Abstract
In this work, Gallium Arsenide (GaAs) films growth via Close Space Vapor Transport (CSVT) technique on n-type Silicon (Si) substrates (100) and its nitridation effect in the ammonia () environment is reported. The GaAs films were grown at 800, 900, and 1000 C, and the nitridation process was carried out at 900 C with an : gasses ratio. The GaAs films with and without nitridation process were analyzed using X-ray diffraction (XRD), Raman spectroscopy, Diffuse Reflectance Spectroscopy, and Scanning Electron Microscopy with Energy Dispersive X-ray analysis (SEM-EDX). Grazing incidence X-ray diffraction measurements of GaAs films nitrided confirm a polycrystalline GaN wurtzite structure with preferential orientation along (002), and additionality, a crystallographic plane (310) of low intensity is observed in corresponding to . The average quantification results in weight (Wt. %) of GaAs films nitrided was determined by EDS; Ga∼79, N∼17.1, O∼2 and As∼1.8 Wt. %. The presence of GaN, , Si, and GaAs modes were found by Raman measurements, demonstrating a partial nitriding. The band gap estimation by diffuse reflectance was between 3.2 and 3.38 eV such values are close to that reported for bulk GaN (3.4 eV). The presence of oxygen in the structure could be related to substrates or the GaAs source.
1. Introduction
The wafers growth method of GaAs by excellence in the microelectronic industry is liquid-encapsulated Czochralski (LEC) and vertical Bridgman (VB) [1,2,3]. However, for optoelectronic devices, other growth methods of GaAs thin films exist, such as Molecular Beam Epitaxy (MBE) [4], Metal-Organic Chemical Vapor Deposition (MOCVD) [5], Sputtering [6], Chemical Vapor Deposition (CVD) [7], Close Space Vapor Transport (CSVT) [8], Vertical Gradient Freeze (VGF) [9] and hydride vapor phase epitaxy (HVPE) [10]. In addition, GaAs is a direct band gap semiconductor of eV (Group III-V) [11] that has a stable Zinc–Blende crystal structure, with lattice constant a = 5.653 Å and a melting point of 1240 C [12]. Therefore, GaAs is an interesting material in the optoelectronic area for the development of Light emission diodes (LEDs), Solar cells, heterojunction bipolar transistors (HBTs), High Electron mobility transistors (HEMTs), and Monolithic microwave integrated circuit (MMICs) [8].
In this regard, GaAs films often are used as a starting point material in the generation of GaN films. The common methods used to grow GaN since the 1990s are Halide Vapor Phase Epitaxy (HVPE) [13], Plasma Enhanced Chemical Vapor Deposition (PECVD) [14], Sodium flux method (Na-flux), MOCVD [15], MBE [16], and Halide Free Vapor Phase Epitaxy (HFVPE) [17]. The structural quality of GaN is an important factor in care if the material will be used in electronic components that exhibit electroluminescence phenomena. Therefore different techniques exist to grow GaN with particular improvements to required quality [18].
Another less common method of synthesis of GaN is ammonothermal-like, which is used in this work to nitride GaAs films. The ammonothermal method is defined as employing a heterogeneous reaction in ammonia as one homogenous fluid in a supercritical state [19]. Little is known about the transport, dissolution, and mechanism in the internal process of ammonothermic crystal growth. This is the reason why there are many intermediate species as a result, which are strongly influenced by the materials with which they started. However, it is a crystal growth suitable for a large range of different materials, such as nitrides, amides, imides, ammoniates, and non-nitrogen compounds like hydroxides, where the control of the main parameters (pressure, temperature, and mineralizer concentration) impact in the crystal quality [20,21].
The GaN is a direct band-gap semiconductor thermodynamically stable with a band gap between 3.27 and 3.47 eV belonging to group III-V, which presents two phases; cubic and hexagonal structure [22]. For the Wurtzite structure with a basic hexagonal symmetry phase of bulk GaN, the lattice parameters are Å and Å (ICDD-PDF 50-0792). Thus GaN is an attractive material that operates in a large regime, from green to ultraviolet radiation, for the fabrication of many optoelectronic devices; LEDs, detectors in blue and UV regions, as well as electronic high-frequency devices. On the other hand, the cubic structure of GaN has a high electron drag rate, improving the efficiency in high-power devices [23]. In this case, substrates in which GaN is grown represent an important aspect of the properties of the material, especially for heteroepitaxial growth. GaN deposited on substrates whose lattice parameters are different (Si, SiC, and sapphire) could reflect poor mismatching in devices that contain this material [23]. However, silicon is one of the most developed semiconductor materials in the microelectronic industry, so a study of the Si-substrate effect on the microstructure of GaAs films nitrided is very important.
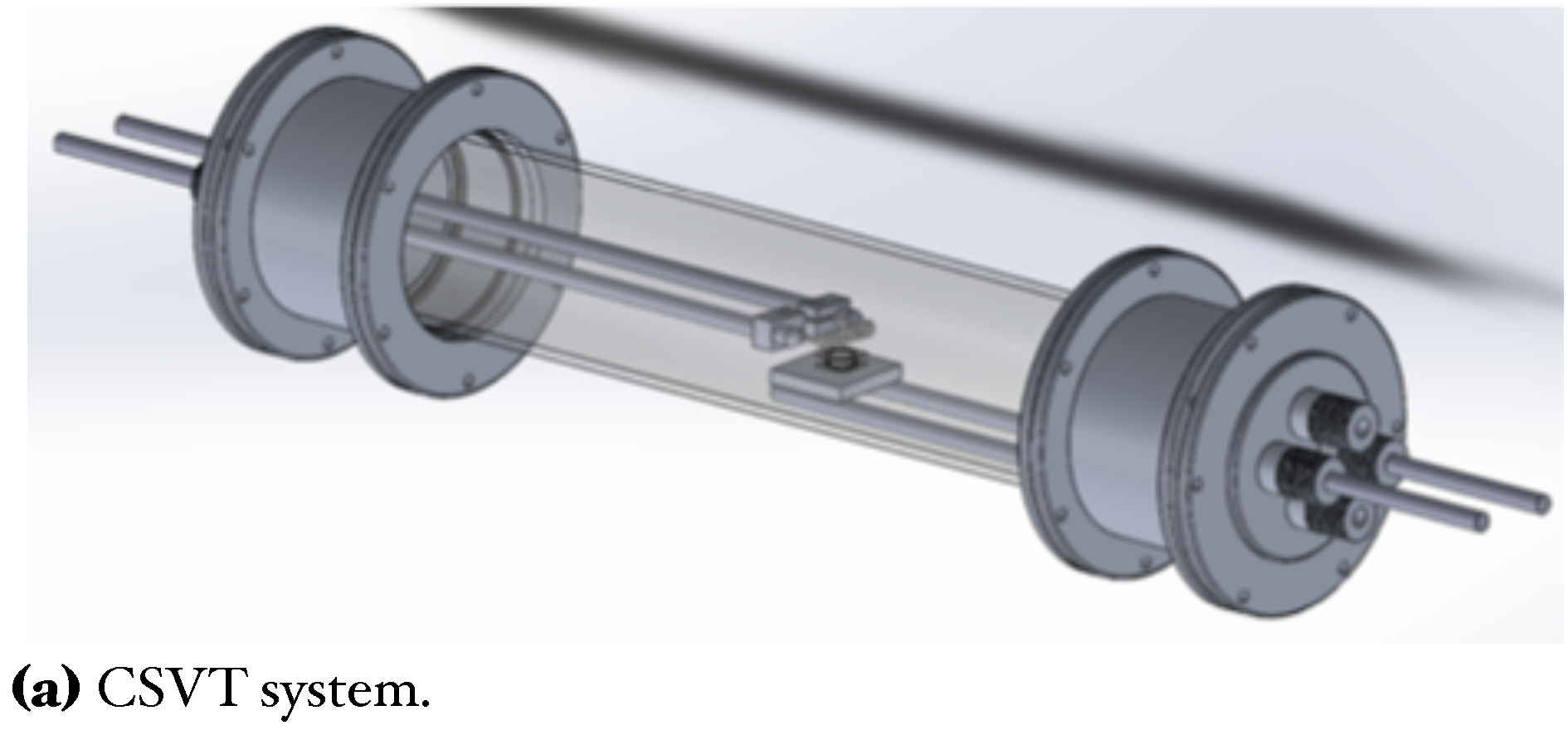
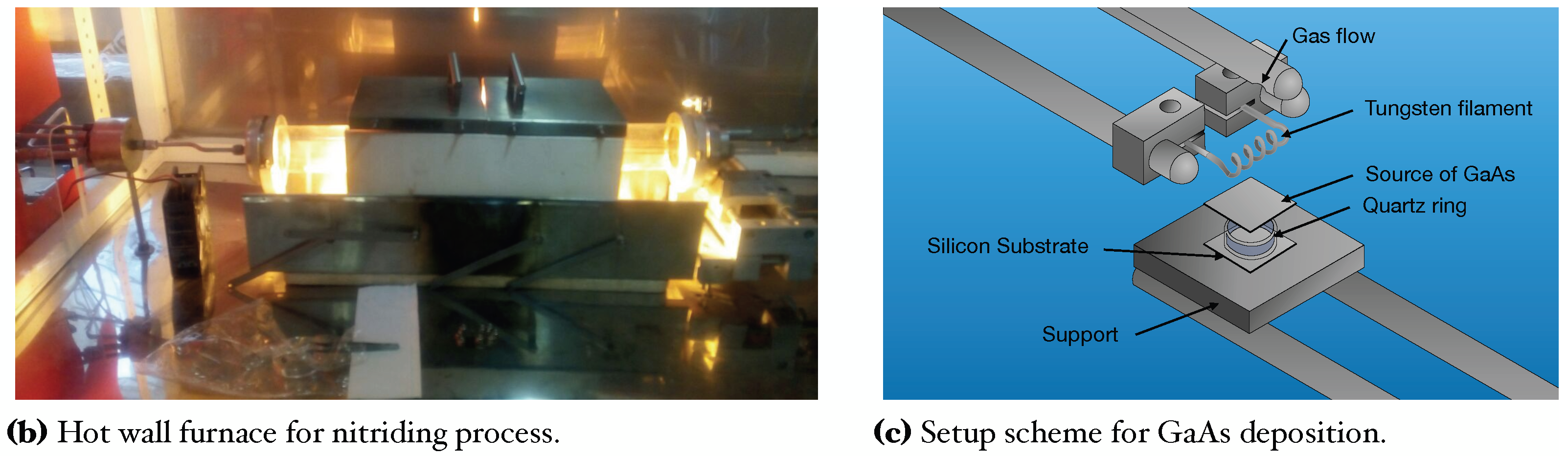
In this work, GaAs films deposited on Si substrates were obtained with the CSVT system (Figure 1a). This technique is based on the evaporation of a solid source (GaAs wafer) instead of using expensive gasses; this also allows many deposits with only one target [8,24]. Afterward, we used an ammonothermal-like process to nitride the GaAs films using a ratio in a horizontal hot-wall furnace to obtain GaN films (Figure 1b). This process has been used by other authors using to high temperatures (>1000 C), like typically used in MOVPE (Metal Organic Vapor Phase Epitaxy) techniques [18,25].
