Abstract
The development of effective, feasible, stable, and inexpensive electrocatalysts has been a great challenge in the field of overall water splitting (WS). Herein, a bifunctional electrocatalyst (BF ECS), FeCo2S4 nanowire (FCS NWs/Ni)/nickel (Ni) foam, with superior HER/OER activity and stability was designed and fabricated using a hydrothermal method. In addition, this efficient method can be used for the synthesis of other bimetallic MCo2S4 sulfides (M = Cu, Zn, Mn, etc.). Electrochemical experiments showed the as-synthesized FCS NWs/Ni exhibited overpotentials of 350.5, 203.7, 115.97, and 62.6 mV (0.05, 0.1, 0.2, and 1 M KOH) at the current density of −10 mA cm−2 for HER, including small overpotentials of 1.51, 1.36, 1.24, and 1.11 V (10 mA cm−2) in a 0.05, 0.1, 0.2, and 1 M KOH solution for OER. The FCS NWs/Ni has a splendid electrocatalytic performance which is related to the synergistic effect of cobalt, iron, and sulfur. Specifically, it has excellent electrical conductivity, a higher specific capacity, and a rich redox state of iron, cobalt, and sulfur elements. The results demonstrate a promising method for the design and fabrication of metal BF ECS for overall water splitting.
1. Introduction
Bifunctional (BF) electrocatalysts (ECS) for the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) have good application prospects in the field of energy storage [1,2]. In recent years, platinum-based, iridium-based, and ruthenium-based electrocatalysts have been considered the best choice for high-performance HER and OER electrocatalysts [3]. However, the wide application of precious metals as electrocatalysts is limited by the high cost and scarcity of resources. In addition, the HER catalyst reacts slowly in an alkaline solution and the OER catalyst reacts slowly in an acidic solution [4]. Considering the low-cost effect, the HER and OER of the working electrode catalyst should react at the same pH as the integrated electrolytic solutions [5]. Therefore, abundant attempts have been made to develop bifunctional electrocatalysts which can effectively catalyze the water splitting of HER and OER [6]. For instance, transition metal compounds (Fe [7], Co [8], Ni [9], and Mn [10]) have been widely researched both theoretically and experimentally. Regrettably, the development and application of these materials are hindered by their inherently low ECS performance.
Currently, broad ranges of non-precious metal ECS based on transition metal oxides (MxOy) [11] and double metal oxides (AB2O4) have gained attention due to their high ECT activity [12,13]. Li et al. [14] reported that the MnFe2O4/Fe hybrid nanoparticles possess superior ECT performance in alkaline media. Furthermore, Jin et al. [15] reported that the BF ECT activities of metal oxides are mainly determined by the σ*-orbital (eg) occupation of metal cations in the octahedral centers. Among several cobalt oxides [16], the surface Co3+ are referred to as ECT active centers for HER/OER. Obviously, Co3+ cations are used in the position of B3+ and the double metal oxide expression changes to MCo2O4. Hyunsik Im et al. demonstrated that CuCo2O4 is an efficient OER ECS in strong alkaline KOH media [17]. Unfortunately, its rate and cycle stability are hampered by low electrical conductivity. It has been proven that the mechanical and thermal stability of metal sulfides, rather than metal oxides, benefit from their diversified stoichiometry and crystal structures [18]. In plentiful chalcogenides, bimetallic sulfides (MCo2S4) have been identified as the most promising catalysts, owing to the synergistic effect of two different metal ions. They exhibited excellent catalysis and have a richer redox state for many chemical conversions compared to monometallic sulfides and their electrical conductivity is also greater. Arumugam S. et al. [19] fabricated NiCo2S4 for OER/HER with excellent activity and stability compared to Ni3S2 and NiCo2O4, which is consistent with the above description. Moreover, Ni2+ and Co3+ metal ions occupy tetrahedral and octahedral positions, respectively, which surround a close-range sulfide ion. Zhu et al. [20] reported that NiCo2S4 possesses an extremely small energy band and that its conductivity is much better than NiCo2O4 (about 100 times) and single-metal oxides (about four-order of magnitude higher). Zhang et al. [21] reported that NiCo2S4 has more octahedral catalytic active sites of Co3+ than the NiCo2O4 crystal structure. Sun et al. [22] reported that CuCo2S4 exhibited high-efficiency OER properties in alkaline solutions. Hence, MCo2S4 has a great potential for BF ECT activity. Hao et al. reported that a Fe ion in MCo2S4 could provide better electrical conductivity, while the sulfur element in redox could provide a richer redox state and improve conductivity [23]. Promisingly, the Fe element in the earth’s crust is abundant (reserves of non-precious metals in the earth’s crust: Fe >> Ni > Cu > Co > Mo). Therefore, by benefiting from the high-efficiency synergy between the Co ion, Fe ion, and S ion, the FCS remains promising in the area of HER/OER. Moreover, it is very important to exploit a successful template for improved ECT activities, and Ni foam can be widely used as a template because it can provide more reactive sites to contact with electrolyte ions [19].
Herein, the FCS NWs/Ni was fabricated by a hydrothermal method followed by exclusive vulcanization. The as-fabricated FCS NWs/Ni foams were used as flexible electrodes for HER/OER. Moreover, we investigated the effects of the onset-potential, Tafel slope, and concentration of OH− in HER/OER. As a result, the FCS NWs/Ni showed splendid ECT activity for both HER and OER. Our work provides an interesting route to designing and fabricating stable and efficient ECS based on non-precious metals for OER/HER.
