Abstract
This review covers over fifty Pt(II) monomeric complexes with a wide combination of η3-ligands of the composition Pt(η3-X3L)(PL), (X3 = N3; S3; Te3; ONO; CNC, SeNSe; ONC; ONS; CNS; NNC, NNS; NNSe, SOS; SBS; NON; SSO). The η3-ligand with monodentate PL displays distorted square-planar geometry about Pt(II) atoms. The structural parameters (Pt-L, L-Pt-L) are analyzed and discussed, with a particular emphasis on the distortion of square-planar geometry about Pt(II) atoms, as well as of the trans-influence. There is a relation between the membered nature of the metallocycles and the distortion of square-planar geometry about the Pt(II) atoms. The distortion increases as indicated by parameter τ4 in the following order: 0.023 (6+6) < 0.024 (^+5) < 0.040 (5+6) < 0.062 (5+5).
1. Introduction
The chemistry of platinum is particularly important in the areas of catalysis and biochemistry. There are numerous published structural studies on platinum complexes that have been classified and analyzed [1]. The high affinity of platinum(II) ions for phosphorous enables it to bind effectively to organophosphines. Organophosphines as soft P-donor ligands are very useful for building a wide variety of platinum complexes. Recently, we classified and analyzed structural data of monomeric organoplatinum complexes with PtP3C inner coordination spheres [2]. Another review covers structural data of monomeric Pt(II) coordination complexes with inner coordination spheres, including PtP4, PtP3X, and PtP2X2, in which P-donor ligands are monodentate organomonophosphines [3].
This study aims to correlate the following structural parameters available for Pt(η3-X3L)(PL): (X3 = N1,N2,N3; S1,S2,S3; Te1,Te2,Te3; O1,N1,O2; O1,N1,C1; O1,N1,S1; N1,N2,C1; N1,N2,S1; N1,N2,Se1; N1,C1,N2; C1,N1,C2; C1,N1,S1; S1,C1,S2; S1,B1,S2; S1,S2,O1; Se1,N1,Se2.
2. Pt(η3-X3L)(PL) Derivatives
There are over fifty examples in which the inner coordination spheres about the Pt(II) atoms of the Pt(η3-X3L)(PL) type are formed by variable combinations of donor atoms of tridentate ligands. Each η3-ligand creates two metallocyclic rings. The complexes based on membered metallocyclic rings can be divided into four groups.
2.1. 6+6-Membered Metallocyclic Rings
There are only three examples in which a η3-ligand creates such rings (Table 1). In [Pt(η3-C22H11F6N3O2–O1,N1,O2)(PPh3)] (at 173 K) [4], the η3-ligand forms a metallocyclic ring of the O1C3N1C3O2 type with common ligating N1 atoms. The values of the chelate L-Pt-L angles are 90.6° (O1-Pt-N1) and 90.2° (N1-Pt-O2). The O1C2NN1C3O2 type with the respective chelate angles of 88.2° (O1-Pt-N1) and 90.0° (N1-Pt-O2) was found in [Pt(η3-C14H10N2O3–O1,N1,O2)(PPh3)] (at 150 K) [5]. The remaining L-Pt-L angles open in the following order (mean values): 88.1° (O2-Pt-P) < 89.0° (O1-Pt-P) < 176.0° (N1-Pt-P) < 177.7° (O1-Pt-O2). The monodentate PPh3 displayed square-planar geometry about each Pt(II) atom. The Pt-L bond distance increased in the following order (mean values): 1.995 Å (Pt-O1 trans to O2) < 1.996 Å (Pt-O2) < 2.010 Å (Pt-N1) < 2.254 Å (Pt-P).

Table 1.
Structural data for Pt(η3-X3)(Y) derivatives. a—6+6-membered metallocyclic rings.
For the complex [Pt{η3-C12H24S3-S1,S2,S3}(PPh3)]BF4, the η3-ligand creates a pair of six-membered metallocyclic rings of the S1C3S2C3S3 type (as shown in Figure 1) [6]. The values of the chelate angles are 87.1° (S1-Pt-S2) and 89.5° (S2-Pt-S3). The remaining L-Pt-L bond angles open in the following order: 91.1° (S1-Pt-P) < 92.3° (S3-Pt-P) < 171.0° (S2-Pt-P) < 176.3° (S1-Pt-S3). The Pt-L bond distance increases in the following order: 2.330 Å (Pt-S1) < 2.332 Å (Pt-P) < 2.336 Å (Pt-S3) < 2.339 Å (Pt-S2 trans to P). Noticeably, the trans-X1-Pt-X3 bond angles are somewhat bigger than the trans-X2-Pt-P bond angles (Table 1).
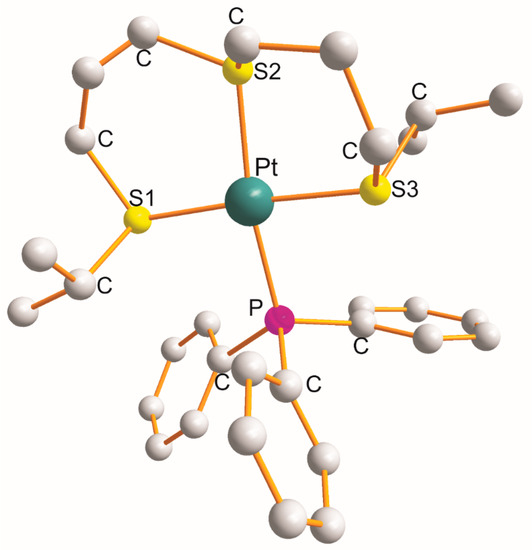
Figure 1.
Structure of [Pt{η3-C12H24S3-S1,S2,S3}(PPh3)] [6].
2.2. 6+5-Membered Metallocyclic Rings
There are five examples that will be discussed in this section, namely [Pt(η3-C16H14N2OS2–O1,N1,S1)(PPh3)] [7], [Pt(η3-C16H13N3O3S2–O1,N1,S1)(PPh3)] (at 200K) [7], [Pt(η3-C8H8N3OS–O1,N1,S1)(PPh3)] toluene [8], [Pt(η3-C9H9N3OS–O1,N1,S1)(PPh3)] (at 100 K) (Figure 2) [9], and [Pt(η3-C18H16N2OS2–O1,N1,S1)(PPh3)] [7] (Table 2). In each of them, the η3-ligand creates six- and five-membered metallocyclic rings with a common ligating N1 atom of the O1C3N1NCS1 type. The values of the respective chelate angles (mean values) are 92.3° (O1-Pt-N1) and 84.6° (N1-Pt-S1). The remaining L-Pt-L bond angles open in the following order (mean values): 90.7° (O1-Pt-P) < 92.4° (S1-Pt-P) < 175.8° (N1-Pt-P) < 175.9° (O1-Pt-S1). Interestingly, the mean values of both trans-O1-Pt-S1 and N1-Pt-P angles are equal. The Pt-L bond distance increases (mean values) in the following order: 2.028 Å (Pt-O1 trans to S1) < 2.035 Å (Pt-N1 trans to P) < 2.244 Å (Pt-S1) < 2.259 Å (Pt-P).
