Abstract
Protein crystallization is nowadays performed at the micro to macro scale in academia and industry, being particularly interesting for pharmaceutical applications. Protein crystallization offers an attractive alternative to chromatography as a downstream processing step in the biotechnology industry, but also in the food and chemical industries. Monitoring of the protein crystallization process is required to understand the crystal growth mechanism and to obtain the information necessary for efficient process control, which needs to comply with the critical quality attributes of the product. Since a wide range of monitoring techniques have already been developed and established, this review focuses on the recent advances of selected techniques in monitoring protein crystallization processes such as the focused beam reflectance method (FBRM), and machine learning-based image analysis for solid-phase monitoring, as well as the spectroscopic methods for liquid-phase monitoring, such as attenuated total reflectance Fourier transform infrared (ATR-FTIR) and UV/Vis spectroscopy.
1. Introduction
The industrial production of biopharmaceuticals has increased rapidly in recent years [1], which has accentuated the bottleneck in the efficient downstream processing of biomolecules. Here, the designation of “anything but chromatography” evolved, with one potential alternative being crystallization [1]. Protein crystallization is a natural phenomenon that occurs when molecules in solution are in a supersaturated state, until the solubility line is reached through the metastable state. The supersaturated state can be artificially achieved by increasing the molecule’s concentration while decreasing the temperature, by evaporation or by adding an antisolvent. This increased protein density strengthens the electrostatic and hydrophobic interactions. For a correct setting of the physical and chemical process parameters, the interacting proteins eventually pass through intermediate nucleation steps and arrange spatially in a periodic manner [2], forming crystals.
Crystals from macromolecules such as proteins, DNA, viruses and other large organic molecules are produced for diverse academic and industrial purposes. While protein crystallization has been broadly applied for structure determination, recent trends include a consideration of it in the context of downstream processing for product separation, purification, and formulation. Here, the high integrity and concentration levels of purified protein crystals, the long shelf life, as well as the practical scalability of the crystallization process, are attractive for industrial applications [2].
In general, crystallization processes can be carried out as batch, semi-batch or continuous processes regardless of scale [3,4]. Nevertheless, batch processes are often preferred in the pharmaceutical industry since inline process control can be achieved effortlessly. Therefore, the final crystals’ critical quality attributes such as crystal size, shape and homogeneity can be controlled [5,6]. Despite the batch process being conceptionally simple, there are currently no standard crystallization conditions that can be applied. Since each protein is intrinsically different in regard to its complex structure, the protein’s biochemical characteristics are affected [7]. It was demonstrated that protein crystallization processes and their success are very sensitive even down to single amino acid exchanges [8,9,10,11,12,13]. In addition, the crystallization process is challenging to control [14] and often needs to be optimized empirically, especially as nucleation, which is the step prior to crystal growth, is a stochastic phenomenon influenced by various parameters such as supersaturation, agitation, presence of impurities, and non-ideal mixing conditions [14]. Therefore, crystallization optimization procedures, as well as active process control in industrial settings, require reliable inline monitoring capabilities. More specifically, it is important to monitor the crystallization processes’ critical quality attributes in order to find the ideal crystallization conditions and to enable higher process stability and product quality.
Monitoring (preparative) crystallization processes of small molecules have already been reviewed extensively [6,14,15,16,17] and crystallization platforms with combinations of monitoring devices are commonly available [18]. Additionally, process analytical technologies (PAT) for the quality control of protein crystallization with a focus on monitoring with Raman spectroscopy were recently discussed [17]. Thus, Raman spectroscopy is not covered in this overview.
However, studies monitoring small molecules cannot be completely transferred to the crystallization of biomolecules such as proteins. This is because this process is highly variable due to their complex biological structure and heterogeneity, and the biological variation of the raw material [19,20].
In this work, recent advances focusing on the applicability of monitoring tools for protein crystallization processes are reviewed. Here, the focused beam reflectance measurement (FBRM) and the automated analysis of microphotographs by machine learning (ML)-based image analysis are discussed as non-destructive solid-phase monitoring methods. Additionally, spectroscopic approaches, often coupled with supersaturation state control, are reviewed for liquid-phase monitoring applications.
2. Solid-Phase Monitoring in Protein Crystallization Processes
Monitoring crystal morphology, porosity and size distribution during the crystallization process is relevant, as these characteristics are directly linked to the following downstream processing steps: filtration, sedimentation, and fluidization [21]. Crystal morphology, the grade of polymorphism, the degree of agglomeration, and impurities in the crystal lattice are also relevant variables for the pharmaceutical industry, for these characteristics influence the purity, stability, dissolution properties [22], and shelf life [2] of the final products.
2.1. Laser Backscattering; Focused Beam Reflectance Measurement (FBRM)
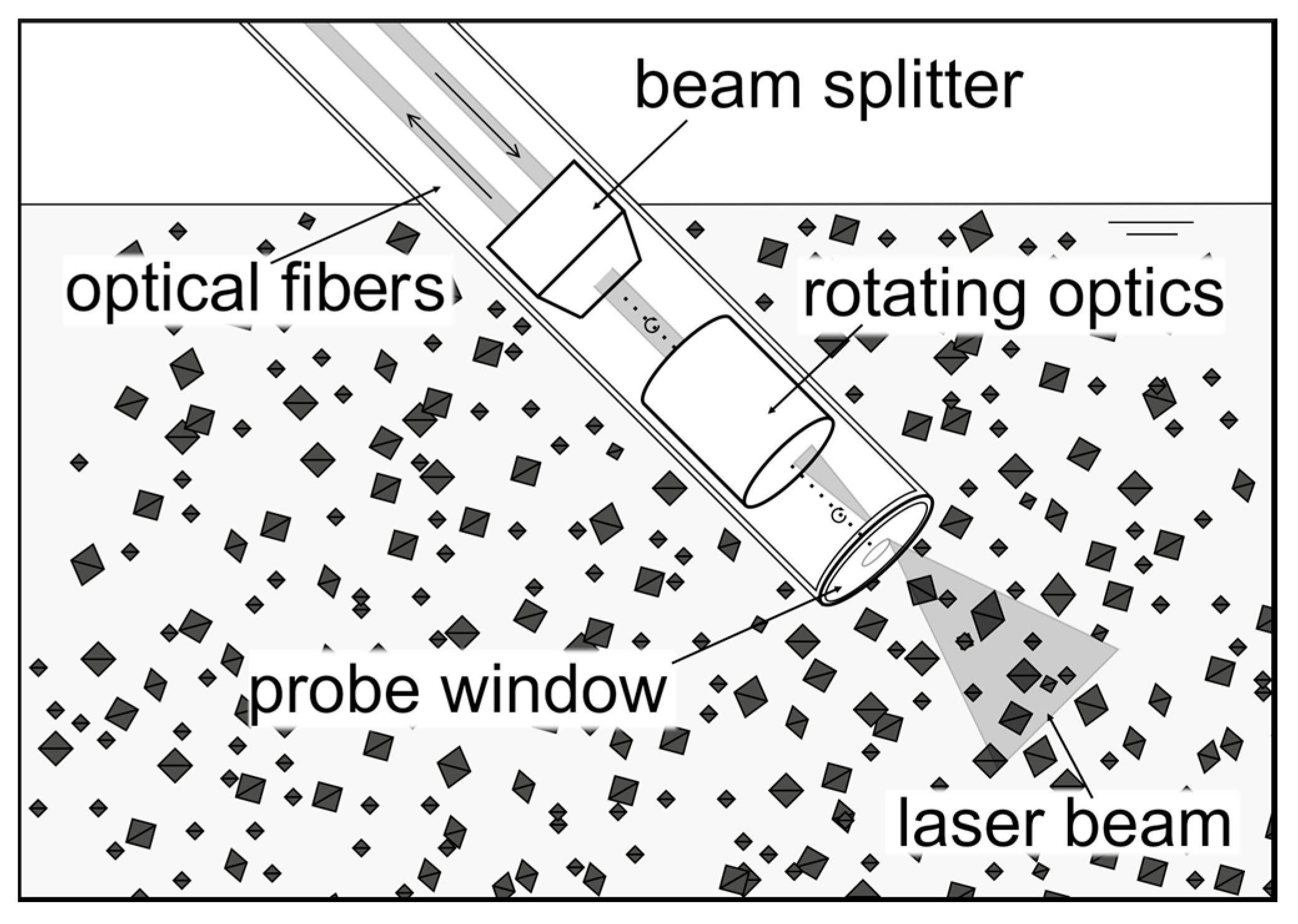
If crystal size is of primary interest during the protein purification process, FBRM is largely applied as an in situ measurement tool. FBRM also provides information about nucleation, growth, dissolution, and polymorphic transformation, in addition to the size distribution data of the crystal population in the particle size range of 0.1–1000 µm [23,24]. FBRM can be applied as an inline measurement for chord length distribution (CLD), which reflects in real time the size and shape of the protein, as well as the number of crystals dispersed within the reactor (schematically illustrated in Figure 1).