2. Experimental Section
2.1. Materials Preparation
The chemical reagents used in the experiments are shown in Table 1. All experiments were carried out under the surrounding experimental conditions. In addition, the cleaning method of the Ni foam substrate is consistent with the previous work of our research group [24].

Table 1.
Reagents used during the experiment.
2.2. Synthesis Process
The FCS NWs/Ni was synthesized with a brief hydrothermal method [24]. Co(NO3)2•6H2O (0.9 mmol) and Fe(NO3)3•9H2O (0.18 mmol) were dissolved in deionized water (30 mL) and dispersed uniformly by magnetic stirring. Then NH4F (2.7 mmol) and urea (2.7 mmol) were added to the mixture while it was still being stirred. After stirring for 0.5 h, the solution was transferred into an autoclave and then the dried Ni foam was soaked in it. The sealed autoclave was put into a hydrothermal oven, maintained at 120 °C for 14 h. Afterward, the sample was cleaned carefully with acetone, alcohol, and ultrapure water several times (all about 30 mL each time), and then placed in a vacuum oven (60 °C for 12 h). The dried Ni foam was immersed into 0.1 M Na2S solutions, sealed, and placed into a hot-air oven (120 °C for 10 h). After the vulcanization, the sample was carefully taken out and cleaned in the same way as previously mentioned. Eventually, the dried sample was obtained from a vacuum oven (60 °C after 12 h).
2.3. Characterization and Electrochemical Measurements
The microscopic morphologies of the FCS NWs/Ni were revealed by scanning electron microscopy (SEM, VEGA3 SBH, Tescan, Brno, Czech Republic). The X-ray diffraction (XRD) patterns were performed using Kratos Analytical Ltd. (Cu Ka radiation, D/MAX-2500). The entire electrochemical test was performed in a three-electrode system with various concentrations of KOH electrolyte (0.05 M, 0.1 M, 0.2 M, and 1 M), using an electrochemical workstation (CHI660D, CH Instruments, Inc., Austin, TX, USA). The as-prepared FCS NWs/Ni foam acted as the working electrode, the saturated calomel electrode (SCE) was used as the reference electrode, and the platinum plate was used as the cathode. During the test process, the electrolyte was stirred with a magnet while slowly introducing nitrogen. The LSV (linear sweep voltammetry) of the as-fabricated FCS NWs/Ni was tested at a scan rate of 10 mV s−1. Electrochemical Impedance Spectrum (EIS) tests were carried out by applying an alternating current voltage with 5 mV perturbation amplitude and a frequency range from 100 kHz to 0.005 Hz. The chronopotentiometric measurements were used to test its long-term durability. Furthermore, the electrode potential could be transformed into the RHE (reversible hydrogen electrode) potential, which is related to the Nernst equation (the details were in Table 2).

Table 2.
The electrode potential versus the SCE transferred to the RHE (reversible hydrogen electrode) potential.
3. Results and Discussion
3.1. Morphology and Microstructure Characterization
The crystallinity of the FCS NWs/Ni was characterized via XRD. In Figure 1a, the diffraction peaks at 44.5° and 51.8° respond to the crystal orientations of Ni (111) and (200), respectively (JCPDS 04-0850). The 22.28°, 31.58°, 38.18°, 50.26°, and 55.92° diffraction peaks are in agreement with the characteristic peak of FCS [25,26]. Simultaneously, the typical XRD patterns of the precursor of FCS (FeCo2(C2O4)3) possess three strong diffraction peaks (ranging from 30° to 40°) which are consistent with the reported literature [20]. SEM images of FCS NWs/Ni are shown in Figure 1b–d. Figure 1d displays the entire surface of the Ni foam. It was evenly covered by numerous FCS NWs. This nanostructure possesses two advantages: (i) it better penetrates the electrolyte and (ii) it provides a large specific surface area. Thus, the transmission of electrons and ions between the nanowires and the conductive substrate can be greatly improved.
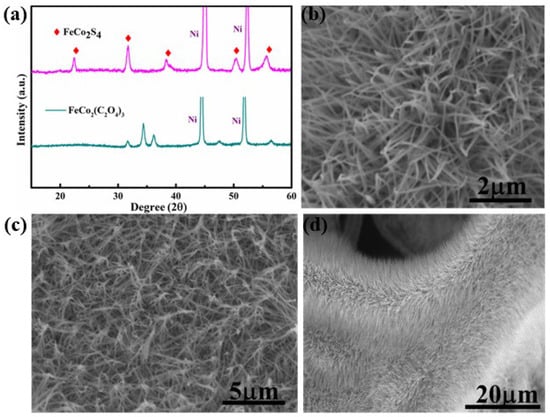
Figure 1.
(a) XRD patterns of the FeCo2S4 nanowire and the precursor of FeCo2S4 (FeCo2(C2O4)3). (b–d) SEM images of the FeCo2S4 nanowire with different magnifications.