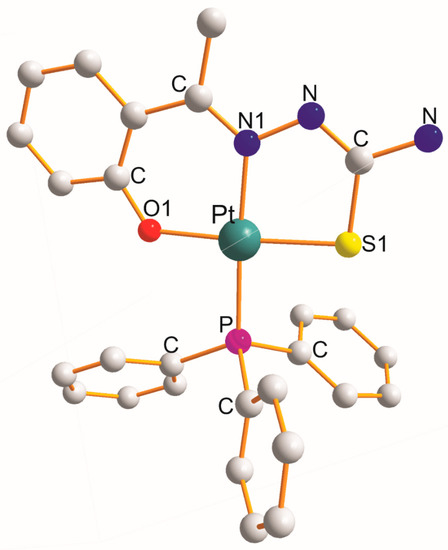
Figure 2.
Structure of [Pt(η3-C9H9N3OS–O1,N1,S1)(PPh3)] [9].

Table 2.
Structural data for Pt(η3-X3)(Y) derivatives. a—6+5-membered metallocyclic rings.
2.3. 5+6-Membered Metallocyclic Rings
There are four complexes mentioned in this section, namely [Pt(η3-C12H10N4–N1,N2,N3)(PPh3)] (at 100 K) [10], [Pt(η3-C13H9NO2–O1,N1,O2)(PPh3)] [11], [Pt(η3-C12H16N2O4Se2–Se1,N1,Se2){P(η1-C11H19O5)(Ph)2}] [12], and [Pt(η3-C29H20F6S2O–S1,S2,O1)(PPh3)] (at 100 K) [13], and their structural parameters are gathered in Table 3. The structure of [Pt(η3-C12H10N4–N1,N2,N3)(PPh3)] [10] is shown in Figure 3 as an example. Each η3-ligand creates five and six metallocyclic rings. The donor atoms of the respective η3-ligands play a role in the size of the L-Pt-L chelate angles. These angles increase in the following sequences:

Table 3.
Structural data for Pt(η3-X3)(Y) derivatives. a—5+6-membered metallocyclic rings.
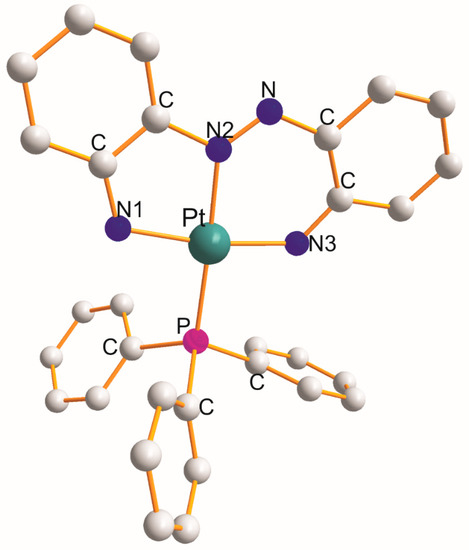
Figure 3.
Structure of [Pt(η3-C12H10N4–N1,N2,N3)(PPh3)] [10].
N1C2N2NC2N3—81.7° (N1-Pt-N2) and 89.6° (N2-Pt-N3);
O1C2N1C3O2—2.4° (O1-Pt-N1) and 94.8° (N1-Pt-O2);
Se1C2N1NC2Se2—83.3° (Se1-Pt-N1) and 98.3° (N1-Pt-Se2);
S1C2S2C3O1—90.2° (S1-Pt-S2) and 99.2° (S2-Pt-O1).
The monodentate PL displayed distorted square-planar geometry about Pt(II) atoms. The Pt-L bond distance to PL increased in the following order: 2.025 Å (Pt-N2) < 2.064 Å (Pt-N1) < 2.078 Å (Pt-N1) < 2.277 Å (Pt-S2). The order follows the above-mentioned sentence for the Pt-L (L is a common central ligating atom between five and six-rings).
2.4. 5+5-Membered Metallocyclic Rings
There are thirty-nine compounds in which each η3-ligand creates two five-membered metallocyclic rings. These complexes based on variable combinations of atoms involved in the chelate angles can be divided into twelve groups. Structural data are given in Table 4.

Table 4.
Structural data for Pt(η3-X3)(Y) derivatives. a—5+5-membered metallocyclic rings.
The structure of [Pt(η3-C33H24P2S2–S1,C1,S2)(PPh3)].CH2Cl2 [14] is shown in Figure 4. The η3-ligand creates two five-membered metallocyclic rings with a common C1 atom of the S1PCC1CPS2 type with chelate angles of 87.9° (S1-Pt-C1) and 87.7° (C1-Pt-S2). This is the only example of this type. The PPh3 demonstrated distorted square-planar geometry about Pt(II) atoms. The remaining L-Pt-L bond angles open in the following order: 89.7° (S1-Pt-P) < 94.2° (S2-Pt-P) < 173.8° (S1-Pt-S2) < 176.9° (C1-Pt-P). The Pt-L bond distance increases in the following order: 2.020 Å (Pt-C1) < 2.316 Å (Pt-S2) < 2.332 Å (Pt-S1) < 2.322 Å (Pt-P).
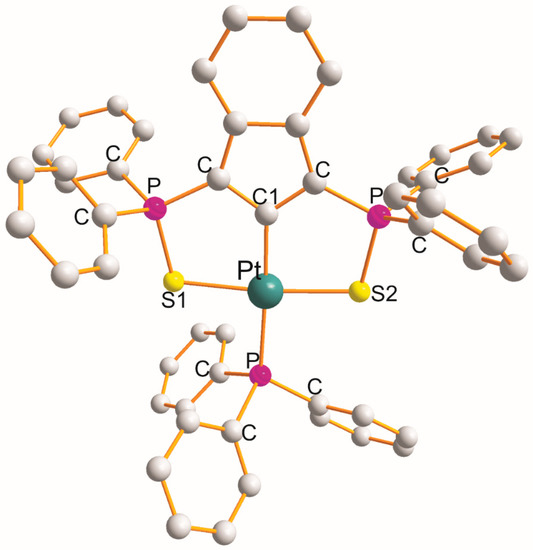
Figure 4.