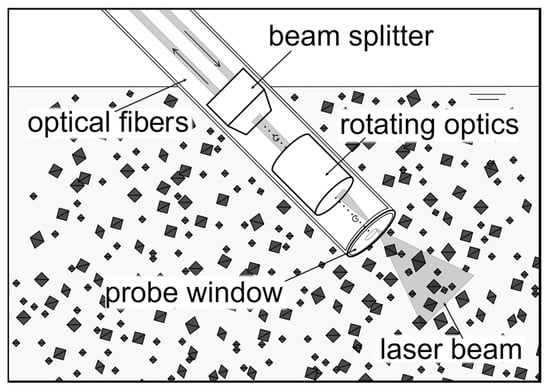
Figure 1.
Schematic sketch of a focused beam reflectance measurement (FBRM) probe in a crystal solution. The probe is inserted at an angle to ensure particle flow across the probe window. The rotating laser beam rapidly scans the particles flowing past the probe window. The backscattered light provides the chord length distribution data on the scattered particles (crystals are not in scale to probe). The sketch was generated using draw.io.
Since FBRM analyzes backscattering from a rotating monochromatic laser beam, it is able to be operated even in concentrated slurries. Therefore, additional dilution steps or external sampling, which are required in online and offline measurements, are not required [24]. This method is based on the reflection of scattered laser light which is encountered in the particle system, so it is highly dependent on the optical properties of the particles’ surface. FBRM is an alternative to offline particle size measurement methods, such as laser diffraction or sieve analysis [24]. While FBRM is already established for monitoring the crystallization of small molecules [24,25,26,27,28], only a few publications have applied it to biological macromolecules.
Similarly to other protein crystallization monitoring techniques, FBRM is mainly used in batch processes, with few exceptions that have been carried out in the continuous mode. This mode of operation through a tubular crystallizer was applied for the selective crystallization of L-glutamic acid [3]. In this study, FBRM was employed for in situ measurement and control of the size distribution of the growing crystals, enabling the selective production of the α-form of the amino acid with a uniform mean size of 130 µm. As a limitation of FBRM was the output form, which was the particle counts and chord length distribution, the evaluation of crystal size distribution became possible, as shown when Pandit et al. [29] demonstrated the applicability of inversion models to convert chord length distribution data from low-aspect-ratio crystals received from FBRM to crystal size distribution data, using purified lysozyme, recombinant human insulin, and vitamin B12 solutions as model biopharmaceuticals crystallizing in a stirred batch vessel in accordance with Smejkal et al. [30]. Within this study, the crystal size distribution was monitored in real time for low-aspect-ratio crystals with tetragonal (lysozyme), rhombohedral (recombinant human insulin), and polyhedral (vitamin B12) shapes [29]. FBRM is also a relevant method for detecting the preliminary step of crystallization, nucleation, as many other methods fail at detecting the initial nuclei in a large crystallizer [31]. As the impact of direct nucleation on crystal size distribution data was already revealed before [32], the parameter estimation of the secondary nucleation kinetics of biomolecules was determined in a simulation by Unno et al. [33]. Using structurally less complex L-arginine for the establishment of a model to predict the kinetic parameters of nucleation for predetermining crystal size distribution data, FBRM was used as a final validation step. Despite the advantages of FBRM, it was demonstrated that in the case of high-aspect-ratio (>4) crystals, e.g., needle-shaped crystals, there is an intrinsic limitation to using FBRM for length-based crystal size measurement [29], as infinitely long length crystals were assumed for the model (invalid for the aspect ratio of 1). Additionally, this shape evolves during crystallization and FBRM relies on a single size descriptor [34]. In addition, the robustness and accuracy of the data-driven model need to be improved in order for it to be considered as a quantitative monitoring tool for crystal size distribution [28,29]. It must be pointed out that one major challenge for FBRM is the occurrence of aggregation and/or precipitation in protein crystallization processes [29], which might interfere with the focused laser beam and affect the pulse intensity and duration of the backscattered light.
2.2. Microscopic Image Analysis
Crystal size, a random variable, is difficult to characterize during crystallization processes. Noise factors during crystallization such as precipitation, aggregation or thin-film effects affect the identification and shape definition of crystals through traditional detection methods, as described in Gan et al. [35]. As an example, FBRM is easily influenced by crystal properties or operating conditions [18]. As early as in the 1990s, this problem was tackled by using microscopy [36]. While being non-invasive and cost-effective, microscopic photography and the automated image evaluation of protein crystallization techniques remain challenging.
The results of optical microscopy imaging depend on the illumination of the sample and the system’s refractive index, as well as on sample composition with regard to noise through aggregation, precipitation, and heterogeneous particles [37].
Additionally, morphology might vary for proteins that crystallize in different crystal systems, or due to changes in the crystallization conditions, as can be seen in Figure 2. For bright-field imaging, the crystals appear mainly transparent and their edges are only partially visible, therefore complicating common image analysis methods. On the other hand, imaging methods exist that are more selective towards the crystallized phase of the crystallization slurry. For example, second-order nonlinear optical imaging [38] or its complement two-photon-excited UV fluorescence microscopy [39] has many advantages when it comes to robustness in the presence of noise, e.g., being able to distinguish between protein crystals and salt crystals. However, even recent iterations require elaborate experimental setups and data acquisition times of multiple seconds per image [40], rendering them impractical for use in stirred industrial bioreactors. Additionally, image analysis and the extraction of crystal size distributions are still left as challenges, especially in systems of high protein crystal density.