3.2. HER/OER Performance
Interestingly, since Ni foam has excellent conductivity, FCS NWs/Ni can be used as an electrode for HER and OER. The geometric surface area of these electrodes was 1 × 1 cm2. The electrocatalytic HER activity of the FCS NWs/Ni was assessed in a KOH solution with high-purity N2 and magnetic stirring. Figure 2a demonstrates the polarization curves with a scan of 10 mV s−1. The cathode current of each sample was dramatically increased by increasing the negative potential. As shown in Figure 2a, the FCS NWs/Ni can provide an extraordinarily low overpotential (η) of 62.6 mV (1 M KOH) with a current density of −10 mA cm−2. Meanwhile, the tests of FCS NWs/Ni were conducted at −10 mA cm−2 under 0.05 M, 0.1 M, and 0.2 M KOH environments; the overpotential was 350.5 mV, 203.7 mV, and 115.97 mV, respectively. For comparison, the overpotential of the bare Ni foam was 328.5 mV (−10 mA cm−2, 0.05 M KOH). While the cathodic current density achieved −20 mA cm−2, the FCS NWs/Ni foam in 1 M KOH only required an overpotential of 158.6 mV, smaller than 524.5 mV (0.05 M), 334.7 mV (0.1 M), and 227.97 mV (0.2 M). The Tafel slope is dependent on the intrinsic kinetics of the catalyst, as shown in Figure 2b. Generally, a smaller Tafel slope indicates that a higher current density can be achieved at a lower overpotential [27]. The Tafel slope b can be obtained by the equation [28]: η = a + b log j. Interestingly, the FCS NWs/Ni electrode shows a Tafel slope of 71.24 mV dec−1 (1 M KOH), which is much lower than that in 0.05 M KOH (177.3 mV dec−1), 0.1 M KOH (161.9 mV dec−1), 0.2 M KOH (141.9 mV dec−1), and the bare Ni foam in 0.05 M KOH (221.3 mV dec−1). The performance of the FCS NWs/Ni electrode is much better than most HER electrocatalysts, which were reported previously, in KOH solutions (Table 3). In addition, the inherent HER performance of FCS NWs/Ni is regulated by the OH− concentration. Meanwhile, typical HER results in different concentrations of OH− (C[OH−]) were summarized and compared in Figure 3a. It is revealed that with the improvement of C[OH−], the HER property is greatly enhanced. The higher electrocatalytic performance of the FCS NWs/Ni is mainly attributed to the remarkable synergy between Co, Fe, and S, and that the Ni foam can promote the entry of electrolyte ions at the active site [19]. Hence, the small Tafel slope and the low HER onset potential suggest that FCS NWs/Ni is a qualified candidate to act as an HER electrocatalyst [29]. The ADT (accelerated durability tests) of FCS NWs/Ni in KOH solutions with various concentrations were tested at the same scan rate of 100 mV s−1 for 1000 cycles. In Figure 2c and Figure 4, we can see that the polarization curves of FCS NWs/Ni have almost no obvious attenuation after 1000 cycles. Compared to other concentrations of KOH electrolytes, the polarization curve for the 0.1 M KOH electrolyte exhibited a more noticeable divergence (Figure 4b). This divergence can be attributed to the accidental shedding of the active material during HER reaction. In addition, as shown in Figure 2d, the cathodic current density for the FCS NWs/Ni catalyst achieves a small degradation after 17 h in 0.05 M KOH with a −0.8 V overpotential.
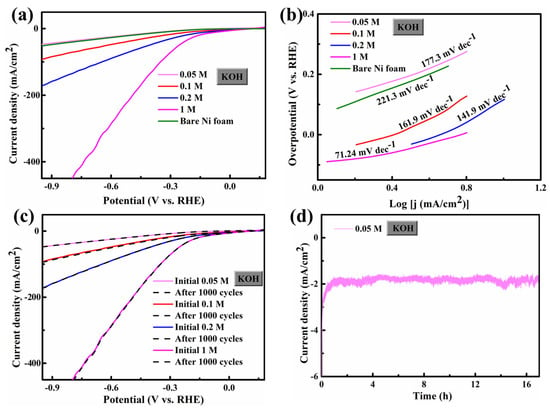
Figure 2.
(a) Polarization curves obtained with bare Ni foam in 0.05 M KOH and FeCo2S4 nanowire in different electrolytes (0.05, 0.1, 0.2, and 1 M KOH); (b) the bare Ni foam and FeCo2S4 nanowires corresponding to Tafel diagrams; (c) photozation curves of the initial LSV polarization curve of FeCo2S4 nanowires after 1000 cycles; and (d) the chronoamperometric response of a FeCo2S4 nanowire at a constant potential of −0.8 V. All experiments were conducted for HER.
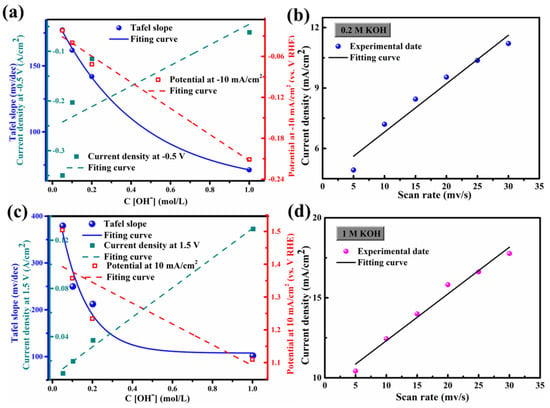
Figure 3.
(a) The current density of −10 mV/cm2 and the Tafel slope calculated at a potential of −0.5 V, which is used as a function of the concentration of OH−(C[OH−]) with all experiments were performed for HER; (b) The current density at 1.25 V vs. RHE of FeCo2S4 nanowire in 0.2 M KOH; (c) the slope of Tafel of 10 mA/cm2 current density and 1.5 V potential calculated as a function of the concentration of OH− (C[OH−]); all experiments were conducted for OER; and (d) 1 M KOH achieved at different scan (5, 10, 15, 20, 25, and 30 mV/s).