Structure of [Pt(η3-C33H24P2S2–S1,C1,S2)(PPh3)] [14].
In another two complexes, namely [Pt(η3-C12H12N2Te3–Te1,Te2,Te3)(PPh3)].C6H6 and [Pt(η3-C10H8N2Te3–Te1,Te2,Te3)(PPh3)] [15], which are isostructural, the η3-ligand creates a pair of five-membered metallocyclic rings with common central ligating Te2 atoms of the Te1CNTe2NCTe3 type. The mean values of the respective angles are 92.2 (±6)° (Te1-Pt-Te2) and 92.0 (±6)° (Te2-Pt-Te3). The PPh3 ligand demonstrated distorted square-planar geometry about each Pt(II) atom. The remaining L-Pt-L bond angles open in the following order (mean values): 88.1 (±1.2)° (Te3-Pt-P) ~ 88.1 (±2.31)° (Te1-Pt-P) < 173.3 (±8)° (Te1-Pt-Te3) < 173.4 (±2.1)° (Te2-Pt-P). The Pt-L bond distance increases in the following order (mean values): 2.283 (±1) Å (Pt-P) < 2.571 (±2) Å (Pt-Te2) < 2.591 (±3) Å (Pt-Te1) < 2.592 (±20) Å (Pt-Te3).
In another two complexes, namely Pt(η3-C12H9N2S2B–S1,B1,S2)(PPh3)].0.06CH2Cl2 [16] and Pt(η3-C13H14N5S3B–S1,B1,S2)(PPh3)] [17], each η3-ligand creates a pair of five-membered metallocyclic rings with a common central ligating B1 atom of the S1CNB1NCS2 type. The values of the respective chelate angles are (mean values): 80.4 (±6)° (S1-Pt-B1) and 85.7 (±8)° (B1-Pt-S2). The PPh3 demonstrated distorted square-planar geometry about the Pt(II) atom. The remaining L-Pt-L bond angles open in the following order (mean values): 95.9 (±5)° (S2-Pt-P) < 99.3 (±5)° (S1-Pt-P) < 162.4 (±1.0)° (S1-Pt-S2) < 174.4 (±2.2)° (B1-Pt-P). The Pt-L bond distance increases in the following order (mean values): 2.110 (±19) Å (Pt-B1) < 2.284 (±10) Å (Pt-S2) < 2.301 (±3) Å (Pt-S1) < 2.382 (±2) Å (Pt-P).
Distorted square-planar geometry about the Pt(II) atoms in [Pt(η3-C28H24N4Se2–N1,N2,Se1)(PPh3)].0.5H2O [18] was achieved by η3-ligands with PPh3. The η3-ligand forms a pair of five-membered metallocyclic rings with a common central ligating N2 atom of the N1CNN2NCSe1 type. The values of the respective chelate angles are 78.7° (N1-Pt-N2) and 82.3° (N2-Pt-Se1). The remaining L-Pt-L bond angles increase in the following sequence: 98.6° (Se1-Pt-P) < 100.0° (N1-Pt-P) < 162.0° (N1-Pt-S1) < 173.4° (N2-Pt-P). The Pt-L bond distance increases in the order 1.980 Å (Pt-N2) < 2.020 Å (Pt-N1) < 2.272 Å (Pt-P) < 2.368 Å (Pt-Se1).
In [Pt(η3-C28H24N4S2–N1,N2,S1)(PPh3)].0.5thf [19], the η3-ligand creates two five-membered metallocyclic rings with common central ligating N2 atoms of the N1CNN2NCS1 type with the chelate angles of 78.5° (N1-Pt-N2) and 81.6° (N2-Pt-S1). The PPh3 demonstrated an inner coordination sphere about the Pt(II) atoms. The remaining L-Pt-L bond angles open in the following order: 99.7° (S1-Pt-P) < 99.9° (N1-Pt-P) < 162.2° (N1-Pt-S1) < 172.5° (N2-Pt-P). The Pt-L bond distance increases in the following sequence: 1.987 Å (Pt-N2) < 2.031 Å (Pt-N1) < 2.266 Å (Pt-S1) < 2.279 Å (Pt-P).
Distorted square-planar geometry about the Pt(II) atoms in [Pt(η3-C17H12N2O–O1,N1,C1)(PPh3)] [20] was achieved by η3-ligands and PPh3. The η3-ligand creates two five-membered metallocycles with a common central ligating N1 atom of the O1C2N1NCC1 type with the chelate angles of 80.9° (C1-Pt-N1) and 79.0° (N1-Pt-C1). The remaining L-Pt-L bond angles open in the following order: 99.8° (O1-Pt-P) < 100.1° (C1-Pt-P) < 159.9° (O1-Pt-C1) < 178.9° (N1-Pt-P). The Pt-L bond distance increases in the following sequence: 1.997 Å (Pt-N1) < 2.004 Å (Pt-C1) < 2.109 Å (Pt-O1) < 2.254 Å (Pt-P).
There are four complexes, namely [Pt(η3-C9H9N3S–C1,N2,S1)(PPh3)] [21], [Pt(η3-C10H11N3S–C1,N1,S1)(PPh3)] [22], [Pt(η3-C12H19Cl2N3O2S3–C1,N1,S1)(PPh3)].2Me2SO [23], and [Pt(η3-C9H7Cl2N3S–C1,N1,S1){(η3-C6H12N3)}] [23], in which each η3-C1N1S1 donor ligand creates two five-membered metallocycles with common central ligating N1 atoms of the C1C2N1NCS1 type. The mean values of the chelate angles are 80.1 (±2.2)° (C1-Pt-N1) and 82.2 (±1.0)° (N1-Pt-S1). The remaining L-Pt-L bond angles open in the following order (mean values): 97.2 (±1.1)° (C1-Pt-P) < 98.8 (±2.2)° (S1-Pt-P) < 162.8 (±1.4)° (C1-Pt-S1) < 174.5 (±5.9)° (N1-Pt-P). The Pt-L bond distance increases in the following order (mean values): 2.032 (±14) Å (Pt-C1) < 2.033 (±4) Å (Pt-N1) < 2.229 (±6) Å (Pt-P) < 2.332 (±8) Å (Pt-S1).