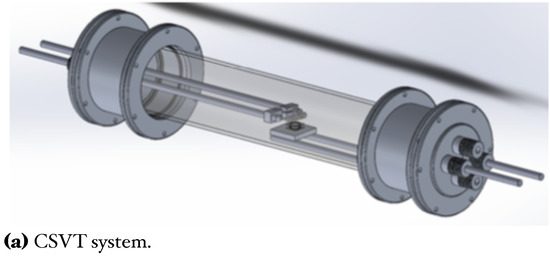
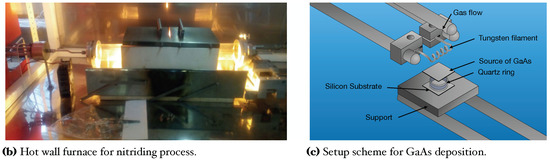
Figure 1.
Schemes and photography of the deposition and nitriding systems.
2. Experimental Details
Silicon substrates n-type (100), = 1−10 cm were used for GaAs film growth. Likewise, a solid source (2 × 2 cm) of GaAs (111) n-type of =100 cm was used as a generator of vapor precursors in the closed cavity. For this, a tungsten filament inside the reaction chamber reaches a temperature around ∼2000 C to provide the proper temperature in both; GaAs solid source and silicon substrate (800 C, 900 C, and 1000 C) [8,24]. Before the growth, silicon substrates and solid sources were cleaned in an ultrasonic bath with xylene, acetone, methanol, and deionized water, successively by 5 min for each solution. After Si substrates were cleaned for 1 min in HF (10%) to remove native oxide. Figure 1a, shows the CSVT system where GaAs films were grown. For the evaporation of GaAs compounds from a solid source, flow (110 sccm) of chromatographic grade passes through to tungsten filament, maintaining a pressure of 1 atmosphere inside the reaction chamber. The chamber reaction consists of a horizontal quartz tube hermetically sealed which contains important parameters such as tungsten filament, quartz ring, solid source, and silicon substrate (see Figure 1c). The quartz ring thickness creates the necessary conditions to make a temperature gradient between the solid source and Si substrate, generating precursors in the vapor phase inside the cavity, which travels from the solid source to the Si substrate. The transport process occurs by temperature and concentration gradients; this is because the solid source of GaAs exceeds the temperature of Si substrate [8,24].
It is worth mentioning that the distance between the solid source (GaAs) and the tungsten filament is kept constant at 7 mm. On the other hand, the distance between the solid source (GaAs) and the silicon substrate is adjusted by the thickness of the quartz ring; 1 mm, 2 mm, and 3 mm, to obtain a temperature around ∼1000, ∼900, and ∼800 C respectively. The deposit time was 5 min for all samples. Table 1 shows parameters of GaAs film growth by using the CSVT technique.

Table 1.
Parameters of GaAs films before and after nitriding, EDS quantification results, and profilometry results.
In the nitriding process, GaAs films are exposed to active radicals of hydrogen and ammonia at high temperatures (900 C). The difficulty in getting a film of GaN is the binding of molecule that makes it impossible to make active radicals of nitrogen, however using , the density of radicals supposes an increase due to the difference between binding energy ( 9.76 eV and 2.46 eV) making possible an active molecular nitrogen radicals [20,26]. The growth of GaN on the surface occurs by the migration of Ga to the surface and reactions with active nitrogen radicals that come from the decomposition of , forming the GaN compound. Likewise, annealing promotes the evaporated Arsenide (As). The growth of GaN depends on the penetration and the diffusion of active radicals of nitrogen, where these radicals of nitrogen take vacancies left by the As and interstices due to small radio (lattice parameter decrease for vacancies occupied). [20,26]. The nitriding process at an annealing furnace consists of 4 steps. 1. Purge; serve to clean the reaction chamber, ensuring an environment of (laminar flux of 2100 sccm) at 1 atm. 2. Substrate pre-heat; Samples GaAs/Si are pre-heat (300 C) to favor the growth of GaN films and diminish thermal effects due to high temperatures; for this, a ramp is programmed to reach 300 C with a laminar flow of 2100 sccm by 3 min. 3. Incorporation of precursors; Through a mass flow controller is applied a ratio (1/100) of 21/2100 sccm to the reaction chamber, and the second ramp is programmed to reach a temperature of at 500 C (GaAs/Si) by 3 min. 4. Nitriding; finally, the third ramp is programmed to reach 900 C during 30 min. Figure 1b shows the furnace where the nitriding process was carried out.
The GaAs films and GaAs films nitrided were characterized by X-ray diffractions using a BRUKER model AXS D8 “Discovery” diffractometer with radiation of Cu ( Å). Samples were measured with of grazing angle in the range from to . The analysis of crystallite size is calculated by the Scherrer equation. Micro-Raman measurements of samples were performed using the model Thermo Scientific DXR with laser excitation of 633 nm and 8 mW of power. The SEM measurements were performed by a Schottky Field Emission Scanning Electron Microscope model JSM-7800F (JOEL) 5 kV of power in several maximizations. Finally, band gap energy was measured from a Thermo Scientific Evolution 201 UV-VIS spectrometer using the Kubelka–Munk relation. The thickness measurements were performed by Dektak 150 profilometer, with 5 mg of contact force in a diamond-tipped (stylus).
3. Results
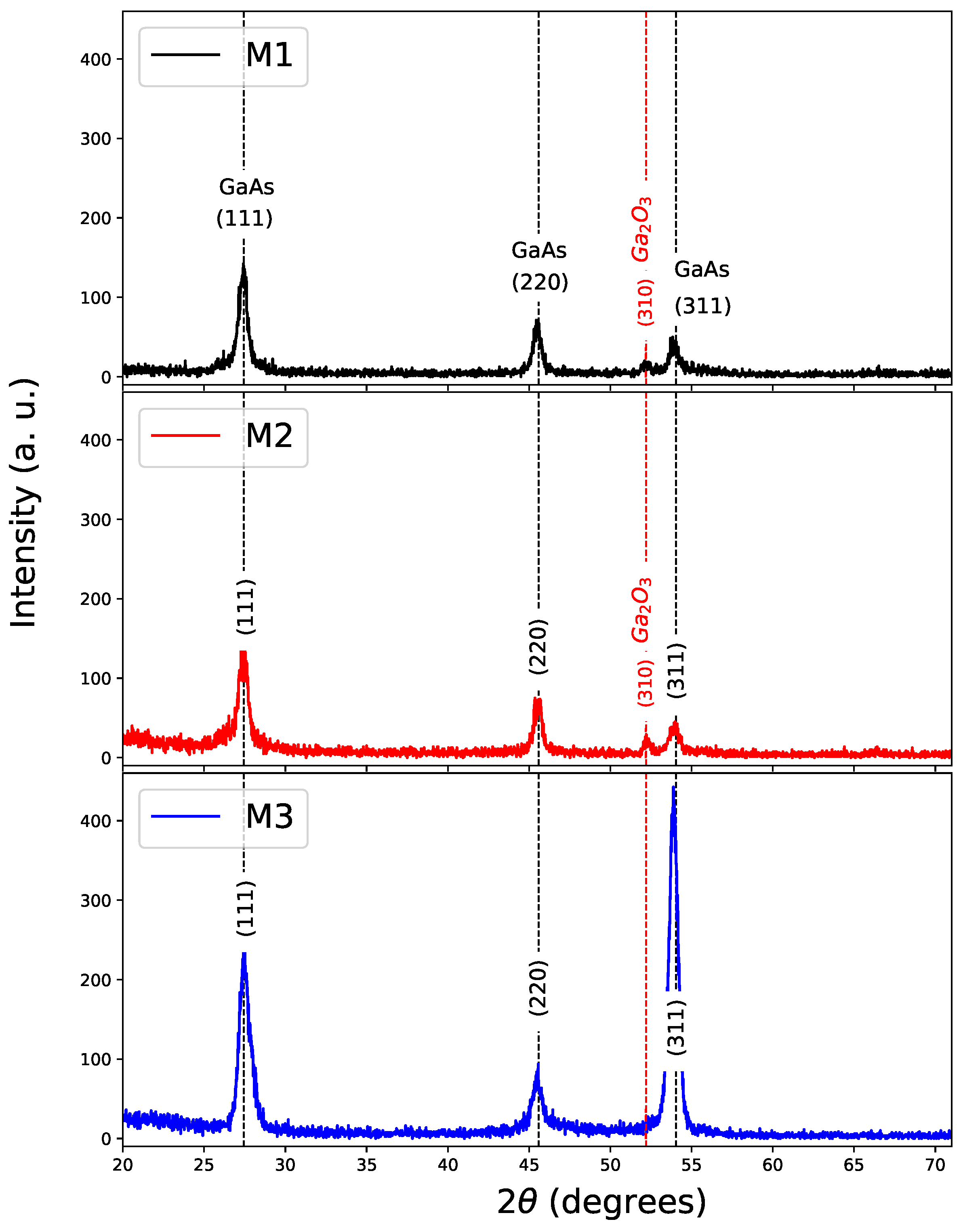
XRD patterns were used to identify crystalline phases of the GaAs films with and without nitriding. In Figure 2, XRD patterns of GaAs films grown by CSVT are shown. It can be observed that GaAs films on the silicon substrates grown at 800 C (M1), 900 C (M2), and 1000 C (M3) are polycrystalline and have a Zinc-blende structure. The reflections correspond to the (111), (220), and (311) planes with angles of , 45.35, and 53.72 respectively [27].
