Abstract
The electrochemical sensing potential of two isostructural one-dimensional nickel(II) and cobalt(II) coordination polymers with 4,4′-bipyridine (4,4′-bpy) and 6-oxonicotinate (6-Onic), namely, {[Ni(4,4′-bpy)(H2O)4](6-Onic)2×2H2O}n and {[Co(4,4′-bpy)(H2O)4](6-Onic)2×2H2O}n, was investigated along with the polymers’ potential applications in the catalytic oxidation of methanol. The highly oxidative species from redox pairs Ni(II)/Ni(III) and Co(II)/Co(III) in these compounds represent catalytically active centres for oxidation of small molecules. A glassy carbon electrode (GCE) modified with a Ni polymer showed stability and reproducibility in 0.1 M NaOH, while the oxidation current inc 2reased with the increasing methanol concentration, suggesting that the Ni-polymer-modified electrode possess good sensing ability with respect to methanol. The GC electrode modified with the Co polymer is not reproducible and cannot be used for electroanalytical purposes under these experimental conditions. The GC electrode modified with the Ni polymer was successfully applied in the determination of methanol. This method showed favourable linear concentration dependence with a good sensitivity of 2.65 and 11.0 mA mM−1, a wide concentration range (0.001–4 mM), and a detection limit of 0.8 μM, which indicates its excellent application potential for methanol oxidation and thus its determination.
1. Introduction
Coordination polymers have attracted a great deal of attention in the last decade [1,2,3,4] because of their important roles in catalytic processes for the polymerization of organic compounds, such as polyethylene and polypropylene, and in hydrometallurgical processes, e.g., the extraction of metals such as nickel, cobalt, or copper from their ores. A novel purpose with regard to the study of coordination polymers is the development of novel catalysts that can be used in electrochemistry. The electrochemical properties and behaviour of coordination polymers have been neglected and poorly reported in the literature but have recently attracted increasing interest. Cyclic voltammetry is the most commonly used electrochemical technique for characterizing coordination polymers, offering information about the redox activity of metal ions and ligands [5,6], while also providing insight into the potential application of coordination polymers as suitable materials for sensor development [7,8,9,10,11,12,13,14].
This new type of material is worth exploring for the manufacture of more efficient materials. As active sites, transition metal ions in coordination polymers influence the properties and performance of many electronic and optoelectronic devices. Mesoporous structures made of cross-linked coordination complexes are likely to be a promising choice in many industries and are well suited as sensing materials because of the expected faster electron transfer reaction associated with their use. Some of the potential applications of coordination polymers as electrocatalytic centres have been known for several decades. One of the first attempts to use them dates to 1980, which involved the reduction of carbon dioxide using macrocycles of nickel and cobalt [15]. Since then, the potential of this type of compound has received little attention in the literature; nevertheless, some of the examples that exist include the reduction of carbon dioxide via binuclear copper complexes [16], the electrocatalytic reduction of oxygen and carbon dioxide via Co(II)-Schiff-based polypyrrole complexes [17], the preparation of metal–polypyrrole films with electrocatalytic properties [18], the detection of nitric oxide via tetrasulfonated Ni-phthalocyanine [19], the determination of sulphide using nickel hydroxide electrodes [20], the activation of a Ni platform for the electrochemical measurement of phosphate [21], the development of non-enzymatic amperometric glucose sensors modified with nanonickel oxide [22], and the oxidation of aliphatic alcohols via copper(II) Schiff base complexes containing pyrrole [23]. A very interesting study from 2021 provided new perspectives with respect to coordination polymers by proposing the use of copper oxide nanoparticles for the induction of antibacterial activity and the effective removal of industrial dyes [24].
In recent years, several research groups have worked intensively on the development of platforms for the electrooxidation of methanol using various coordination polymers (MOFs (metal–organic frameworks)), such as the electrooxidation of methanol via Cu-BTC MOFs [25] or the electrooxidation of methanol using nanoporous cobalt MOFs [26]. Methanol has attracted a great deal of interest because of its potential use in direct methanol fuel cells [27]. The importance of methanol also lies in its potential to generate clean energy for smart electronic devices or small vehicles [28]. Direct methanol fuel cells (DMFCs) have attracted considerable attention due to their less complex configuration and suitability for small, portable devices such as chargers for cell phones, laptops, and cameras. They have the potential to replace batteries because methanol has a remarkably higher specific energy density than the best rechargeable batteries and Li polymer or Li ion polymer systems [27]. Methanol is a liquid at room temperature, inexpensive, and available on an industrial scale; the infrastructure for its production and distribution are already in place. Perhaps one of the obstacles preventing its commercialization is the high excess potential associated with the direct electrooxidation of methanol [29,30,31].
Methanol’s major obstacle is its slow oxidation kinetics, and in many cases, the catalysts used in this regard are not environmentally friendly materials. These problems should be solved prior to its commercial application, not only in terms of the choice of material for the methanol oxidation catalyst but also through a sound understanding of the kinetics of methanol oxidation. The study of the kinetics, mechanisms, and catalytic activity of several materials have been reported [32,33,34,35,36].
Some nickel and cobalt compounds have been prepared and used with excellent results. Ni(II) and Co(II) Schiff-based complexes and coordination polymers are the most commonly used compounds for the electrooxidation and electrochemical sensitization of methanol [32]. The main principles enabling these potential applications is the presence of highly oxidative species in these materials, acting as catalytic active centres towards short-chain or small molecules.
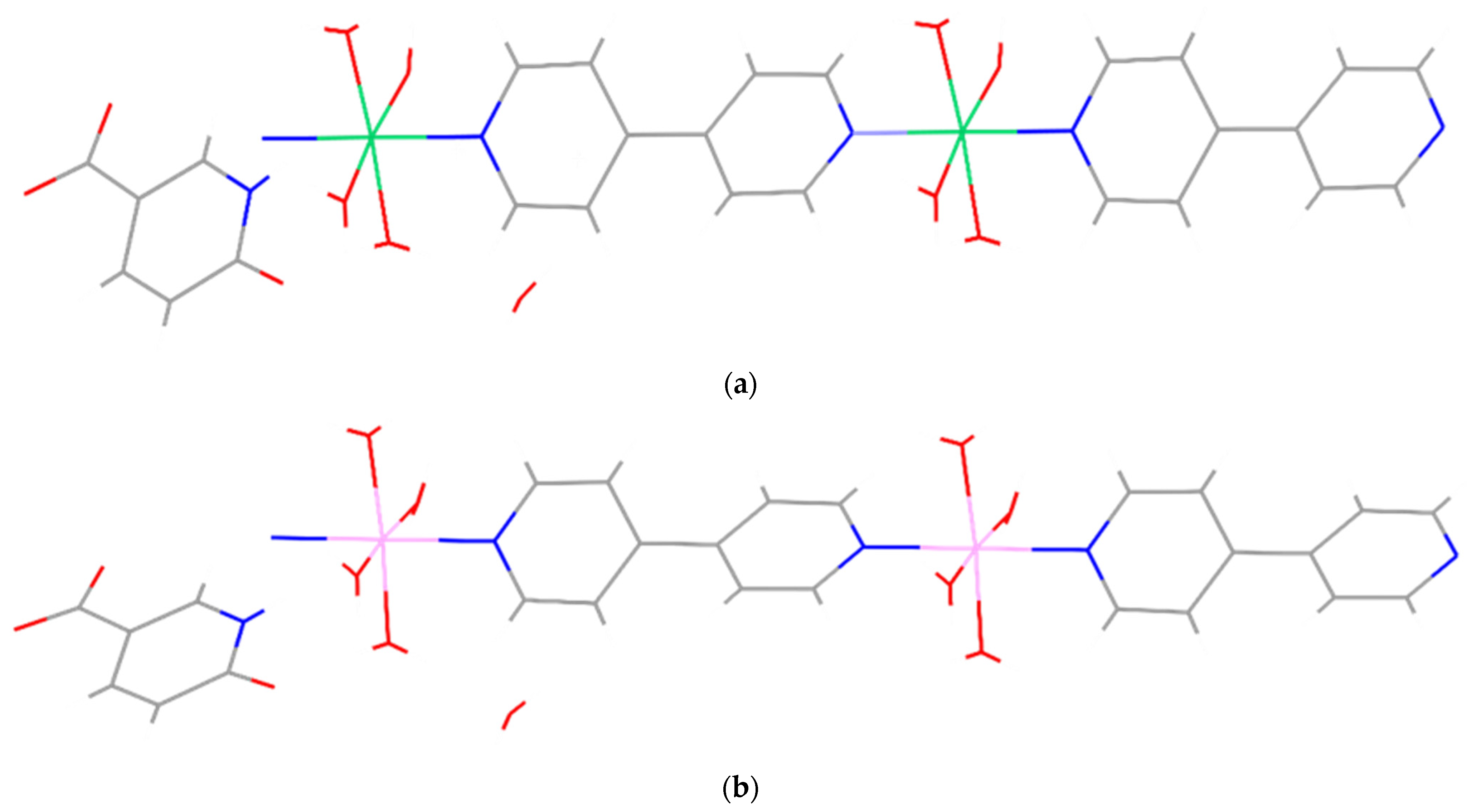
Previously, we hydrothermally prepared two isostructural, one-dimensional nickel(II) and cobalt(II) coordination polymers with 4,4′-bipyridine (4,4′-bpy) and 6-oxonicotinate (6-Onic): {[Ni(4,4′-bpy)(H2O)4](6-Onic)2×2H2O}n and {[Co(4,4′-bpy)(H2O)4](6-Onic)2×2H2O}n. The asymmetric units of both coordination polymers contain the corresponding metal ions, four coordinated water molecules, two 6-oxonicotinate anions, a 4,4′-bipyridine ligand, and two lattice water molecules. Hence, their molecular structures comprise infinite one-dimensional polymeric {[Ni(4,4′-bpy)(H2O)4]2+}n or {[Co(4,4′-bpy)(H2O)4]2+}n cations, which are accompanied by two 6-oxonicotinate anions and two lattice water molecules per repeating polymeric unit [37] (Figure 1). Although isostructural, these coordination polymers showed different electrochemical behaviours, which was evident in their different reactivity toward a Fe(III)/Fe(II) system, as a consequence of the different electronic configurations of the metal(II) ions [37].
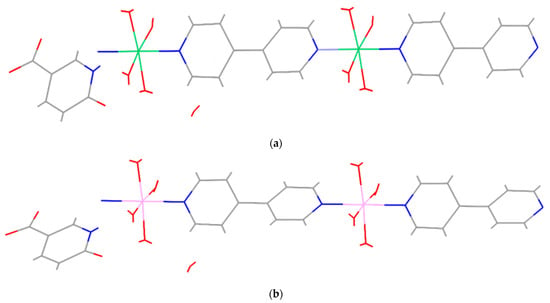
Figure 1.
MERCURY-style plots of {[Ni(4,4′-bpy)(H2O)4](6-Onic)2×2H2O}n (a) and {[Co(4,4′-bpy)(H2O)4](6-Onic)2×2H2O}n (b) showing the infinite one-dimensional polymeric chains of {[Ni(4,4′-bpy)(H2O)4]2+}n and {[Co(4,4′-bpy)(H2O)4]2+}n cations, 6-oxonicotinate anions, and lattice water molecules.
We were interested in conducting further electrochemical studies of the prepared Ni(II) and Co(II) polymers to decipher the origin of the electrochemical differences in the above isostructural system. Therefore, we performed detailed voltametric and impedance studies on these two coordination polymers to explore their sensing potential toward selected organic compounds, e.g., methanol, and to reveal their possible applications for the catalytic oxidation of methanol.