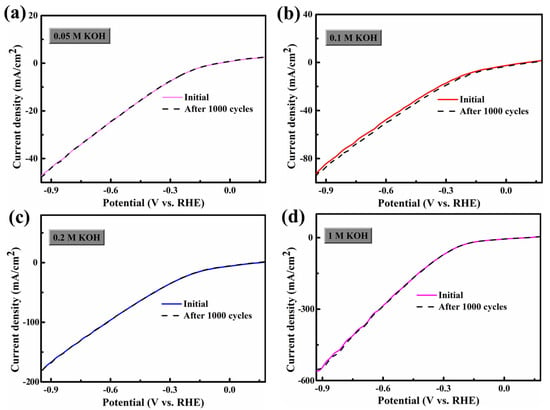
Figure 4.
Polarization curves for FeCo2S4 nanowire with an initial LSV polarization curve after 1000 cycles in different electrolytes: (a) 0.05 M KOH, (b) 0.1 M KOH, (c) 0.2 M KOH, and (d) 1 M KOH. All experiments were carried out for HER.
The test conditions for the OER activity of FCS NWs/Ni are the same as those for HER. Figure 5a shows that the FCS NWs/Ni has a low overpotential (η) of 1.11 V (10 mA cm−2 in 1 M KOH); it is obviously lower than 1.51 V (0.05 M), 1.36 V (0.1 M), 1.24 V (0.2 M), and 1.72 V (bare Ni foam in 0.05 M KOH). Furthermore, it is lower than most of the other OER electrocatalysts, which were reported previously (as shown in Table 3). When the current density of the FCS NWs/Ni electrode increased to 20 mA cm−2 in 1 M KOH, the overpotential was only 1.25 V, which is lower than 1.69 V (0.05 M), 1.50 V (0.1 M), and 1.38 V (0.2 M). The peak of bifunctional activity is influenced by the octahedral centers of the electrocatalyst [15]. In tetrahedrons and octahedrons, a dense array of large S2− anions with iron and cobalt metal cations allows FCS NW to have excellent performance at the active octahedral position of the Co3+ cation [21]. Nevertheless, FCS showed a significant oxidation peak at 1.6 V (vs. RHE) which can be attributed to the surface reaction, as shown in the following equation.
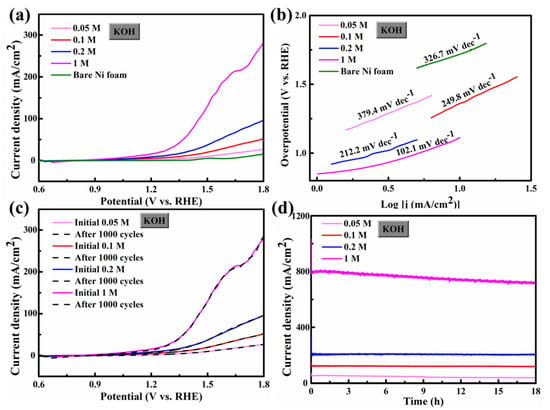
Figure 5.
(a) LSV curves for FeCo2S4 nanowire and bare Ni foam for OER in 0.05, 0.1, 0.2, 1 M KOH; (b) The corresponding Tafel plots; (c) Polarization curves for FeCo2S4 nanowire with an initial LSV polarization curve after 1000 cycles; and (d) the chronoamperometric response kept an account of FeCo2S4 at a constant applied potential (0.8 V).
The Tafel slope was used to assess OER kinetics, as shown in Figure 5b. The Tafel slope of FCS NWs/Ni in 1 M KOH was 102.1 mV dec−1, smaller than the electrode in all other electrolytes (0.05 M: 379.4 mV dec−1, 0.1 M: 249.8 mV dec−1, and 0.2 M: 212.2 mV dec−1), including bare Ni foam in 0.05 M KOH (326.7 mV dec−1). The smaller Tafel slope also indicates the efficient electron and ion transform. The ADT test conditions of FCS NWs/Ni for the OER are the same as for the HER. In Figure 5c, the polarization curves of the FCS anodic current almost have no reduction after 1000 cycles. Figure 5d showed the chronoamperometric response of FeCo2S4 at a constant applied potential (0.8 V). After 18 h of testing, there was only a slight decrease in current density. As for the case of 1 M KOH electrolyte, the current density decreased by about 8.5%, which might result from the irreversible phase transformation of metal sulfides to metal oxide/oxyhydroxide [26,30]. Furthermore, the intrinsic OER properties of FCS were affected by the concentration of OH− (Figure 3c). The OER performance was hugely improved with increased C[OH−]; the required overpotential changed almost linearly with C[OH−].
