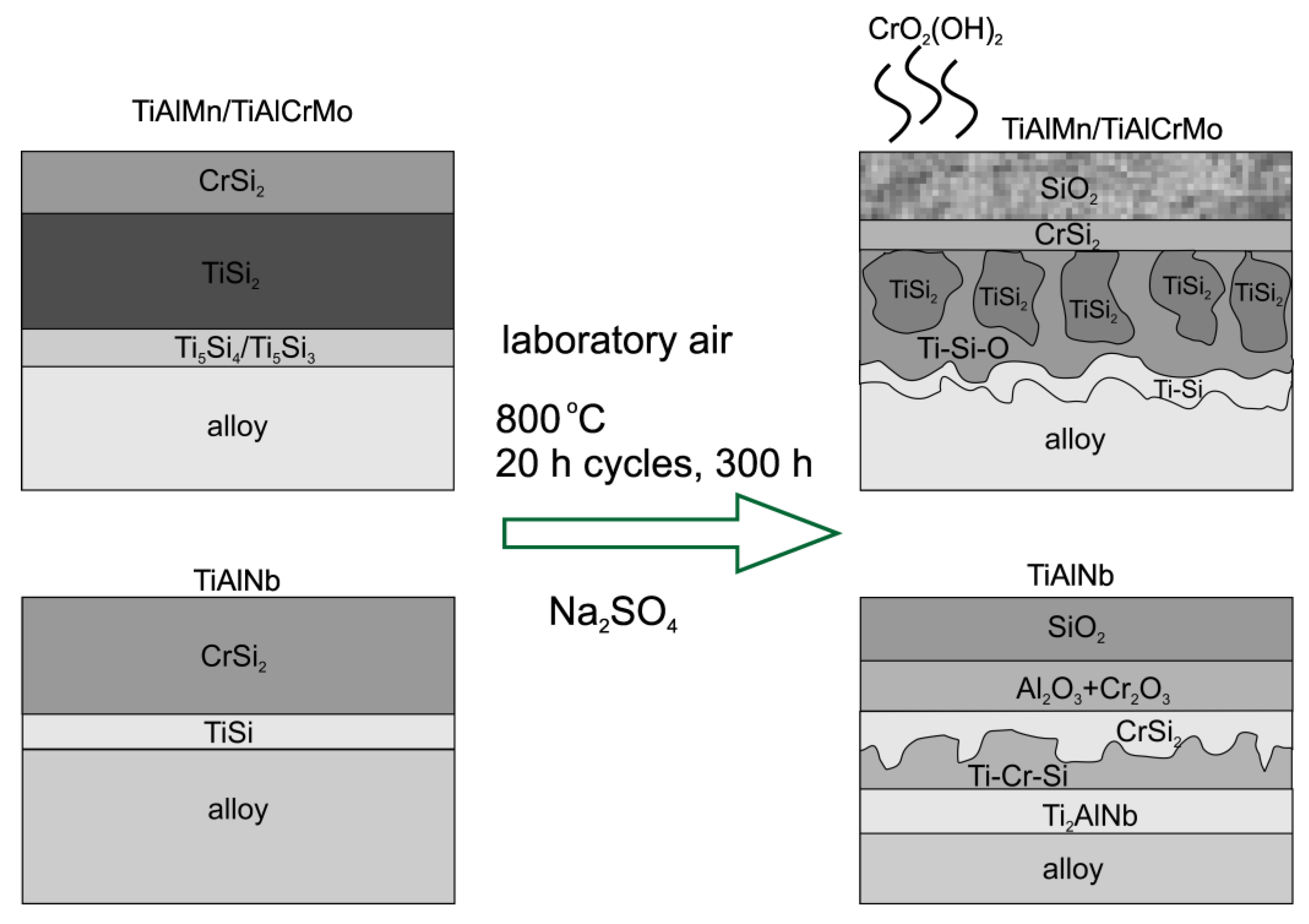
4. Conclusions
- Easier formation of a thicker and more uniform SiO2 barrier layer resulting from the oxidation of CrSi2;
- Cr-promoted growth of an Al2O3-rich inner layer of the scale in the case of the titanium aluminide alloy, TiAlNb;
- Better stability of the Cr-Si coatings in thermal cycling conditions and the progressive release of silicon, owing to the multilayer and multiphase composition.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amigó-Borrás, V.; Lario-Femenía, J.; Amigó-Mata, A.; Vicente-Escuder, A. Titanium, Titanium Alloys and Composites. In Encyclopedia of Materials: Metals and Alloys; Caballero, F.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 179–199. [Google Scholar] [CrossRef]
- Mouritz, A.P. Titanium Alloys for Aerospace Structures and Engines. In Introduction to Aerospace Materials; Mouritz, A.P., Ed.; Woodhead Publishing: Cambridge, UK, 2012; Chapter 9; pp. 202–223. [Google Scholar] [CrossRef]
- Polmear, I.; StJohn, D.; Nie, J.F.; Qian, M. (Eds.) Titanium Alloys. In Light Alloys; Butterworth-Heinemann: Oxford, UK, 2017; Chapter 7; pp. 369–460. [Google Scholar] [CrossRef]
- Kermanpur, A.; Sepehri Amin, H.; Ziaei-Rad, S.; Nourbakhshnia, N.; Mosaddeghfar, M. Failure analysis of Ti6Al4V gas turbine compressor blades. Eng. Fail. Anal. 2008, 15, 1052–1064. [Google Scholar] [CrossRef]
- Xu, K.; Wang, J.; Gao, Y.; Yang, F.; Pan, Y. Deformation measurement and simulation of Ti–6Al–4V blades induced by shot peening process. Int. J. Adv. Manuf. Technol. 2023, 126, 5017–5032. [Google Scholar] [CrossRef]
- Yan, Z.; Zhu, L.; Yang, Z.; Xue, P. Study on the geometrical dimensions and mechanical properties of Ti-6Al-4V alloy blade by laser metal deposition. Int. J. Adv. Manuf. Technol. 2021, 114, 695–707. [Google Scholar] [CrossRef]
- Agarwal, K.M.; Singhal, A.; Kapoor, A.; Bhatia, D. Simulated analysis of Ti-6Al-4V processed through equal channel angular pressing for biomedical applications. Mater. Sci. Energy Tech. 2021, 4, 290–295. [Google Scholar] [CrossRef]
- Rodriguez, G.M.; Bowen, J.; Zelzer, M.; Stamboulis, A. Selective modification of Ti6Al4V surfaces for biomedical applications. RSC Adv. 2020, 10, 17642–17652. [Google Scholar] [CrossRef]