In this study, the direct catalytic oxidation of methanol on the surface of a Ni-BPY/6-Onic electrode in 0.1 M of NaOH was facilitated via the presence of Ni(II)/Ni(III). This process does not take place at the Co-BPY/6-Onic electrode. The electrode modified with the Ni(II) polymer showed a wide working range, a low detection limit, and an excellent sensitivity towards methanol determination compared with similar modified electrodes from the literature [18,32].
2. Materials and Methods
2.1. Preparation of Ni(II) and Co(II) Coordination Polymers
The Ni(II) and Co(II) coordination polymers were prepared according to a recently published procedure [37]. The appropriate amount of Ni(II) or Co(II) nitrate hexahydrate was dissolved in distilled water, 6-hydroxynicotinic acid was dissolved in distilled water, and 4,4′-bipyridine was dissolved in ethanol and mixed appropriately (molar ratio 1:2:1, respectively Ni(II) or Co(II): 6-OHnic: 4,4′-bpy). The pH levels of the final solutions were adjusted to 7 by adding sodium hydroxide. The coordination polymers were prepared using the conventional solvothermal method, heated in a Teflon-lined autoclave for 72 h at 130 °C, and then left to cool slowly to room temperature over 24 h. The green precipitate of {[Ni(4,4′-bpy)(H2O)4](6-Onic)2×2H2O}n and pink precipitate of {[Co(4,4′-bpy)(H2O)4](6-Onic)2×2H2O}n were obtained, collected by filtration, washed with water, and dried in a desiccator over CaCl2. The clear solutions remaining after filtration were left to evaporate slowly at room temperature until green crystals of Ni(II) polymer and pink crystals of Co(II) polymer, suitable for electrode preparation and spectroscopic measurements, had formed.
The IR spectra (KBr pellets) (Figures S3 and S4) were collected using a Shimadzu IRAffinity-1 FT-IR spectrometer operating in the 4000–400 cm−1 range and used to confirm the identity and purity of the coordination polymers during the preparation of the working electrodes. The assignation of the bands in the IR spectra is summarized in Table S2. The crystal structures of the Ni(II) and Co(II) coordination polymers and their respective spectroscopic (IR) and thermal characterization (TGA-DTA) data were published previously [37].
2.2. Preparation of Working Electrodes
Coordination polymers of Ni(II) and Co(II) (5 mg) were dispersed in 2 mL of N,N-dimethylformamide (DMF), and the mixtures were treated in an ultrasonic bath for 1 h to obtain homogeneous suspensions. The working electrode was glassy carbon (GC) electrode with an electrode diameter of 5 mm that was modified with a suspension of the respective coordination polymers (Table 1). Prior to its modification, the GC electrode was pre-treated mechanically (to form a mirror coating), ultrasonically (in ultrapure water and ethanol for 5 min), and chemically (soaked in a 0.5 M nitric acid solution). A homogeneous suspension (20 µL) was transferred dropwise to the GC electrode’s surface and dried in an oven for 30 min at a temperature of 50 °C to produce a layer on the GC electrode’s surface. This procedure was repeated to obtain more layers.

Table 1.
The abbreviations of compounds and terms.
2.3. Electrochemical Measurements
All electrochemical experiments were performed in a standard three-electrode cell (using a Pt-counter electrode and Ag/AgCl/3M KCl as a reference electrode).
The electrochemical impedance spectroscopy (EIS) measurements and activity of both coordination polymers were evaluated using Solartron SI 1287 electrochemical interface and a Solartron SI 1255 frequency response analyser, which were controlled by a personal computer and by the Zplot computer program, while the Zview program was used for data processing. The EIS measurements were taken at open-circuit potential (EOCP) over the frequency range of 100 kHz to 10 mHz and 5 mV amplitude. Modelling of the spectra with an appropriate electrical equivalent circuit (EEC) was performed using a complex non-linear least-squares method (CNLS algorithm); the values of the elements of the proposed circle show the Hi-square value (χ2) of the order of magnitude of 10−4 (0.5–3% errors).
Cyclic voltammograms were developed using a potentiostat (Autolab PGSTAT 302N) connected to a PC operating with GPES 4.9 Software (Eco Chemie Autolab PGSTAT30 Potentiostat / Galvanostat, Eco Utrecht, Netherlands) and were recorded in a broader range (from −1.0 to 1.0 V vs. Ag/AgCl; scan rate-50 mV/s) with different numbers of layers of the prepared polymers (1, 2, 5 and 10 layers).
The different parameters for square wave voltametric (SWV) measurements (deposition potential, Edep; deposition time, tdep; frequency, f; pulse amplitude, ΔEp; and step potential, ΔEs,) were optimized in order to develop an electrochemical procedure for determination of methanol. All the experiments were performed using a recording range from 0.370 to 0.800 V and with Edep = 0.370 V, tdep = 120 s, f = 20 Hz, ΔEp = 100 mV, and ΔEs = 20 mV.
2.4. Morphological Study-Surface Examination
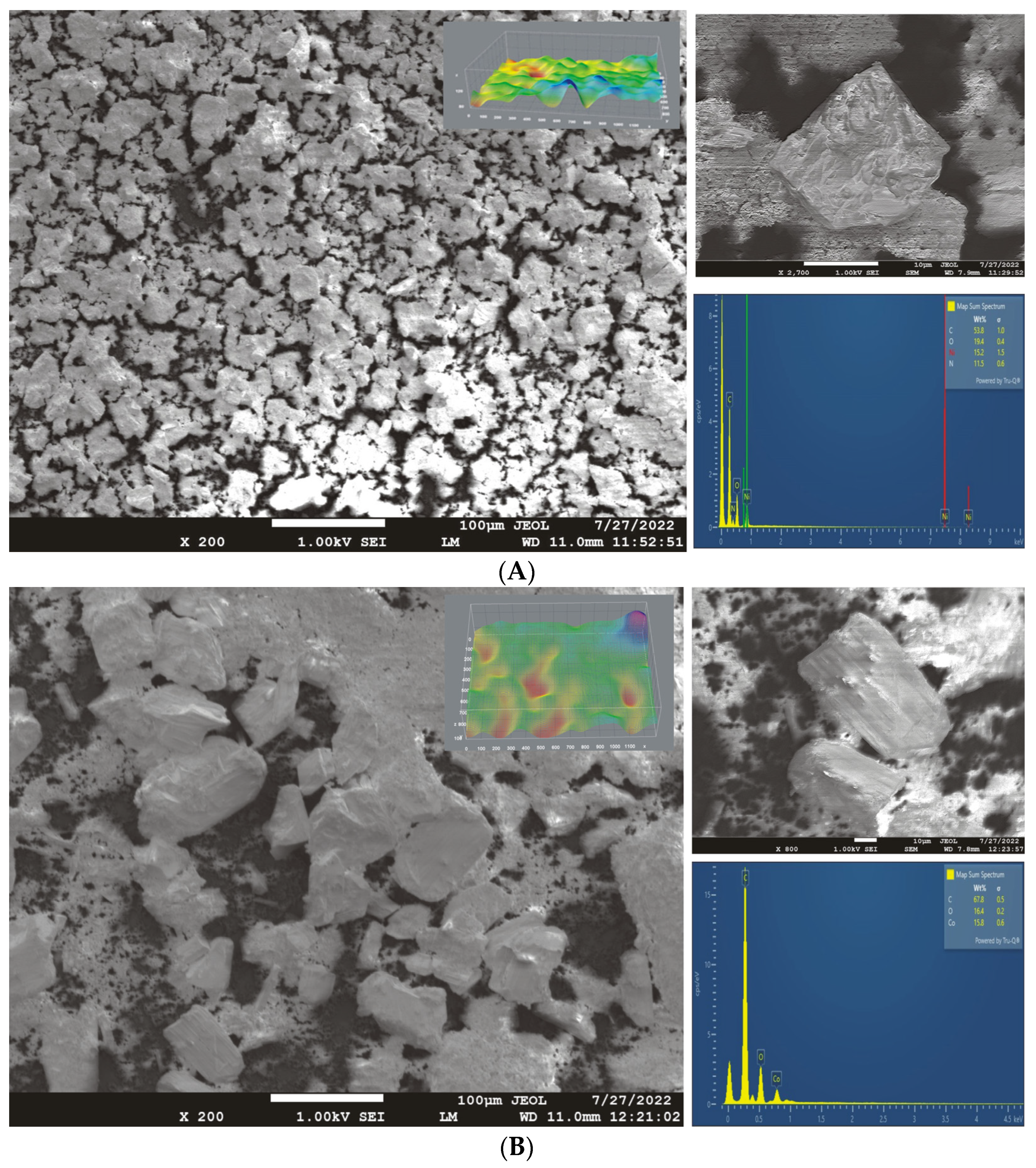
We carried out scanning electron microscopy (SEM) with energy-dispersive spectroscopy (EDS) analysis (JEOL JSM-7610F Plus) at 0.5 kV SEI on the GC electrodes modified with two layers of the prepared polymers after performing cyclic voltammetry measurements (from −1.0 to 1.0 V vs. Ag/AgCl with scan rate of 50 mV/s and 10 cycles, briefly washed in distilled water, and dried). The electrochemically treated electrodes were visually characterized using high-definition SEM. The images were evaluated using ImageJ software. A cross-sectional view was generated from the SEM images and displayed as a ZY or ZX plane. The images show a smooth and uniform surface with visible layers, while EDS analysis yielded chemical information about surface composition.
3. Results and Discussion
3.1. Electrochemical Impedance Spectroscopy
Since coordination polymers have low levels of electrical conductivity, they are rarely used as electrochemical sensors, and the design of high-performance sensors based on these materials remains a difficult task. Electrochemical impedance spectroscopy (EIS) is a widely used, non-invasive, in situ characterization method for studying the multiphase transport of electrons, ions, and gases as well as the coupling of interfacial reactions in porous electrodes. The interpretation of EIS data requires the modelling and fitting of results. The fitting procedure primarily determines the type and amount of information that can be obtained and thus the effectiveness of the EIS method. In addition, EIS is performed by employing a three-electrode configuration to better understand the electrochemical behaviour of the electrode/electrolyte interface [38,39].