Figure 6 shows the CV (cyclic voltammetric) curves of FCS NWs/Ni electrolytes in 0.05 M, 0.1 M, 0.2 M, and 1 M KOH. The scanning rates were from 5 mV s−1 to 30 mV s−1. The distorted rectangular-like shape of FCS NWs/Ni was similar to NiCo2S4 reported previously in the literature [20]. Furthermore, the CV curve remained in good shape, indicating that the electron and ion transfer rates are fast enough [31]. In Figure 3b,d, we can see that the fitting curve of FCS NWs/Ni at the potential of 1.25 vs. RHE (in 0.2 M and 1 M KOH) with different scan rates (5, 10, 15, 20, 25, and 30 mV s−1). These excellent properties indicate that FCS NWs/Ni has great potential for application in OER. The remarkable conductivity represents a fast electron transfer rate and accelerated reaction kinetics. Figure 7 showed the Polarization curves for the FeCo2S4 nanowire with an initial LSV polarization curve and after 1000 cycles. The curves almost overlapped completely, indicating the stability of OER performance of the FeCo2S4 nanowire. The EIS measurements of FCS NWs/Ni were conducted, in 0.05 M, 0.1 M, 0.2 M, and 1 M KOH, as shown in Figure 8. Figure 8a–c, reveals that the FeCo2S4 in 1 M KOH had a smaller EIS compared to the three other concentrations of KOH solution and that it increased slightly after cycling. In addition, as shown in Figure 8d, the current density curve had a small attenuation at an overpotential of 0.8 V (0.1 M KOH). Therefore, all the above results reveal that the hybrid structure of FCS NWs/Ni has stupendous potential in the overall water-splitting field.


Table 3.
The performance of the FeCo2S4 electrode compared with the OER/HER electrocatalysts reported previously in KOH solutions.
Table 3.
The performance of the FeCo2S4 electrode compared with the OER/HER electrocatalysts reported previously in KOH solutions.
| Materials | Electrolyte | J (mA/cm2) | Overpotential (V) | Tafel Slope (mV dec−1) | References | |
|---|---|---|---|---|---|---|
| HER | FeCo2S4/Ni foam | 1 M KOH 0.2 M KOH 0.1 M KOH 0.05 M KOH | 10 10 10 10 | 0.0626 0.11597 0.2037 0.35052 | 71.24 141.9 161.9 177.3 | This work |
| OER | FeCo2S4/Ni foam | 1 M KOH 0.2 M KOH 0.1 M KOH 0.05 M KOH | 10 10 10 10 | 1.1124 1.237 1.3603 1.50748 | 102.1 212.2 249.8 379.4 | This work |
| HER/ OER | Ni3Se2/Ni foam | 1 M KOH | 10 100 | 0.097 0.353 | 79 144 | [32] |
| HER/ OER | NiSe2 NSs-120 | 1 M KOH 1 M KOH | 10 40 | 0.207 1.562 | 186.5 109.4 | [33] |
| HER/ OER | NiCo2S4/CC | 1 M KOH | 50 50 | 0.263 0.31 | 141 89 | [34] |
| OER | CuCo2O4 | 1 M KOH | 20 | 0.29 | 117 | [17] |
| HER | CoOx@CN | 1 M KOH | 10 | 0.232 | … | [35] |
| OER | FeCoW oxyhydroxides | 1 M KOH | 10 | 0.191 | … | [36] |
| HER | β-InSe | 1 M KOH | 10 | 0.483 | 135 | [37] |
| OER | CuCo2S4/CF | 1 M KOH | 60 | 0.259 | 110 | [38] |
| HER/ OER | NiSe2 NCs | 1 M KOH | 10 10 | 0.54 0.25 | 139 38 | [39] |
FeOOH + OH− → FeO(OH)2 + e−
FeO(OH)2 + OH− → [FeO(OH)2]+ + e−
[FeO(OH)2]+ + 2OH− → [FeO]+ + O2 + 2H2O + 2e−
[FeO]+ + OH− → FeOOH
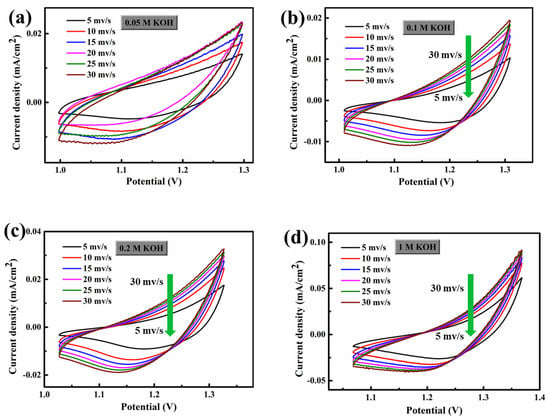
Figure 6.
Electrochemical cycle voltammograms of the FeCo2S4 nanowire in different electrolytes (a) 0.05 M KOH, (b) 0.1 M KOH, (c) 0.2 M KOH, and (d) 1 M KOH under the conditions of different potential scanning rates. The potential range of the selected no-faradic current was 0–0.3 V.
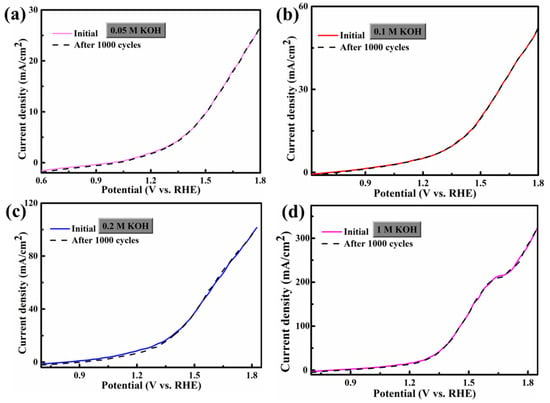
Figure 7.
Polarization curves for the FeCo2S4 nanowire with an initial LSV polarization curve after 1000 cycles in different electrolytes: (a) 0.05 M KOH, (b) 0.1 M KOH, (c) 0.2 M KOH, and (d) 1 M KOH. All experiments were carried out for OER.