In [Pt(η3-C20H31N4S2–N1,N2,S1)(PPh3)].EtOH [24], the η3-ligand forms two five-membered metallocycles with common central ligating N2 atoms of the N1C2N2NCS1 type, with the chelate angles of 79.0° (N1-Pt-N2) and 82.0° (N2-Pt-S1). The remaining L-Pt-L angles open in the following order: 96.0° (S1-Pt-P) < 100.0° (N1-Pt-P) < 161.0° (N1-Pt-S1) < 173.0° (N2-Pt-P). The Pt-L bond distance increases in the following order: 2.000 Å (Pt-N2) < 2.042 Å (Pt-N1) < 2.268 Å (Pt-P) < 2.270 Å (Pt-S1).
Distorted square-planar geometry in [Pt(η3-C18H12N2O–O1,N1,C1)(PPh3)].MeCN [25] was achieved by η3-ligands with PPh3. The η3-ligand forms two five-membered metallocycles with a common central ligating N1 atom of the O1CNN1C2C1 type with the chelate angles of 76.0° (O1-Pt-N1) and 81.8° (N1-Pt-S1). The remaining angles open in the following sequence: 99.1° (C1-Pt-P) < 103.2° (O1-Pt-P) < 157.7° (O1-Pt-C1) < 176.4° (N1-Pt-P). The Pt-L bond distance increases in the following order: 1.995 Å (Pt-N1) < 2.025 Å (Pt-C1) < 2.138 Å (Pt-O1) < 2.244 Å (Pt-P).
The structure of [Pt(η3-C12H21N2–N1,C1,N2){P(C6H4SO3)3}]2-[Pt(η3-C12H21N6)(H2O)]2 consists of complex anions and complex cations [26]. In a complex anion, a η3-ligand via N1,C1,N2 atoms with PL ligands achieves distorted square-planar geometry about Pt(II) atoms. This η3-ligand creates a pair of five-membered metallocycles with a common central ligating C1 atom of the N1C2C1C2N2 type. The values of the chelate angles are 80.5° (N1-Pt-C1) and 79.9° (C1-Pt-N2). The remaining L-Pt-L bond angles open in the following sequence: 97.6° (N2-Pt-P) < 102.6° (N1-Pt-P) < 159.8° (N1-Pt-N2) < 176.8° (C1-Pt-P). The Pt-L bond distance increases in the following order: 1.971 Å (Pt-C1) < 2.130 Å (Pt-N1) < 2.131 Å (Pt-N2) < 2.363 Å (Pt-P).
In the following eleven complexes, each η3-ligand creates a pair of five-membered metallocycles with a common central ligating N2 atom of the N1C2N2C2N3 type: [Pt(η3-C30H35N5–N1,N2,N3)(PPh3)] (Sanning et al. 2015) [27], [Pt(η3-C11H3F6N7–N1,N2,N3){P(Me)(Ph2)}] [28], [Pt(η3-C15H11N3–N1,N2,N3){P(η1-C14H19O5)Ph2}].2SO3CF3.2MeCO [29], [Pt(η3-C25H19N5–N1,N2,N3)(PPh3)].3CH2Cl2 [30], [Pt(η3-C29H33N7–N1,N2,N3)(PPh3)].CH2Cl2 [30], [Pt(η3-C15H11N3–N1,N2,N3)(PPh3)].2SO3CF3 [31], [Pt(η3-C22H15N7O–N1,N2,N3)(PPh3)] [27], [Pt(η3-C11H3F6N7–N1,N2,N3)(PPh3)] [27], [Pt(η3-C12H6F6N7O–N1,N2,N3)(PPh3)] [27], [Pt(η3-C17H21N7–N1,N2,N3)(PPh3)] [27], and [Pt(η3-C18H23N7O–N1,N2,N3)(PPh3)] [27]. The monodentate PL displayed distorted square-planar geometry about each Pt(II) atom. The total mean values of the chelate angles are 78.9 (±1.0)° (N1-Pt-N2) and 79.0 (±1.0)° (N2-Pt-N3). The remaining L-Pt-L bond angles open in the following order (total mean values): 99.5 (±3.0)° (N1-Pt-P) < 102.9 (±2.8)° (N3-Pt-P) < 157.6 (±1.1)° (N1-Pt-N3) < 175.7 (±1.5)° (N2-Pt-P). The Pt-L bond distance increases in the following order (total mean values): 2.012 (±20) Å (Pt-N1) < 2.012 (±32) Å (Pt-N2) < 2.025 (±32) Å (Pt-N3) < 2.264 (±24) Å (Pt-P).
There are five complexes, namely [Pt(η3-C14H10N3–N1,N2,C1)(PPh3)].ClO4 (at 173 K) [32], [Pt(η3-C14H10N3–N1,N2,C1)(PPh3)].ClO4 (at 193K) [32], [Pt(η3-C14H10N3–N1,N2,C1)(PPh3)].ClO4 (at 245 K) [33], [Pt(η3-C24H20N3–N1,N2,C1)(PPh3)].ClO4 [34], and [Pt(η3-C28H23N2–N1,N2,C1)(Pcy3)].ClO4.MeCN [35], in which each η3-ligand creates a pair of five-membered metallocycles with a common central ligating N2 atom of the N1C2N2C2C1 type. The structure f [Pt(η3-C14H10N3–N1,N2,C1)(PPh3)] [32] is shown in Figure 5. The total mean values of the respective chelate angles are 78.4 (±0.6)° (N1-Pt-N2) and 80.7 (±1.0)° (N2-Pt-C1). The remaining L-Pt-L bond angles open in the following order (total mean values): 99.0 (±3.5)° (C1-Pt-P) < 103.1 (±2.5)° (N1-Pt-P) < 158.0 (±1.4)° (N1-Pt-N2) < 172.7 (±5.5)° (N2-Pt-P). The Pt-L bond distance increases in the following order (total mean values): 2.021 (±30) Å (Pt-C1) < 2.030 (±24) Å (Pt-N2) < 2.121 (±18) Å (Pt-N1) < 2.242 (±32) Å (Pt-P).
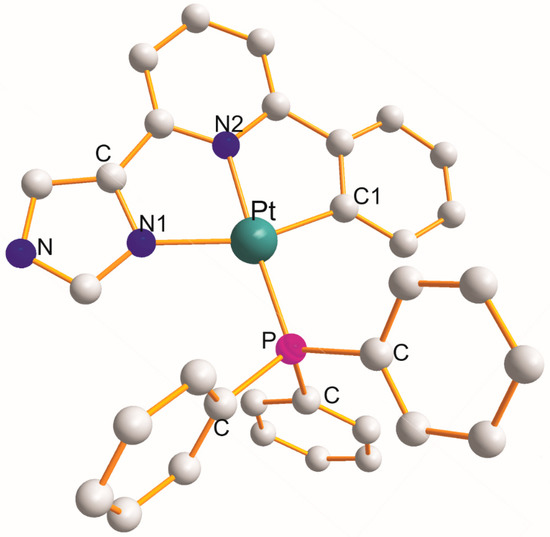
Figure 5.