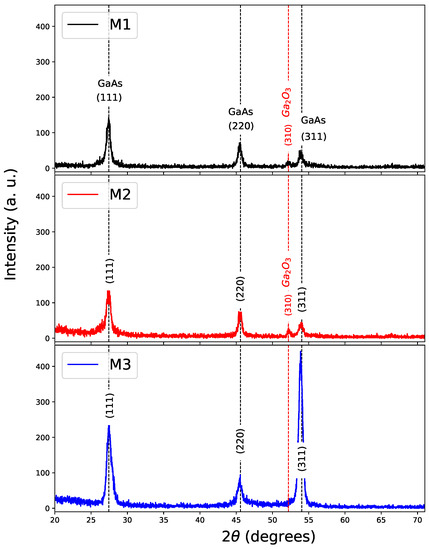
Figure 2.
XRD of samples with GaAs Films. M1 (800 C), M2 (900 C), and M3 (1000 C) in the substrate.
Although the growth temperature does not promote a clear trend towards a preferential direction except for (111), the appearance of a diffraction plane (310) can be observed in corresponding to of cubic phase and it is notorious for M1 and M2 at 800 C and 900 C, respectively. Table 2 shows growth parameters for each sample and crystallite size for the direction (111), obtaining crystallite size between 10 to 14 nm by using Scherrer formula [28]. The lattice parameter is very close to that reported for bulk GaAs of 5.654 Å [27,29].

Table 2.
Lattice Parameter and crystallite size of GaAs films grown by CSVT.
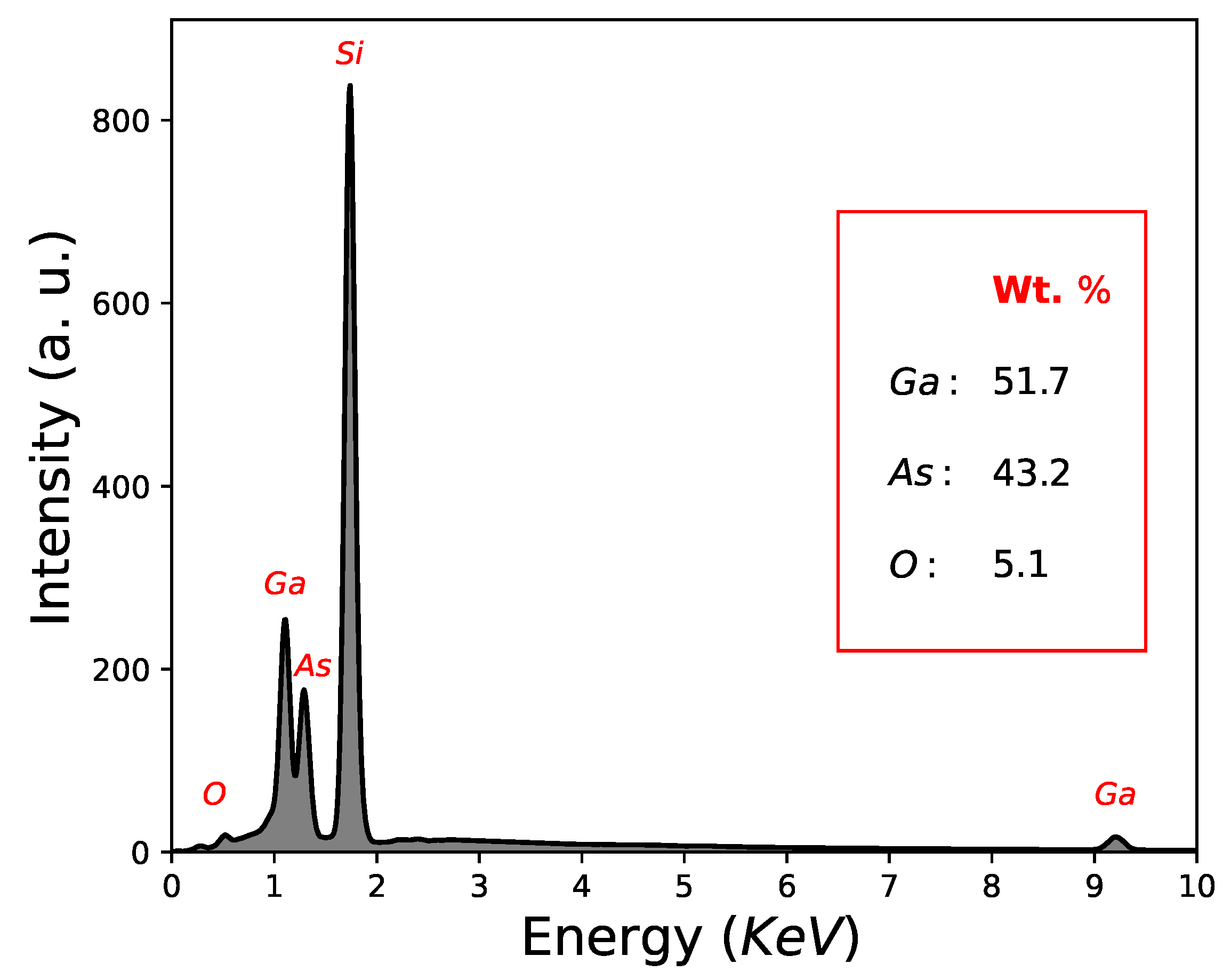
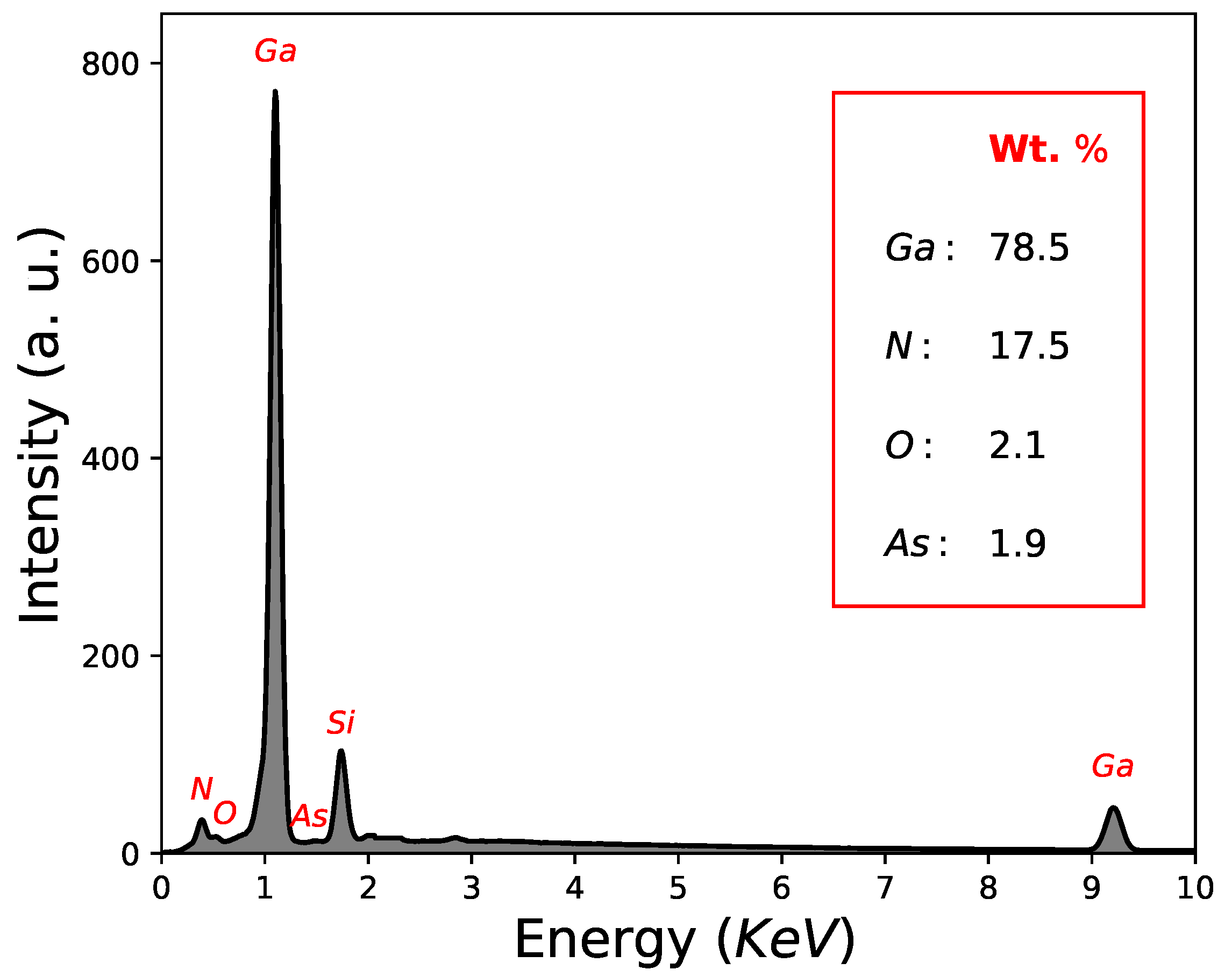
Another important factor is the quantification results in weight (Wt. %) determined by EDS; As = 43.2 Wt. %, Ga = 51.7 Wt. % and O = 5.1 Wt. %, as can be seen in Figure 3 and Table 1. In this way, GaAs film growth using the CSVT technique is demonstrated, although with a low oxygen concentration. It is worth mentioning that the signal of Si-substrate in the quantification results in weight (Wt. %) by EDS was not considered.