- Milovanović, A.; Sedmak, A.; Grbović, A.; Mijatović, T.; Čolić, K. Design Aspects of Hip Implant Made of Ti-6Al-4V Extra Low Interstitials Alloy. Procedia Struct. Integr. 2020, 26, 299–305. [Google Scholar] [CrossRef]
- Kustono, D.; Andoko, R.W.; Puspitasari, P.; Kurniawan, G.A.; Putra, A.D. Simulation of knee implants made of Ti6Al4V material during walking. MATEC Web Conf. 2018, 204, 07015. [Google Scholar] [CrossRef]
- De la Garza-Ramos, M.A.; Estupiñan-Lopez, F.H.; Gaona-Tiburcio, C.; Beltrán-Novelo, L.G.; Zambrano-Robledo, P.; Cabral-Miramontes, J.; Almeraya-Calderón, F. Electrochemical Behavior of Ti6Al4V Alloy Used in Dental Implants Immersed in Streptococcus gordonii and Fusobacterium nucleatum Solutions. Materials 2020, 13, 4185. [Google Scholar] [CrossRef] [PubMed]
- Gurrappa, I. Characterization of titanium alloy Ti-6Al-4V for chemical, marine and industrial applications. Mater. Charact. 2003, 51, 131–139. [Google Scholar] [CrossRef]
- Zheng, X.; Zhuang, X.; Lei, Y.; Chu, Z.; Xu, J.; Gao, L.; Sun, X. Corrosion Behavior of the Ti–6Al–4V Alloy in Sulfate-Reducing Bacteria Solution. Coatings 2020, 10, 24. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, D.; Wang, E.; Yan, F.; Xiang, L.; Xie, Z. Corrosion Degradation Behaviors of Ti6Al4V Alloys in Simulated Marine Environments. Coatings 2022, 12, 1028. [Google Scholar] [CrossRef]
- Williams, J.C.; Boyer, R.R. Opportunities and Issues in the Application of Titanium Alloys for Aerospace Components. Metals 2020, 10, 705. [Google Scholar] [CrossRef]
- Takahashi, K.; Mori, K.; Takebe, H. Application of Titanium and its Alloys for Automobile Parts. MATEC Web Conf. 2020, 321, 02003. [Google Scholar] [CrossRef]
- Singh, P.; Pungotra, H.; Kalsi, N.S. On the characteristics of titanium alloys for the aircraft applications. Mater. Today Proc. 2017, 4, 8971–8982. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Nag, S.; Suzuki, A.; Weimer, M.J. TiAl alloys in commercial aircraft engines. Mater. High Temp. 2016, 33, 549–559. [Google Scholar] [CrossRef]
- Moore, S. 3D Printing Titanium in Aerospace Manufacturing Applications. AZOM 2022. 24 September. Available online: https://www.azom.com/article.aspx?ArticleID=19272 (accessed on 24 April 2023).
