The Crystal Structure of Manganotychite, Na6Mn2(CO3)4(SO4), and Structural Relations in the Northupite Group
Abstract
1. Introduction
2. Materials and Methods
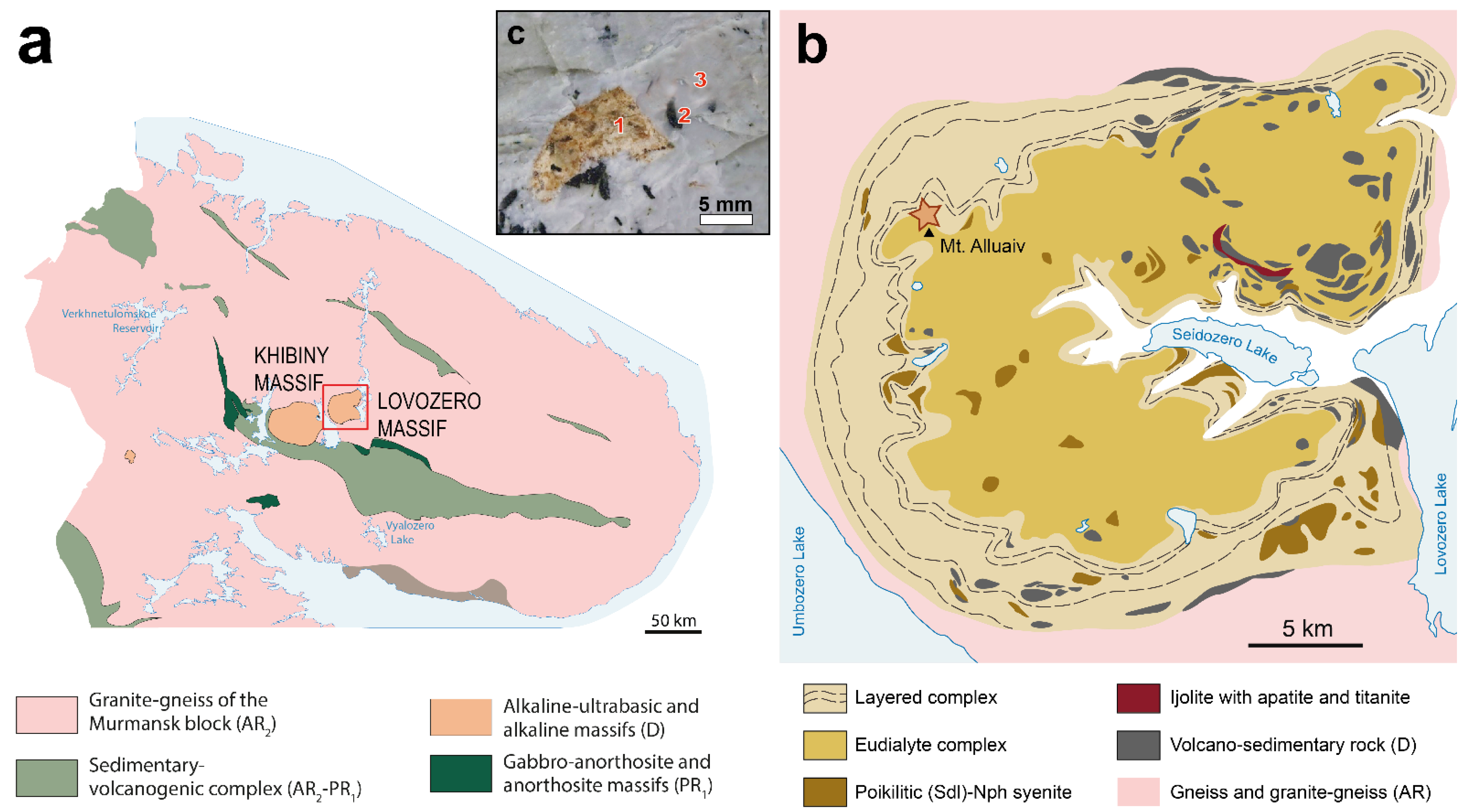
2.1. Sample Description and Composition
2.2. Single-Crystal X-ray Diffraction
3. Results
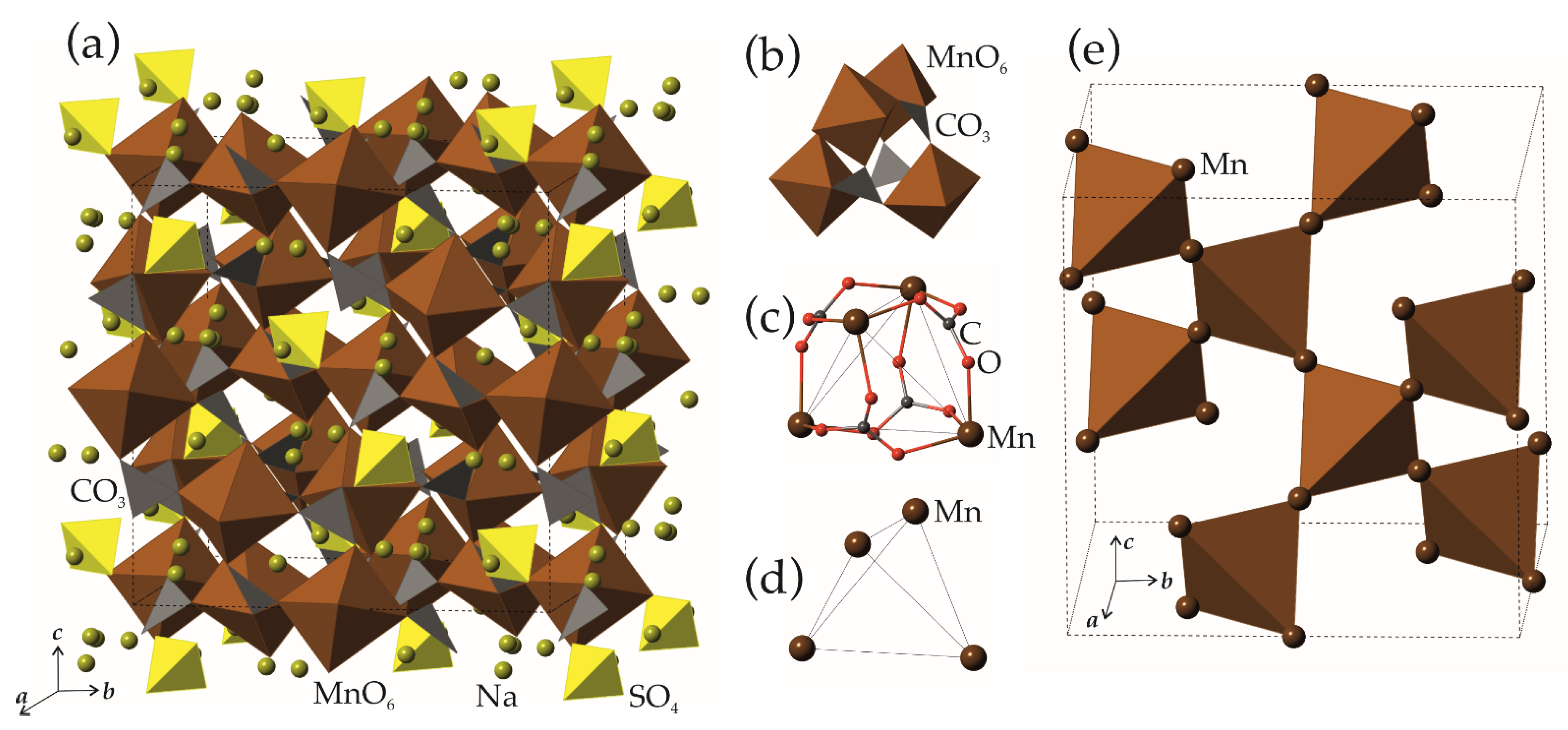
3.1. Structure Description
3.2. X-ray Diffraction versus Raman Spectroscopic Study
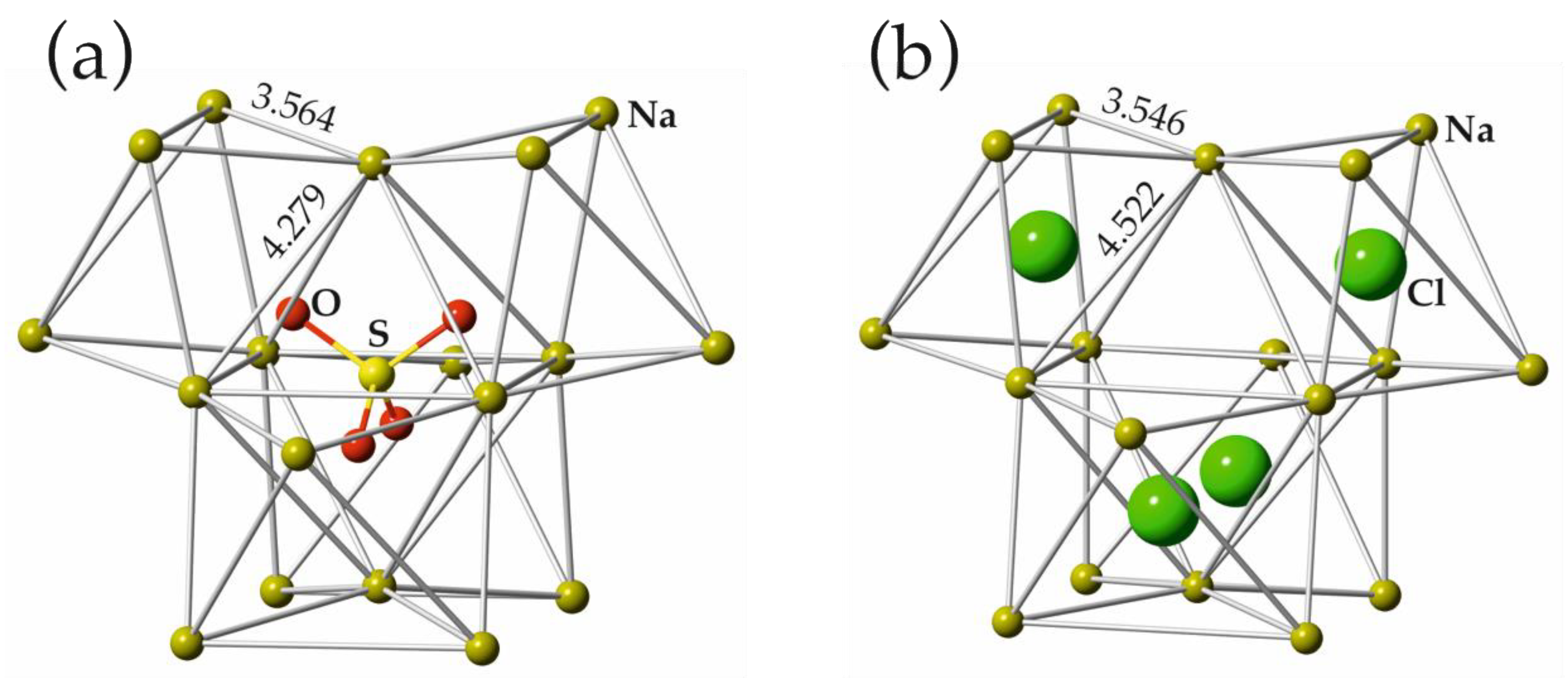
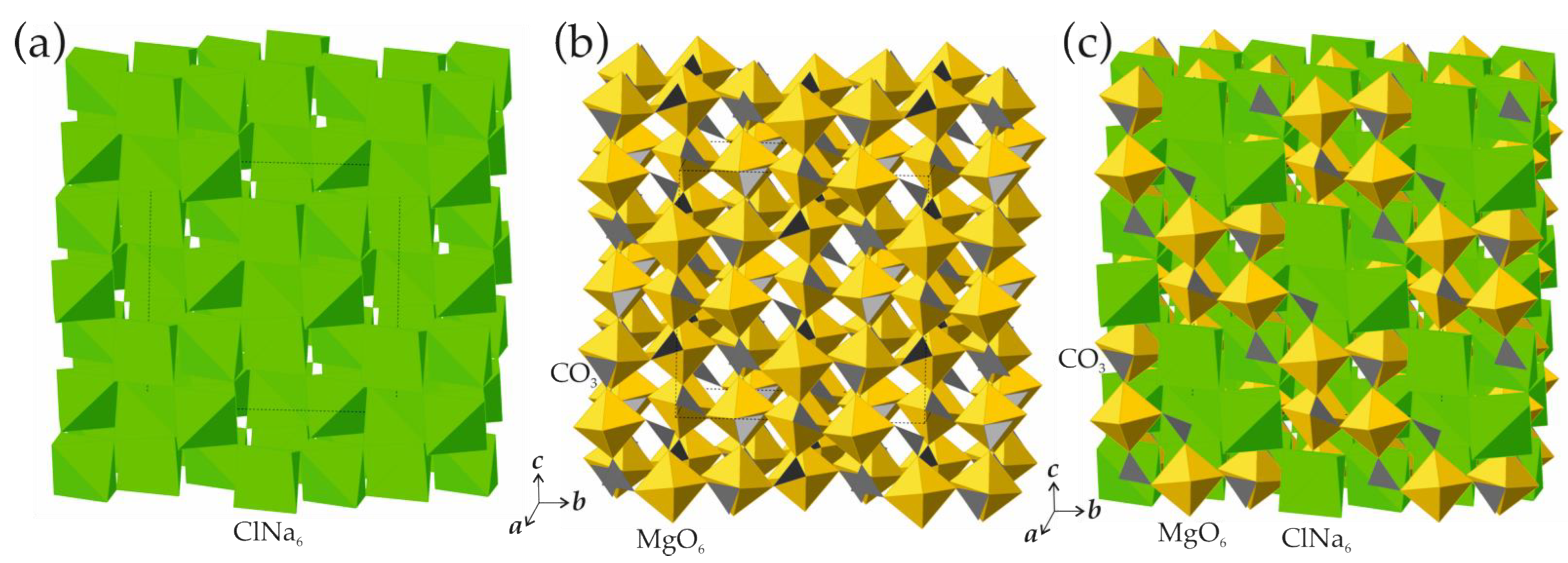
3.3. Structural Relations
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foote, W.M. Preliminary note on a new alkali mineral. Am. J. Sci. 1895, 150, 480–481. [Google Scholar] [CrossRef]
- Pratt, J.H. On northupite; pirssonite, a new mineral; Gaylussite and hanksite from Borax Lake, San Bernardino County, California. Am. J. Sci. 1896, 152, 123–135. [Google Scholar] [CrossRef]
- Penfield, S.L.; Jamieson, G.S. On tychite, a new mineral from Borax Lake, California, and on its artificial production and its relations to northupite. Am. J. Sci. 1905, 20, 217–224. [Google Scholar] [CrossRef]
- Penfield, S.L.; Jamieson, G.S. Über Tychit, ein neues Mineral vom Boraxsee in Californien, seine künstliche Darstellung und seine Beziehungen zum Northupit. Z. Krystallogr. Miner. 1906, 41, 235–242. [Google Scholar] [CrossRef]
- Fahey, J.J.; Mrose, M.E. Saline minerals of the Green River formation, with a section on X-ray powder data for saline materials of the Green River formation. USGS Prof. Paper 1962, 405, 50. [Google Scholar]
- Marchesini, M.; Barresi, A. The Wadi Natrun evaporite deposits, Western Desert, Buhayra Governate, Egypt. Mineral. Rec. 2019, 50, 723–748. [Google Scholar]