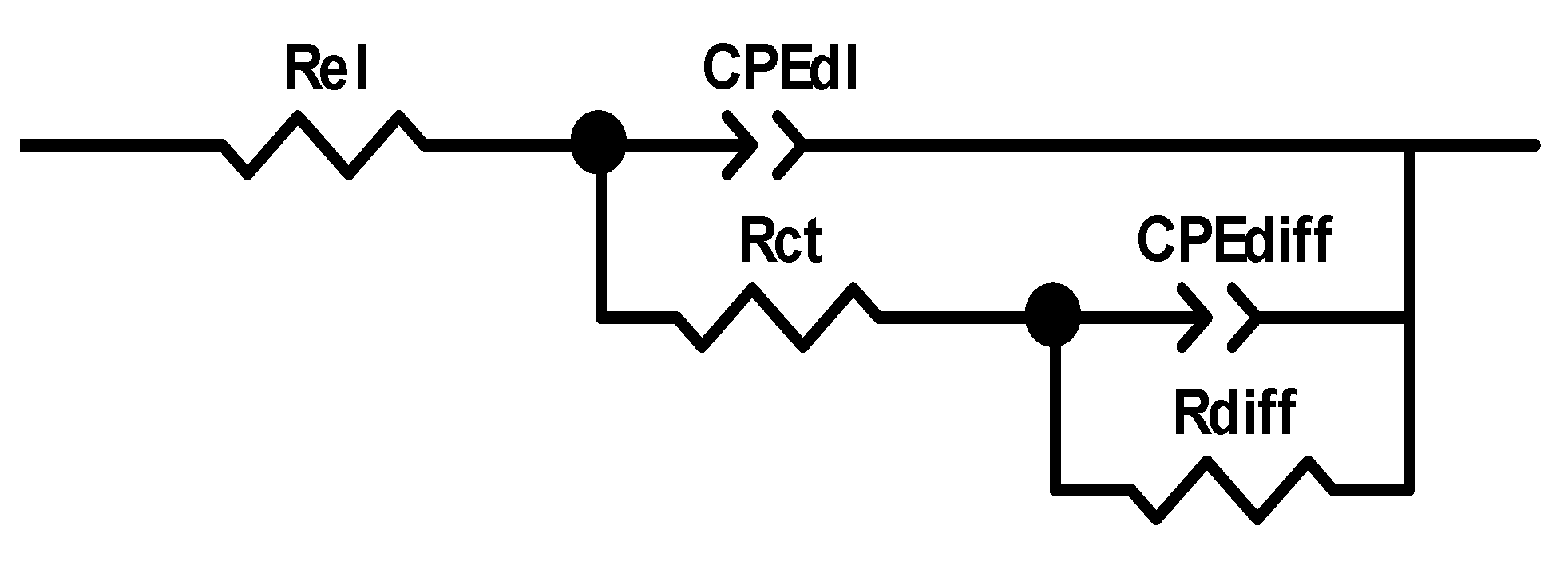
The recorded EIS data were modelled using the electrical equivalent circuit (EEC) shown in Scheme 1. A constant phase element (CPE) was used instead of a non-ideal capacitor [38]. In the EEC, Rel denotes electrolyte resistance, while CPEdl denotes the constant phase element, whose value is attributed to double-layer capacitance and Rct, which refers to the charge-transfer resistance. The values of the CPEdl and Rct depend on the insulating and barrier properties at the electrode/electrolyte interface. The CPEdiff and Rdiff values represent dimensional diffusion through a finite thickness layer or AC signal permeability through finite-length pores.
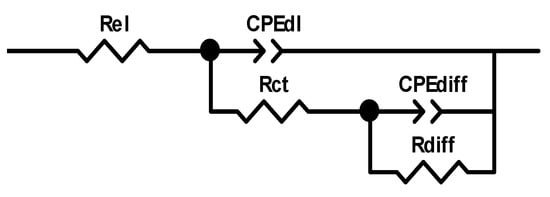
Scheme 1.
An EEC used to fit experimental data. (Rel-electrolyte resistance; CPEdl-constant phase element, related to double layer capacitance and Rct-charge-transfer resistance; CPEdiff-constant phase element, concerning diffusion through a finite thickness layer; and Rdiff-diffusion resistance).
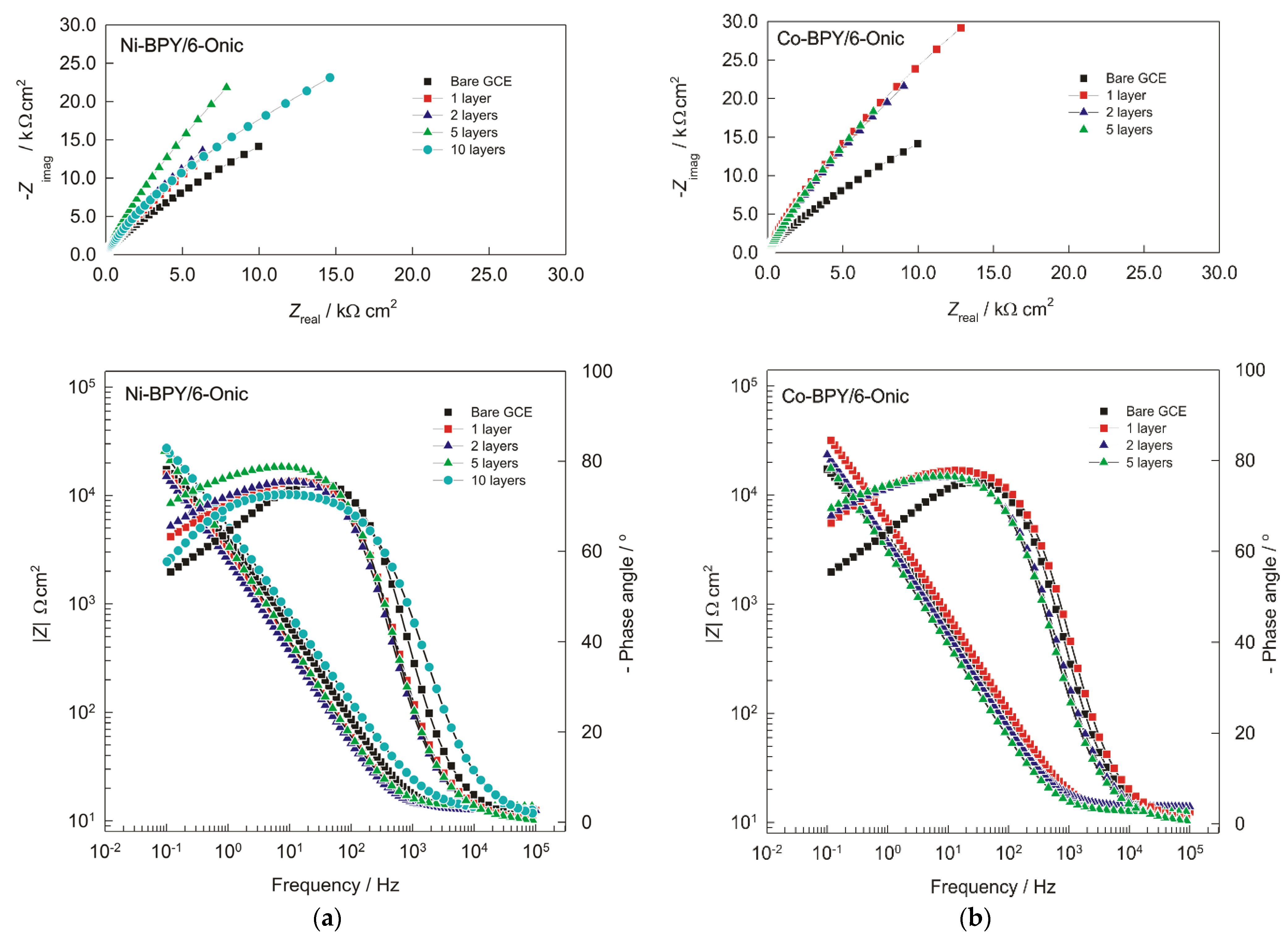
The impedance spectra were recorded in the form of Bode and Nyquist plots and presented in Figure 2. By fitting the electrochemical impedance spectra to the EEC, the value of each electrical element was obtained. The impedance parameters are given in Table 2.
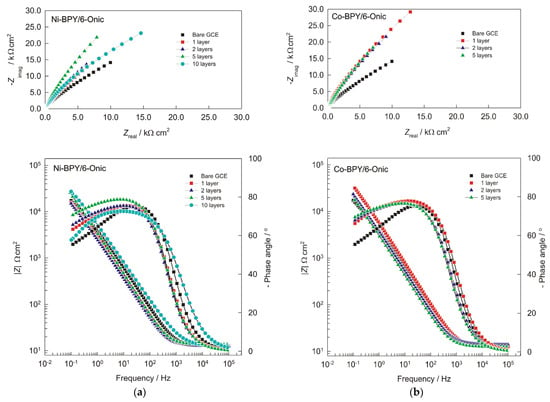
Figure 2.
The Nyquist plot (imaginary impedance (Zimag) versus real impedance (Zreal)) and Bode plots (two plots with same frequency at abscissa and the impedance and phase shift at ordinate) of the GC electrode, which was modified with (a) Ni(II) and (b) Cu(II) polymers in 0.1 mol L−1 of NaOH solution (with 1, 2, 5, and 10 layers) and recorded at Eocp.

Table 2.
Numerical values of impedance parameters for unmodified and modified glassy carbon (GC) electrodes in 0.1 mol L−1 NaOH solution (1, 2, 5, and 10 layers) recorded at Eocp.
Two time constants can be observed in the Bode plot, which are very similar for both modifications of the GC electrode. In the high-frequency region, there is one non-dominant semicircle whose diameter is related to the charge transfer resistance at the Ni-BPY/6-Onic or Co-BPY/6-Onic/electrolyte interface. Compared with the unmodified GC electrode, the resistance Rct in the case of the Ni polymer shows that the Ni polymer is a nonconducting compound with a passivating tendency. For the one-, two-, and five-layer modified electrodes, the adsorbed layer inhibits charge transfer from 1435 Ω cm2 up to 10,931 Ω cm2. The second-time constant in the low-frequency region indicates the presence of a diffusion process (n ≈ 0.5), which led to the higher diffusion resistance values. Both the total impedance and Rdiff values increase with an increasing number of layers up to the fifth layer. The 10-layer modified electrode with the Ni(II) polymer shows deviation of the results due to the weak adsorption of the film on the electrode. The electrolyte resistance was 12.5 Ω cm2. In the case of the Co(II) polymer, the EIS response was not affected by the number of modification layers, so the response for the 10-layer modified electrode with the Co(II) polymer clearly refers to a blocked electrode surface. Therefore, the results are not applicable to the modifications with a larger number of layers (not shown in the Nyquist and Bode plots).
3.2. Electrochemical Behaviour of the GC Electrodes Modified with the Ni(II) and Co(II) Polymers
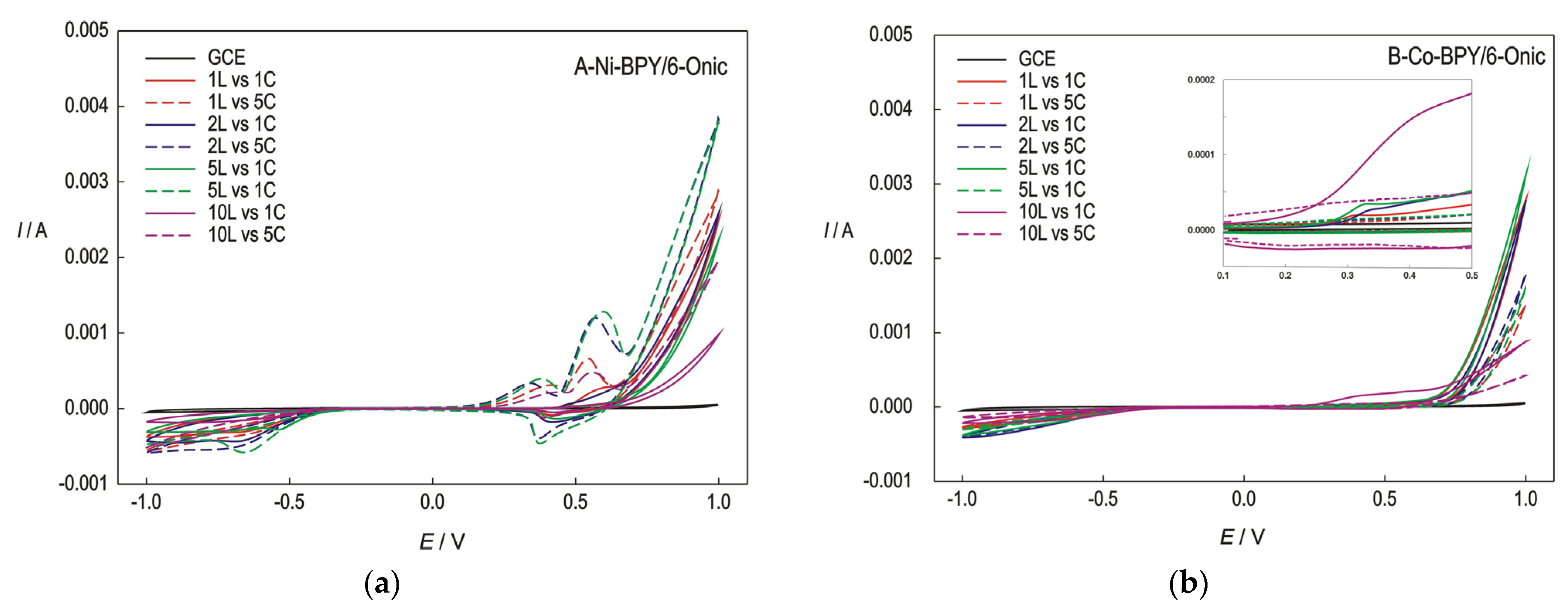
The cyclic voltammograms (CVs) obtained for the electrodes modified with different numbers of layers of the Ni(II) and Co(II) polymers are presented in Figure 3.