- Bewlay, B.; Weimer, M.; Kelly, T.; Suzuki, A.; Subramanian, P. The Science, Technology, and Implementation of TiAl Alloys in Commercial Aircraft Engines. MRS Proc. 2013, 1516, 49–58. [Google Scholar] [CrossRef]
- Smialek, J.L.; Jacobson, N.S. Oxidation of High-Temperature Aerospace Materials. In High Temperature Materials and Mechanisms, 1st ed.; Bar-Cohen, Y., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; Chapter 5; pp. 95–162. [Google Scholar] [CrossRef]
- Gurrappa, I.; Yashwanth, I.V.S.; Mounika, I.; Murakami, H.; Kuroda, S. The Importance of Hot Corrosion and Its Effective Prevention for Enhanced Efficiency of Gas Turbines. In Gas Turbines—Materials, Modeling and Performance; Gurrappa, I., Ed.; InTechOpen: London, UK, 2015; Chapter 3. [Google Scholar] [CrossRef]
- Rapp, R.A.; Zhang, Y.S. Hot corrosion of materials: Fundamental studies. JOM 1994, 46, 47–55. [Google Scholar] [CrossRef]
- Yang, P.; Bu, Z.; An, Y.; Zhou, H.; Li, Y.; Chen, J. A systematic study on Na2SO4-induced hot corrosion behavior of plasma-sprayed La2(Zr0.75Ce0.25)2O7 coating. Surf. Coat. Tech. 2022, 429, 127979. [Google Scholar] [CrossRef]
- ASCENT Environmental Cost-Benefit Analysis of Ultra Low Sulfur Jet Fuels. Available online: https://ascent.aero/partner-27 (accessed on 14 March 2023).
- Hu, S.; Finklea, H.; Liu, H. A review on molten sulfate salts induced hot corrosion. J. Mater. Sci. Technol. 2021, 90, 243–254. [Google Scholar] [CrossRef]
- Waeytens, M.; Syed, A.U.; Roberts, T.; Martinez, F.D.; Gray, S.; Nicholls, J.R. A microscopy study of nickel-based superalloys performance in type I hot corrosion conditions. Mater. High Temp. 2023. [Google Scholar] [CrossRef]
- Valenti, L.E.; Bonnet, L.V.; Galiano, M.R.; Giacomelli, C.E. A simple strategy to prepare hybrid coating on titanium (Ti6Al4V). Surf. Coat. Tech. 2022, 431, 128017. [Google Scholar] [CrossRef]
- Sánchez-Bodón, J.; Andrade Del Olmo, J.; Alonso, J.M.; Moreno-Benítez, I.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies. Polymers 2021, 4, 165. [Google Scholar] [CrossRef]