Structure of [Pt(η3-C14H10N3–N1,N2,C1)(PPh3)] [32].
In the following eight complexes, each η3-ligand creates a pair of five-membered metallocycles with a common central ligating N1 atom of the C1C2N1C2C2 type: [Pt(η3-C17H11N–C1,N1,C2){P(η1-C14H19O5)(Ph)2}].CH2Cl2 [29], [Pt(η3-C17H9F2N–C1,N1,C2){P(η1-o-tolyl)3}].CHCl3 (at 150 K) [36], [Pt(η3-C17H9F2N–C1,N1,C2){P(CH2Ph)3}] (at 150 K) [36], [Pt(η3-C18H11F2N–C1,N1,C2){P(CH2Et)2}] (at 150K) [37], [Pt(η3-C20H15NO2–C1,N1,C2)(PPh3)] (at 150 K) [38], [Pt(η3-C18H11F2N–C1,N1,C2){P(CH2Ph)3}].CHCl3 (at 150 K) [39], [Pt(η3-C12H11F2N–C1,N1,C2)(PMe3)] (at 150 K) [40], and [Pt(η3-C17H11N–C1,N1,C2)(PPh3)] (Figure 6) (at 100 K) [41]. The values of the respective chelate angles are 79.7 (±3)° (C1-Pt-N1) and 80.1 (±3)° (N1-Pt-C2). The remaining L-Pt-L bond angles open in the following order: 99.5 (±4.2)° (C2-Pt-P) < 100.0 (±4.5)° (C1-Pt-P) < 158.5 (±1.4)° (C1-Pt-C2) < 171.5 (±4.5)° (N1-Pt-P). The Pt-L bond distance increases in the following order (total mean values): 2.026 (±21) Å (Pt-N1) < 2.068 (±17) Å (Pt-C1) < 2.078 (±6) Å (Pt-C2) < 2.249 (±32) Å (Pt-P).
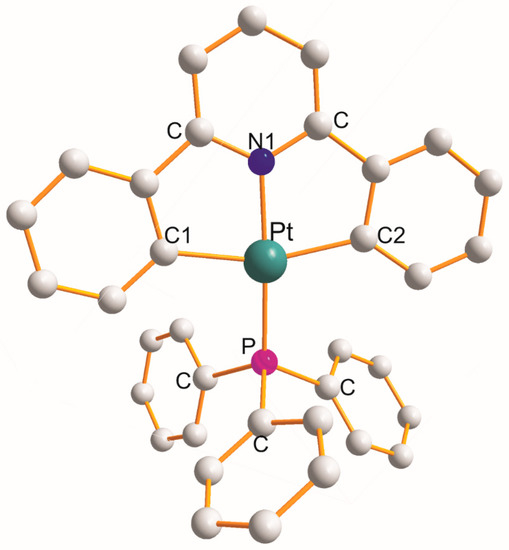
Figure 6.
Structure of [Pt(η3-C17H11N–C1,N1,C2)(PPh3)] [41].
Each η3-ligand creates two five-membered metallocyclic rings. The donor atoms of the respective η3-ligands play a role in the size of the L-Pt-L chelate angles. These angles increase in the following sequences:
N1C2N2C2N3: 78.8° (N1C2N2), 78.9°(N2C2N3) [12,26,27,28,30,31]
O1CNN1C2C1: 76.0° (O1CNN1), 81.8° (N1C2C1) [25]
N1C2N2C2C1: 78.4° (N1C2N2), 80.2° (N2C2C1) [32,33,34,35]
C1C2N1C2C2: 79.7° (C1C2N1), 80.2° (N1C2C2) [29,36,37,38,39,40,41]
N1CNN2NCS1: 78.5° (N1CNN2), 81.6° (N2NCS1) [19]
N1C2C1C2N2: 80.5° (N1C2C1), 79.9° (C1C2N2) [18]
N1CNN2NCSe1: 78.7° (N1CNN2), 82.3° (N2NCSe1) [26]
O1CNN1NCC1: 80.9° (O1CNN1), 79.0° (N1NCC1) [20]
N1C2N2NCS1: 79.0° (N1C2N2), 82.0°(N2NCS1) [24]
C1C2N1NCS1: 77.9° (C1C2N1), 83.2°(N1NCS1) [21,22,23]
S1CNB1NCS2: 79.8° (S1CNB1), 85.0° (B1NCS2) [16,17]
S1PCC1CPS2: 87.9° (S1PCC1), 87.7° (C1CPS2) [14]
Te1CNTe2NCTe3: 91.6° (Te1CNTe2), 91.4° (Te2NCTe3) [15]
3. Conclusions
This review includes over fifty monomeric Pt(II) coordination complexes with the composition of Pt(η3-X3L)(PL). There are 14 types of η3-ligand coordination via donor atoms (X3 = N1N2N3; S1S2S3; Te1Te2Te3; O1N1O2; C1N1C2; Se1N1Se2; O1N1C1; O1N1S1; C1N1S1; N1N2C1, N1N2S1; N1N2Se1; S1O1S2; S1B1S2; N1O1N2; S1S2O1) and each η3-ligand with monodentate PL displays distorted square-planar geometry about each Pt(II) atom.
Each tridentate ligand creates two metallocyclic rings. Based on the metallocycles, these complexes can be divided into the following four groups with 6+6-; 6+5-; 5+6-; and 5+5-membered metallocyclic rings:
- 6+6-membered metallocycles of the O1C3N1C3O2; O1C2NN1C3O2 and S1C3S2C3S3 types with common N1 and S2 atoms (three examples) (Table 1);
- 6+5-membered metallocycles of the O1C3N1NCS1 type with common central ligating N1 atoms (five examples) (Table 2);
- 5+6-membered metallocycles of the O1C2N1C3O2; N1C2N2NC2N3; Se1C2N1NC2Se2 and S1C2S2C3O1 types with common central N1, ligating N2 and S2 atoms (four examples) (Table 3);
- 5+5-membered metallocycles of the S1PCC1CPS2; Te1CNTe2NCTe3 (2 examples); S1CNB1NCS2 (2 examples); N1CNN2NCSe1; N1CNN2NCS1; O1C2N1NCC1; C1C2N1NCS1 (4 examples); N1C2N2NCS1; O1CNN1C2C1; N1C2C1C2N2; N1C2N2C2N3 (11 examples); N1C2N2C2C1 (5 examples); and C1C2N1C2C2 (8 examples) types with common central ligating C1, Te2, B1, N2 and N1 atoms (39 examples) (Table 4).