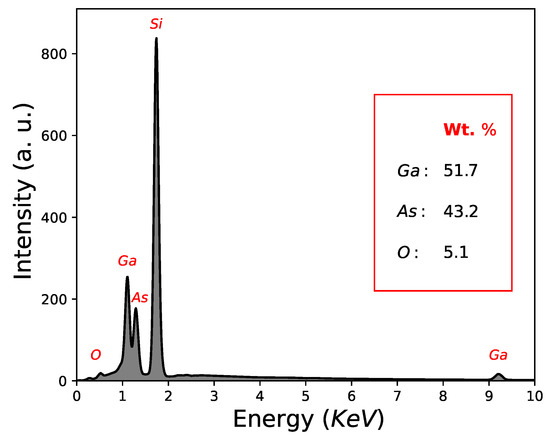
Figure 3.
Quantification results by EDS corresponding to sample M2.
Similar results were reported for GaAs films on Quartz substrates by using the CSVT technique with directions (111), (220), and (311) in [8]. In this case, direction (310) referring to was not reported. Likewise, Bernal Correa et al. reported polycrystalline films of GaAs deposited using sputtering with favorable growth in two directions (111) and (220) [28]. It is important to reaffirm that the growth process using the CSVT method was performed in an environment of free of oxide groups; however, small contributions were observed in samples M1 and M2 (Figure 2).
The GaAs film growth on the silicon substrate is carried out in a system in which there is a tungsten filament that reaches high temperatures (2000 C). We assume that high temperature in the tungsten filament causes reactive hydrogen (H°) that attacks the quartz ring. This could explain the possible source of oxygen that gives rise to the formation of present in the X-ray diffraction spectra and EDS measurements. This effect is supported in different works, where the attack of a quartz solid source () placed 3 mm from the filament tungsten generates a large amount of SiO(g) compounds for the formation of SRO or films [30,31]. In this case, the distance between the filament and the quartz ring is around 7 mm; however, we believe that it still attacks the ring quartz responsible for the oxygen present in the GaAs samples. Additionally, the presence of oxygen in the solid source (GaAs) and the silicon substrate due to native oxide could also be pointed out as a possibility.
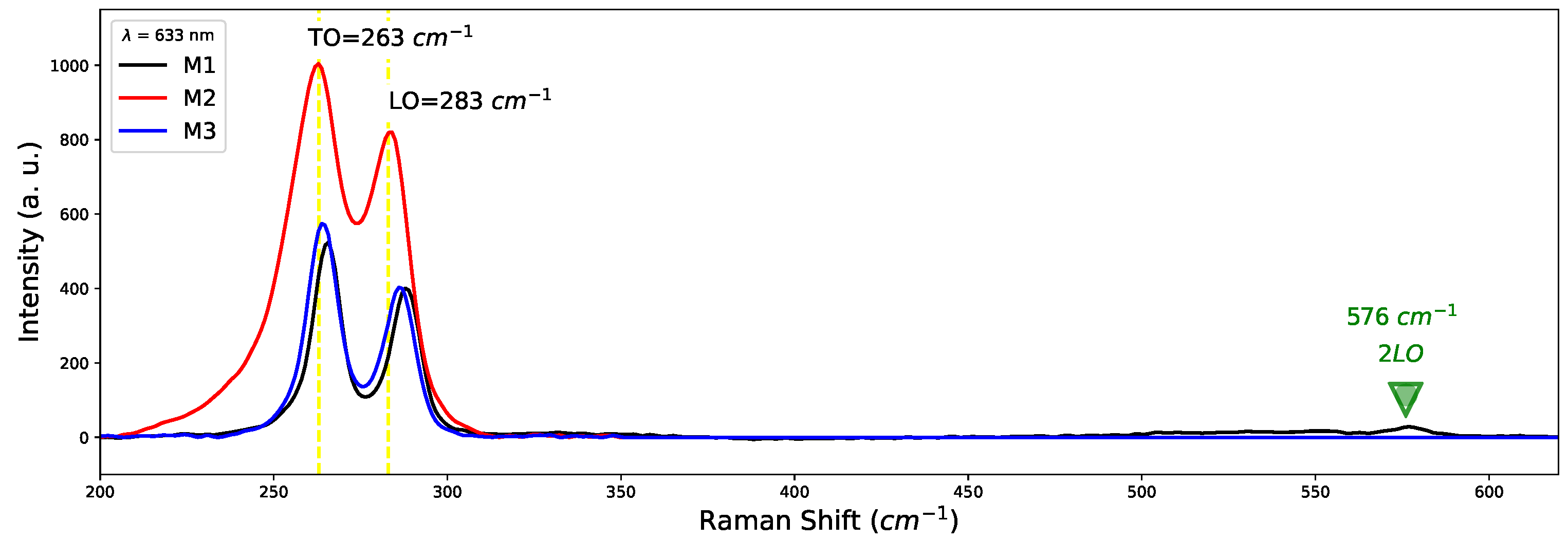
The existence of crystallographic planes of confirms the existence of oxygen in samples. We think that oxygen atoms enter the GaAs lattice to replace arsenic, even knowing that the difference in atomic radius is not the same; As (115 pm) and oxygen (73 pm) there are no significant changes in the crystal structure since the lattice parameters are very close to those reported for bulk GaAs. Figure 4 shows Raman spectra of samples M1 (800 C), M2 (900 C) and M3 (1000 C). Two characteristic shoulders close to 261 cm and 285 cm attributed to TO and LO modes of GaAs are observed. Such modes related to are responsible for the appearance of the growth direction plane (111) in XRD patterns; this effect was also observed by Bernal et al. [28]. Furthermore, a band at 576 cm corresponding to the 2LO mode of GaAs appears for sample M1. On the other hand, no signals o modes were observed regarding the Silicon substrate. Due to the average thickness of the GaAs samples (∼643 nm), the Raman penetration depth is insufficient to generate interaction with the Silicon substrate.
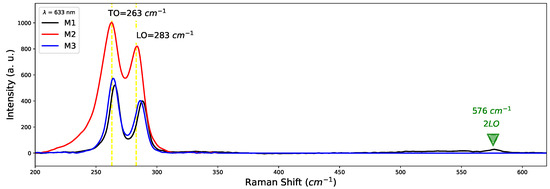
Figure 4.
Raman spectra of samples M1, M2, M3 of GaAs grown by CSVT technique.