- Yang, L.; Gao, F.; Zhou, Z.; Jia, Y.; Du, Y.; Wang, J.; Qiao, Y.; Zhu, S.; Wang, F. Oxidation behavior of the AlN coatings on the TiAl alloy at 900 °C. Corros. Sci. 2023, 211, 110891. [Google Scholar] [CrossRef]
- Gruner, H. Thermal Spray Coatings on Titanium. In Titanium in Medicine. Engineering Materials; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Bononi, M.; Colombini, E.; Giovanardi, R.; Veronesi, P.; Tranquillo, E. Coating of Titanium Substrates with ZrO2 and ZrO2-SiO2 Composites by Sol-Gel Synthesis for Biomedical Applications: Structural Characterization, Mechanical and Corrosive Behavior. Coatings 2019, 9, 200. [Google Scholar] [CrossRef]
- Jacobs, M.; De Vos, Y.; Middelkoop, V. Thickness controlled SiO2/TiO2 sol-gel coating by spraying. Open Ceram. 2021, 6, 100121. [Google Scholar] [CrossRef]
- Costa, M.Y.P.; Venditti, M.L.R.; Cioffi, M.O.H.; Voorwald, H.J.C.; Guimarães, V.A.; Ruas, R. Fatigue behavior of PVD coated Ti–6Al–4V alloy. Int. J. Fatigue 2011, 33, 759–765. [Google Scholar] [CrossRef]
- Ewald, A.; Glückermann, S.K.; Thull, R.; Gbureck, U. Antimicrobial titanium/silver PVD coatings on titanium. BioMed. Eng. Online 2006, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Delmas, M.; Ucar, M.; Ressier, L.; Pons, M.; Vahlas, C. MOCVD processed platinum–aluminum coatings on titanium alloys. Surf. Coat. Tech. 2004, 188–189, 49–54. [Google Scholar] [CrossRef]
- Wu, L.K.; Wu, J.J.; Wu, J.Y.; Yan, H.Y.; Jiang, M.Y.; Cao, F.H. Hot corrosion behavior of electrodeposited SiO2 coating on TiAl alloy. Corros. Sci. 2020, 174, 108827. [Google Scholar] [CrossRef]
- Pambudi, M.J.; Basuki, E.A.; Prajitno, D.H. Improving Hot Corrosion Resistance of Two Phases Intermetallic Alloy α2-Ti3Al/γ-TiAl with Enamel Coating. AIP Conf. Proc. 2017, 1805, 070003. [Google Scholar] [CrossRef]
- Hu, Y.T.; Zheng, L.; Yan, H.J.; Wu, L.K.; Lin, X.J.; Cao, F.H.; Jiang, M.Y. Improving hot corrosion resistance of aluminized TiAl alloy by anodization and pre-oxidation. Trans. Nonferrous Met. Soc. China 2021, 3, 193–206. [Google Scholar] [CrossRef]