- Yu, K.-H.; Cao, Y.-C.; Qiu, L.-W.; Sun, P.-P.; Yang, Y.-Q.; Qu, C.-S.; Li, Y.-W.; Wan, M.; Su, Y.-G. Brine Evolution of Ancient Lake and Mechanism of Carbonate Minerals during the Sedimentation of Early Permian Fengcheng Formation in Mahu Depression, Junggar Basin, China. Nat. Gas Geosci. 2016, 27, 1248–1263. [Google Scholar]
- Kasedde, H.; Kirabira, J.B.; Bäbler, M.U.; Tilliander, A.; Jonsson, S. Characterization of brines and evaporites of Lake Katwe, Uganda. J. Afr. Earth Sci. 2014, 91, 55–65. [Google Scholar] [CrossRef]
- Khomyakov, A.P.; Malinovskii, Y.A.; Sandomirskaya, S.M. Ferrotychite, Na6Fe2(SO4)(CO3)4, a new mineral. Zap. Vses. Mineral. Obshch. 1981, 110, 600–603. (In Russian) [Google Scholar]
- Khomyakov, A.P.; Bakhchisaraitsev, A.Y.; Martynova, A.V.; Parashchenko, T.M. Manganotychite Na6Mn2(SO4)(CO3)4—A new mineral. Zap. Vses. Mineral. Obshch. 1990, 119, 46–49. (In Russian) [Google Scholar]
- Sharygin, I.S.; Golovin, A.V.; Korsakov, A.V.; Pokhilenko, N.P. Tychite in Mantle Xenoliths from Kimberlites: The First Find and a New Genetic Type. Dokl. Earth Sci. 2016, 467, 270–274. [Google Scholar] [CrossRef]
- Potapov, S.V.; Sharygin, I.S.; Konstantinov, K.M.; Danilov, B.S.; Shcherbakov, Y.D.; Letnikov, F.A. Melt Inclusions in Chromium Spinel of Kimberlites of the Zapolyarnaya Pipe, Upper Muna Field, Siberian Craton. Dokl. Earth Sci. 2022, 504, 271–275. [Google Scholar] [CrossRef]
- Gossner, B.; Koch, I. Über das Kristallgitter von Langbeinit, Northupit und Hanksit. Z. Kristallogr. Miner. Petrogr. 1931, 80, 455–464. [Google Scholar]