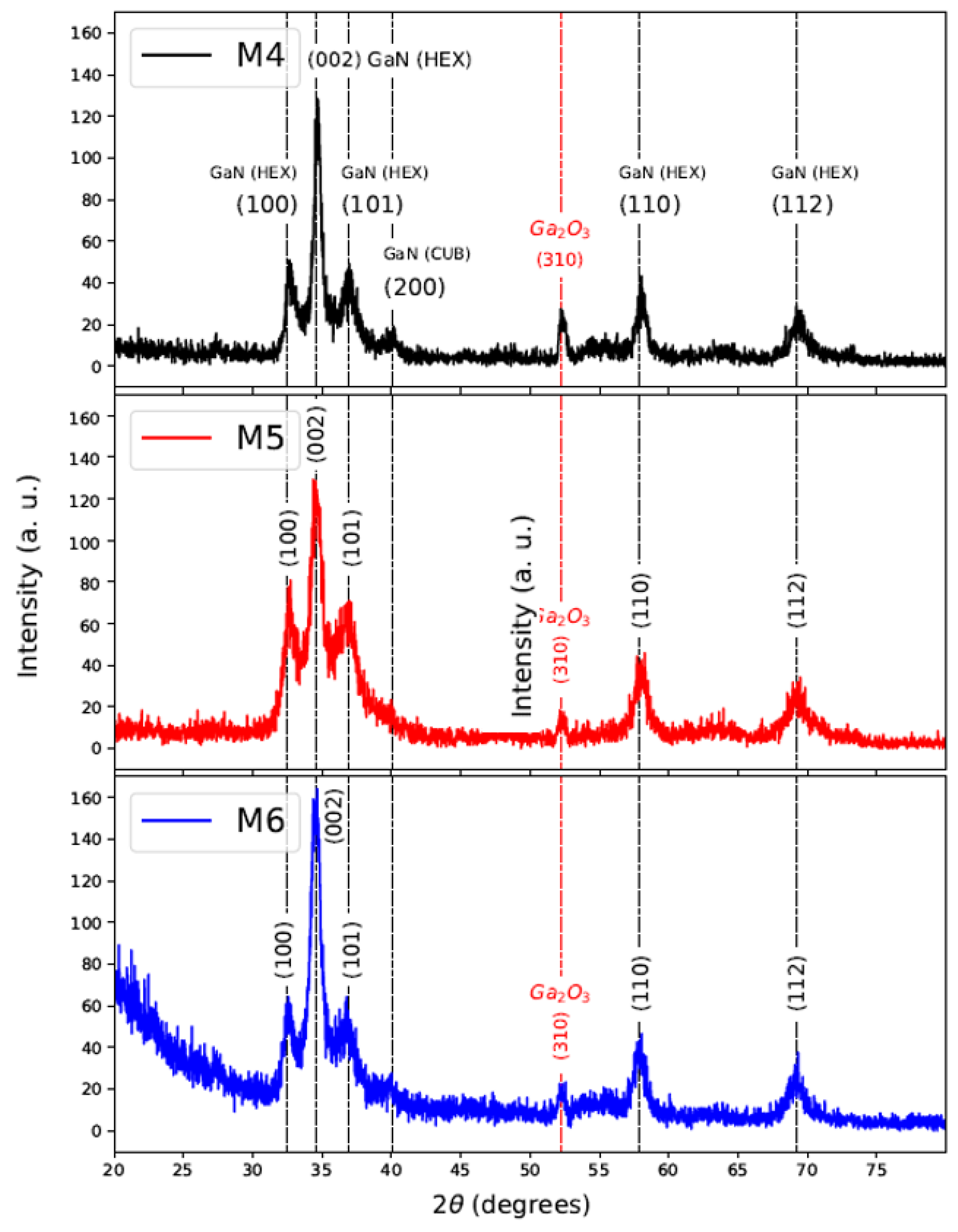
For the nitriding process, samples of GaAs/Si (M1, M2, and M3) were exposed to the ammonia environment for 30 min. The XRD patterns of nitrided samples named M4, M5, and M6 are shown in Figure 5. It can be seen diffraction planes of GaN related to a wurtzite structure at (100), (002), (101), (110), and (112) located at position : , and respectively. The diffraction plane (310) of low intensity also can be observed in corresponding to , and it is notorious in M4 and M5 samples. The positions of the diffraction peaks agree with the powder diffraction file ICDD-PDF number 50-0792 of GaN. Table 3, shows FWHM and crystallite size of the growth direction (002), obtaining a crystallite size between 9 to 12 nm using Scherrer formula [28].
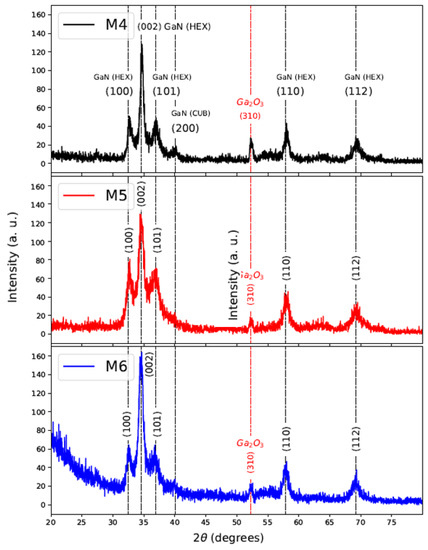
Figure 5.
XRD of Nitrided samples M4, M5, and M6.

Table 3.
Parameters of nitriding samples on Si substrates.
To calculate the lattice constants a=b and c of a hexagonal structure, it is necessary to calculate the interplanar spacings of the growth directions (100) and (002) [22].
where Å. From Equation (1) we obtain the values of Å and Å in agreement with the powder diffraction file: 50-0792.
The use of the Miller indices for the growth directions (110) and (002) in Equation (2) allows us to obtain the lattice parameters a and c as shown in Equations (3) and (4).
The results for lattice constants are shown in Table 3 and agree with ICDD-PDF Nº 50-0792 where a = b = 3.189 Å and c = 5.186 Å. It is observed that the value for the lattice constant a is less than the aforementioned reference and since its calculation comes from the interplanar spacing , it indicates that the samples M4–M6 have compression stress in the plane (100).
From these results, it can be inferred that the nitriding process at 900 C during 30 min, promotes GaN polycrystalline of phase hexagonal dominant. For samples M4–M6 also prevails the direction (310) corresponding to . In the case of sample M4 (previously obtained at 800 C), a diffraction plane with low intensity in ∼40º associated with GaN cubic phase (200) is observed; therefore, the appearance of this cubic phase depends on the growth temperature of GaAs by CSVT.
Another important factor is the quantification results in weight (Wt. %) determined by EDS, which yielded percentages of Wt. %, Wt. % for M4 sample; Wt. %, Wt. % for M5 sample and Wt. %, Wt. % for M6. Likewise, the existence of oxygen and Arsenic around ∼2 Wt. % for all samples as can be seen in Figure 6 and Table 1. The reduction of oxygen in GaAs films nitrided (∼2 Wt. %) with respect to the initial samples (GaAs as deposited by CSVT) can be explained by the process carried out at 900 C, where oxygen groups volatilize. On the other hand, EDS reveals the existence of As around 2 Wt. %; however, the non-existence of GaAs-type growth directions in nitrided films (Figure 5) may be due to its location near the substrate (in the bottom), which is impossible to detect when films are measured with grazing angle.
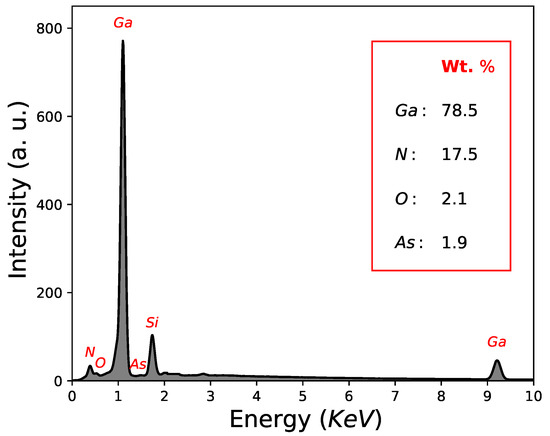
Figure 6.
Elemental composition via EDS of sample M4.
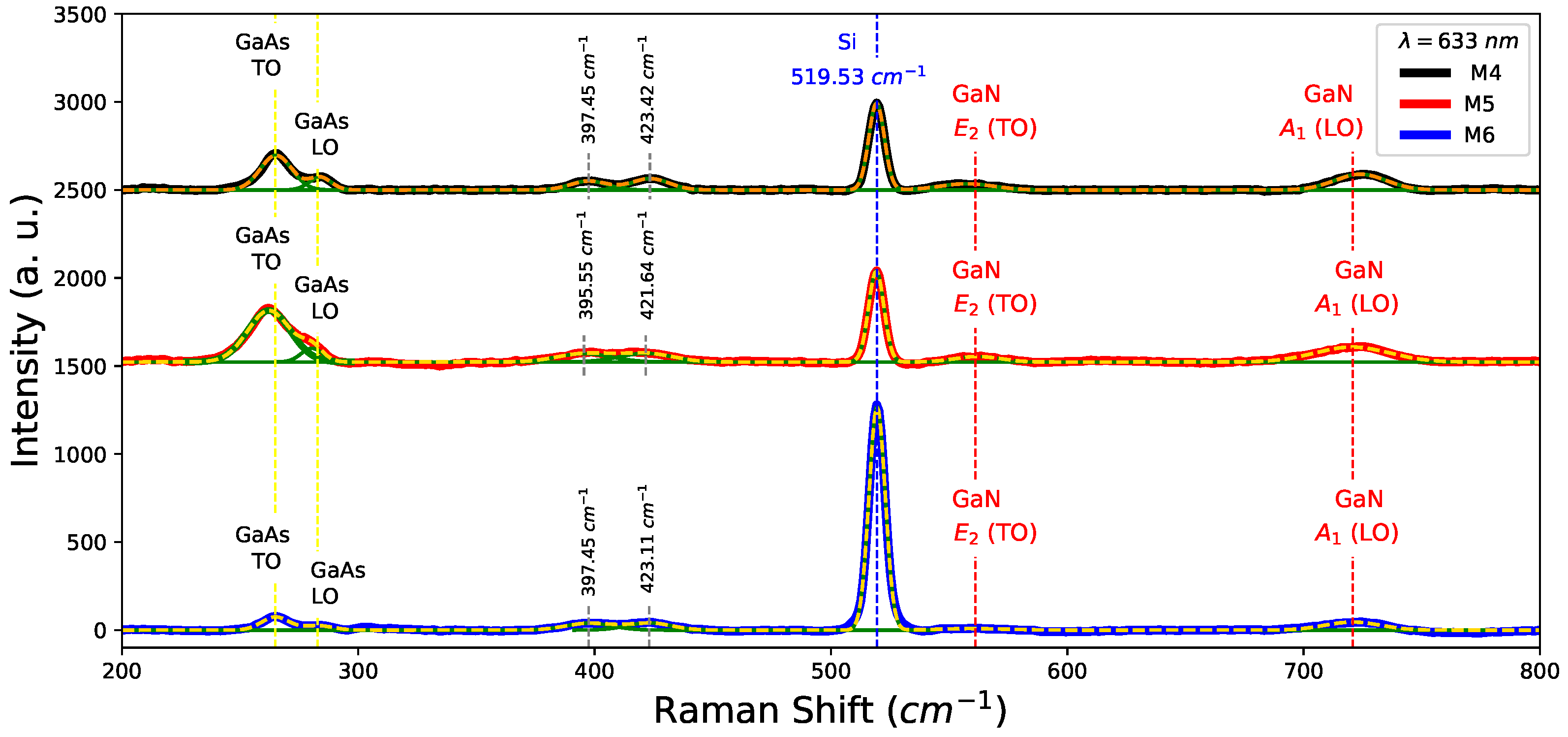
To corroborate the presence of GaAs in nitrided samples, Raman spectra were measured in samples M4, M5, and M6, as shown in Figure 7. Peaks associated with modes GaN, and around 561 cm and 721 cm respectively were detected [32]. In fact, Table 4 lists the position of GaN modes for nitrided samples. On the other hand, TO and LO modes around 263 cm and 284 cm confirm the presence of GaAs in the nitrided samples (M4, M5, and M6) as can be seen in Figure 7. Likewise, changes in the shoulder LO which is comparatively less intense than its predecessor in Figure 4, mainly in the decreasing LO mode contribution, appear to vanish for M5 and M6 samples. The peak in 519.53 cm associated Si-substrate is observed in all samples, although it is more noticeable for the M6 sample. This effect is due to the existence of decreasing thickness for this sample in comparison with M4 and M5. Finally, the existences of groups in nitrided films were found at 397 cm and 423 cm attributed to bending vibrations [33].