- Li, Y.Q.; Xie, F.Q.; Yang, S.L. Microstructure and hot corrosion resistance of Si-Al-Y coated TiAl alloy. J. Cent. South Univ. 2020, 27, 2530–2537. [Google Scholar] [CrossRef]
- Rubacha, K.; Godlewska, E.; Mars, K. Behaviour of a silicon-rich coating on Ti-46Al-8Ta (at. %) in hot-corrosion environments. Corros. Sci. 2017, 118, 158–167. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, Y.; Wang, Y.; Niu, Y.; Xin, L.; Li, Y.; Su, J.; Zhu, S.; Wang, F. Corrosion Behaviors of Nitride Coatings on Titanium Alloy in NaCl-Induced Hot Corrosion. Acta Metall. Sin. 2021, 34, 1434–1446. [Google Scholar] [CrossRef]
- Mitoraj, M.; Mars, K.; Matuła, M.; Godlewska, E. Hot corrosion behaviour of Cr-Si coated titanium alloys. Ann. Chim. Mat. 2015, 39, 141–148. [Google Scholar] [CrossRef]
- Ziaja, W.; Motyka, M.; Kubiak, K.; Sieniawski, J. Primary creep bahaviour of two-phase titanium alloy with various microstructure. Arch. Metall. Mater. 2016, 61, 683–688. [Google Scholar] [CrossRef]
- Mitoraj-Królikowska, M.; Godlewska, E. Silicide coatings on Ti-6Al-1Mn (at.%) alloy and their oxidation resistance. Surf. Coat. Tech. 2018, 334, 491–499. [Google Scholar] [CrossRef]
- Harper, M.A.; Rapp, R.A. Codeposited Chromium and Silicon Diffusion Coatings for Fe-Base Alloys via Pack Cementation. Oxid. Met. 1994, 42, 303–333. [Google Scholar] [CrossRef]
- Massalski, T.B.; Okamoto, H.; Subramanian, P.R.; Kacprzak, L. (Eds.) Binary Alloy Phase Diagrams, 2nd ed.; ASM International: Almere, The Netherlands, 1990. [Google Scholar] [CrossRef]
- Cockeram, B.V.; Rapp, R.A. The Kinetics of Multilayered Titanium-Silicide Coatings Grown by the Pack Cementation Method. Metal. Mater. Trans. A 1995, 26A, 777–791. [Google Scholar] [CrossRef]
- Barin, I.; Knacke, O.; Kubaschewski, O. Thermochemical Properties of Inorganic Substances: Supplement; Springer: Berlin, Germany, 1977; Volume 1. [Google Scholar]