- Shiba, H.; Watanabé, T. Les structures des cristaux de northupite, de northupite bromée et de tychite. Comptes Rend Hebd Acad. Sci. 1931, 193, 1421–1423. [Google Scholar]
- Watanabé, T. Les structures cristallines de la northupite 2MgCO3.2Na2CO3.2NaCl et de la tychite 2MgCO3.2Na2CO3.Na2SO4. Sci. Papers Inst. Phys. Chem. Res. 1933, 21, 40–62. [Google Scholar]
- Dal Negro, A.; Giuseppetti, G.; Tadini, C. Refinement of the crystal structure of northupite: Na3Mg(CO3)2Cl. Tscherm. Miner. Petrogr. Mitt. 1975, 22, 158–163. [Google Scholar] [CrossRef]
- Schmidt, G.R.; Jacqueline, R.; Yang, H.; Downs, R.T. Tychite, Na6Mg2(SO4)(CO3)4: Structure Analysis and Raman Spectroscopic Data. Acta Crystallogr. 2006, E62, i207–i209. [Google Scholar] [CrossRef]
- Malinovskii, Y.A.; Baturin, S.V.; Belov, N.V. The crystal structure of the Fe-tychite. Dokl. Akad. Nauk SSSR 1979, 249, 1365–1368. [Google Scholar]
- Palaich, S.E.M.; Manning, C.E.; Schauble, E.; Kavner, A. Spectroscopic and X-ray Diffraction Investigation of the Behavior of Hanksite and Tychite at High Pressures, and a Model for the Compressibility of Sulfate Minerals. Am. Miner. 2013, 98, 1543–1549. [Google Scholar] [CrossRef]
- Sidorov, M.; Kompanchenko, A.; Fomina, E.; Kozlov, E. Raman Spectroscopic Study of Northupite Group Minerals (Tychite, Manganotychite, and Ferrotychite). Zap. Ross. Mineral. Obshch. 2022, 151, 94–101. (In Russian) [Google Scholar]