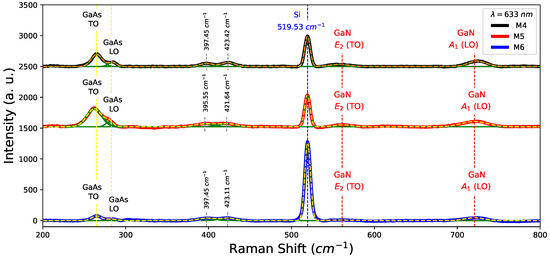
Figure 7.
Raman spectra for the nitrided samples M4, M5, M6.

Table 4.
Peaks position of the modes in Raman spectra of each nitrided sample.
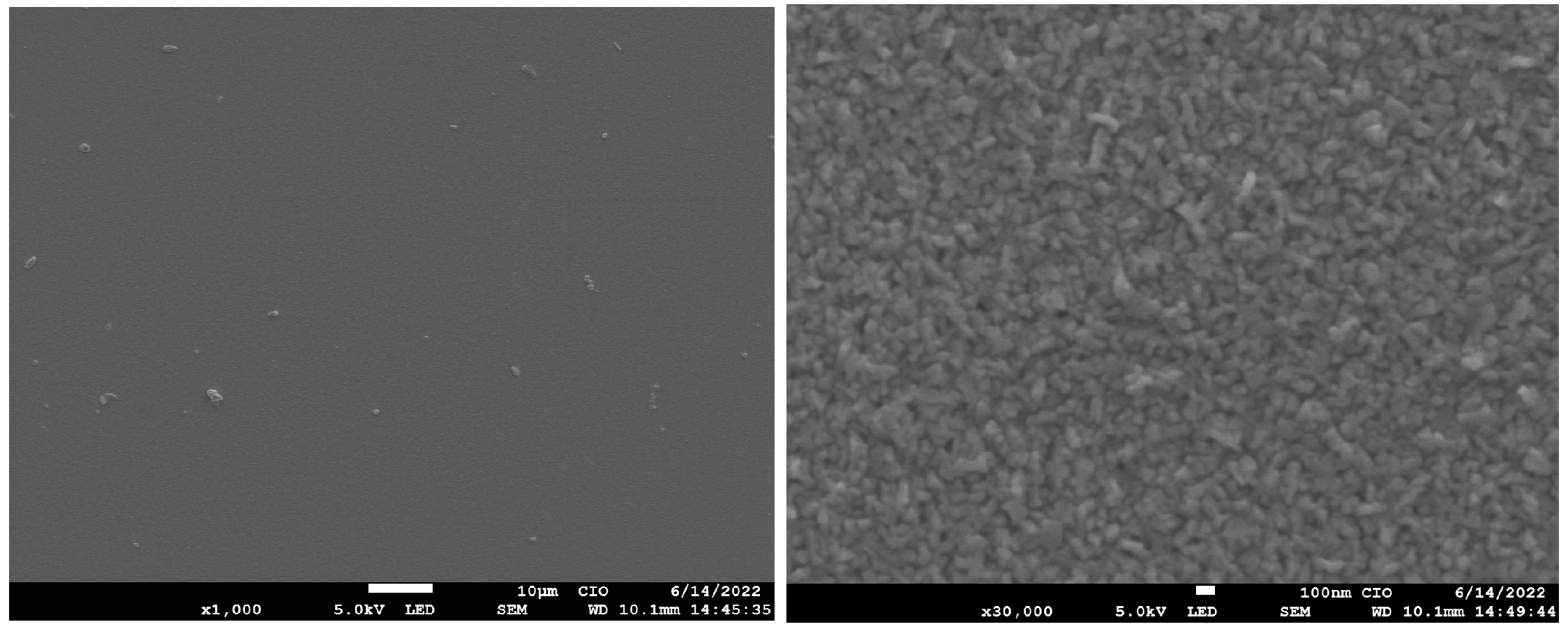
Figure 8 shows an SEM micrograph of magnification (×1000 and ×30,000) of GaAs film (M2), which observes homogeneous morphology on the surface with granular and stack structures rice-like (Figure 8 right). It is important to mention that SEM micrographs of GaAs films grown on quartz substrates using the same technique [8] show cracks along them. In this work, such an effect on silicon substrates was not observed. Cracks in materials observed by SEM measurements are indicative of losses whenever the purpose is luminescence, making our parameters of growth with the potential to luminescence devices [32,34].
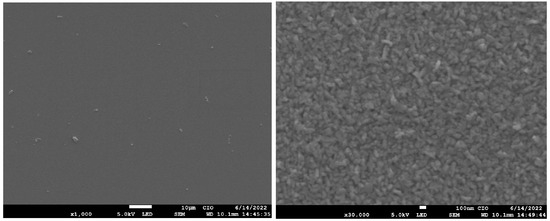
Figure 8.
Micrograph SEM of the sample of GaAs (M3).
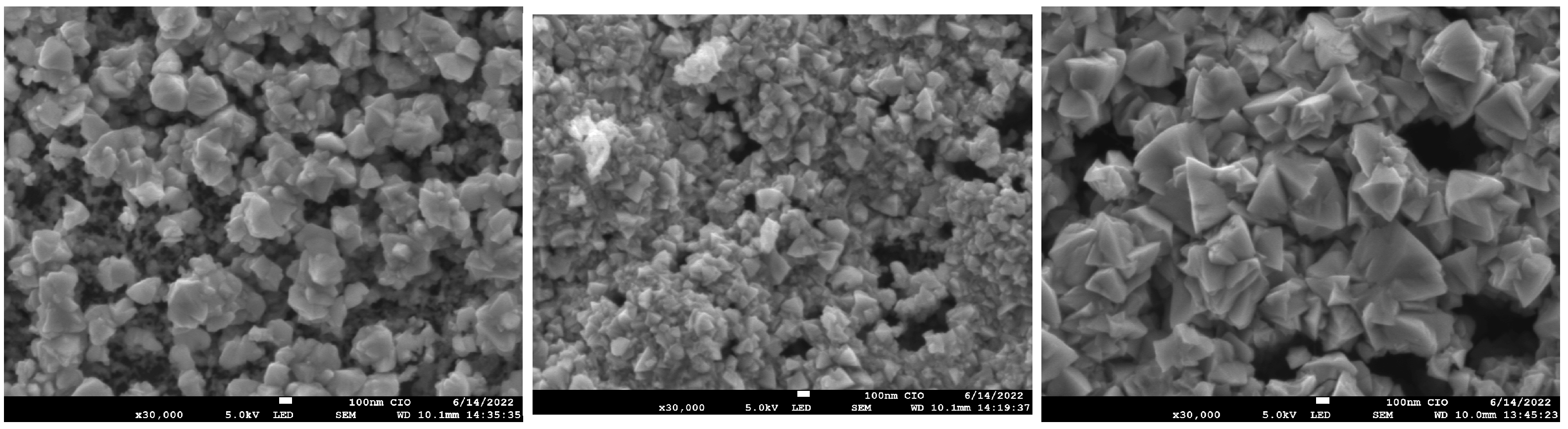
Figure 9 shows the top-view SEM images of magnification (×30,000) of GaAs films nitrided (M4, M5, M6). The surface in samples consists of randomly oriented grains with sizes of the order of several hundred nanometers. These grains increase in size for sample M6, previously deposited by CSVT at 1000 C, which could explain the increase in crystallite size measured by the Scherrer formula (see Table 3).
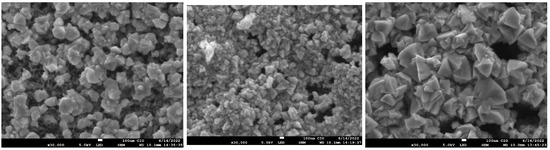
Figure 9.