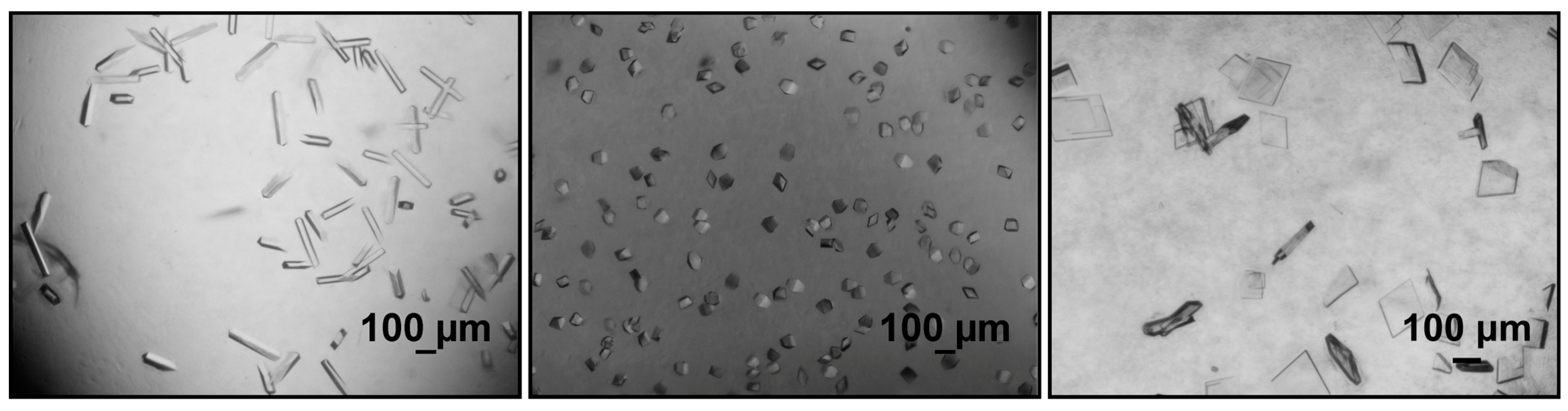
Figure 2.
Bright-field photomicrographs of batch crystallization studies with purified protein, resulting in different crystal shapes. Lactobacillus brevis alcohol dehydrogenase variants (left, middle) crystallize in needle-shaped and cubes, respectively. Nostoc sp. PCC 7120 ene-reductase variant (right) crystallizes in platelets (photomicrographs provided by the Chair of Biochemical Engineering, Technical University of Munich, unpublished results).
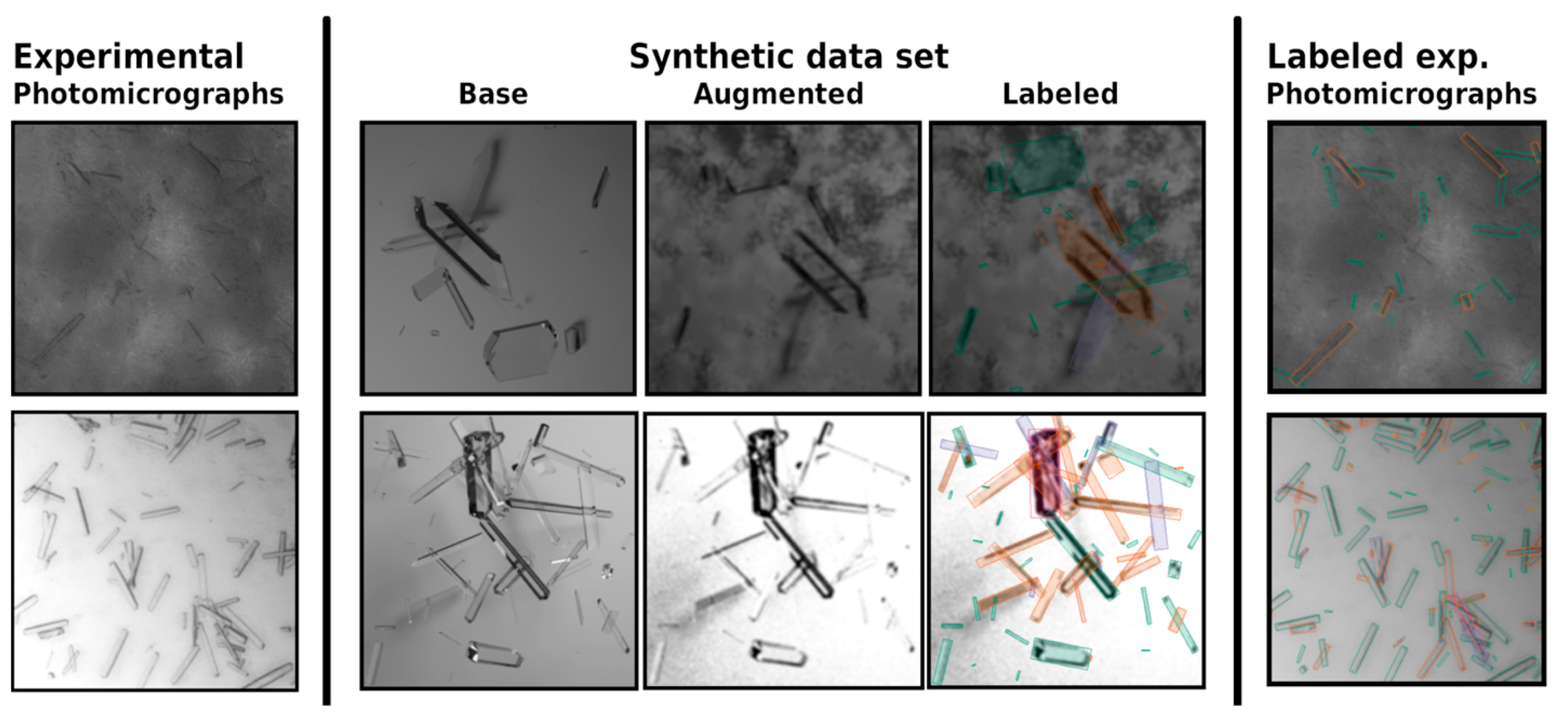
Initial work on image analysis was based on image preprocessing, segmentation, thresholding, and filtering steps that would detect crystal objects in the absence of noise in the images if their shape was known prior to the experiment [36,41]. The SHARC and M-SHARC algorithms were introduced as an alternative to segmentation-based approaches. They rely on a pattern matching strategy, in which nearby parallel edges are matched and analyzed together, with M-SHARC storing multiple internal crystal wire-frame models to compare against [42,43]. For these manually implemented algorithms, susceptibility to noise in images, and the partial visibility of crystal edges in low-contrast situations, as well as the required prior knowledge about the expected crystal shape remain challenges. With the success of deep learning [44] and convolutional neural networks in image analysis applications, novel tools for the detection of crystals from images started being developed. Object detection of individual crystals using deep learning was first introduced using mask region-based convolutional neural networks (Mask R-CNN) by He et al. [45] for α- and β-forms of L-glutamic acid [46,47]. Two drawbacks of this model were the use of non-oriented detection boxes leading to decreasing detection performance for dense systems of elongated crystals and the use of a relatively slow two-stage detector model. The latter issue was improved in later work by using a one-stage detector model based on RetinaNet [48,49]. Additionally, research focusing on the areal detection scenarios from satellite images progressed oriented object detection (OOD). The first application of an OOD model in protein crystals was performed by Wu et al. [18] using the single-shot alignment network (S2A-Net) by Han et al. [50] for a model system of taurine, experimentally validating the trained model through FBRM. However, modern deep learning-based approaches train models with a large number of parameters distributed across multiple successive layers. Using supervised learning to train these networks means that large labeled data sets need to be accessible. The number of images in this data set, their variety, labeling quality, and overall similarity to microscopic images in a production environment contribute to the trained models’ performance. With limited resources, these requirements cannot be met using human labeling. Instead, Bischoff et al. [37] designed a synthetic data set of 332,558 photorealistic images of protein crystals in suspension. While individual crystals in the data set were designed to be unbiased with respect to the crystals’ sizes, their aspect ratio, and angle within the image, the implementation of specialized data augmentations introduces synthetic noise to more accurately modeled realistic experimental photomicrographs (see Figure 3). Synthetic noise includes protein-specific noise (visible aggregation, precipitation, and heterogeneous particles), as well as instrument-dependent noise (brightness gradients, reflections, and contrast). These noise attributes were addressed by using blurring, brightness/contrast variations, edges distortions, rounded corner overlay, thin-film interference patterns, Perlin noise, per-object highlights, and random splines on the images to generate an augmented synthetic data set [37].