- Zheng, Y.; Ellern, A.; Kögerler, P. A Spin-Frustrated Cobalt(II) Carbonate Pyrochlore Network. Acta Crystallogr. 2011, C67, i56–i58. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Zheng, Y.; Xiao, Y.; Bedanta, S.; Senyshyn, A.; Simeoni, G.G.; Su, Y.; Rücker, U.; Kögerler, P.; Brückel, T. Coexistence of Magnetic Order and Spin-Glass-like Phase in the Pyrochlore Antiferromagnet Na3Co(CO3)2Cl. Phys. Rev. B 2013, 87, 214406. [Google Scholar] [CrossRef]
- Nawa, K.; Okuyama, D.; Avdeev, M.; Nojiri, H.; Yoshida, M.; Ueta, D.; Yoshizawa, H.; Sato, T.J. Degenerate Ground State in the Classical Pyrochlore Antiferromagnet Na3Mn(CO3)2Cl. Phys. Rev. B 2018, 98, 144426. [Google Scholar] [CrossRef]
- Khomyakov, A.P. Mineralogical features of alkaline pegmatites of the Khibino-Lovozero Province. In Development of Mineralogy and Geochemistry and Their Relation to the Science of Minerals; Nauka: Moscow, Russia, 1983; pp. 66–82. (In Russian) [Google Scholar]
- Panikorovskii, T.L.; Mikhailova, J.A.; Pakhomovsky, Y.A.; Bazai, A.V.; Aksenov, S.M.; Kalashnikov, A.O.; Krivovichev, S.V. Zr-Rich Eudialyte from the Lovozero Peralkaline Massif, Kola Peninsula, Russia. Minerals 2021, 11, 982. [Google Scholar] [CrossRef]
- Agilent Technologies. CrysAlisPro; Version 1.171.36.20; Tokyo, Japan, 2012. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, F.C.; Krivovichev, S.V.; Burns, P.C. The Crystal Chemistry of Sulfate Minerals. Rev. Miner. Geochem. 2000, 40, 1–112. [Google Scholar] [CrossRef]
- Alexandrov, E.V.; Blatov, V.A.; Kochetkov, A.V.; Proserpio, D.M. Underlying Nets in Three-Periodic Coordination Polymers: Topology, Taxonomy and Prediction from a Computer-Aided Analysis of the Cambridge Structural Database. CrystEngComm 2011, 13, 3947–3958. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Filatov, S.K. Structural Principles for Minerals and Inorganic Compounds Containing Anion-Centered Tetrahedra. Am. Mineral. 1999, 84, 1099–1106. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Minerals with antiperovskite structure: A review. Z. Kristallogr. 2008, 223, 109–113. [Google Scholar] [CrossRef]
- Bailey, M.S.; Shen, D.Y.; McGuire, M.A.; Fredrickson, D.C.; Toby, B.H.; DiSalvo, F.J.; Yamane, H.; Sasaki, S.; Shimada, M. The Indium Subnitrides Ae6In4(InxLiy)N3-z (Ae = Sr and Ba). Inorg. Chem. 2005, 44, 6680–6690. [Google Scholar] [CrossRef]
- Pathak, M.; Stoiber, D.; Bobnar, M.; Ovchinnikov, A.; Ormeci, A.; Niewa, R.; Höhn, P. Synthesis, Characterization, and Chemical Bonding Analysis of the Lithium Alkaline-Earth Metal Gallide Nitrides Li2(Ca3N)2[Ga4] and Li2(Sr3N)2[Ga4]. Z. Anorg. Allg. Chem. 2017, 643, 1557–1563. [Google Scholar] [CrossRef]
- Leonard, S.R.; Snyder, B.S.; Brewer, L.; Stacy, A.M. Structure Determinations of Two New Ternary Oxides: Ti3PdO and Ti4Pd2O. J. Solid State Chem. 1991, 92, 39–50. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Topological complexity of crystal structures: Quantitative approach. Acta Crystallogr. 2012, A68, 393–398. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity of minerals: Information storage and processing in the mineral world. Miner. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Krivovichev, V.G.; Hazen, R.M.; Aksenov, S.M.; Avdontceva, M.S.; Banaru, A.M.; Gorelova, L.A.; Ismagilova, R.M.; Kornyakov, I.V.; Kuporev, I.V.; et al. Structural and chemical complexity of minerals: An update. Mineral. Mag. 2022, 86, 183–204. [Google Scholar] [CrossRef]