- Prasad, S.; Paul, A. Growth mechanism of phases by interdiffusion and diffusion of species in the niobium–silicon system. Acta Mater. 2011, 59, 1577–1585. [Google Scholar] [CrossRef]
- Hu, D.; Huang, A.J.; Wu, X. On the massive phase transformation regime in TiAl alloys: The alloying effect on massive/lamellar competition. Intermetallics 2007, 15, 327–332. [Google Scholar] [CrossRef]
- Yuan, B.; Li, Y.; Qiao, M.; Zhou, C. Vapor phase codeposition of Cr an dSi on Nb-base insitu composites by pack cementation process. Prog. Nat. Sci. Mater. Inter. 2013, 23, 198–204. [Google Scholar] [CrossRef]
- Mitoraj-Królikowska, M.; Godlewska, E. Hot corrosion behaviour of (γ + α2)-Ti-46Al-8Nb (at. %) and α-Ti-6Al-1Mn (at. %) alloys. Corros. Sci. 2017, 115, 18–29. [Google Scholar] [CrossRef]
- Brewer, L.; Lamoreaux, R.H. The Mo-O System (Molybdenum-Oxygen). Bull. Alloy. Phase Diagr. 1980, 1, 85–89. [Google Scholar] [CrossRef]
- Micco, G.; Nassini, H.E.; Bohé, A.E. Kinetics of Molybdenym Oxidation Between 375 and 500 °C. In Molybdenum and its Compounds; Saji, V.S., Lopatin, S.I., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2014; Chapter 17; pp. 313–338. [Google Scholar]
- Yang, Y.; Pu, H.; Di, J.; Zhang, S.; Hu, J.; Zang, Y.; Gao, C.; Chen, C. Influences of temperature gradient and distance on the morphologies of MoS2 domains. AIP Adv. 2018, 8, 085218. [Google Scholar] [CrossRef]
- Chang, Y.N.; Wei, F.I. High-temperature chlorine corrosion of metals and alloys. J. Mater. Sci. 1991, 26, 3693–3698. [Google Scholar] [CrossRef]
- Mitoraj, M.; Godlewska, E.M. Oxidation of Ti-46Al-8Ta in air at 700 °C and 800 °C under thermal cycling conditions. Intermetallics 2013, 34, 112–121. [Google Scholar] [CrossRef]
- Si, P.Z.; Wang, H.X.; Jiang, W.; Lee, J.G.; Choi, C.J.; Liu, J.J. Synthesis, structure and exchange bias in Cr2O3/CrO2/Cr2O5 particles. Thin Solid Film. 2011, 519, 8423–8425. [Google Scholar] [CrossRef]
- Singh, G.P.; Ram, S.; Eckert, J.; Fecht, H.J. Synthesis and morphological stability in CrO2 single crystals of a half-metallic ferromagnetic compound. J. Phys. Conf. Ser. 2009, 144, 012110. [Google Scholar] [CrossRef]