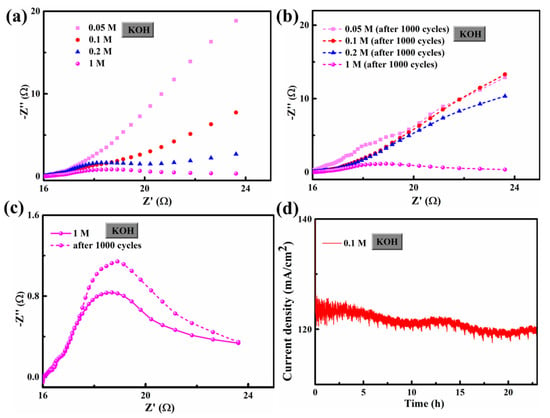
Figure 8.
(a) Initial and (b) after 1000 cycles electrochemical impedance spectra of FeCo2S4 nanowires in 0.05 M, 0.1 M, 0.2 M, and 1 M KOH at an applied potential (0.4 V); (c) electrochemical impedance spectra for FeCo2S4 with initial curves and after 1000 cycles; and (d) the chronoamperometric response kept an account of FeCo2S4 at a constant applied potential of 0.8 V (0.1 M KOH).
4. Conclusions
In this study, a smart FCS NWs/Ni hybrid structure was prepared as a non-noble metal electrocatalyst in different alkaline solutions for OER/HER. The unique design of the microstructure endowed the FCS NWs/Ni with a high ion/electron transport capacity and a large specific surface area. Moreover, its excellent conductivity, higher specific capacity, and rich redox state are attributed to its iron, cobalt, and sulfur elements, respectively. The FCS NWs/Ni catalysts achieved 10 mA cm−2 at HER overpotential of 62.6 mV (1 M KOH) and 129.56 mA cm−2 at OER overpotential of 1.5 V (1 M KOH). Lastly, the method presented in this work will instruct the future reasonable design and development of efficient bifunctional non-precious metal electrocatalysts to be widely used in the field of electrochemical hydrogen and oxygen production in water electrolytic systems.
Author Contributions
J.T. contributed to the experiment and wrote the paper; the data was processed and analyzed by S.L. (Shuhua Liu), Y.L. (Yanmo Liao), H.Q. and Y.W.; Y.L. (Yu Liu), H.C., M.T. and S.L. (Sanjie Liu) conducted the experiment and discussed the results; Z.Q., C.L. and X.Q. proposed the study conception and were responsible for part of the experimental data, data discussion, and grammar revision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 11504312), the Provincial Natural Science Foundation of Hunan (No. 2021JJ30298), the Science and Technology Program of Hunan Province, China (Grant No. 2019TP1014), and the Changjiang Scholars and Innovative Research Team in University (IRT_17R91).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guo, Y.; Park, T.; Yi, J.W.; Henzie, J.; Kim, J.; Wang, Z.; Jiang, B.; Bando, Y.; Sugahara, Y.; Tang, J. Nanoarchitectonics for Transition-Metal-Sulfide-Based Electrocatalysts for Water Splitting. Adv. Mater. 2019, 31, 1807134. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Wang, X.W.; Fan, Y.; Wang, Q.; Lu, Y.; Li, J.; Shao, L.; Luo, J.L.; Fu, X.Z. Efficient bifunctional electrocatalysts for solid oxide cells based on the structural evolution of perovskites with abundant defects and exsolved CoFe nanoparticles. J. Power Sources 2021, 482, 228981. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, T.; Zhao, B.; Cai, W.; Liu, Y.; Jiao, S.; Li, Q.; Cao, R.; Liu, M. Recent Progress in Electrocatalysts for Acidic Water Oxidation. Adv. Energy Mater. 2020, 10, 2000478. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, H.; Sun, J.; Fan, Q.; Dan, L.; Xie, L.; Fang, Y.; Bao, J.; Yong, L.; Ying, Y. Hierarchical Cu@CoFe layered double hydroxide core-shell nanoarchitectures as bifunctional electrocatalysts for efficient overall water splitting. Nano Energy 2017, 41, 327–336. [Google Scholar]
- Ledendecker, M.; Krick Calderón, S.; Papp, C.; Steinrück, H.P.; Antonietti, M.; Shalom, M. The Synthesis of Nanostructured Ni5P4 Films and their Use as a Non-Noble Bifunctional Electrocatalyst for Full Water Splitting. Angew. Chem. Int. Ed. 2015, 54, 12361–12365. [Google Scholar] [CrossRef]
- Wang, B.; Ye, Y.; Xu, L.; Quan, Y.; Wei, W.; Zhu, W.; Li, H.; Xia, J. Space-Confined Yolk-Shell Construction of Fe3O4 Nanoparticles Inside N-Doped Hollow Mesoporous Carbon Spheres as Bifunctional Electrocatalysts for Long-Term Rechargeable Zinc-Air Batteries. Adv. Funct. Mater. 2020, 30, 2005834. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Ye, Y.; Aslam, Z.; Brydson, R.; Liang, C. Fe-N-Doped Mesoporous Carbon with Dual Active Sites Loaded on Reduced Graphene Oxides for Efficient Oxygen Reduction Catalysts. ACS Appl. Mater. Interfaces 2018, 10, 2423–2429. [Google Scholar] [CrossRef]