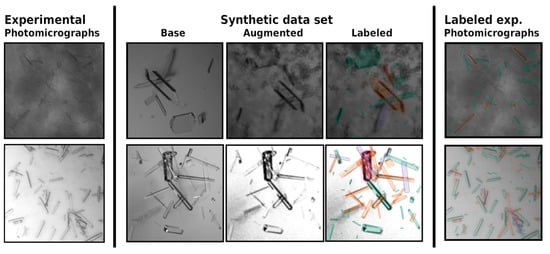
Figure 3.
Experimental photomicrographs (left) as basis of an augmented synthetic data set (middle) generated in accordance with Bischoff et al. [37]. Using this synthetic data set, a S2A-Net model was trained that could detect crystals in the original experimental photomicrographs (right), even when noise was present. The trained model directly output the crystal size distributions (length and width) compared in the original publication for undiluted (top) and diluted (bottom) samples.
The trained models were again based on S2A-Net, with slight modifications to the initial layers that improved the models’ capabilities to detect small crystals within the image. Overall performance was evaluated using an augmented validation data set, as well as using a joint evaluation of diluted and undiluted and therefore noisy samples from a Lactobacillus kefir alcohol dehydrogenase (LkADH) crystallization process in 5 mL of a stirred crystallizer. No statistically significant differences were observed between the diluted and undiluted samples during protein crystallization in an E. coli cell lysate, demonstrating the high potential to be used as a low-cost, inline protein crystal size distribution data monitoring tool for impure sources. However, the included example of evaluated real bright-field photomicrographs in Figure 3 hints at a drawback of the presented method. The labeled noisy photomicrograph (right) contains a few unlabeled crystals that might have been included by human labeling. The reason for the absence of these labels is not apparent, a drawback that is sometimes referred to as the explainability problem for deep neural networks. Additional training that adjusts the behavior of the model for particular systems requires additional computational resources and time-consuming fine-tuning steps and data acquisition.
The software of the endoscopic probes is able to take photomicrographs usually at >1–6 Hz [51,52,53], providing 2D pictures in real time. Nagy et al. [15] have already listed the diversity of endoscopic probes for imaging developed by industry and academia in 2013. Since in situ microscopy probes are commonly available today from a diversity of companies, the image-based monitoring tool is proving to be a promising concept for industrial protein crystallization in combination with deep learning image analysis for challenging protein crystallization process conditions such as those involving aggregates, precipitated proteins, heterogeneous particles, and low-resolution imaging devices [37].
The previous work of Wu et al. [18] furthermore demonstrated the potential of combining different monitoring modalities. Here, image analysis provided information from the solid phase, while Raman spectroscopy provided information from the liquid phase. The joint information helped to estimate the nucleation kinetics as well as crystal growth kinetics. Based on the results obtained in an aqueous taurine solution saturated at 30 °C and 40 °C, this approach proved to be more sensitive and provided more reproducible nucleation detection compared to FBRM.
3. Liquid-Phase Monitoring in Protein Crystallization Processes
Spectroscopy is a technique used for analyzing molecules by observing how light and matter interact. By examining the absorption and emission rate of a sample, the sample’s constituents, properties, and volume can be characterized. Spectroscopy is known to be a powerful technique in monitoring processes. It has been widely used to determine and monitor detailed information about proteins’ primary, secondary, tertiary, and quaternary structures [23], using different regions of the electromagnetic spectrum, such as the ultraviolet (UV; 190–380 nm), visible (Vis; 380–750 nm), or mid-infrared (MIR; 2.5–30 µm) light regions [54]. Using visible or near-infrared (NIR; 750 nm–2.5 μm) radiation, Raman spectroscopy is a non-invasive inline method which provides data of the chemical composition and molecular structure of small and large (bio)molecules. Recently, Raman spectroscopy was discussed as a monitoring and controlling tool for biopharmaceutical applications [17]. For further information on the inline downstream monitoring of protein crystallization by Raman spectroscopy, interested readers are encouraged to read the comprehensive review of Esmonde-White et al. [17].
In this overview, two selected types of spectroscopy with relevance to industrial applications are discussed with respect to their properties, monitoring capabilities and the various applications in monitoring protein crystallization processes.
3.1. Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) Spectroscopy
Infrared spectroscopy is a technique based on the coupling of electromagnetic radiation within the IR wavelength range and the associated vibrational modes of molecules. IR spectroscopy is an important method for chemical analysis and research on functional materials, as well as biological tests. It has been improved and developed to allow the study of molecular conformation, biochemical reactions, protein function, and crystal structures, for example [55]. Vibrational spectroscopy is significant because of the non-invasive and non-destructive form of measurement in reactors [56].
In the biopharmaceutical industry, FTIR spectroscopy was formerly employed as an at-line PAT method in downstream processing for the determination of product and byproduct content, as well as for the determination of the content of host cell proteins. It has been clearly proven that it can differentiate and quantify certain proteins inline based on their secondary structure [57].
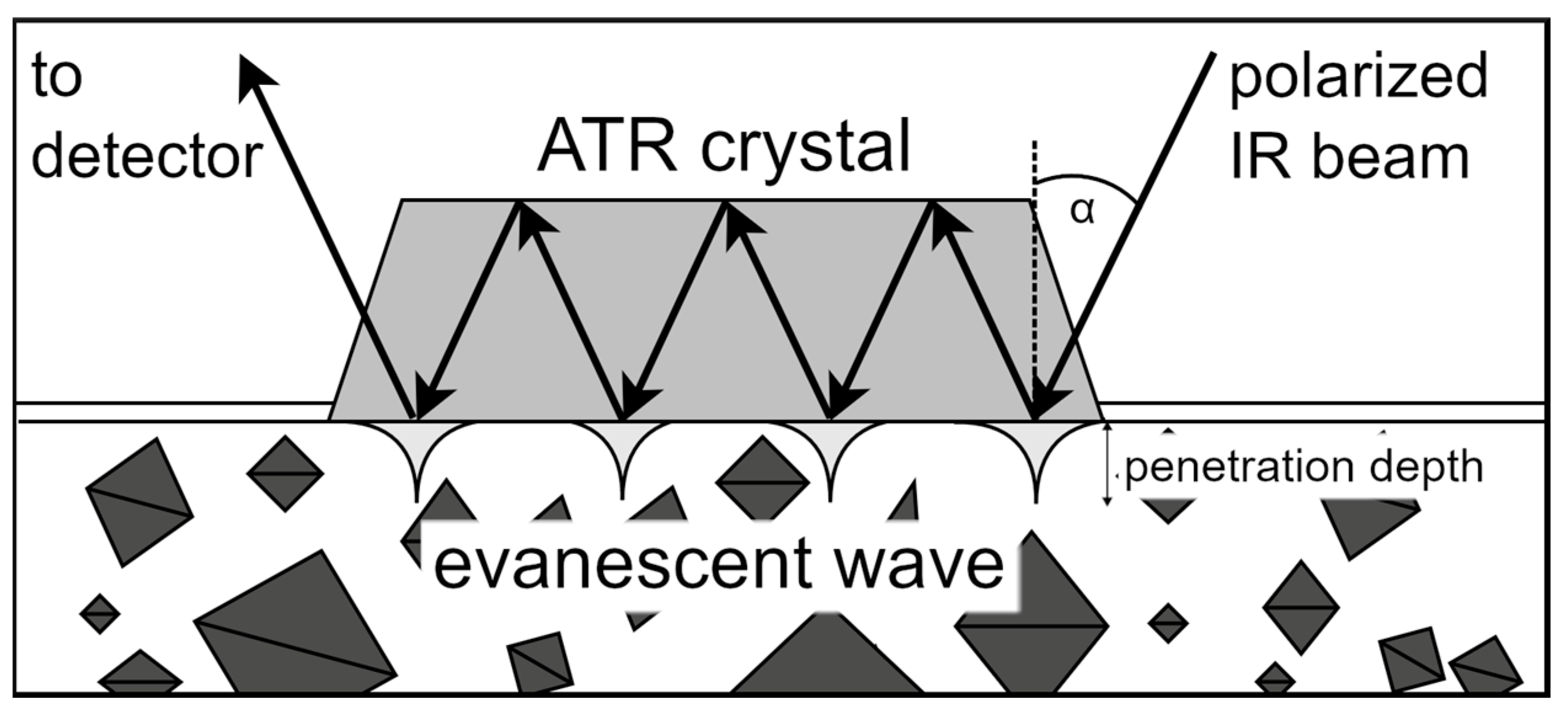
ATR-FTIR spectroscopy is particularly applicable in studying aqueous samples due to the interaction of MIR light with the sample within a few microns of the surface of the internal reflection element [58,59], as schematically depicted in Figure 4. As an ATR probe tolerates suspended particles or bubbles, it is able to measure slurries with high solid content [60]. Studies have demonstrated that ATR-FTIR can be used as an online supersaturation monitoring method for the stirred crystallization of small molecules such as D-mannitol [61], ibuprofen [62], and L-glutamic acid [63,64].