- Cheng, M.; Lu, Z.; Zhang, Z.; Yu, Z.; Liu, S.; Chen, C.; Li, Y.; Liu, Y.; Shi, J.; Xiong, R. Manipulation of film quality and magnetic properties of CrO2 (100) films on TiO2 substrates with carrier gas and growth temperature. RSC Adv. 2018, 8, 1562–1568. [Google Scholar] [CrossRef]
- Sousa, P.M.; Dias, S.A.; Conde, O.; Silvestre, A.J.; Branford, W.R.; Morris, B.; Yates, K.A.; Cohen, L.F. Influence of Growth Temperature and Carrier Flux on the Structure and Transport Properties of Highly Oriented CrO2 on Al2O3 (0001). Chem. Vapor Depos. 2007, 13, 537–545. [Google Scholar] [CrossRef]
- Solecka, M.; Kusinski, J.; Kopia, A.; Rozmus-Górnikowska, M.; Radziszewska, A. High-Temperature Corrosion of Ni-Base Alloys by Waste Incineration Ashes. Acta. Phys. Pol. A 2016, 130, 1045–1048. [Google Scholar] [CrossRef]
- Luo, M.; Ortiz, A.L.; Guo, F.; Shi, Z.; Li, L.; Ren, Y.; Zhang, X.; Chen, Z.; Shaw, L.L.; Chen, W. Mechanical activation enhanced solid-state synthesis of NaCrO2 cathode material. Materialia 2019, 5, 100172. [Google Scholar] [CrossRef]
- Fryburg, G.C.; Miller, R.A.; Kohl, F.J.; Stearns, C.A. Volatile Products in the Corrosion of Cr, Mo, Ti, and Four Superalloys Exposed to O2 Containing H2O and Gaseous NaCI. J. Electrochem. Soc. 1977, 124, 1738–1743. [Google Scholar] [CrossRef]
- Opila, E.J.; Myers, D.J.; Jacobson, N.S.; Nielsen, I.M.B.; Johnson, D.F.; Olminsky, J.K.; Allendorf, M.D. Theoretical and Experimental Investigation of the Thermochemistry of CrO2(OH)2(g). J. Phys. Chem. A 2007, 111, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Huang, T.; Song, P.; Chen, R.; Zheng, B.; Wang, C.; Li, C.; Lu, J. CrO2(OH)2 volatilization rate and oxidation behavior prediction of the NiCr coating in air-H2O environment at 650 °C. Corros. Sci. 2021, 182, 109303. [Google Scholar] [CrossRef]