- Luque-Centeno, J.M.; Martínez-Huerta, M.V.; Sebastián, D.; Lemes, G.; Pastor, E.; Lázaro, M.J. Bifunctional N-doped graphene Ti and Co nanocomposites for the oxygen reduction and evolution reactions. Renew. Energy 2018, 125, 182–192. [Google Scholar] [CrossRef]
- Devaguptapu, S.V.; Hwang, S.; Karakalos, S.; Zhao, S.; Gupta, S.; Su, D.; Xu, H.; Wu, G. Morphology Control of Carbon-Free Spinel NiCo2O4 Catalysts for Enhanced Bifunctional Oxygen Reduction and Evolution in Alkaline Media. ACS Appl. Mater. Interfaces 2018, 9, 44567–44578. [Google Scholar] [CrossRef]
- Liu, K.; Li, J.; Li, J.; Wang, J.; Zhao, D.; Jiang, J.; Qian, D. A facile and low-cost synthesis of MnOx/Mn2N-N-C nanohybrid as an advanced oxygen reduction electrocatalyst. Mater. Res. Bull. 2018, 104, 60–64. [Google Scholar] [CrossRef]
- Wang, D.; Luo, D.; Zhang, Y.; Zhao, Y.; Zhou, G.; Shui, L.; Chen, Z.; Wang, X. Deciphering interpenetrated interface of transition metal oxides/phosphates from atomic level for reliable Li/S electrocatalytic behavior. Nano Energy 2021, 81, 105602. [Google Scholar] [CrossRef]
- Zhou, Y.; Xi, S.; Wang, J.; Sun, S.; Wei, C.; Feng, Z.; Du, Y.; Xu, Z.J. Revealing the Dominant Chemistry for Oxygen Reduction Reaction on Small Oxide Nanoparticles. ACS Catal. 2017, 8, 673–677. [Google Scholar] [CrossRef]
- Ting, B.; Hui, Z.; Jiang, Y.; Jin, C.; Wu, J.; Hong, Y.; Deren, Y. Epitaxial Growth of Twinned Au-Pt Core-Shell Star-Shaped Decahedra as Highly Durable Electrocatalysts. Nano Lett. 2015, 15, 7808–7815. [Google Scholar]
- Wu, X.; Niu, Y.; Feng, B.; Yu, Y.; Huang, X.; Zhong, C.; Hu, W.; Li, C.M. Mesoporous Hollow Nitrogen-Doped Carbon Nanospheres with Embedded MnFe2O4/Fe Hybrid Nanoparticles as Efficient Bifunctional Oxygen Electrocatalysts in Alkaline Media. ACS Appl. Mater. Interfaces 2018, 10, 20440–20447. [Google Scholar] [CrossRef] [PubMed]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shaohorn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Chem. Inform. 2012, 43, 1383–1385. [Google Scholar] [CrossRef]
- Hogg, S.A. Oxidation states of Co and Fe in Ba1−xSrxCo1−yFeyO3−δ (x,y = 0.2–0.8) and oxygen desorption in the temperature range 300–1273 K. Phys. Chem. Chem. Phys. 2009, 11, 3090–3098. [Google Scholar]
- Aqueel Ahmed, A.T.; Hou, B.; Chavan, H.S.; Jo, Y.; Cho, S.; Kim, J.; Pawar, S.M.; Cha, S.; Inamdar, A.I.; Kim, H. Self-Assembled Nanostructured CuCo2O4 for Electrochemical Energy Storage and the Oxygen Evolution Reaction via Morphology Engineering. Small 2018, 14, 1800742. [Google Scholar] [CrossRef]
- Shi, J.; Li, X.; He, G.; Zhang, L.; Li, M. Electrodeposition of high-capacitance 3D CoS/graphene nanosheets on nickel foam for high-performance aqueous asymmetric supercapacitors. J. Mater. Chem. A 2015, 3, 20619–20626. [Google Scholar] [CrossRef]
- Sivanantham, A.; Ganesan, P.; Shanmugam, S. Bifunctional Electrocatalysts: Hierarchical NiCo2S4 Nanowire Arrays Supported on Ni Foam: An Efficient and Durable Bifunctional Electrocatalyst for Oxygen and Hydrogen Evolution Reactions. Adv. Funct. Mater. 2016, 26, 4660. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, S.; Wu, J.; Shi, X.; Zhu, B.; Meng, X. Wearable High-Performance Supercapacitors Based on Silver-Sputtered Textiles with FeCo2S4@NiCo2S4 Composite Nanotube-Built Multitripod Architectures as Advanced Flexible Electrodes. Adv. Energy Mater. 2017, 7, 1601234. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Cui, G.; Zhang, A.; Zhou, X.; Xu, H.; Gu, L. NiCo2S4 sub-micron spheres: An efficient non-precious metal bifunctional electrocatalyst. Nanoscale 2014, 6, 3540–3544. [Google Scholar] [CrossRef]
- Yang, L.; Xie, L.; Ren, X.; Wang, Z.; Liu, Z.; Du, G.; Asiri, A.M.; Yao, Y.; Sun, X. Hierarchical CuCo2S4 nanoarrays for high-efficient and durable water oxidation electrocatalysis. Chem. Commun. 2017, 52, 78–81. [Google Scholar] [CrossRef]
- Hao, Z.; He, X.; Li, H.; Trefilov, D.; Song, Y.; Li, Y.; Fu, X.; Cui, Y.; Tang, S.; Ge, H. Vertically Aligned and Ordered Arrays of 2D MCo2S4@Metal with Ultrafast Ion/Electron Transport for Thickness-Independent Pseudocapacitive Energy Storage. ACS Nano 2020, 14, 12719–12731. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhang, Z.; Qi, X.; Ren, X.; Liu, S.; Chen, Q.; Huang, Z.; Zhong, J. Hydrothermally synthesized FeCo2O4 nanostructures: Structural manipulation for high-performance all solid-state supercapacitors. Ceram. Int. 2018, 4, 120–127. [Google Scholar] [CrossRef]