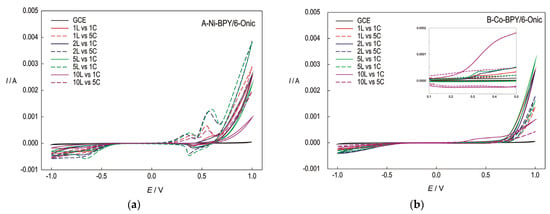
Figure 3.
The CVs recorded at the GC electrode modified with the Ni(II) polymer (a) and the Co(II) polymer (b) in 0.1 M of NaOH for the first and fifth cycles (1C and 5C), concerning the modified electrode with 1, 2, 5, and 10 layers (1L, 2L, 5L, and 10L); scan rate-50 mV/s.
The CVs were recorded in a wide potential range in order to gain an insight into the behaviour of the polymers. The voltammograms for the electrodes modified with the Ni(II) polymer, which were recorded in a pure electrolyte solution, revealed the presence of a smaller oxidation current peak around 0.4 V. This peak was not revealed in the first cycle, but after a cathodic reduction, it seemed that the Ni(II) ions present in the polymer may be reduced to form metallic nickel, Ni(0). So, the first, smaller peak corresponds to the anodic oxidation of nickel from oxidation state 0 to +2 due to the negative cathodic reduction of the electrode material. This reaction is unfavourable for the complex since a structural change could occur when Ni changes its oxidation state to zero. The reduction of Ni(II) to Ni(0) is probably accompanied by the decomposition of the Ni polymer. This reaction can be avoided by lowering the potential limit of the cathode branch to −0.5 V.
The second, larger peak around 0.5 V corresponds to the oxidation of Ni(II) to Ni(III); consequently, the reduction current peak around 0.4 V shows a reversal reaction of the electrode material corresponding to reaction (1):
Ni(II) polymer ⇆ Ni(III) polymer + e−
When the applied cathodic potential was −0.4 V, the cyclic voltammograms did not reveal a peak corresponding to the oxidation of Ni(0) to Ni(II). The stability of the unmodified and modified electrodes was investigated by conducting 30 consecutive scans at a scan rate of 50 mV/s. It was observed that the modified electrode (with up to five layers) exhibited a general contraction of the oxidation peak current (<5% up to the 30th cycle) in the potential range of −0.4 V to 1.0 V, indicating the very high stability of the polymer in a somewhat narrower potential range. A wider potential range slowly leads to the destruction of the modified electrode, indicating possible destruction due to the hydrogen evolution reaction (HER) or the reduction of nickel to its elemental state.
In the electrode modified with the Co(II) polymer, the anodic peak appeared only in the first cycles, indicating an irreversible oxidation of the electrode material (2) and, finally, a reduction of cobalt to its elemental state when the applied potential shifted toward the negative direction. In the narrower potential range from −0.4 V to 1.0 V, the Co(II) polymer is unstable, with an overall contraction in oxidation peak current (>20% from 1st to 3rd cycle).
Co(II) polymer → Co(III) polymer + e−
The increase in the current of the anodic branch at a potential higher than 0.7 V, which is analogous to the second reaction, is pronounced in the first cycle and is somewhat weaker in the other cycles, indicating that the oxidation of the organic species of the electrode material oxidized by the gradual decomposition of the Co(III)/BPY/6-Onic complex had transpired.
3.3. SEM/EDS Analysis
To obtain information on the influence of an electrochemical treatment on the changes in the composition and structure of the polymers, SEM/EDS analysis was performed, for which the results are presented in Figure 4. First, the surfaces were modified with two polymer layers, and cyclic voltammetry was performed in 0.1 M of NaOH with a potential ranging from −1.0 to 1.0 V vs. Ag/AgCl at a scan rate of 50 mV/s (10 cycles), after which the surfaces were briefly washed in distilled water and dried. An SEM characterization of the electrode was performed together with EDS analysis.
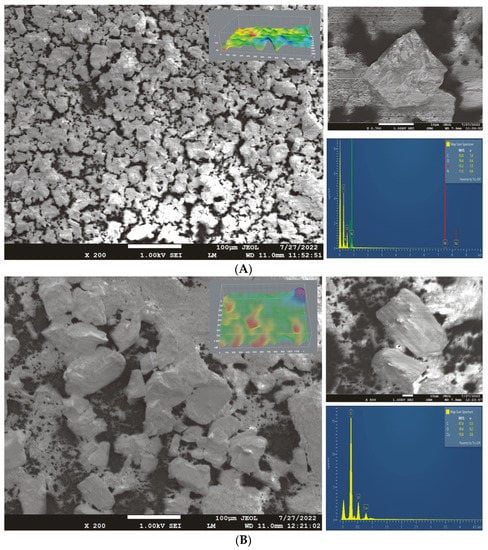
Figure 4.
The SEM/EDS analysis of the GC electrodes modified with (A) Ni(II) polymer and (B) Co(II) polymer after performing cyclic voltammetry in 0.1 M of NaOH (scan rate: 50 mV/s).
The surface morphologies of the Ni(II) and Co(II) modified GC electrodes are presented in Figure 4A,B and were evaluated using ImageJ software. In the case of the Ni(II) polymer electrode, the particles were slightly inhomogeneously distributed throughout the entire electrode surface, with a small amount of uncovered surface (pure GC). The inhomogeneity of the surface and the non-uniformly distributed crystals on the surface may indicate that the suspension (prior to the taking CV measurements) needs to be better homogenised before being dispersed on the GC electrode’s surface. The time for ultrasonic homogenization should be extended to avoid the appearance of larger complex crystals on the surface. In addition, this change may be a result of a decreasing level of adhesion due to the voltametric measurements or due to an alteration in the structure and properties of the Ni(II) polymer.
Using EDS, we characterized the composition of the particles and the composition distribution (Figure 4). The EDS diagrams for (A) the Ni(II) polymer and (B) the Co(II) polymer following the cyclic voltammetry measurements are also shown in Figure 4. The EDS analysis confirmed the stability of the Ni(II) polymer on the surface even under such harsh conditions. The voltametric behaviour of Ni-BPY/6-onic is reminiscent of nickel(II) hydroxide electrodes in alkaline solutions, which can be converted to NiOOH upon oxidation in an alkaline solution. The structure of the polymer was somewhat changed after cyclic voltammetry, which is evident from the ratio of nickel to nitrogen content, which should be almost equal. The ratio of nitrogen is slightly lower than that of nickel, indicating the partial decomposition of the complex. According to Tommaso, in a nickel–tetraazaannulene complex, the Ni(II) centres are no longer bonded to the polymeric skeleton upon electrochemical treatment, but they remain inside the catalytic layer [40]. In our case, the assumption is the same for a small part of the polymer: the Ni centre remains in the catalytic layer. A lower ratio of oxygen than the theoretical value also indicates that the formation of nickel hydroxide or nickel oxide has not occurred. More than 70% of the polymer stays in the polymer chain as a part of Ni-BPY/6-Onic.
In the case of the Co(II) polymer, it can be concluded from the SEM images that the electrode surface is inhomogeneous and not completely covered by the complex. The mass ratios of the elements obtained by EDS analysis indicate changes in the structure that occurred during the cyclization of the electrode. A significantly lower oxygen content than the theoretical one (it should be about three times that of cobalt) indicates the decomposition of the complex. The decomposition of the complex was also confirmed by the fact that the detector did not indicate the nitrogen content, even though it should be close to the cobalt content. Although the nitrogen peak is thought to be between oxygen and carbon, its concentration is not high enough to be detected.
In the SEM image (upper right corner of Figure 4A,B), the following crystal dimensions were measured: (A) the Ni(II) polymer-X: 24.306 μm, Y: 22.083 μm, and D: 32.840 μm; (B) the Co(II) polymer-X: 68.437 μm, Y: 65.977 μm, and D: 95.061 μm. As can be seen from the crystal dimensions, the obtained Co(II) polymer crystals are about three times larger at the surface after cyclization, resulting in high levels of surface roughness and inhomogeneity of the film. The elemental distribution and table-of-elements ratio are presented in the Supplementary Materials in Figures S1 and S2 and Tables S1 and S2.
3.4. Electrochemical Behaviour of the GC Electrodes Modified with Ni(II) and Co(II) Polymers towards Methanol
The electrochemical sensing performance or electrocatalytic ability of the glassy carbon electrode modified with the Ni(II) polymer (Figure 5) and the Co(II) polymer (Figure 6) towards methanol oxidation was evaluated via cyclic voltammetry in 0.1 M of NaOH in the potential range of 0.1 V to 0.9 V. The methanol oxidation at bare glassy carbon electrodes in basic media is very poor (see inset in Figure 6).
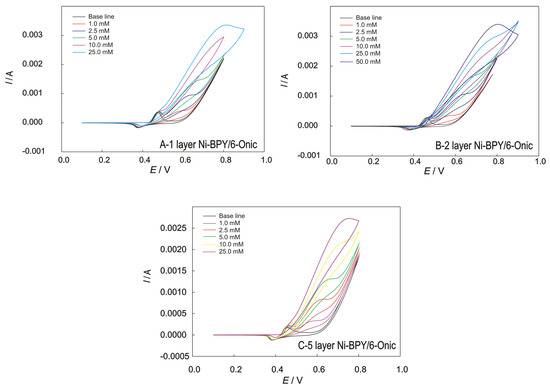
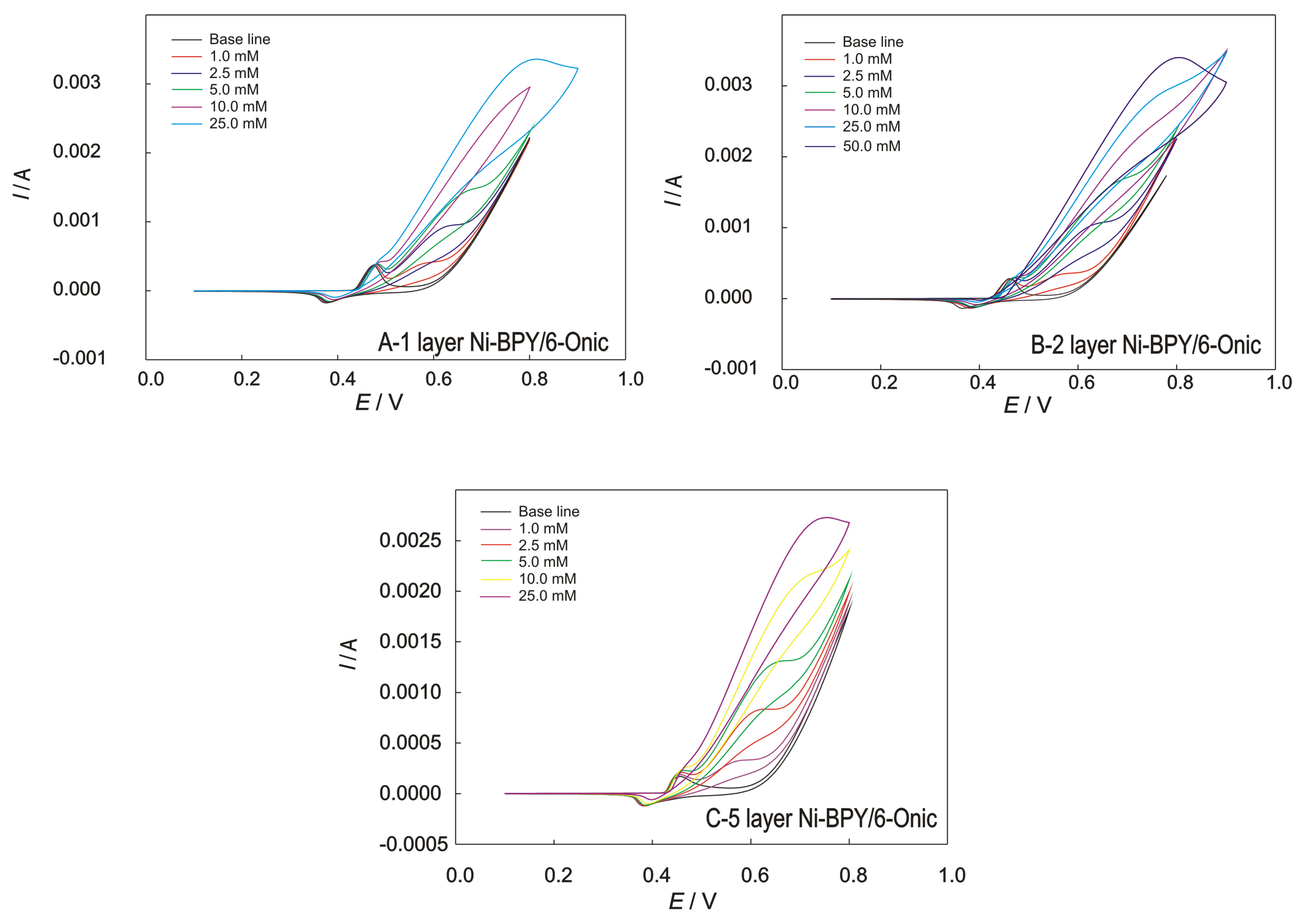
Figure 5.