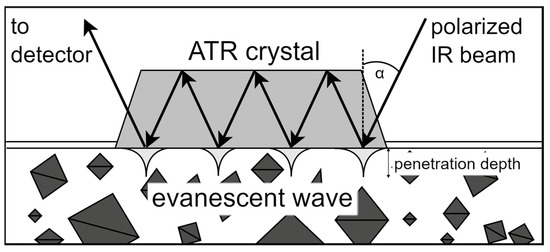
Figure 4.
Schematic illustration of ATR-FTIR with a multiple-bounce ATR cell. Polarized IR light is sent into the crystal at a defined angle (α) to bounce through the crystal. The resulting evanescent waves contain chemical and vibrational information about the molecules in the solution and deliver it to the detector, whereby penetration depth (0.5–5 µm) is correlated to the wave number and thus the absorbance intensity, influencing the sensitivity [65,66]. The sketch was generated using draw.io.
For seed crystallization, measuring the solution concentration with ATR-FTIR spectroscopy is highly effective for detecting seed points, as it has the ability to distinguish between salt and protein crystals due to the highly localized amide II absorbance (spectral region of 1585–1505 cm−1) on the image [58,67]. After seeding, the particle number and size distribution can be measured with an in situ/online particle size and counting device to determine seeding effectiveness [68]. Despite this, several studies successfully demonstrated the measurement of protein crystal growth for lysozyme, thaumatin, and lobster alpha crustacyanin [59] using ATR-FTIR spectroscopy in hanging-drop experiments [58,59,69], mainly for crystallization condition screening [58]. Here, protein crystal growth can be detected by an increase in the absorbance of the amide II band with a simultaneous decrease in the band from the bending mode of water (spectral region of about 1640 cm−1) with optical imaging methods being used as validation tools for crystal growth [59]. Additionally, this method allows the detection of microcrystals due to its high spatial resolution [57,59]. So far, ATR-FTIR has been successfully used in micro approaches (hanging drop), but not yet in preparative protein crystallization. However, it is already being used in other industrial crystallization processes in combination with feedback loop control strategies, which follow simple heuristic operating policies. For detailed explanations, the method of supersaturation control/concentration feedback control is further described in Gao et al. [6]. Furthermore, in situ concentration measurements of ATR-FTIR spectroscopy and UV/Vis spectroscopy have been mostly used in combination with feedback control techniques to monitor crystallization processes [70]. For Öner et al. [71], the measurements provided by these spectroscopic techniques provided information about the solute concentration in the crystallizer, as well as the current solubility of the suspension. Combining this information with a data-driven control strategy, a constant supersaturation state can be maintained by manipulating variables such as temperature or antisolvent feed rate. Furthermore, the same combination of ATR-FTIR spectroscopy and feedback controls was used by Montes et al. [70] in a promising model-based evaluation of a data-driven control strategy for crystallization processes using radial basis functions, predictive control and soft sensor control.
3.2. UV/Vis Spectroscopy
UV/Vis spectroscopy measures the absorption of proteins in the range between 240–340 nm based on the content of aromatic amino acids, in particular L-tryptophan and L-tyrosine. Hence, for analyzing crystallization parameters such as nucleation, polymorphic transition, and supersaturation changes, UV/Vis spectroscopy is used, relying on the qualitative and quantitative relationship between the protein structure and its composition, as well as its UV light absorption spectrum [72,73]. For correlating the measured spectral intensity with the concentration of the solution, the full-spectrum quantitative analysis technology of chemometrics is used [6]. Wegner et al. [20] successfully managed to combine UV/Vis spectroscopy and chemometrics by creating a high-throughput crystallization screening technique to quickly quantify individual species in a multicomponent matrix, composed of lysozyme, ribonuclease A, and cytochrome C, by allowing the calculation of process performance indicators in the early stage of process development. The combination of partial least regression models with UV/Vis spectroscopy creates a highly accurate and conceptionally simple tool with spectral analysis offering selective quantification and yield calculation within minutes [20]. Protein phase transition can be specifically detected, with growing protein crystals being easily distinguishable from salt crystals, resulting in a promising method for monitoring the protein concentration of the protein of interest in a multicomponent matrix during protein crystallization processes, with a future potential application In protein crystallization from an E. coli cell lysate.
The use of ATR-UV/Vis spectroscopy in pharmaceutical crystallization as a monitoring and concentration-measuring tool has been a subject of studies since the late 2000s when Abu Bakar et al. [32] studied the impact of direct nucleation control on crystal size distribution in pharmaceutical crystallization processes. ATR can improve the quality of UV/Vis spectroscopy by the identification of solution-mediated transformations in suspended solids under isothermal conditions [74].
With the recent development of ATR-UV/Vis in situ concentration measurement methods [75], supersaturation control or concentration feedback control can be applied to crystallization processes on laboratory and industrial scales. Here, the solution concentration measured in real time by ATR-FTIR and ATR-UV/Vis, combined with temperature and solubility data, can calculate the current supersaturation state to maintain the target supersaturation set point in the phase diagram by adjusting the operating temperature [6,76]. Thus, to avoid an uncertain nucleation zone or undersaturation, the crystal growth process can be controlled by following an operating trajectory, which is specified in the crystal phase diagram and results in a cooling curve. By means of a standard tracking control system, concentration feedback control can be implemented on the industrial scale [6].
Based on the information provided by ATR-FTIR and ATR-UV/Vis spectroscopy measurements, state variables of the crystallization processes can be controlled. For example, Griffin et al. [77] used ATR-FTIR and FBRM to expand the mass–count framework for crystal size control and colloidal assembly by inferring the total crystal mass via a mass balance. Simone et al. [73] used ATR-UV/vis and FBRM in combination with Raman and NIR spectroscopy to monitor polymorphic transformations of a small molecule and to establish real-time control of the crystal shape. These combinations of different monitoring tools are promising in monitoring complex processes such as protein crystallization.