- Tang, S.; Zhu, B.; Shi, X.; Wu, J.; Meng, X. General Controlled Sulfidation toward Achieving Novel Nanosheet-Built Porous Square-FeCo2S4-Tube Arrays for High-Performance Asymmetric All-Solid-State Pseudocapacitors. Adv. Energy Mater. 2017, 7, 1601985. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, X.; Ge, S.; Zhang, Q.; Zhang, X.; Li, W.; Cui, Y. Hierarchical FeCo2S4@CoFe layered double hydroxide on Ni foam as bifunctional electrocatalyst for overall water splitting. Catal. Sci. Technol. 2020, 10, 1292–1298. [Google Scholar] [CrossRef]
- Qiao, H.; Liu, H.; Huang, Z.; Ma, Q.; Luo, S.; Li, J.; Liu, Y.; Zhong, J.; Qi, X. Black Phosphorus Nanosheets Modified with Au Nanoparticles as High Conductivity and High Activity Electrocatalyst for Oxygen Evolution Reaction. Adv. Energy Mater. 2020, 10, 2002424. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef]
- Menezes, P.; Panda, C.; Loos, S.; Bunscheibruns, F.; Walter, C.; Schwarze, M.; Deng, X.; Dau, H.; Driess, M. A structurally versatile nickel phosphite acting as a robust bifunctional electrocatalyst for overall water splitting. Energy Environ. Sci. 2018, 5, 1287–1298. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Hähnel, A.; Naumann, V.; Lin, C.; Azimi, S.; Schweizer, S.L.; Maijenburg, A.W.; Wehrspohn, R.B. Bifunctional Heterostructure Assembly of NiFe LDH Nanosheets on NiCoP Nanowires for Highly Efficient and Stable Overall Water Splitting. Adv. Funct. Mater. 2018, 28, 1706847. [Google Scholar] [CrossRef]
- Liu, B.; Yang, M.; Yang, D.; Chen, H.; Li, H. Graphene-like porous carbon nanosheets for ultra-high rate performance supercapacitors and efficient oxygen reduction electrocatalysts. J. Power Sources 2020, 456, 227999. [Google Scholar] [CrossRef]
- Xu, R.; Wu, R.; Shi, Y.; Zhang, J.; Zhang, B. Ni3Se2 nanoforest/Ni foam as a hydrophilic, metallic, and self-supported bifunctional electrocatalyst for both H2 and O2 generations. Nano Energy 2016, 24, 103–110. [Google Scholar] [CrossRef]
- Jie, Z.; Liu, Y.; Zhen, Z.; Huang, Z.; Chen, X.; Ren, X.; Long, R.; Xiang, Q.; Zhong, J. Hierarchical NiSe2 sheet-like nano-architectures as an efficient and stable bifunctional electrocatalyst for overall water splitting: Phase and morphology engineering. Electrochim. Acta 2018, 279, 195–203. [Google Scholar]
- Kang, J.; Wood, J.D.; Wells, S.A.; Lee, J.H.; Liu, X.; Chen, K.S.; Hersam, M.C. Solvent Exfoliation of Electronic-Grade, Two-Dimensional Black Phosphorus. ACS Nano 2015, 9, 3596–3604. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, J.; Su, D.; Wei, Z.; Pang, Z.; Wang, Y.; Am, J. In situ Cobalt-Cobalt Oxide/N-Doped Carbon Hybrids as Superior Bifunctional Electrocatalysts for Hydrogen and Oxygen Evolution. Chem. Soc. 2015, 137, 2688–2694. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, X.; Voznyy, O.; Comin, R.; Bajdich, M.; Garcíamelchor, M.; Han, L.; Xu, J.; Liu, M.; Zheng, L. Homogeneously dispersed multimetal oxygen-evolving catalysts. Sci. Found. China 2016, 352, 51. [Google Scholar] [CrossRef]
- Elisa, P.; Emanuele, L.; Sebastiano, B.; Danil, W.B.; Antonio, P.; Bekir, G.; Songül, D.; Mirko, P.; Silvia, G.; Reinier, O.N.; et al. Liquid-Phase Exfoliated Indium-Selenide Flakes and Their Application in Hydrogen Evolution Reaction. Small 2018, 26, 1800749. [Google Scholar]
- Hu, Z.; Li, Q.; Lei, B.; Zhou, Q.; Xiang, D.; Lyu, Z.; Hu, F.; Wang, J.; Ren, Y.; Guo, R. Water-Catalyzed Oxidation of Few-Layer Black Phosphorous in a Dark Environment. Angew. Chem. Int. Ed. 2017, 56, 9131–9135. [Google Scholar] [CrossRef]
- Kwak, I.H.; Im, H.S.; Dong, M.J.; Kim, Y.W.; Park, K.; Lim, Y.R.; Cha, E.H.; Park, J. CoSe2 and NiSe2 Nanocrystals as Superior Bifunctional Catalysts for Electrochemical and Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2016, 8, 5327–5334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).