Micrograph SEM of samples M4, M5, M6.
It is important to mention that cracks were not observed on the surface of nitrided samples so that the silicon substrate can favor the formation of GaN films. Reference [32], shows a type grain similar to GaN on Si substrates, where changes in morphology from tiny grains become wider and intertwine as temperature increases. Such deposits are the most efficient for reducing leakage current in solar cells with morphology with wide and intertwinings grains similar to the M6 micrograph. In fact, the M6 sample previously deposited at 1000 C by CSVT with subsequent nitrided at 900 C was the sample that presented the best characteristics since the diffraction plane (310) corresponding to is minimal, and diffraction plane (200) attributed to the cubic phase of GaN disappears. On the other hand, Raman measurements indicate that GaAs modes in nitrided sample decrease (Figure 7), so we suggest a better nitriding in the GaAs film (M6). This assertion can be explained by analyzing the band gap energy.
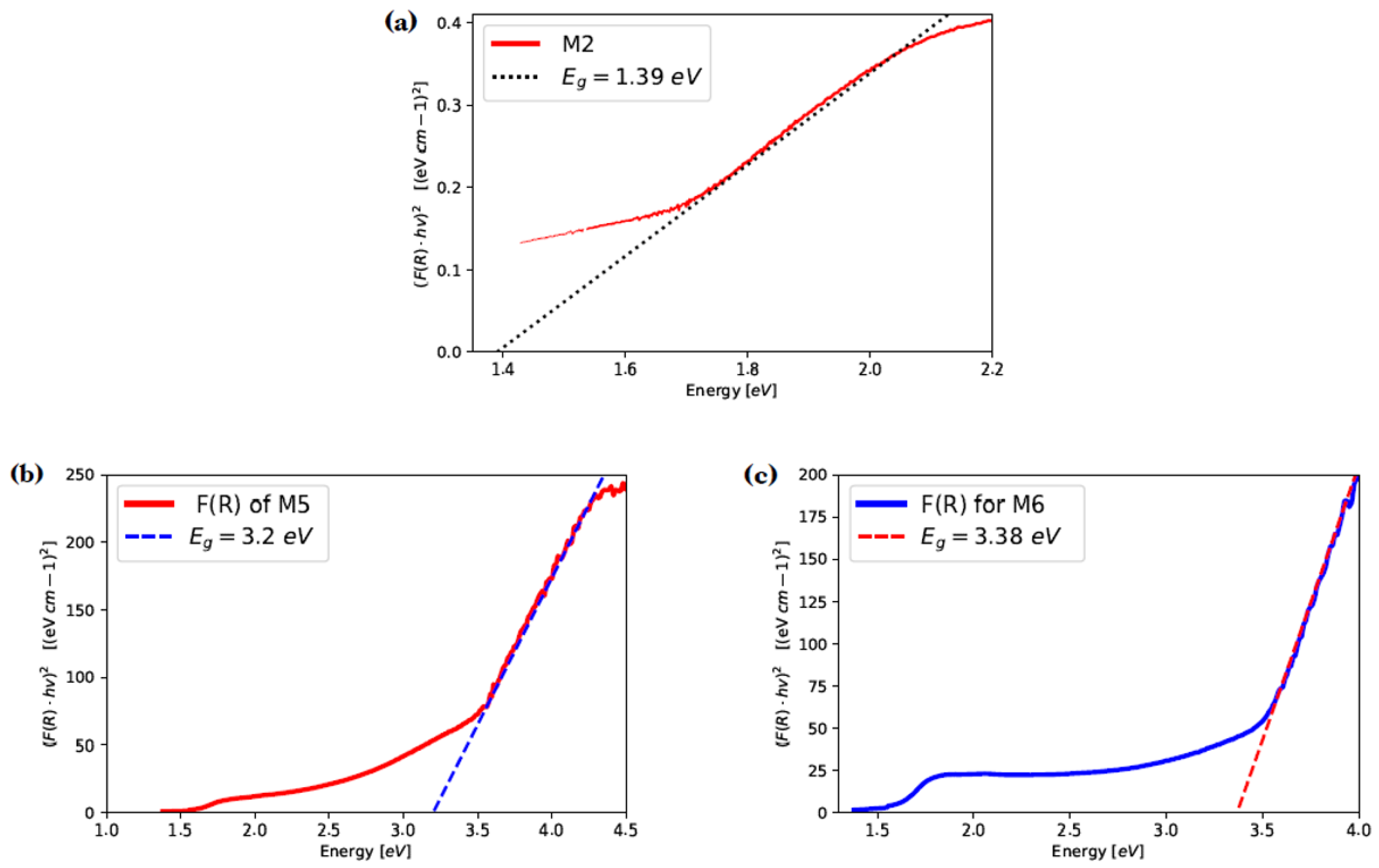
The Kubelka–Munk model is commonly used to analyze Diffuse Reflectance Spectroscopy results and estimate the optical band gap energy of semiconductor materials. Using the diffuse reflectance data and Kubelka–Munk theory, it determines the function F(R). The slope resulting from linear regression in F(R) points out the estimated value when it intersects the energy axis. Figure 10a shows the function F(R) and the estimated value for GaAs film (M2) of 1.39 eV, which is close to the corresponding bulk GaAs band gap energy of 1.42 eV [8].
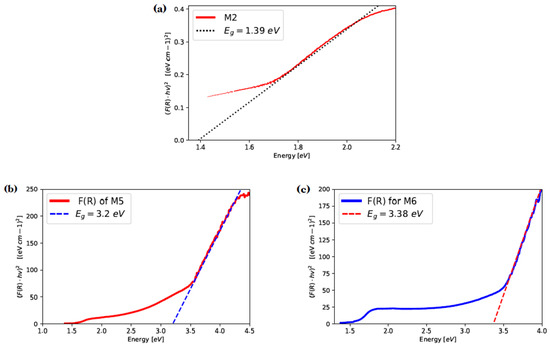
Figure 10.
Kubelka–Munk transformed reflectance spectra of samples M2, M5 and M6. (a) Kubelka–Munk transformed reflectance spectra of sample M2; (b) Kubelka–Munk transformed reflectance spectra of sample M5; (c) Kubelka–Munk transformed reflectance spectra of sample M6.
For estimate band gap energy values for GaAs films nitride (samples M5 and M6), the F(R) function and the linear part of the plot extrapolation with the x-axis were determined (Figure 10b,c). The band gap estimates were eV and eV for M5 and M6, respectively, which are close to the direct band energy of bulk GaN ( eV). Such difference may be due to groups present in the GaAs films nitrided, although it is important to mention that the sample that has a band gap close to the bulk GaN is the sample M6 [35].
4. Conclusions
In this article, we discuss results on the structural, morphological, and optical properties of GaAs films nitrided on Si substrates obtained previously by the CSVT at ∼800 C, ∼900 C and ∼1000 C. XRD confirms polycrystalline films of GaAs on Si substrates with a low concentration of oxygen (5.1 Wt. %). In addition, diffraction planes of low intensity at (310) correspond to and are reported in GaAs films. Analysis by profilometry reveals an average thickness of the GaAs film greater than 640 nm, which is why the Raman study with an incidence of 633 nm shows the LO and TO modes of GaAs only. SEM measurements reveal no cracks and homogeneous morphology in the surface of GaAs films. The estimation of the bandgap (1.39 eV) is close to bulk GaAs.
On the other hand, when GaAs films are nitrided at 900 C for 30 min, the diffraction directions XRD of a hexagonal structure dominant corresponding to the GaN structure is observed. In addition, a phase of low intensity corresponding to is observed, and a cubic GaN appears for a growth temperature of 800 C. In fact, the sample previously deposited at 1000 C by CSVT with subsequent nitrided is the sample that presented the best characteristics since the diffraction plane (310) corresponding to is minimal, and the diffraction plane (200) attributed to the cubic phase of GaN disappears. The thickness obtained from the analysis by profilometry is less than 342 nm. On the other hand, Raman measurements indicate that responsible modes of GaAs decrease. The origin of the nitrided in GaAs films is explained as a process that substitutes arsenic for nitrogen; the existence of O and As in nitrided samples makes it be considered as a non-complete diffusive–substitutive method, and since with grazing beam in XRD does not show traces of GaAs or products with As, it is to be considered that the GaAs is at the bottom of the GaN films. Raman spectroscopy confirms the associated modes to GaAs, , Si, and GaN. Despite the presence of in the network, the estimated gap is not far from that reported for bulk GaN.