- Bunting, E.N. Phase equilibria in the system Cr2O3-Al2O3. Bur. Stand. J. Res. 1931, 6, 948. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, H.; Yu, J.; Zhang, H.; Gao, H.; Chen, Q. Crystal structure and properties of Al2O3-Cr2O3 solid solutions with different Cr2O3 contents. Ceram. Int. 2018, 44, 1356–1361. [Google Scholar] [CrossRef]
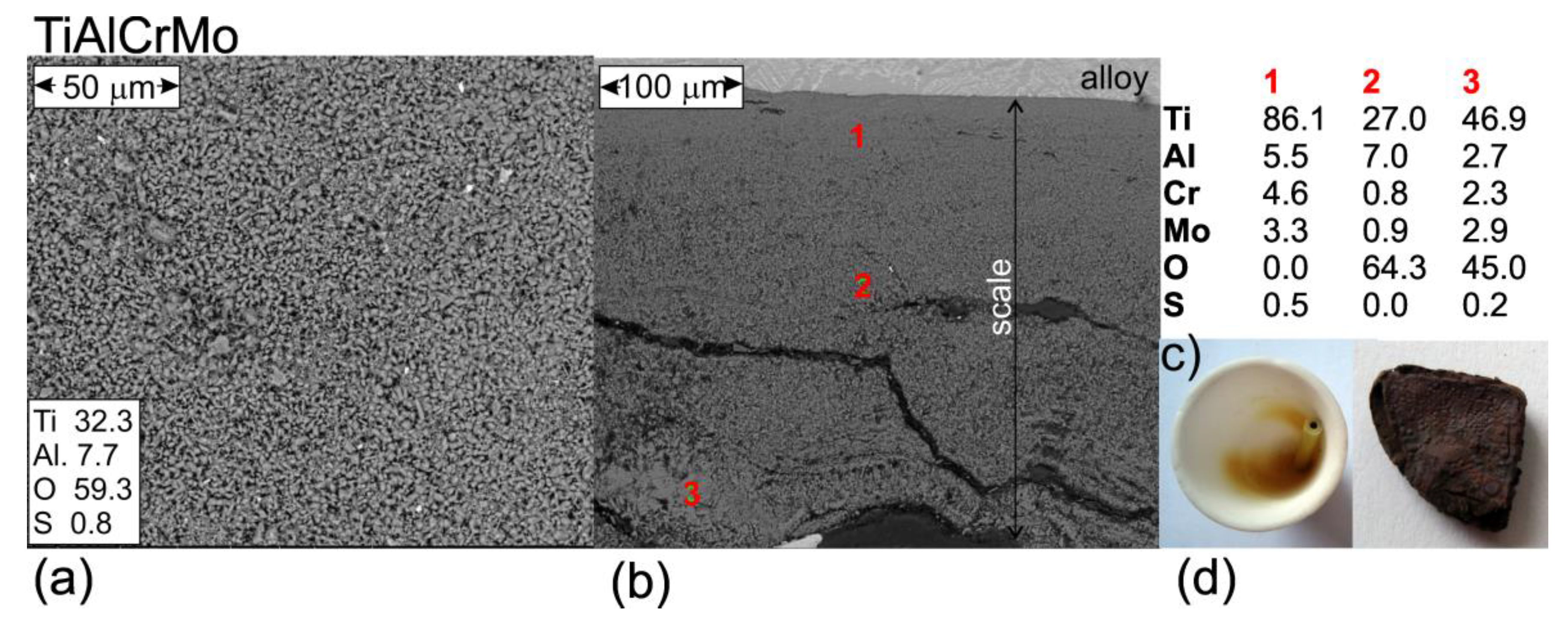
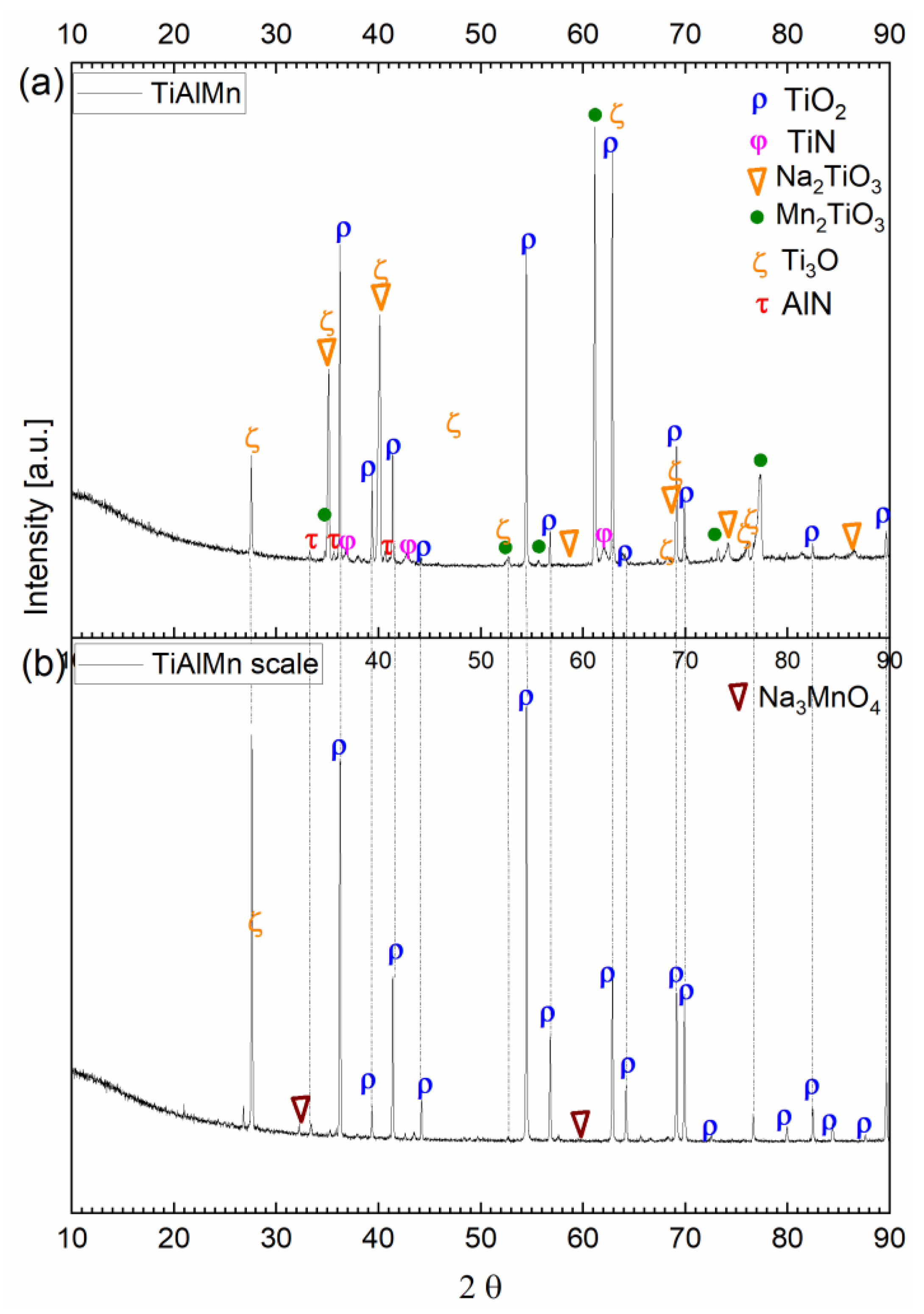
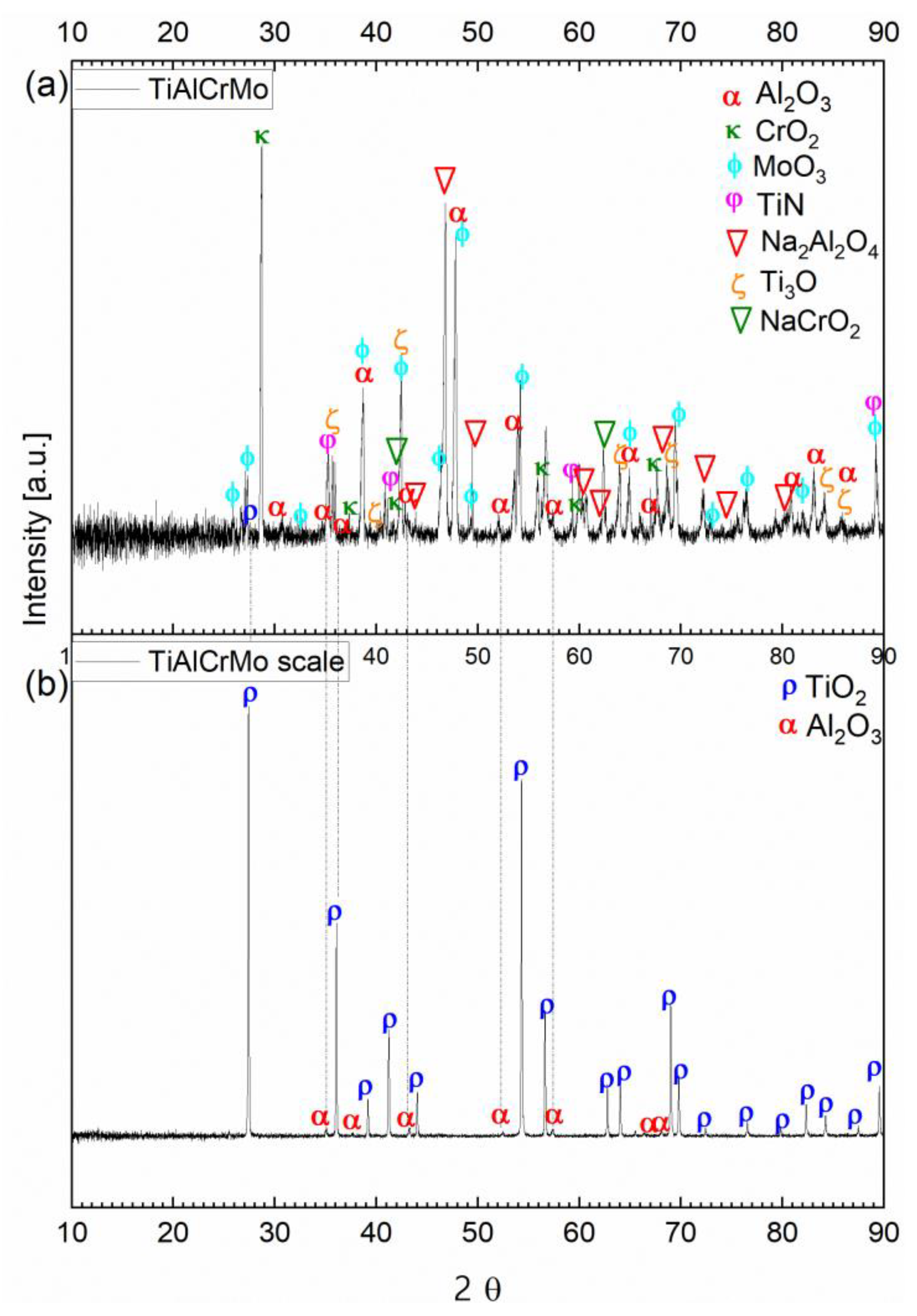
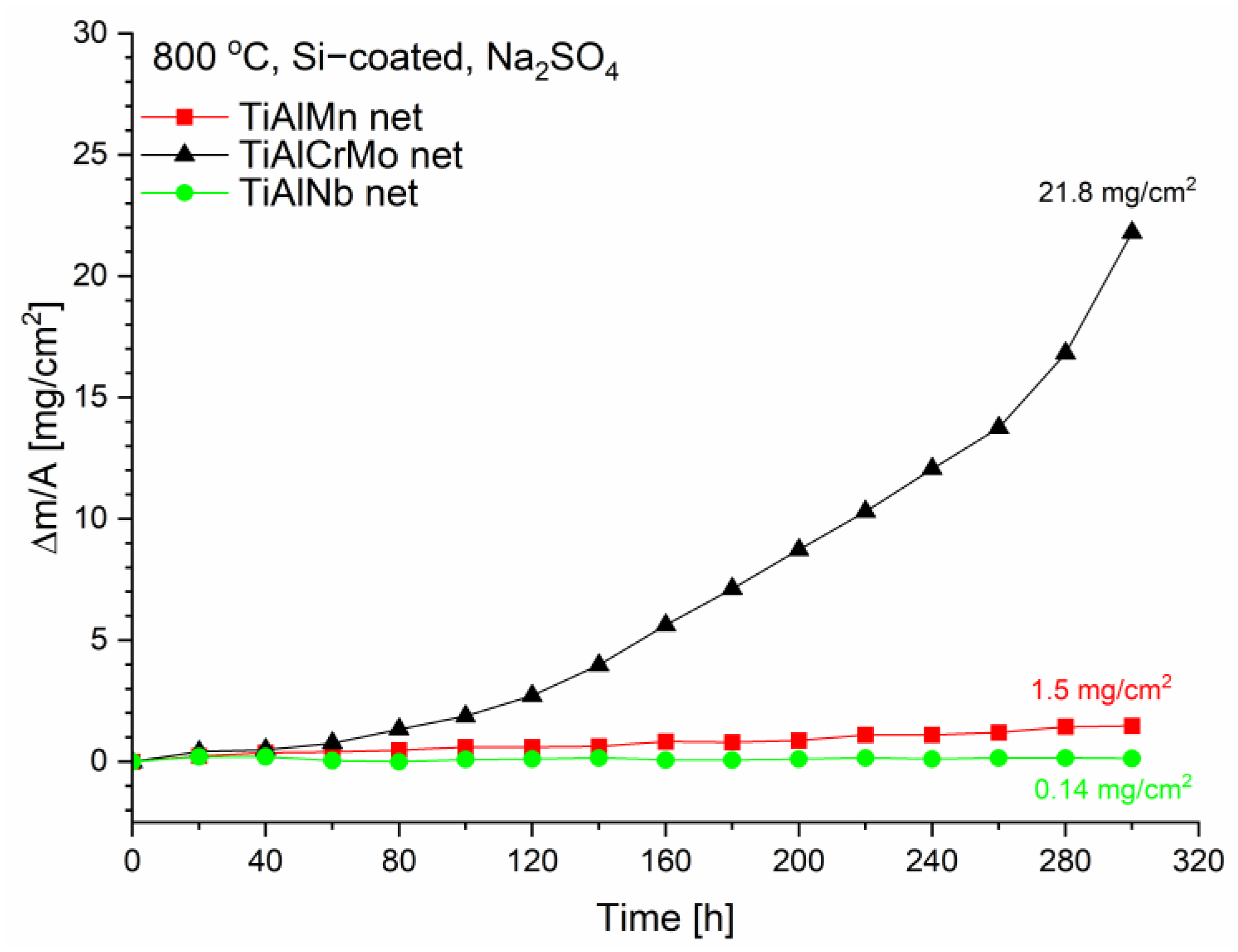


| Alloy | Ti [at%] | Al [at%] | Cr [at%] | Mn [at%] | Mo [at%] | Nb [at%] |
|---|---|---|---|---|---|---|
| Ti-5Al-2Mn (OT4-1) | 93.0–96.6 | 3.0–4.5 | - | 1.7–2.3 | - | - |
| EDS | 92.0 ± 1.8 | 4.4 ± 1.3 | - | 3.2 ± 1.0 | - | - |
| Ti-12Al-2Cr-2Mo (VT3-1) | 84.8–88.9 | 9.5–12.0 | 0.7–1.8 | - | 1.0–1.4 | - |
| EDS | 83.7 ± 1.4 | 10.8 ± 1.1 | 2.4 ± 0.9 | - | 2.7 ± 0.5 | - |
| Ti-46Al-8Nb | 46.1–46.4 | 45.9–46.3 | - | - | - | 7.6–7.9 |
| EDS | 51.7 ± 3.9 | 39.7 ± 3.4 | - | - | - | 8.6 ± 0.4 |
| Alloy | PVD Chromizing [mg/cm2] | CVD Siliconizing [mg/cm2] | CVD Siliconizing after PVD [mg/cm2] |
|---|---|---|---|
| Cr-Coating | Si-Coating | Cr-Si-Coating | |
| TiAlMn | 0.85 ± 0.09 | 1.75 ± 0.63 | 1.96 ± 0.26 |
| TiAlCrMo | 0.86 ± 0.14 | 1.97 ± 0.23 | 2.37 ± 0.27 |
| TiAlNb | 0.84 ± 0.16 | 1.60 ± 0.60 | 2.09 ± 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitoraj-Królikowska, M. Influence of Silicon and Chromium on the Na2SO4-Induced Hot Corrosion Behavior of Titanium Alloys. Crystals 2023, 13, 948. https://doi.org/10.3390/cryst13060948
Mitoraj-Królikowska M. Influence of Silicon and Chromium on the Na2SO4-Induced Hot Corrosion Behavior of Titanium Alloys. Crystals. 2023; 13(6):948. https://doi.org/10.3390/cryst13060948
Chicago/Turabian StyleMitoraj-Królikowska, Marzena. 2023. "Influence of Silicon and Chromium on the Na2SO4-Induced Hot Corrosion Behavior of Titanium Alloys" Crystals 13, no. 6: 948. https://doi.org/10.3390/cryst13060948
APA StyleMitoraj-Królikowska, M. (2023). Influence of Silicon and Chromium on the Na2SO4-Induced Hot Corrosion Behavior of Titanium Alloys. Crystals, 13(6), 948. https://doi.org/10.3390/cryst13060948