The CVs recorded at the GC electrode modified with Ni(II) polymer in 0.1 M of NaOH for (A) 1, (B) 2, and (C) 5 layers; scan rate of 50 mV/s, with the addition of methanol.
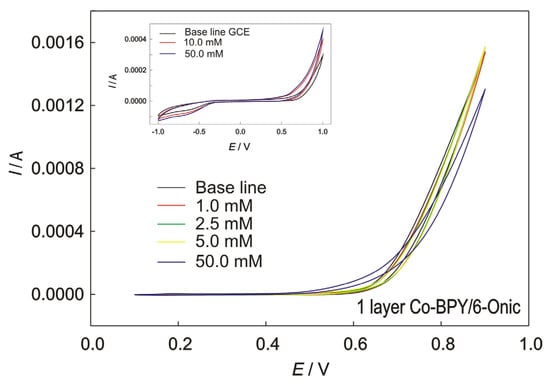
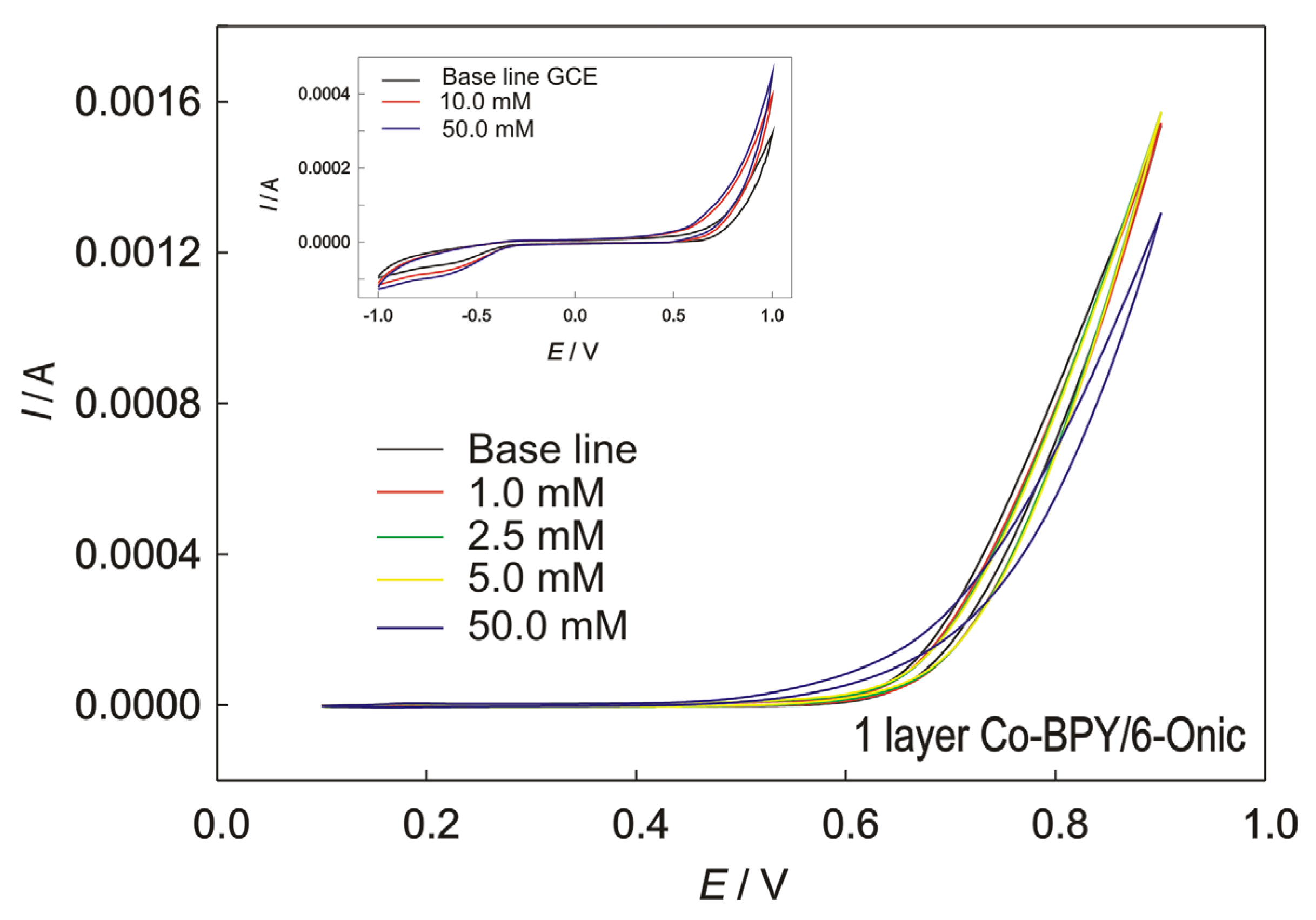
Figure 6.
The CVs recorded at GC electrode, modified with the Co(II) polymer in 0.1 M of NaOH, for 1 layer; scan rate 50 mV/s, with the addition of methanol. Inset: Bare GC electrode with and without addition of methanol.
The reduced potential range enabled the existence of an Ni(II)/Ni(III) centre and prevented the degradation of the Ni(II) polymer. The somewhat narrower potential range exhibited a highly stabile modified surface. The wider potential range, especially in the cathodic direction, slowly leads to the destruction of the modified electrode, indicating the possibility of destruction via the hydrogen evolution reaction (HER) or the reduction of nickel to its elemental state.
An increase in the current was observed with an increasing methanol concentration for the electrodes modified with one, two, and five layers (Figure 5). As the total layer thickness increases (over two layers), the current decreases, which is in accordance with the results of the EIS measurements and can be attributed to the saturation of the surface, resulting in impaired diffusion and electron transfer. The Ni-BPY/6-Onic electrode consisting of two layers showed the most promising behaviour towards methanol determination. The voltammograms of the Ni-BPY/6-Onic electrode in 0.1 M of NaOH showed oxidation/reduction peaks, namely, Eox = 0.47 V and Ered = 0.35 V, in the absence of methanol, which can be attributed to the reaction of the redox couple Ni(II)/Ni(III) in the polymer. In the presence of methanol, the potential of the anodic peak of Ni(II)/Ni(III) is slightly shifted toward the positive direction, clearly suggesting an interaction between the methanol and the coordination polymer at the electrode’s surface. Another oxidation peak starts to appear at E = 0.55 V, which corresponds to the oxidation of methanol. When excessive concentrations are added, the oxidation peak currents increase, and the oxidation potential shifts to the more positive potential, suggesting that the diffusion process is disrupted at a high concentration.