Author Contributions
E.A.V.-T. and A.C. wrote, conceived and designed the experiments; G.G.-S. validation and designed the experiments: R.R.-T., C.M.-R., E.R.-A. and M.A.V.-A., R.G.-I. reviews, provided resources and systems; J.M.G.-J. and F.M.-M. formal analysis and provided resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors wish to thank María Cristina Zorrilla Cangas, an academic technician from Universidad Nacional Autónoma de México, Instituto de F, Mexico, for their help in Raman’s measurements.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Schlesinger, T. Gallium Arsenide. In Encyclopedia of Materials: Science and Technology; Buschow, K.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 3431–3435. [Google Scholar] [CrossRef]
- Rudolph, P.; Jurisch, M. Bulk growth of GaAs An overview. J. Cryst. Growth 1999, 198, 325–335. [Google Scholar] [CrossRef]
- Yugova, T.G.; Chuprakov, V.A.; Sanzharovsky, N.A.; Yugov, A.A.; Martynov, I.D.; Knyazev, S.N. Effect of a Travelling Magnetic Field on the Parameters of Tellurium-Doped Gallium Arsenide Single Crystals Grown by the Czochralski Method. Crystallogr. Rep. 2022, 67, 1099–1104. [Google Scholar] [CrossRef]
- Heckingbottom, R.; Davies, G.; Prior, K. Growth and doping of gallium arsenide using molecular beam epitaxy (MBE): Thermodynamic and kinetic aspects. Surf. Sci. 1983, 132, 375–389. [Google Scholar] [CrossRef]
- Nishizawa, J.; Kurabayashi, T. Mechanism of gallium arsenide MOCVD. Vacuum 1990, 41, 958–962. [Google Scholar] [CrossRef]
- Szymoński, M.; Bhattacharya, R.S. The sputtering of gallium arsenide at elevated temperatures. Appl. Phys. 1979, 20, 207–211. [Google Scholar] [CrossRef]
- Manasevit, H.M. Single-Crystal Gallium Arsenide on Insulating Substrates. Appl. Phys. Lett. 1968, 12, 156–159. [Google Scholar] [CrossRef]
- Cruz Bueno, J.J.; García Salgado, G.; Balderas Valadez, R.F.; Luna López, J.A.; Nieto Caballero, F.G.; Díaz Becerril, T.; Rosendo Andrés, E.; Coyopol Solís, A.; Romano Trujillo, R.; Morales Ruiz, C.; et al. Effect of the Gaseous Atmosphere in GaAs Films Grown by Close-Spaced Vapor Transport Technique. Crystals 2019, 9, 68. [Google Scholar] [CrossRef]
- Birkmann, B.; Rasp, M.; Stenzenberger, J.; Müller, G. Growth of 3” and 4” gallium arsenide crystals by the vertical gradient freeze (VGF) method. J. Cryst. Growth 2000, 211, 157–162. [Google Scholar] [CrossRef]
- Oshima, R.; Ogura, A.; Shoji, Y.; Makita, K.; Ubukata, A.; Koseki, S.; Imaizumi, M.; Sugaya, T. Ultra-High-Speed Growth of GaAs Solar Cells by Triple-Chamber Hydride Vapor Phase Epitaxy. Crystals 2023, 13, 370. [Google Scholar] [CrossRef]
- Ferhat, M.; Zaoui, A. Structural and electronic properties of III-V bismuth compounds. Phys. Rev. B 2006, 73, 115107. [Google Scholar] [CrossRef]
- Adachi, S. Physical Properties of III-V Semiconductor Compounds; Wiley-VCH: Hoboken, NJ, USA, 1992. [Google Scholar]
- Fujito, K.; Kubo, S.; Nagaoka, H.; Mochizuki, T.; Namita, H.; Nagao, S. Bulk GaN crystals grown by HVPE. J. Cryst. Growth 2009, 311, 3011–3014. [Google Scholar] [CrossRef]
- Humphreys, T.P.; Sukow, C.A.; Nemanich, R.J.; Posthill, J.B.; Rudder, R.A.; Hattangady, S.V.; Markunas, R.J. Microstructural and Optical Characterization of GaN Films Grown by PECVD on (0001) Sapphire Substrates. MRS Online Proc. Libr. (OPL) 1989, 162, 531. [Google Scholar] [CrossRef]
- Papamichail, A.; Kakanakova-Georgieva, A.; Sveinbjörnsson, E.Ö.; Persson, A.R.; Hult, B.; Rorsman, N.; Stanishev, V.; Le, S.P.; Persson, P.O.Å.; Nawaz, M.; et al. Mg-doping and free-hole properties of hot-wall MOCVD GaN. J. Appl. Phys. 2022, 131, 185704. [Google Scholar] [CrossRef]
- Moustakas, T.; Lei, T.; Molnar, R. Growth of GaN by ECR-assisted MBE. Phys. Condens. Matter 1993, 185, 36–49. [Google Scholar] [CrossRef]
- Kucharski, R.; Sochacki, T.; Lucznik, B.; Bockowski, M. Growth of bulk GaN crystals. J. Appl. Phys. 2020, 128, 050902. [Google Scholar] [CrossRef]
- García-Salgado, G.; Cruz-Bueno, J.; Ramírez-González, F.; Gastellou, E.; Nieto-Caballero, F.; Rosendo-Andrés, E.; Luna-López, J.; Coyopol-Solís, A.; Romano-Trujillo, R.; Morales-Ruiz, C.; et al. GaN obtained on quartz substrates through the nitridation of GaAs films deposited via CSVT. J. Alloys Compd. 2021, 887, 161469. [Google Scholar] [CrossRef]
- Richter, T.M.M.; Niewa, R. Chemistry of Ammonothermal Synthesis. Inorganics 2014, 2, 29–78. [Google Scholar] [CrossRef]
- Elke Meissner, R.N. (Ed.) Ammonothermal Synthesis and Crystal Growth of Nitrides; 0933-033X; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Kharisov, B.I.; Oxana, V. Less-Common Methods of the “Direct Synthesis” Area Direct Synthesis of Metal Complexes; Elsevier Science Publishing: Amsterdam, The Netherlands, 2018; pp. 415–433. [Google Scholar]
- Fathy, M.; Gad, S.; Anis, B.; Kashyout, A.E.H.B. Crystal Growth of Cubic and Hexagonal GaN Bulk Alloys and Their Thermal-Vacuum-Evaporated Nano-Thin Films. Micromachines 2021, 12, 1240. [Google Scholar] [CrossRef]
- Runton, D.; Trabert, B.; Shealy, J.; Vetury, R. History of GaN: High-Power RF Gallium Nitride (GaN) from Infancy to Manufacturable Process and Beyond. Microw. Mag. IEEE 2013, 14, 82–93. [Google Scholar] [CrossRef]
- Kobayashi, R.; Fujii, K.F.K.; Hasegawa, F.H.F. Etching of GaAs by Atomic Hydrogen Generated by a Tungsten Filament. Jpn. J. Appl. Phys. 1991, 30, L1447. [Google Scholar] [CrossRef]
- Beloruchev, L.V.; Dembovskii, V.V.; Morshtein, I.M. Method of determining the degree of dissociation of ammonia in different gaseous nitriding processes. Met. Sci. Heat Treat. 1968, 10, 227–228. [Google Scholar] [CrossRef]
- Sukach, G.A.; Kidalov, V.V.; Kotlyarevsky, M.B.; Potapenko, E.P. Structure and composition of gallium nitride films produced by processing gallium arsenide single crystals in atomic nitrogen. Tech. Phys. 2003, 48, 437–440. [Google Scholar] [CrossRef]
- JCPDS—ICDD. Powder Diffraction Files, Swarthmore, PA, Card No. 32-0389, PDF-2 Database (2000). Available online: https://scholar.google.com/scholar_lookup?hl=es-MX&publication_year=2000&pages=%00empty%00&author=JCPDSICDD&isbn=%00null%00&title=Powder+Diffraction+Files#d=gs_cit&t=1680218075343&u=%2Fscholar%3Fq%3Dinfo%3AKZri3gX175wJ%3Ascholar.google.com%2F%26output%3Dcite%26scirp%3D0%26hl%3Des (accessed on 27 February 2023).
- Bernal Correa, R.; Montes Monsalve, J.; Pulzara Mora, A.; López López, M.; Cruz Orea, A.; Cardona, J. Polycristalline growth of zinc blende gallium arsenide layers by R.F. magnetron sputtering. Superf. Vac. 2004, 27, 102–106. [Google Scholar]
- Blakemore, J.S. Semiconducting and other major properties of gallium arsenide. J. Appl. Phys. 1982, 53, R123–R181. [Google Scholar] [CrossRef]
- Coyopol, A.; García-Salgado, G.; Díaz-Becerril, T.; Juárez, H.; Rosendo, E.; López, R.; Pacio, M.; Luna-López, J.A.; Carrillo-López, J. Optical and Structural Properties of Silicon Nanocrystals Embedded in SiOx Matrix Obtained by HWCVD. J. Nanomater. 2012, 2012, 368268. [Google Scholar] [CrossRef]
- Coyopol, A.; Díaz-Becerril, T.; García-Salgado, G.; Juárez-Santisteban, H.; López, R.; Rosendo-Andrés, E. Evolution on the structural and optical properties of SiOx films annealed in nitrogen atmosphere. J. Lumin. 2014, 145, 88–93. [Google Scholar] [CrossRef]
- Saron, K.M.A.; Ibrahim, M.; Hashim, M.R.; Taha, T.A.; Elfadill, N.G.; Mkawi, E.M.; Allam, N.K. Leakage current reduction in n-GaN/p-Si (100) heterojunction solar cells. Appl. Phys. Lett. 2021, 118, 023902. [Google Scholar] [CrossRef]
- Sulikowski, B.; Olejniczak, Z.; Corberán, V.C. Faujasite Catalysts Promoted with Gallium Oxide: A Physicochemical Study. J. Phys. Chem. 1996, 100, 10323–10330. [Google Scholar] [CrossRef]
- Dadgar, A.; Alam, A.; Riemann, T.; Bläsing, J.; Diez, A.; Poschenrieder, M.; Strassburg, M.; Heuken, M.; Christen, J.; Krost, A. Crack-Free InGaN/GaN Light Emitters on Si(111). Phys. Status Solidi (A) 2001, 188, 155–158. [Google Scholar] [CrossRef]
- Chen, Z.; Jaramillo, T. The Use of UV-Visible Spectroscopy to Measure the Band Gap of a Semiconductor; Department of Chemical Engineering, Stanford University: Stanford, CA, USA, 2017. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).