The Pt-P (trans to the common central atom) bond distance increases in the following sequence (total mean values): 2.237 Å (N1) < 2.259 Å (N2) < 2.283 Å (Te2) < 2.293 Å (S2) < 2.348 Å (C1) < 2.356 Å (B1), which corresponds quite well with the trans influence of the respective central common donor atoms. The Pt-X (trans to P) bond distance increases in the following sequence (total mean values): 1.995 Å (C1) < 2.014 Å (N2) < 2.030 Å (N1) < 2.109 Å (B1) < 2.308 Å (S2) < 2.574 Å (Te2).
There is a cooperative effect between the covalent radius of the respective donor atoms, the Pt-L bond distance, and chelate rings, as the former grows, the Pt-L bond distance increases and the chelate angles open, as can be observed below (Table 5).

Table 5.
Cooperative effects of the respective data.
In transition metal complexes, the oxidation state plays a leading role in the geometry formed and platinum is no exception. In four coordinates, Pt(II) prefers square-planar geometry. The utility of a simple metric to assess molecule shape and degree of distortion, as well as to exemplify the τ4 parameter for square-planar geometry, is demonstrated by the following equations [42]:
For square planar geometry
For tetrahedral geometry
The values of the τ4 parameter range from 0.00 for perfect square planar geometry to 1.00 for perfect tetrahedral geometry, since 360 − 2(109.5) = 141.
The total mean values of trans-α-L-Pt-L (L represents terminal ligating atoms of the respective η3-ligand) trans-β-L-Pt-P (L is a common central atom) bond angles, as well as of parameter τ4, reflect the membered nature of the respective metallocycles, as can be observed from the data in the following summary (Table 6).

Table 6.
Selected data of trans angle and parameter τ4 of metallocycles.
As can be observed, while the β-L-Pt-P angles are almost constant, the α-L-Pt-L angles are slowly increase with the membered metallocycles in the following sequence: 5+5- < 5+6- < 6+5- < 6+6-. The distortion of the square-planar geometry about Pt(II) atoms decreases in the same sequence, as indicated by parameter τ4 (0.062 (5+5) > 0.040 (5+6) > 0.024 (6+5) > 0.023 (6+6)).
The coexistence of two or more species that differ only by the degree of distortion of the M-L bond distance and L-M-L bond angles is typical of the general class of distortion isomerism [43]. Over 160 platinum complexes exist following the analysis and classification of isomers [44]. This includes distortion (65%), cis-trans (30%), mixed isomers (cis-trans and distortion), and ligand isomerism.
The complex [Pt(η3-C15H11N3–N1,N2,N3)(PPh3)].2SO3CF3 [31] contains two crystallographically independent molecules within the same crystals (Table 4). These molecules differ by the degree of distortion of Pt-L and L-Pt-L, with the values of parameter τ4 of 0.070 and 0.082, respectively. Below is a classic example of distortion isomers [43].
As part of the X-ray analysis, [Pt(η3-C14H10N3–N1,N2,C1)(PPh3)].ClO4 was measured at 173 K and 193 K [32], and 295 K [33] (Table 4). It was found that the temperature had an influence on the structural parameters. When the temperature drops, the distortion grows, as indicated by parameter τ4 in the following order: 0.065 (at 173 K) < 0.068 (at 193 K) < 0.079 (at 295 K). These molecules, as well as independent molecules, are classical distortion isomers [43].
During the collection and organization of the data, it has become evident that some original papers are lacking important information such as atom coordinates and the analysis of intermolecular distances. Because of these limitations, we believe that a review such as this can continue to serve a useful function by centralizing the available material and delineating areas worthy of further investigation.
Author Contributions
Conceptualization, M.M. and P.M.; methodology M.M and P.M.; writing–original draft preparation, M.M. and P.M.; data curation, M.M.; writing–review and editing, V.M.; supervision, M.M. and P.M.; funding acquisition, P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the projects VEGA 1/0514/22, KEGA 027UK-4/2020, and APVV-15-0585.
Data Availability Statement
Data supporting reported results can be found at author M.M.
Acknowledgments
This work was supported by Faculty of Pharmacy, Comenius University Bratislava.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| C8H8N3OS | (salicylaldehyde thiosemicarbazone) |
| C9H9N3S | (2-(((amino(sulfido)methylene)hydrazono)methyl)-5-methylphenyl) |
| C10H8N2Te3 | (1,1′-tellanyl)bis(5-pyridine-2-tellurolate) |
| C10H11N3S | (4-methylacetophenone thisemicarbazone) |
| C11H3F6N7 | (2,6-bis(3-(trifluoromethyl)-1H-1,2,4-triazol-5-yl)pyridinate) |
| C11H11N | (2,2′-(pyridine-2,6-diyl)diphenyl) |
| C12H24S3 | (2,12-Diisopropyl-3,7,11-trithiadecane) |
| C12H6F6N7O | (4-methoxy-2,6-bis(3-(trifluoromethyl)-1H-1,2,4-triazol-5-yl)) pyridinate) |
| C12H10N2Te3 | (1,1′-tellanyl)bis(3-methyl-1λ5-pyridine-2-tellurolate) |