4. Conclusions
As stated in Scott et al. [78], the focus of industrial research for advances in the in-process characterization of suspensions and slurries has been on particle size measurement, using primarily optical and imaging techniques. Machine learning resources can improve the combination of monitoring protein crystallization processes with optical endoscopic probes using image analysis for the real-time monitoring of protein crystal growth. Recent research in the field of protein crystallization is mainly based on detecting the critical quality attributes of crystals in real time, adjusting the final product for the required purposes by applying sophisticated crystallization process control algorithms. Here, spectroscopic methods are the most commonly used, and are often combined with control strategies such as supersaturation or concentration feedback control. The combination of UV/Vis spectroscopy with chemometrics is opening up the possibility of a promising application for monitoring selected protein concentrations in a multicomponent matrix.
Overall, the solid-phase and liquid-phase monitoring techniques discussed here (could) contribute to efficient protein crystallization process control, since protein crystallization is an attractive alternative to chromatography as a downstream processing step in the biotechnology, food, and chemical industries.
Author Contributions
B.W., D.B., I.C.V., S.M.F. and D.W.-B. contributed to the conception and design of the review paper; I.C.V. analyzed the literature with respect to the introduction, FBRM, and ML-based microscopic image analysis; S.M.F. focused the literature compilation on ATR-FTIR and UV/Vis spectroscopy; B.W. coordinated the literature research and evaluation; B.W. and D.B. designed the figures; B.W., D.B., I.C.V., S.M.F. and D.W.-B. wrote the manuscript. All the authors contributed to the critical revision and final approval of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Research Foundation (DFG), project number WE2715/14-2, within the framework of priority program SPP 1934.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Rose Jacobs and Natashia Msibi (both at the TUM Language Center), Anna-Lena Heins (Biochemical Engineering, TUM), Franziska Glasl (Center for Key Competences, TUM), and Kristin Preuß (Library, TUM) for their support in academic writing, collaborative scientific editing, virtual teamwork and digital collaboration, and literature research, respectively. The support of Brigitte Walla and Daniel Bischoff of the TUM Graduate School (Technical University of Munich, Germany) is acknowledged as well.
Conflicts of Interest
The authors declare no conflict of interest.
References
- dos Santos, R.; Carvalho, A.L.; Roque, A.C.A. Renaissance of protein crystallization and precipitation in biopharmaceuticals purification. Biotechnol. Adv. 2017, 35, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, D. Large-scale crystallization of proteins for purification and formulation. Bioprocess Biosyst. Eng. 2015, 38, 1209–1231. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wu, Y.; Gong, J.; Wang, J.; Rohani, S. Continuous crystallization of α-form L-glutamic acid in an MSMPR-Tubular crystallizer system. J. Cryst. Growth 2019, 507, 344–351. [Google Scholar] [CrossRef]
- Zhang, T.; Nagy, B.; Szilágyi, B.; Gong, J.; Nagy, Z.K. Simulation and experimental investigation of a novel supersaturation feedback control strategy for cooling crystallization in semi-batch implementation. Chem. Eng. Sci. 2020, 225, 115807. [Google Scholar] [CrossRef]
- Simon, L.L.; Simone, E.; Oucherif, K.A. Crystallization process monitoring and control using process analytical technology. Comput. Aided Chem. Eng. 2018, 41, 215–242. [Google Scholar]
- Gao, Y.; Zhang, T.; Ma, Y.; Xue, F.; Gao, Z.; Hou, B.; Gong, J. Application of PAT-based feedback control approaches in pharmaceutical crystallization. Crystals 2021, 11, 221. [Google Scholar] [CrossRef]
- Schmidt, S.; Havekost, D.; Kaiser, K.; Kauling, J.; Henzler, H.-J. Crystallization for the Downstream Processing of Proteins. Eng. Life Sci. 2005, 5, 273–276. [Google Scholar] [CrossRef]
- Longenecker, K.L.; Garrard, S.M.; Sheffield, P.J.; Derewenda, Z.S. Protein crystallization by rational mutagenesis of surface residues: Lys to Ala mutations promote crystallization of RhoGDI. Acta Crystallogr. D Biol. Crystallogr. 2001, 57 Pt 5, 679–688. [Google Scholar] [CrossRef]
- Derewenda, Z.S. The use of recombinant methods and molecular engineering in protein crystallization. Methods 2004, 34, 354–363. [Google Scholar] [CrossRef]
- Cooper, D.R.; Boczek, T.; Grelewska-Nowotko, K.; Pinkowska, M.; Sikorska, M.; Zawadzki, M.; Derewenda, Z. Protein crystallization by surface entropy reduction: Optimization of the SER strategy. Acta Crystallogr. Sect. D Biol. Crystallogr. 2007, 63, 636–645. [Google Scholar] [CrossRef]
- Derewenda, Z.S.; Godzik, A. The “Sticky Patch” Model of Crystallization and Modification of Proteins for Enhanced Crystallizability. Protein Crystallogr. Methods Protoc. 2017, 1607, 77–115. [Google Scholar] [CrossRef]
- Grob, P.; Huber, M.; Walla, B.; Hermann, J.; Janowski, R.; Niessing, D.; Hekmat, D.; Weuster-Botz, D. Crystal Contact Engineering Enables Efficient Capture and Purification of an Oxidoreductase by Technical Crystallization. Biotechnol. J. 2020, 15, e2000010. [Google Scholar] [CrossRef] [PubMed]
- Walla, B.; Bischoff, D.; Janowski, R.; Eichen, N.V.D.; Niessing, D.; Weuster-Botz, D. Transfer of a Rational Crystal Contact Engineering Strategy between Diverse Alcohol Dehydrogenases. Crystals 2021, 11, 975. [Google Scholar] [CrossRef]
- Nagy, Z.K.; Fujiwara, M.; Braatz, R.D. Monitoring and Advanced Control of Crystallization Processes. In Handbook of Industrial Crystallization; Cambridge University Press (CUP): Cambridge, UK, 2019; pp. 313–345. [Google Scholar]
- Nagy, Z.K.; Fevotte, G.; Kramer, H.; Simon, L.L. Recent advances in the monitoring, modelling and control of crystallization systems. Chem. Eng. Res. Des. 2013, 91, 1903–1922. [Google Scholar] [CrossRef]
- Prasad, R.; Dalvi, S.V. Sonocrystallization: Monitoring and controlling crystallization using ultrasound. Chem. Eng. Sci. 2020, 226, 115911. [Google Scholar] [CrossRef]
- Esmonde-White, K.A.; Cuellar, M.; Lewis, I.R. The role of Raman spectroscopy in biopharmaceuticals from development to manufacturing. Anal. Bioanal. Chem. 2021, 414, 969–991. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y. A Study of The Deep Learning-based Monitoring and Efficient Numerical Modeling Methodologies for Crystallization Processes. Electron. Thesis Diss. Repos. 2021, 7813, 1–224. [Google Scholar]
- Rathore, A.S.; Bhambure, R.; Ghare, V. Process analytical technology (PAT) for biopharmaceutical products. Anal. Bioanal. Chem. 2010, 398, 137–154. [Google Scholar] [CrossRef]
- Wegner, C.H.; Zimmermann, I.; Hubbuch, J.R. Rapid Analysis for Multicomponent High-Throughput Crystallization Screening: Combination of UV−Vis Spectroscopy and Chemometrics. Cryst. Growth Des. 2022, 22, 1054–1065. [Google Scholar] [CrossRef]
- Abbas, A.; Nobbs, D.; Romagnoli, J.A. Investigation of on-line optical particle characterization in reaction and cooling crystallization systems. Current state of the art. Meas. Sci. Technol. 2002, 13, 349–356. [Google Scholar] [CrossRef]