- Kampf, A.R.; Rossman, G.R.; Ma, C.; Belmonte, D.; Biagioni, C.; Castellaro, F.; Chiappino, L. Ramazzoite, [Mg8Cu12(PO4)(CO3)4(OH)24(H2O)20][(H0.33SO4)3(H2O)36], the first mineral with a polyoxometalate cation. Eur. J. Mineral. 2018, 30, 827–834. [Google Scholar] [CrossRef]
- Elliott, P.; Giester, G.; Rowe, R.; Pring, A. Putnisite, SrCa4Cr3+8(CO3)8SO4(OH)16·25H2O, a new mineral from Western Australia: Description and crystal structure. Mineral. Mag. 2014, 78, 131–144. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Polyoxometalate clusters in minerals: Review and complexity analysis. Acta Crystallogr. 2020, B76, 618–629. [Google Scholar] [CrossRef]
- Hazen, R.M.; Morrison, S.M. On the paragenetic modes of minerals: A mineral evolution perspective. Am. Miner. 2022, 107, 1262–1287. [Google Scholar] [CrossRef]
| Temperature/K | 293(2) |
| Crystal system | cubic |
| Space group | Fd |
| a/Å | 14.0015(3) |
| Volume/Å3 | 2744.88(18) |
| Z | 8 |
| Dcalc, g/cm3 | 2.788 |
| μ/mm−1 | 2.071 |
| F(000) | 2245.0 |
| Crystal size/mm3 | 0.18 × 0.14 × 0.14 |
| Radiation | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 8.234 to 65.960 |
| Index ranges | −15 ≤ h ≤ 21, −16 ≤ k ≤ 12, −20 ≤ l ≤ 8 |
| Reflections collected | 1340 |
| Independent reflections | 388 [Rint = 0.0182, Rsigma = 0.0166] |
| Data/restraints/parameters | 388/0/26 |
| Goodness-of-fit on F2 | 1.129 |
| Final R indices [I ≥ 2σ (I)] | R1 = 0.0201, wR2 = 0.0532 |
| Final R indices [all data] | R1 = 0.0214, wR2 = 0.0536 |
| Largest diff. peak/hole/e Å−3 | 0.24/−0.51 |