The presence of Ni(III) on the surface enhances the oxidation of methanol according to the following equation:
Ni(II) polymer + CH3OH → Ni(III) polymer + oxidation product
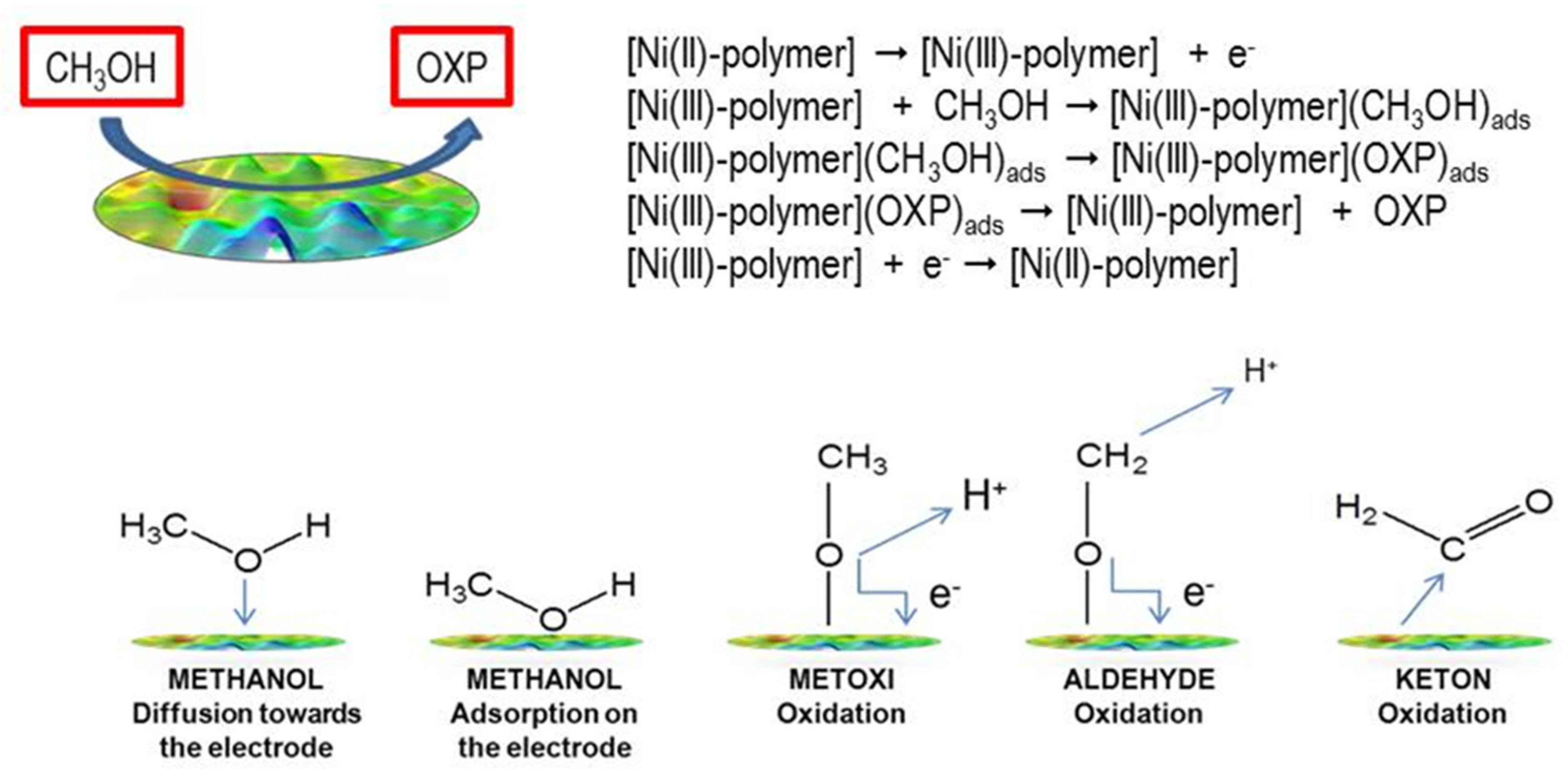
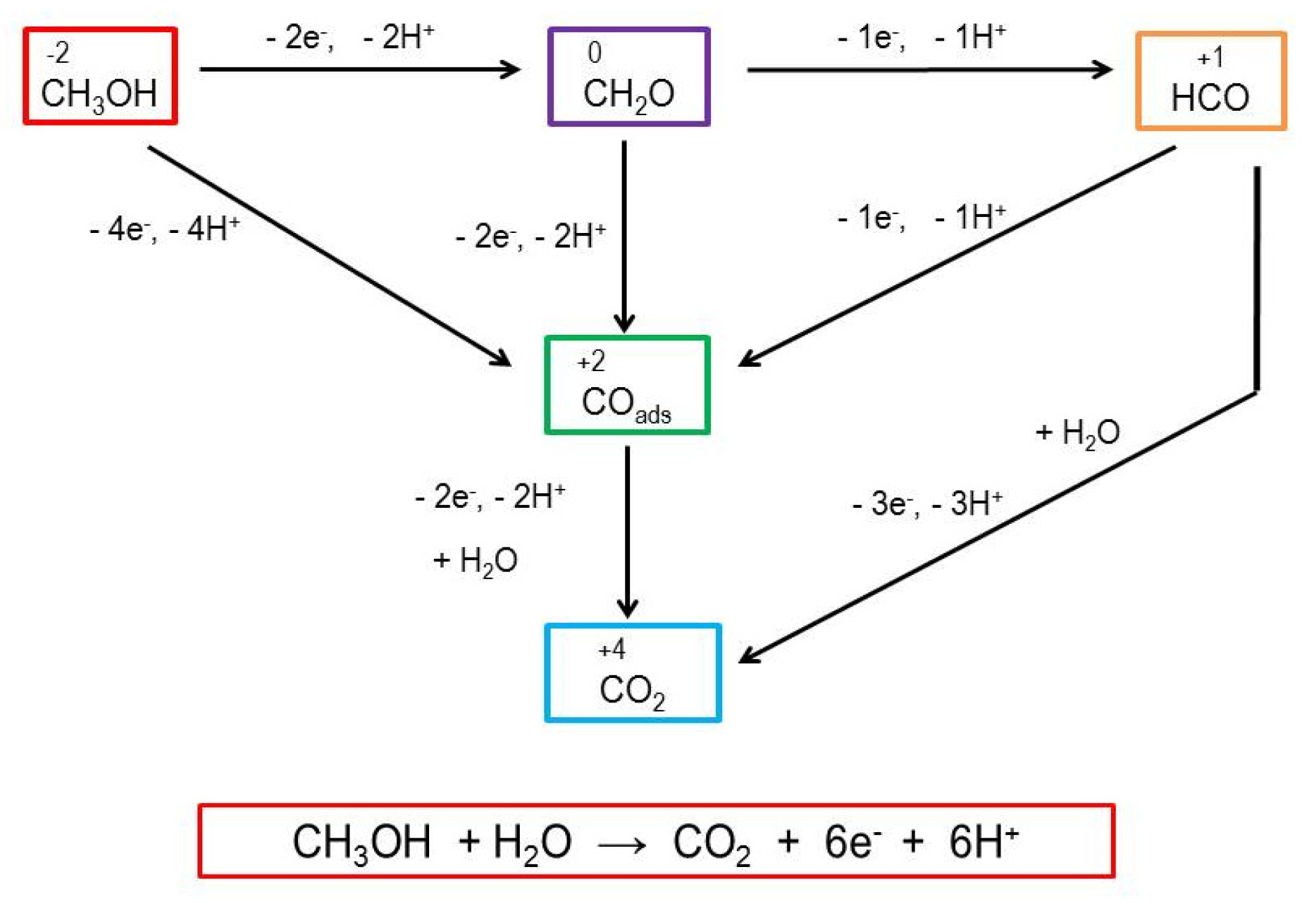
This mechanism is based on the observation of the Ni(II)/Ni(III) redox pair via cyclic voltammetry and its activity in the presence of methanol [32]. The oxidation of Ni(II) to Ni(III) in aqueous media is somewhat inhibited due to the strong hydration of nickel(II) ions [41]. However, this oxidation is facilitated by 4,4-bipyridine’s coordination to nickel(II). With regard to pH, the mechanism of the electrooxidation of methanol can follow an acidic or an alkaline pathway. In acid electrooxidation, there are several electric potential limitations [42,43,44]. In an alkaline medium, methanol’s oxidation kinetics are faster than in an acidic medium. Therefore, the use of alkaline media is a viable approach for the electrocatalytic oxidation of methanol. The mechanism and steps of methanol oxidation are shown in Scheme 2. In the upper part of Scheme 2, the proposed mechanism is shown, wherein we assume that oxidation takes place at the electrode modified with the Ni(II) polymer. In the lower part of the Scheme 2, the mechanism is very complex and still insufficiently understood, and the reaction products are very difficult to detect, even with modern analytical techniques. It has been suggested that the elementary steps leading to the formation of CH2O could begin with the adsorption of a methanol molecule via the oxygen atom, leading to the formation of methoxide species (H3CO)ad. This reaction involves the elimination of a hydrogen atom (as H+), which donates an electron to the metal:
[CH3OH → (CH3O)ad + H+ + e−]
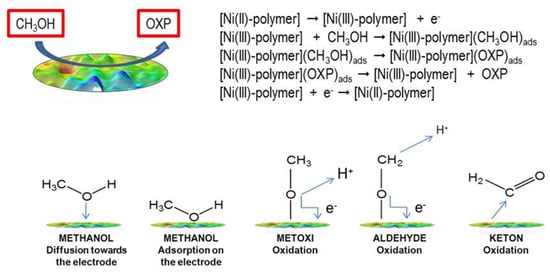
Scheme 2.
Upper part-two-electron reaction in the electrooxidation of methanol at the electrode’s surface; lower part-possible step-by-step reaction.
In the second step, (H3CO)ad can eliminate another H atom from the CH3 group to form CH2O, which is then desorbed from the electrode’s surface:
[(CH3O)ad → CH2O + H+ + e−]
Different multistep reactions of methanol oxidation have been reported [36], thus proving the complexity of the mechanism (Scheme 3). According to Chung [42], the full oxidation of methanol results in carbon dioxide in a six-electron-mediated reaction. The same author investigated the electrooxidation of methanol on a Pt electrode, which exhibited electrocatalytic activity towards the oxidation of methanol (methanol oxidation reaction-MOR) at 1.1 V vs RHE. As an intermediate in the MOR reaction, carbon monoxide is formed and strongly adsorbed to the surface of the Pt catalyst, causing the formation of Pt-COad and the deactivation of the catalyst via poisoning [42].
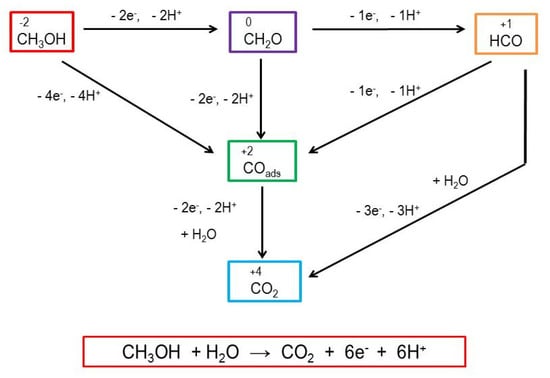
Scheme 3.
The possible step-by-step, six-electron reaction in the electrooxidation of methanol at the electrode surface [36,42].
In our case, the MOR did not cause surface deactivation due to the adsorption of intermediate products, thereby indicating the electrocatalytic properties of the synthesized polymers with respect to the MOR.
The Co-BPY/6-Onic electrode (Figure 6) showed no catalytic activity towards the MOR, suggesting the inertness of cobalt(II) in the Co(II) polymer. The methanol oxidation reaction at the electrode modified with the Co(II) polymer was not enhanced.
The cyclic voltammograms of the bare GC electrode to which with methanol was added are shown in the inset of Figure 6. The voltametric curve of the bare GC electrode (Figure 6) to which methanol was not added shows the oxygen oxidation reaction characteristic of glassy carbon activity. When methanol was added, no significant current increase was observed, even in excessive quantities, which could be attributed to the methanol oxidation reaction at the bare electrode. Slight potential shifts indicated the oxidation of other species on the electrode’s surface.
A comparison of the results shown in Figure 5 and Figure 6 indicates that the Ni(II) polymer can significantly improve the electrocatalytic process at the glassy carbon electrode. The excellent improvement of the methanol oxidation reaction at the electrode modified with the Ni(II) polymer can also be seen in the significant reduction in the anodic potential to 0.55 V, which can be compared to the value of 0.66 V recorded at the unmodified electrode. After searching the literature, we also determined that there was a satisfactory catalytic effect on the oxidation potential. In an alkaline medium, the potential for the electrooxidation of methanol on the surface of Pt [42] or on GO/Cu-MOF [25] is much more positive: approximately 0.900 V. The highly positive potential for electrooxidation is still the main obstacle. Li also deals with this topic in her 2023 article and provides a brief overview of the data from the literature, pointing out that the oxidation potential on many Ni(II)-modified electrodes is still very positive (around 1.300 V vs. RHE) [30]. Using similar modifications (Eox = 0.550 V for Ni/Co/ZIF-67 [26], Eox = 0.626 V for Ni(II)-DHS [41], or Eox = 0.650 V for Ni/Cu-PANI [45]), the obtained results show good catalytic potential. The presence of highly oxidative species in these materials, e.g., Ni(II)/Ni(III), which act as catalytic active centres towards methanol, was confirmed [18,40,41,45,46].
3.5. Electroanalytical Determination of Methanol
The cyclic voltammograms (obtained at the Ni-BPY/6-Onic electrode in 0.1 M of NaOH) show that in the presence of different methanol concentrations, a successive addition of methanol increases the anodic current. In this potential range, this process represents an oxidation of methanol in accordance with Scheme 2. In this regard, the optimal number of layers is two.
The optimization of the SWV procedure required a systematic study of parameters; the obtained optimized parameters are presented in the Materials and Methods section. A deposition/accumulation potential more negative than 0.350 V showed weak catalytic activity towards methanol oxidation, while one higher than 0.390 V showed no linear dependence of concentration on current change. Therefore, the optimum potential was determined to be 0.370 V with an accumulation time of 120 s. The selected deposition/accumulation potential suggests that the oxidation of Ni(II) to Ni(III) in the presence of methanol under the selected experimental SWV parameters starts at this potential and ensures the formation of Ni(III) on the surface.