- Lewis, A.; Seckler, M.; Kramer, H.; van Rosmalen, G. Industrial Crystallization: Fundamentals and Application; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Ma, Y.; Wu, S.; Macaringue, E.G.J.; Zhang, T.; Gong, J.; Wang, J. Recent Progress in Continuous Crystallization of Pharmaceutical Products: Precise Preparation and Control. Org. Process. Res. Dev. 2020, 24, 1785–1801. [Google Scholar] [CrossRef]
- Muhaimin, M.; Chaerunisaa, A.Y.; Bodmeier, R. Real-time particle size analysis using focused beam reflectance measurement as a process analytical technology tool for continuous microencapsulation process. Sci. Rep. 2021, 11, 19390. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, T.; Baudry, C.; Hernandez, M.L.E. Optimization of a Crystallization by Online FBRM Analysis of Needle-Shaped Crystals. Org. Process. Res. Dev. 2011, 15, 413–426. [Google Scholar] [CrossRef]
- Pandalaneni, K.; Amamcharla, J. Focused beam reflectance measurement as a tool for in situ monitoring of the lactose crystallization process. J. Dairy Sci. 2016, 99, 5244–5253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, L.; Xu, S.; Du, S.; Dong, W.; Gong, J. Optimization of cooling strategy and seeding by FBRM analysis of batch crystallization. J. Cryst. Growth 2018, 486, 1–9. [Google Scholar] [CrossRef]
- Acevedo, D.; Wu, W.-L.; Yang, X.; Pavurala, N.; Mohammad, A.; O’Connor, T.F. Evaluation of focused beam reflectance measurement (FBRM) for monitoring and predicting the crystal size of carbamazepine in crystallization processes. Crystengcomm 2020, 23, 972–985. [Google Scholar] [CrossRef]
- Pandit, A.V.; Katkar, V.V.; Ranade, V.V.; Bhambure, R. Real-Time Monitoring of Biopharmaceutical Crystallization: Chord Length Distribution to Crystal Size Distribution for Lysozyme, rHu Insulin, and Vitamin B12. Ind. Eng. Chem. Res. 2018, 58, 7607–7619. [Google Scholar] [CrossRef]
- Smejkal, B.; Helk, B.; Rondeau, J.-M.; Anton, S.; Wilke, A.; Scheyerer, P.; Fries, J.; Hekmat, D.; Weuster-Botz, D. Protein crystallization in stirred systems-scale-up via the maximum local energy dissipation. Biotechnol. Bioeng. 2013, 110, 1956–1963. [Google Scholar] [CrossRef]
- Kubota, N. Analysis of the effect of volume on induction time and metastable zone width using a stochastic model. J. Cryst. Growth 2015, 418, 15–24. [Google Scholar] [CrossRef]
- Abu Bakar, M.R.; Nagy, Z.K.; Saleemi, A.N.; Rielly, C.D. The Impact of Direct Nucleation Control on Crystal Size Distribution in Pharmaceutical Crystallization Processes. Cryst. Growth Des. 2009, 9, 1378–1384. [Google Scholar] [CrossRef]
- Unno, J.; Hirasawa, I. Parameter Estimation of the Stochastic Primary Nucleation Kinetics by Stochastic Inte-grals Using Focused-Beam Reflectance Measurements. Crystals 2020, 10, 380. [Google Scholar] [CrossRef]
- de Albuquerque, I.; Mazzotti, M.; Ochsenbein, D.R.; Morari, M. Effect of needle-like crystal shape on measured particle size distributions. AIChE J. 2016, 62, 2974–2985. [Google Scholar] [CrossRef]
- Gan, C.; Wang, L.; Xiao, S.; Zhu, Y. Feedback Control of Crystal Size Distribution for Cooling Batch Crystalli-zation Using Deep Learning-Based Image Analysis. Crystals 2022, 12, 570. [Google Scholar] [CrossRef]
- Pons, M.-N.; Vivier, H. Crystallization monitoring by quantitative image analysis. Anal. Chim. Acta 1990, 238, 243–249. [Google Scholar] [CrossRef]
- Bischoff, D.; Walla, B.; Weuster-Botz, D. Machine learning-based protein crystal detection for monitoring of crystallization processes enabled with large-scale synthetic data sets of photorealistic images. Anal. Bioanal. Chem. 2022, 414, 6379–6391. [Google Scholar] [CrossRef] [PubMed]
- Haupert, L.M.; Simpson, G.J. Screening of protein crystallization trials by second order nonlinear optical imaging of chiral crystals (SONICC). Methods 2011, 55, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.T.; DeWalt, E.L.; Simpson, G.J. Two-photon excited UV fluorescence for protein crystal detection. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 839–846. [Google Scholar] [CrossRef]
- Cheng, Q.-D.; Chung, H.-Y.; Schubert, R.; Chia, S.-H.; Falke, S.; Mudogo, C.N.; Kärtner, F.X.; Chang, G.; Betzel, C. Protein-crystal detection with a compact multimodal multiphoton microscope. Commun. Biol. 2020, 3, 569. [Google Scholar] [CrossRef]
- Patience, D.B.; Rawlings, J.B. Particle-shape monitoring and control in crystallization processes. AIChE J. 2001, 47, 2125–2130. [Google Scholar] [CrossRef]
- Larsen, P.; Rawlings, J.; Ferrier, N. An algorithm for analyzing noisy, in situ images of high-aspect-ratio crystals to monitor particle size distribution. Chem. Eng. Sci. 2006, 61, 5236–5248. [Google Scholar] [CrossRef]
- Larsen, P.; Rawlings, J.; Ferrier, N. Model-based object recognition to measure crystal size and shape distributions from in situ video images. Chem. Eng. Sci. 2007, 62, 1430–1441. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Gkioxari, G.; Dollár, P.; Girshick, R. Mask R-CNN. In Proceedings of the IEEE International Conference on Computer Vision (ICCV), Venice, Italy, 22–29 October 2017. [Google Scholar]
- Gao, Z.; Wu, Y.; Bao, Y.; Gong, J.; Wang, J.; Rohani, S. Image Analysis for In-line Measurement of Multidimensional Size, Shape, and Polymorphic Transformation of l-Glutamic Acid Using Deep Learning-Based Image Segmentation and Classification. Cryst. Growth Des. 2018, 18, 4275–4281. [Google Scholar] [CrossRef]
- Chen, S.; Liu, T.; Xu, D.; Huo, Y.; Yang, Y. Image based Measurement of Population Growth Rate for L-Glutamic Acid Crystallization. In Proceedings of the 2019 Chinese Control Conference (CCC), Guangzhou, China, 27–30 July 2019. [Google Scholar] [CrossRef]
- Lin, T.Y.; Goyal, P.; Girshick, R.; He, K.; Dollár, P. Focal Loss for Dense Object Detection. In Proceedings of the 2017 IEEE International Conference on Computer Vision (ICCV), Venice, Italy, 22–29 October 2017. [Google Scholar]
- Manee, V.; Zhu, W.; Romagnoli, J.A. A Deep Learning Image-Based Sensor for Real-Time Crystal Size Distribution Characterization. Ind. Eng. Chem. Res. 2019, 58, 23175–23186. [Google Scholar] [CrossRef]
- Han, J.; Ding, J.; Li, J.; Xia, G.-S. Align Deep Features for Oriented Object Detection. IEEE Trans. Geosci. Remote Sens. 2021, 60, 1–11. [Google Scholar] [CrossRef]
- Bluma, A.; Höpfner, T.; Rudolph, G.; Lindner, P.; Beutel, S.; Hitzmann, B.; Scheper, T. Adaptation of in-situ microscopy for crystallization processes. J. Cryst. Growth 2009, 311, 4193–4198. [Google Scholar] [CrossRef]
- Kacker, R.; Maaß, S.; Emmerich, J.; Kramer, H. Application of inline imaging for monitoring crystallization process in a continuous oscillatory baffled crystallizer. AIChE J. 2018, 64, 2450–2461. [Google Scholar] [CrossRef]
- Huo, Y.; Liu, T.; Yang, Y.; Ma, C.Y.; Wang, X.Z.; Ni, X. In Situ Measurement of 3D Crystal Size Distribution by Double-View Image Analysis with Case Study on l-Glutamic Acid Crystallization. Ind. Eng. Chem. Res. 2020, 59, 4646–4658. [Google Scholar] [CrossRef]
- Klijn, M.E.; Hubbuch, J. Application of ultraviolet, visible, and infrared light imaging in protein-based biopharmaceutical formulation characterization and development studies. Eur. J. Pharm. Biopharm. 2021, 165, 319–336. [Google Scholar] [CrossRef]
- Baiz, C.R.; Błasiak, B.; Bredenbeck, J.; Cho, M.; Choi, J.-H.; Corcelli, S.A.; Dijkstra, A.G.; Feng, C.-J.; Garrett-Roe, S.; Ge, N.-H.; et al. Vibrational Spectroscopic Map, Vibrational Spectroscopy, and Intermolecular Interaction. Chem. Rev. 2020, 120, 7152–7218. [Google Scholar] [CrossRef]
- Adar, F. Analytical Vibrational Spectroscopy—NIR, IR, and Raman. 2011. Available online: https://www.spectroscopyonline.com/view/analytical-vibrational-spectroscopy-nir-ir-and-raman (accessed on 30 March 2023).