| C12H11N4 | (2-(2-amino)phenyl diazenyl)anilinate) |
| C12H16N2O4Se2 | diethyl-3,3′-(diazone-1,2-diyl-N)bis(2-(hydroseleno)but-2-enoatato) |
| C12H19Cl2N3O2S3 | (2-(1-((amino(sulfido)methylene)hydrazono)ethyl)-4,5-dichlorophenyl) |
| C13H9NO2 | N(α,α′-dioxobenzylidene)anilinate |
| C14H10N2O3 | (2-1(2′-carboxylatophenylazo)-4-methylphenolate) |
| C14H10N3 | (2-(6-(3-1H-pyrazolyl)-2-pyridyl)phenyl) |
| C15H11N3 | (2,2′.6′2″-terpyridine) |
| C16H13N3O3S2 | (4-nitrobenzyl(1-(2-oxidophenyl)ethylidene)carbonodithiohyrazonate) |
| C16H14N2OS2 | (benzyl(1-(2-oxidophenyl)ethylidene)carbodithiohydrazonate) |
| C17H9F2N | (2,6-(4-fluorophenyl)pyridine) |
| C17H11N | (2,2′-(pyridine-2,6-diyl) diphenyl) |
| C17H12N2O | (1-((5-methyl-2-oxodiphenyl)diazinyl)-2-naphtyl) |
| C17H21N7 | (2,6-bis(3-t-butyl-1H-1,2,4-triazol-5-yl))pyridinate) |
| C18H11F2N | (5-fluoro-2-{6-(4-fluorobenzene-2-diyl}-3-methylbenzenide) |
| C18H12N2O | (1-(((oxidanidyl)(phenyl)methylene)amino)imino)methyl-2-2-naphtyl) |
| C18H16N2OS2 | (benzyl(4-oxido-4-phenylbut-3en-2-ylidene)carbonodithiohydrazonate) |
| C18H23N7O | (2,6-bis(3-t-butyl-1H-1,2,4-triazol-5-yl)-4-methoxypyridinate) |
| C20H15NO2 | (2,6-bis(o-phenylene)-4-ethoxycarbonylpyridine) |
| C22H15N7O | (4-methoxy-2,6-bis(3-phenyl-1H-1,2,4-triazol-5-yl)pyridinate) |
| C24H20N3 | (2-(4-(4-dimethylaminophenyl)-2,2′-bipyridin-6-yl)phenyl) |
| C25H19N5 | (2,6-bis(3-(4-methyl)-1H-pyrazol-5yl)pyridinate) |
| C28H23N2 | (3-(4-t-butyl-8-(isoquinolin-3-yl)pyridine-2-yl)-2-naphtyl) |
| C28H24N4S2 | PhSNC(MeC6H4)N-NC(MeC6H4)NSPh |
| C28H24N4Se2 | PhSeNC(MeC6H4)N-NC(MeC6H4)NSePh |
| C29H33N7 | (2,6-bis(3-(adamantam-1-yl)-1H-1,2,4-triazol-5-yl)pyridinate) |
| C30H35N5 | (2,6-bis(3-(adamantam-1-yl)-1H-pyrazol-5-yl) pyridinate) |
| P(C14H19O5)(Ph)2 | (benzo-15-crown [5] diphenylphosphine |
| P(CH2Et)3 | tri-n-propylphosphine |
| P(CH2Ph)3 | tribenzylphsphine |
| P(CH3)Ph2 | methyldiphenylphenylphosphine |
| PMe3 | trimethylphosphine |
| P(tolyl)3 | tris(2-methylphenyl)phosphine |
| Pcy3 | tricyclohexylphosphine |
| PPh3 | triphenylphosphine |
| tfh | tetrahydrofuran |
References
- Holloway, C.E.; McInik, M. Structural Aspects of Platinum Coordination Compounds: Part III. Monomeric square-planar (PtA2XY and PtABXY) and trigonal bipyramidal Pt(II) coordination compounds. Rev. Inorg. Chem. 2004, 24, 135–299. [Google Scholar] [CrossRef]
- Melník, M.; Mikuš, P. Organophosphines in organoplatinum complexes: Structural aspects of PtP3C derivatives. J. Organomet. Chem. 2017, 830, 62–66. [Google Scholar] [CrossRef]
- Melník, M.; Mikuš, P. Organomonophosphines in Pt(II) Coordination Complexes. Part I. Monomeric Square Planar (PtP4, PtP3X and PtP2X2). Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 1764–1780. [Google Scholar] [CrossRef]
- Dahm, G.; Borré, E.; Fu, C.; Bellemin-Laponnaz, S.; Mauro, M. Tridentate Complexes of Palladium(II) and Platinum(II) Bearingbis-Aryloxide Triazole Ligands: A Joint Experimental and Theoretical Investigation. Chem. Asian J. 2015, 10, 2368–2379. [Google Scholar] [CrossRef]
- Halder, S.; Drew, M.G.B.; Bhattacharya, S. Palladium and platinum complexes of 2-(2′-carboxyphenylazo)-4-methylphenol: Synthesis, structure and spectral properties. J. Chem. Sci. 2008, 120, 441–446. [Google Scholar] [CrossRef]
- Loeb, S.; Mansfield, J.R. Platinum(II) complexes of the tridentate thioether ligands RS(CH2)3S(CH2)3SR (R = Et, iPr, Ph). Structures of [PtCl(iPrS(CH2)3S(CH2) 3SiPr)][BF4], [Ptl(PhS(CH2)3S(CH2)2SPh)][BF4], and [Pt(PPh3)(iPrS (CH2)3. Can. J. Chem. 1996, 74, 1377–1390. [Google Scholar] [CrossRef]
- Maia, P.I.D.S.; Fernandes, A.G.D.A.; Silva, J.J.N.; Andricopulo, A.D.; Lemos, S.S.; Lang, E.S.; Abram, U.; Deflon, V.M. Dithiocarbazate complexes with the [M(PPh3)]2+ (M = Pd or Pt) moiety: Synthesis, characterization and anti-Tripanosoma cruzi activity. J. Inorg. Biochem. 2010, 104, 1276–1282. [Google Scholar] [CrossRef]
- Lobana, T.S.; Bawa, G.; Hundal, G.; Zeller, M. The Influence of Substituents at C2 Carbon Atom of Thiosemicarbazones {R(H)C2=N3-N2(H)-C1(=S)-N1H2} on their Dentacy in PtII/PdII Complexes: Synthesis, Spectroscopy, and Crystal Structures. Z. Anorg. Allg. Chem. 2008, 634, 931–937. [Google Scholar] [CrossRef]
- Halder, S.; Butcher, R.J.; Bhattacharya, S. Synthesis, structure and spectroscopic properties of some thiosemicarbazone complexes of platinum. Polyhedron 2007, 26, 2741–2748. [Google Scholar] [CrossRef]
- Mandal, P.; Lin, C.-H.; Brandão, P.; Mal, D.; Felix, V.; Pratihar, J.L. Synthesis, characterization, structure and catalytic activity of (NNN) tridentate azo-imine nickel(II), palladium(II) and platinum(II) complexes. Polyhedron 2016, 106, 171–177. [Google Scholar] [CrossRef]
- Motschi, H.; Nussbaumer, C.; Pregosin, P.S.; Bachechi, F.; Mura, P.; Zambonelli, L. Thetrans-Influence in Platinum (II) Complexes.1H- and13C-NMR. and X-ray structural studies of tridentateSchiff’s base complexes of platinum (II). Helv. Chim. Acta 1980, 63, 2071–2086. [Google Scholar] [CrossRef]