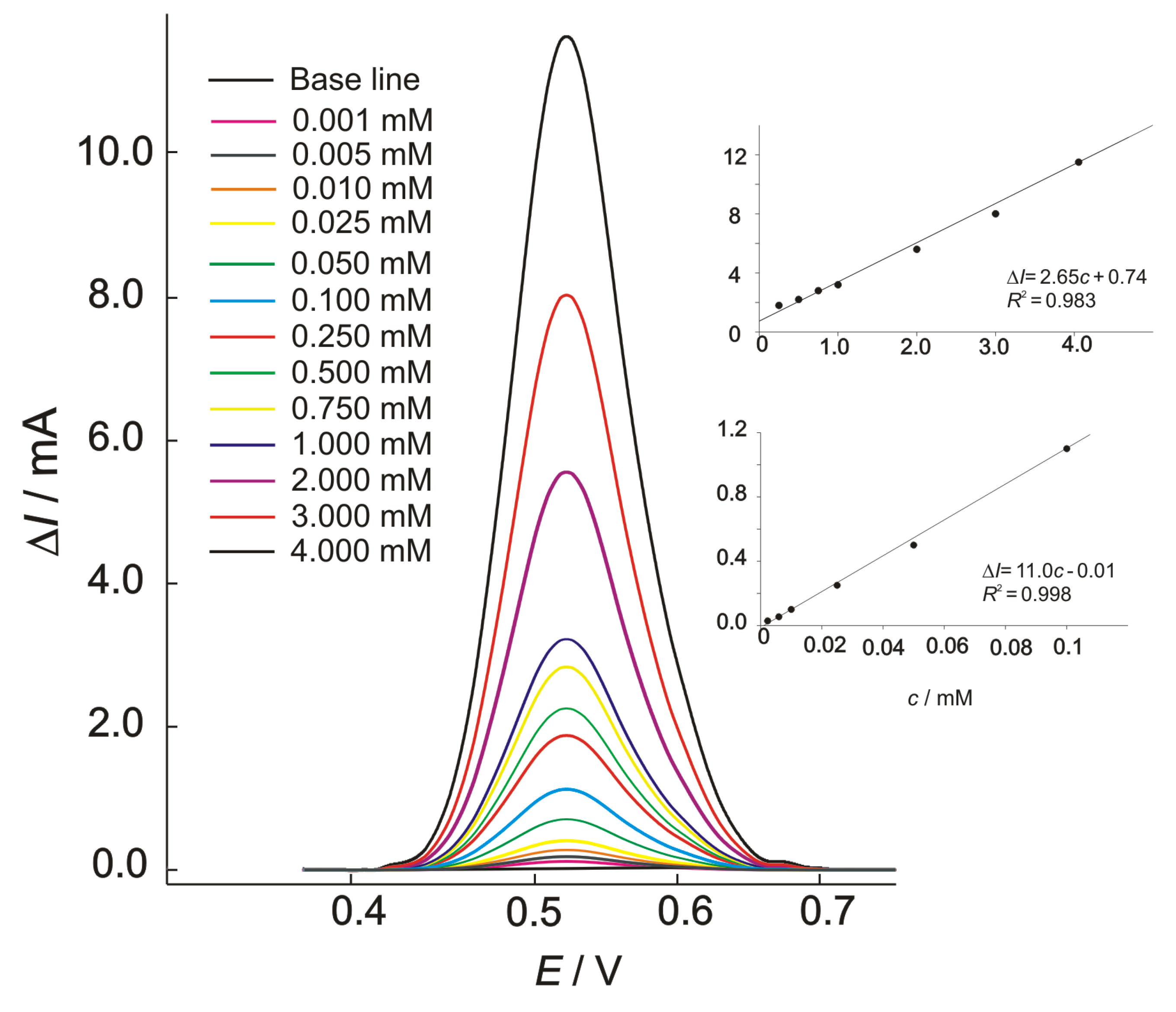
The results of square wave voltammetry conducted in the presence of methanol are shown in Figure 7, with the corresponding calibration plots given as insets. The voltammograms were obtained using a standard addition method, and the presented curves are background-subtracted reduction currents. By the increasing methanol concentration, a linear dependence of an oxidation peak’s current (ΔI) vs. concentration (c) was obtained and represented as equations in the calibration graphs. The calculated detection limit, based on the 3σ criterion, was 0.8 μM. The calibration plots show two different linear concentration ranges, which were both characterized by good sensitivity levels of 2.65 and 11.0 mA mM−1, respectively, for lower (0.001–0.1 mM) and higher (0.1–4.0 mM) concentration ranges.
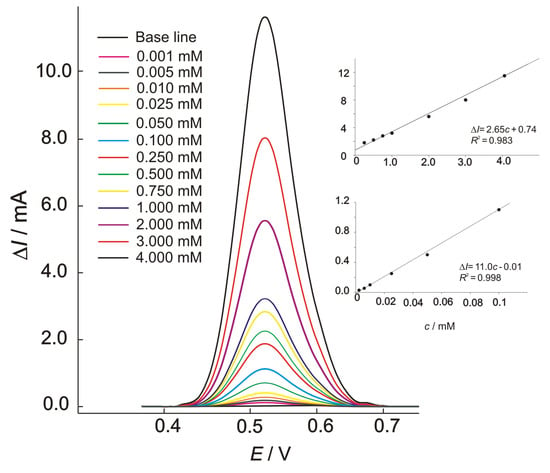
Figure 7.
The square-wave voltammograms for oxidation of methanol at the Ni-BPY/6-Onic electrode in 0.1 M of NaOH for different concentrations of methanol; electrochemical conditions were as follows: Edep = 0.370 V, tdep = 120 s, f = 20 Hz, ΔEp = 100 mV, and ΔEs = 20 mV. INSET: Calibration plots.
Possible interference was studied using the same experimental procedure by adding appropriate amounts of ethanol, glucose, lauric acid, EDTA, vitamin C, L-isoleucine, and glutathione into a solution containing 1.0 mM of methanol. The criterion for interference was a ±5% error in the peak current. The effect of interference on the determination of methanol showed that high-mass molecules, even at very high concentrations, do not interfere with methanol (glucose, lauric acid, EDTA, and glutathione). The addition of an equal concentration of ethanol resulted in a broadening of the oxidation peak and a slight shift of the anodic peak to a more positive value, indicating the simultaneous oxidation of both species.
4. Conclusions
In this study, we have illustrated the wide possibilities of the application of Ni(II) and Co(II) coordination polymers with 4,4-bipyridine and 6-hydroxonicotinic acid, especially in the field of catalysis. The main results are as follows:
- The isostructural Ni(II) and Co(II) coordination polymers exhibited different electrochemical behaviours, as evidenced by their different EIS spectra. The electrochemical stability during cyclization in a wide range of potentials did not show similarities between the studied coordination polymers, which could be interpreted using different electronic configurations of the metal(II) ions.
- In contrast to the Co(II) polymer, the Ni(II) polymer showed a large sensing potential for the electrocatalytic oxidation of methanol. This outstanding feature of {[Ni(4,4′-bpy)(H2O)4](6-Onic)2×2H2O}n is based on the enhancement of catalytic activity related to the redox couple Ni(II)/Ni(III), which leads to a significant enhancement of the analytical signal.
- The anodic current of methanol oxidation at the GC electrode modified with the Ni(II) polymer increased linearly with the concentration, representing a significant improvement in the catalytic application of coordination polymers for the catalytic oxidation and determination of methanol.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst13050718/s1, Figure S1: EDS analysis: nickel, carbon, nitrogen, and oxygen; Table S1: Table-of-elements ratio-theoretical vs. EDS; Figure S2: EDS analysis: cobalt, carbon, nitrogen, and oxygen; Table S2: Table of elements ratio-theoretical vs. EDS; Figure S3: The IR spectrum of {[Ni(4,4′-bpy)(H2O)4](6-Onic)2×2H2O}n; Figure S4: The IR spectrum of {[Co(4,4′-bpy)(H2O)4](6-Onic)2×2H2O}n; Table S3: The selected vibration bands in the IR spectra of {[Ni(4,4′-bpy)(H2O)4](6-Onic)2×2H2O}n and {[Co(4,4′-bpy)(H2O)4](6-Onic)2×2H2O}n.
Author Contributions
Conceptualization, N.V. and I.Š.R.; methodology, N.V. and I.Š.R.; software, N.V. and I.Š.R.; validation, N.V. and I.Š.R.; formal analysis, all authors.; investigation, N.V., I.Š.R., B.-M.K. and V.S.; resources, N.V. and B.-M.K.; data curation, N.V.; writing-original draft preparation, N.V.; writing-review and editing, all authors; visualization, N.V.; supervision, N.V.; project administration, B.-M.K., M.B. (Marijo Buzuk) and M.B. (Maša Buljac); funding acquisition, N.V. and B.-M.K. All authors have read and agreed to the published version of the manuscript.
Funding
The studies in this paper are funded by the institution funds of the Faculty of Chemistry and Technology, University of Split, Croatia.
Data Availability Statement
The data presented in this study will be available on request from the corresponding author.