- Großhans, S.; Rüdt, M.; Sanden, A.; Brestrich, N.; Morgenstern, J.; Heissler, S.; Hubbuch, J. In-line Fourier-transform infrared spectroscopy as a versatile process analytical technology for preparative protein chromatography. J. Chromatogr. A 2018, 1547, 37–44. [Google Scholar] [CrossRef]
- Chan, K.L.A.; Govada, L.; Bill, R.M.; Chayen, N.E.; Kazarian, S.G. Attenuated Total Reflection-FT-IR Spectroscopic Imaging of Protein Crystallization. Anal. Chem. 2009, 81, 3769–3775. [Google Scholar] [CrossRef]
- Glassford, S.E.; Govada, L.; Chayen, N.E.; Byrne, B.; Kazarian, S.G. Micro ATR FTIR imaging of hanging drop protein crystallisation. Vib. Spectrosc. 2012, 63, 492–498. [Google Scholar] [CrossRef]
- Billot, P.; Couty, M.; Hosek, P. Application of ATR-UV Spectroscopy for Monitoring the Crystallisation of UV Absorbing and Nonabsorbing Molecules. Org. Process. Res. Dev. 2010, 14, 511–523. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Glennon, B. Application of in Situ FBRM and ATR-FTIR to the Monitoring of the Polymorphic Transformation of d-Mannitol. Org. Process. Res. Dev. 2005, 9, 884–889. [Google Scholar] [CrossRef]
- Hojjati, H.; Rohani, S. Measurement and Prediction of Solubility of Paracetamol in Water−Isopropanol Solution. Part 1. Measurement and Data Analysis. Org. Process. Res. Dev. 2006, 10, 1101–1109. [Google Scholar] [CrossRef]
- Borissova, A.; Khan, S.; Mahmud, T.; Roberts, K.J.; Andrews, J.; Dallin, P.; Chen, Z.-P.; Morris, J. In Situ Measurement of Solution Concentration during the Batch Cooling Crystallization of l-Glutamic Acid using ATR-FTIR Spectroscopy Coupled with Chemometrics. Cryst. Growth Des. 2008, 9, 692–706. [Google Scholar] [CrossRef]
- Kadam, S.S.; van der Windt, E.; Daudey, P.J.; Kramer, H.J.M. A Comparative Study of ATR-FTIR and FT-NIR Spectroscopy for in-Situ Concentration Monitoring during Batch Cooling Crystallization Processes. Cryst. Growth Des. 2010, 10, 2629–2640. [Google Scholar] [CrossRef]
- Milosevic, M.; Sting, D.; Rein, A. Diamond-composite sensor for ATR spectroscopy. Spectroscopy 1995, 10, 44–49. [Google Scholar]
- Tiernan, H.; Byrne, B.; Kazarian, S.G. ATR-FTIR spectroscopy and spectroscopic imaging for the analysis of biopharmaceuticals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118636. [Google Scholar] [CrossRef]
- Glassford, S.; Chan, K.L.A.; Byrne, B.; Kazarian, S.G. Chemical Imaging of Protein Adsorption and Crystallization on a Wettability Gradient Surface. Langmuir 2012, 28, 3174–3179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shan, B.; Wang, Y.; Zhu, Z.; Yu, Z.-Q.; Ma, C.Y. Progress and Opportunities for Utilizing Seeding Techniques in Crystallization Processes. Org. Process. Res. Dev. 2021, 25, 1496–1511. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Chan, K.L.A. ATR-FTIR spectroscopic imaging: Recent advances and applications to biological systems. Analyst 2013, 138, 1940–1951. [Google Scholar] [CrossRef]
- Montes, F.C.C.; Öner, M.; Gernaey, K.V.; Sin, G. Model-Based Evaluation of a Data-Driven Control Strategy: Application to Ibuprofen Crystallization. Processes 2021, 9, 653. [Google Scholar] [CrossRef]
- Öner, M.; Montes, F.C.; Ståhlberg, T.; Stocks, S.M.; Bajtner, J.E.; Sin, G. Comprehensive evaluation of a data driven control strategy: Experimental application to a pharmaceutical crystallization process. Chem. Eng. Res. Des. 2020, 163, 248–261. [Google Scholar] [CrossRef]
- Saleemi, A.; Rielly, C.; Nagy, Z.K. Automated direct nucleation control for in situ dynamic fines removal in batch cooling crystallization. Cryst. Eng. Comm. 2012, 14, 2196–2203. [Google Scholar] [CrossRef]
- Simone, E.; Saleemi, A.N.; Nagy, Z.K. In Situ Monitoring of Polymorphic Transformations Using a Compo-site Sensor Array of Raman, NIR, and ATR-UV/vis Spectroscopy, FBRM, and PVM for an Intelligent Decision Support System. Org. Process Res. Dev. 2015, 19, 167–177. [Google Scholar] [CrossRef]
- Florence, A.J.; Johnston, A. Applications of ATR UV/vis spectroscopy in physical form characterisation of pharmaceuticals. Spectrosc. Eur. 2004, 16, 24–27. [Google Scholar]
- Ostergaard, I.; Szilagyi, B.; De Diego, H.L.; Qu, H.; Nagy, Z.K. Polymorphic Control and Scale-up Strategy for Antisolvent Crystallization Using Direct Nucleation Control. Cryst. Growth Des. 2020, 20, 2683–2697. [Google Scholar] [CrossRef]
- Fujiwara, M.; Nagy, Z.K.; Chew, J.W.; Braatz, R.D. First-principles and direct design approaches for the control of pharmaceutical crystallization. J. Process Control 2005, 15, 493–504. [Google Scholar] [CrossRef]
- Griffin, D.J.; Grover, M.A.; Kawajiri, Y.; Rousseau, R.W. Mass–count plots for crystal size control. Chem. Eng. Sci. 2015, 137, 338–351. [Google Scholar] [CrossRef]
- Scott, D.M. Recent advances in in-process characterization of suspensions and slurries. Powder Technol. 2022, 399, 117159. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).