| Site | s.o.f. | x/a | y/b | z/c | Uiso |
|---|---|---|---|---|---|
| M | Mn0.87Mg0.13 | ½ | ½ | ½ | 0.0092(2) |
| S | S | 3/8 | 1/8 | 3/8 | 0.0121(2) |
| Na | Na | 3/8 | 0.65892(5) | 3/8 | 0.0193(2) |
| O1 | O | 0.31404(6) | 0.81404(6) | 0.43596(6) | 0.0194(3) |
| O2 | O | 0.48237(6) | 0.52891(5) | 0.34933(5) | 0.0137(2) |
| C | C | 0.47028(7) | 0.47028(7) | 0.27972(7) | 0.0098(3) |
| M–O | 2.1622(8) 6× | Na–O1 | 2.4108(8) 2× |
| S–O | 1.4784(14) 4× | Na–O2 | 2.3880(10) 2× |
| C–O | 1.2855(7) 3× | Na–O1 | 2.4848(7) 2× |
| <Na–O> | 2.4279 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krivovichev, S.V.; Panikorovskii, T.L.; Bazai, A.V.; Sidorov, M.Y. The Crystal Structure of Manganotychite, Na6Mn2(CO3)4(SO4), and Structural Relations in the Northupite Group. Crystals 2023, 13, 800. https://doi.org/10.3390/cryst13050800
Krivovichev SV, Panikorovskii TL, Bazai AV, Sidorov MY. The Crystal Structure of Manganotychite, Na6Mn2(CO3)4(SO4), and Structural Relations in the Northupite Group. Crystals. 2023; 13(5):800. https://doi.org/10.3390/cryst13050800
Chicago/Turabian StyleKrivovichev, Sergey V., Taras L. Panikorovskii, Ayya V. Bazai, and Mikhail Yu. Sidorov. 2023. "The Crystal Structure of Manganotychite, Na6Mn2(CO3)4(SO4), and Structural Relations in the Northupite Group" Crystals 13, no. 5: 800. https://doi.org/10.3390/cryst13050800
APA StyleKrivovichev, S. V., Panikorovskii, T. L., Bazai, A. V., & Sidorov, M. Y. (2023). The Crystal Structure of Manganotychite, Na6Mn2(CO3)4(SO4), and Structural Relations in the Northupite Group. Crystals, 13(5), 800. https://doi.org/10.3390/cryst13050800