Acknowledgments
We are thankful for the scientific research equipment financed by the EU grant, “Functional integration of the University of Split, PMF-ST, PFST and KTFST through the development of the scientific and research infrastructure” (KK.01.1.1.02.0018).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xiang, X.; Pan, F.; Li, Y. Flower-like Bismuth Metal-Organic Frameworks Grown on Carbon Paper as a Free-Standing Electrode for Efficient Electrochemical Sensing of Cd2+ and Pb2+ in Water. Eng. Sci. 2018, 3, 77–83. [Google Scholar] [CrossRef]
- Marandi, F.; Pantenburg, I.; Meyer, G. A New 3D Coordination Polymer of Bismuth with Nicotinic Acid N-Oxide. J. Chem. 2013, 2, 845810. [Google Scholar] [CrossRef]
- Zhao, X.; Xiong, X.; Chen, X.; Li, J.H.J. Synthesis of halide anion-doped bismuth terephthalate hybrids for organic pollutant removal. Appl. Organometal. Chem. 2016, 30, 304–310. [Google Scholar] [CrossRef]
- Selvan, K.S.; Narayanan, S.S. Synthesis, structural characterization and electrochemical studies switching of MWCNT/novel tetradentate ligand forming metal complexes on PIGE modified electrode by using SWASV. Mat. Sci. Eng. C 2019, 98, 657–665. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, J.; Zhang, R.; Shi, H.; Wang, N.; Li, J.; Ma, F.; Zhang, D. Cu-based metal-organic framework as a novel sensing platform for the enhanced electro-oxidation of nitrite. Sens. Actuators B 2016, 222, 632–637. [Google Scholar] [CrossRef]
- Okpara, E.C.; Ogunjinmi, O.E.; Oyewo, O.A.; Fayemi, O.E.; Onwudiwe, D.C. Green synthesis of copper oxide nanoparticles using extracts of Solanum macrocarpon fruit and their redox responses on SPAu electrode. Heliyon 2021, 7, e08571. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Hu, Z.; Wang, G.; Uvdal, K. Coordination polymers for energy transfer: Preparations, properties, sensing applications, and perspectives. Coord. Chem. Rev. 2015, 284, 206–235. [Google Scholar] [CrossRef]
- Zhou, S.F.; Hao, B.B.; Lin, T.; Zhang, C.X. A dual-functional MOF for high proton conduction and sensitive detection of ascorbic acid. Dalton Trans. 2020, 49, 14490–14496. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.-W.; Wang, X.-L.; Lin, H.-Y.; Xu, N.; Wang, X.; Liu, G.-C.; Chang, Z.-H. Two Anderson-type polyoxometalate-based metal-organic complexes with a flexible bis(pyrazine)-bis(amide) ligand for rapid adsorption and selective separation of cationic dyes. Inorg. Chim. Acta. 2020, 513, 119937. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Xu, N.; Chang, Z.; Liu, G.; Lin, H.; Wang, X. Four octamolybdate complexes constructed from a quino-line-imidazole-monoamide ligand: Structures and electrochemical, photocatalytic and magnetic properties. Cryst. Eng. Comm. 2020, 22, 8322–8329. [Google Scholar] [CrossRef]
- Škugor Rončević, I.; Vladislavić, N.; Buzuk, M. Copper(II) Coordination Compound with 2-Oxonicotinate: Synthesis, spectroscopic and electrochemical. Croat. Chem. Acta 2022, 95, 1–10. [Google Scholar] [CrossRef]
- Pang, L.Y.; Wang, P.; Gao, J.J.; Wen, Y.; Liu, H. An active metal-organic anion framework with highly exposed SO42- on {001} facets for the enhanced electrochemical detection of trace Fe3+. J. Electroanal. Chem. 2019, 836, 85–93. [Google Scholar] [CrossRef]
- Huang, Y.; Luo, M.; Li, S.; Xia, D.; Tang, Z.; Hu, S.; Ye, S.; Sun, M.; He, C.; Shu, D. Efficient catalytic activity and bromate minimization over lattice oxygen-rich MnOOH nanorods in catalytic ozonation of bromide-containing organic pollutants: Lattice oxygen-directed redox cycle and bromate reduction. J. Hazard. Mater. 2021, 410, 124545. [Google Scholar] [CrossRef] [PubMed]
- Hok, L.; Lluch Sanchez, E.; Vianello, R.; Kukovec, B.-M.; Popović, Z. Self-assembly of cobalt(II) coordination polymers with differently halosubstituted nicotinate ligands and 4,4′-bipyridine-The effect of the halosubstituent positions on polymer types. Eur. J. Inorg. Chem. 2021, 2021, 1470–1480. [Google Scholar] [CrossRef]
- Fisher, B.J.; Eisenberg, R. Electrocatalytic reduction of carbon dioxide by using macrocycles of nickel and cobalt. J. Am. Chem. Soc. 1980, 102, 7361–7363. [Google Scholar] [CrossRef]
- Haines, R.J.; Wittrig, R.E.; Kubiak, C.P. Molecular approaches to the electrochemical reduction of carbon dioxide. Inorg. Chem. 1994, 33, 4723–4728. [Google Scholar] [CrossRef]
- Losada, J.; Peso, I.d.; Beyer, L.; Hartung, J.; Fernandez, V.; Mobius, M. Electrocatalytic reduction of O2 and CO2 with electropolymerized films of polypyrrole cobalt(II) schiff-base complexes. J. Electroanal. Chem. 1995, 398, 89–93. [Google Scholar] [CrossRef]
- Deronzier, A.; Moutet, J.-C. Polypyrrole films containing metal complexes: Syntheses and applications. Coord. Chem. Rev. 1996, 147, 339–371. [Google Scholar] [CrossRef]
- Maxime, P.; Hervé, L.; Fethi, B. Improvement in the performance of a nickel complex-based electrochemical sensor for the detection of nitric oxide in solution. Sens. Actuators B Chem. 1999, 56, 1–5. [Google Scholar] [CrossRef]
- Giovanelli, D.; Lawrence, N.S.; Jiang, L.; Jones, T.G.J.; Compton, R.G. Electrochemical determination of sulphide at nickel electrodes in alkaline media: A new electrochemical sensor. Sens. Actuators B Chem. 2003, 88, 320–328. [Google Scholar] [CrossRef]
- Cheng, W.-L.; Sue, J.-W.; Chen, W.-C.; Chang, J.-L.; Zen, J.-M. Activated Nickel Platform for Electrochemical Sensing of Phosphate. Anal. Chem. 2010, 82, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Jia, D.; He, Y.; Miao, Y.; Wu, H.-L. Nano nickel oxide modified non-enzymatic glucose sensors with enhanced sensitivity through an electrochemical process strategy at high potential. Biosens. Bioelectron. 2011, 26, 2948–2952. [Google Scholar] [CrossRef] [PubMed]
- Ourari, A.; Aggoun, D.; Ouahab, L. A Novel Copper(II)-Schiff base complex containing pyrrole ring: Synthesis, characterization and its modified electrodes applied in oxidation of aliphatic alcohols. Inorg. Chem. Commun. 2013, 33, 118–124. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; Veeramani, V.; Saravanan, M.; Rajalakshmi, G.; Kaliannan, T.; Al-Misned, F.A.; Pugazhendh, A. Green chemistry route of biosynthesized copper oxide nanoparticles using Psidium guajava leaf extract and their antibacterial activity and effective removal of industrial dyes. J. Environ. Chem. Eng. 2021, 9, 105033. [Google Scholar] [CrossRef]
- Noor, T.; Ammad, M.; Zaman, N.; Iqbal, N.; Yaqoob, L.; Nasir, H. A highly efficient and stable copper BTC metal organic framework derived electrocatalyst for oxidation of methanol in DMFC application. Catal. Lett. 2019, 149, 3312–3327. [Google Scholar] [CrossRef]
- Asadi, F.; Azizi, S.N.; Ghasemi, S. A novel non-precious catalyst containing transition metal in nanoporous cobalt based metal-organic framework (ZIF-67) for electrooxidation of methanol. J. Electroanal. Chem. 2019, 847, 11318. [Google Scholar] [CrossRef]
- Mansor, M.; Timmiati, S.N.; Lim, K.L.; Wong, W.Y.; Kamarudin, S.K.; Nazirah Kamarudin, N.H. Recent progress of anode catalysts and their support materials for methanol electrooxidation reaction. Int. J. Hydrogen Energy 2019, 44, 14744–14769. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Elsaid, K.; Wilberforce, T.; Kamil, M.; Sayed, E.T.; Olabi, A. Environmental aspectsof fuel cells: A review. Sci. Total Environ. 2021, 752, 141803. [Google Scholar] [CrossRef]
- Jafarian, M.; Mahjani, M.G.; Heli, H.; Gobal, F.; Khajehsharifi, H.; Hamedi, M.H. A study of the electro-catalytic oxidation of methanol on a cobalt hydroxide modified glassy carbon electrode. Electrochim. Acta 2003, 48, 3423–3429. [Google Scholar] [CrossRef]
- Li, J. Nickel-organic frameworks as highly efficient catalyst for electrochemical conversion of CH3OH into formic acid. Electrochem. Comm. 2023, 146, 107416. [Google Scholar] [CrossRef]
- Asgari, M.; Ghannadi Maragheh, M.; Davarkhah, R.; Lohrasbi, E.; Nozad Golikand, A. Electrocatalytic oxidation of methanol on the nickel–cobalt modified glassy carbon electrode in alkaline medium. Electrochim. Acta 2012, 59, 284–289. [Google Scholar] [CrossRef]
- Cruz-Navarro, J.A.; Mendoza-Huizar, L.H.; Salazar-Pereda, V.; Cobos-Murcia, J.A.; Colorado-Peralta, R.; Alvarez-Romero, G.A. Progress in the use of electrodes modified with coordination compounds for methanol electro-oxidation. Inorg. Chim. Acta 2021, 520, 120293. [Google Scholar] [CrossRef]
- Vogel, F.; DiNaro Blanchard, J.L.; Marrone, P.A.; Rice, S.F.; Webley, P.A.; Peters, W.A.; Smith, K.A.; Tester, J.W. Critical review of kinetic data for the oxidation of methanol in supercritical water. J. Supercrit. Fluids 2005, 34, 249–286. [Google Scholar] [CrossRef]
- Abdullah, M.M.; Faisal, M.; Ahmed, J.; Harraz, F.A.; Jalalah, M.; Alsareii, S.A. Sensitive Detection of Aqueous Methanol by Electrochemical Route Using Mesoporous α-Fe2O3 Doped CdSe Nanostructures Modified Glassy Carbon Electrode. J. Electrochem. Soc. 2021, 168, 057525. [Google Scholar] [CrossRef]
- Fornaciari, J.C.; Primc, D.; Kawashima, K.; Wygant, B.R.; Verma, S.; Spanu, L.; Mullins, C.B.; Bell, A.T.; Weber, A.Z. A Perspective on the Electrochemical Oxidation of Methane to Methanol in Membrane Electrode Assemblies. ACS Energy Lett. 2020, 5, 2954–2963. [Google Scholar] [CrossRef]
- Yuda, A.; Ashok, A.; Kumar, A. A comprehensive and critical review on recent progress in anode catalyst for methanol oxidation reaction. Catal. Rev. Sci. Eng. 2020, 64, 126–228. [Google Scholar] [CrossRef]
- Škugor Rončević, I.; Vladislavić, N.; Chatterjee, N.; Sokol, V.; Oliver, C.L.; Kukovec, B.-M. Structural and Electrochemical Studies of Cobalt(II) and Nickel(II) Coordination Polymers with 6-Oxonicotinate and 4,4′-Bipyridine. Chemosensors 2021, 9, 352. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance Spectroscopy: Emphasizing, Solid Materials and Systems; John Wiley & Sons Inc.: New York, NY, USA, 1987; p. 301. ISBN 10:0471831220. [Google Scholar]
- Škugor Rončević, I.; Grubač, Z.; Metikoš-Huković, M. Electrodeposition of Hydroxyapatite Coating on AZ91D Alloy for Biodegradable Implant Application. Int. J. Electrochem. Sci. 2014, 9, 5907–5923. [Google Scholar]
- Cataldi Tommaso, R.I.; Centonze, D.; Ricciardi, G. Electrode Modification with a Poly(NiII-tetrame thyldibenzotetraaza[14]annulene) Film. Electrochemical Behavior and Redox Catalysis in Alkaline Solutions. Electroanalysis 1995, 7, 312–318. [Google Scholar] [CrossRef]
- Revenga-Parra, M.; García, T.; Lorenzo, E.; Pariente, F. Electrocatalytic oxidation of methanol and other short chain aliphatic alcohols on glassy carbon electrodes modified with conductive films derived from Ni(II)-(N, N′-Bis(2,5-Dihydroxybenzylidene)-1,2-diaminobenzene). Sens. Actuators B Chem. 2008, 130, 730–738. [Google Scholar] [CrossRef]
- Chung, D.Y.; Lee, K.J.; Sung, Y. Methanol Electro-oxidation on Pt Surface: Revisiting the Cyclic Voltammetry Interpretation. J. Phys. Chem. C. 2016, 120, 9028–9035. [Google Scholar] [CrossRef]
- Mahapatra, S.S.; Datta, J. Characterization of Pt-Pd/C electrocatalyst for methanol oxidation in alkaline medium. Int. J. Electrochem. 2011, 2011, 563495. [Google Scholar] [CrossRef]
- Choban, E.R.; Spendelow, J.S.; Gancs, L.; Wieckowski, A.; Kenis, P.J.A. Membraneless laminar flow-based micro fuel cells operating in alkaline, acidic, and acidic/ alkaline media. Electrochim. Acta 2005, 50, 5390–5398. [Google Scholar] [CrossRef]
- Khouchaf, A.; Takky, D.; El Mahi Chbihi, M.; Benmokhtar, S. Electrocatalytic Oxidation of Methanol on Glassy Carbon Electrode Modified by Metal Ions (Copper and Nickel) Dispersed into Polyaniline Film. J. Mater. Sci. Chem. Eng. 2016, 4, 97–105. [Google Scholar] [CrossRef]
- Garrido-Barros, P.; Grau, S.; Drouet, S.; Benet-Buchholz, J.; Gimbert-Suriñach, C.; Llobe, A. Can Ni Complexes Behave as Molecular Water Oxidation Catalysts? ACS Catal. 2019, 9, 3936–3945. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).