- Arsenyan, P.; Oberte, K.; Rubina, K.; Belyakov, S. Synthesis and application of a new selenoplatinum catalyst. Tetrahedron Lett. 2005, 46, 1001–1003. [Google Scholar] [CrossRef]
- Kano, N.; Kusaka, S.; Kawashima, T. Insertion of platinum and palladium into a sulfur(IV)–sulfur(II) bond of a sulfur-substituted sulfurane. Dalton Trans. 2010, 39, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Monot, J.; Merceron-Saffon, N.; Martin-Vaca, B.; Bourissou, D. SCS indenediide pincer complexes: Zr to Pd and Pt transmetallation. J. Organomet. Chem. 2017, 829, 37–41. [Google Scholar] [CrossRef]
- Chauhan, R.S.; Kedarnath, G.; Wadawale, A.; Muñoz-Castro, A.; Arratia-Perez, R.; Jain, V.K.; Kaim, W. Tellurium(0) as a Ligand: Synthesis and Characterization of 2-Pyridyltellurolates of Platinum(II) and Structures of [Pt{2-Te-3-(R)C5H3N}2Te(PR′3)] (R = H or Me). Inorg. Chem. 2010, 49, 4179–4185. [Google Scholar] [CrossRef]
- Zech, A.; Haddow, M.F.; Othman, H.; Owen, G.R. Utilizing the 8-Methoxycyclooct-4-en-1-ide Unit As a Hydrogen Atom Acceptor en Route to “Metal–Borane Pincers”. Organometallics 2012, 31, 6753–6760. [Google Scholar] [CrossRef]
- Owen, G.R.; Gould, P.H.; Hamilton, A.; Tsoureas, N. Unexpected pincer-type coordination (κ3-SBS) within a zerovalent platinum metallaboratrane complex. Dalton Trans. 2010, 39, 49–52. [Google Scholar] [CrossRef]
- Chivers, T.; McGregor, K.; Parvez, M. Preparation and X-ray Structures of Platinum and Palladium Complexes of the Chalcogen-Substituted Diazenes trans-[PhEN(4-CH3C6H4)CN=NC(4-CH3C6H4)NEPh] (E = S, Se). Inorg. Chem. 1994, 33, 2364–2369. [Google Scholar] [CrossRef]
- Chivers, T.; McGregor, K.; Parvez, M. Preparation and X-ray structures of tridentate (N,N,S) complexes of the diazene trarts-[PhSNC(4-MeC6H4)NNC(4-MeC6H4)NSPh] with platinum and palladium. J. Chem. Soc. Chem. Commun. 1993, 12, 1021–1023. [Google Scholar] [CrossRef]
- Biswas, A.N.; Das, P.; Bagchi, V.; Choudhury, A.; Bandyopadhyay, P. Regiospecific C(naphthyl)–H Bond Activation by Platinum(II)—Isolation, Characterization, Reactivity and TD-DFT Study of the Cycloplatinate Complexes. Eur. J. Inorg. Chem. 2011, 2011, 3739–3748. [Google Scholar] [CrossRef]
- Halder, S.; Paul, P.; Peng, S.-M.; Lee, G.-H.; Mukherjee, A.; Dutta, S.; Sanyal, U.; Bhattacharya, S. Benzaldehyde thiosemicarbazone complexes of platinum: Syntheses, structures and cytotoxic properties. Polyhedron 2012, 45, 177–184. [Google Scholar] [CrossRef]
- Vázquez-García, D.; Fernández, A.; Fernández, J.J.; López-Torres, M.; Suárez, A.; Ortigueira, J.M.; Vila, J.M.; Adams, H. New cyclometallated platinum(II) compounds with thiosemicarbazones: Crystal and molecular structure of [Pt{4-MeC6H3C(Me)=NN=C(S)NH2}(PPh3)]. J. Organomet. Chem. 2000, 595, 199–207. [Google Scholar] [CrossRef]
- Chellan, P.; Land, K.M.; Shokar, A.; Au, A.; An, S.H.; Clavel, C.M.; Dyson, P.J.; de Kock, C.; Smith, P.J.; Chibale, K.; et al. Exploring the Versatility of Cycloplatinated Thiosemicarbazones as Antitumor and Antiparasitic Agents. Organometallics 2012, 31, 5791–5799. [Google Scholar] [CrossRef]
- Matesanz, A.I.; Perles, J.; Souza, P. New palladium and platinum complexes with bioactive 3,5-diacetyl-1,2,4-triazol bis(4-cyclohexyl thiosemicarbazone) ligand: Chemistry, antiproliferative activity and preliminary toxicity studies. Dalton Trans. 2012, 41, 12538–12547. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.B.; Pal, S. Mono- and dinuclear platinum(II) complexes via single and double cycloplatinations of Nʹ-(arylidene)benzohydrazides. J. Organomet. Chem. 2015, 797, 96–100. [Google Scholar] [CrossRef]
- Wieczorek, B.; Snelders, D.J.M.; Dijkstra, H.P.; Versluis, K.; Lutz, M.; Spek, A.L.; Egmond, M.R.; Gebbink, R.J.M.K.; van Koten, G. Coordination Chemistry in Water of a Free and a Lipase-Embedded Cationic NCN-Pincer Platinum Center with Neutral and Ionic Triarylphosphines. Organometallics 2012, 31, 2810–2820. [Google Scholar] [CrossRef]
- Sanning, D.-C.J.; Ewen, M.S.P.R.; Stegemann, M.S.L.; Schmidt, J.; Daniliuc, C.G.; Koch, B.S.T.; Doltsinis, N.L.; Wegner, D.; Strassert, C.A. Scanning-Tunneling-Spectroscopy-Directed Design of Tailored Deep-Blue Emitters. Angew. Chem. Int. Ed. 2015, 54, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, L.; Sanning, J.; Daniliuc, C.G.; Strassert, C.A. Influence of the monodentate ancillary ligand on the photophysical properties of Pt(II) complexes bearing a symmetric dianionic tridentate luminophore. Z. Naturforscung B Chem. Sci. 2016, 71, 1087–1093. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Tang, R.P.-L.; Wong, K.M.-C.; Lu, X.-X.; Cheung, K.-K.; Zhu, N. Synthesis, electronic absorption, emission, and ion-binding studies of platinum(II) C empty set N empty set C and terpyridyl complexes containing crown ether pendants. Chem. Eur. J. 2002, 8, 4066–4076. [Google Scholar] [CrossRef] [PubMed]
- Galstyan, A.; Naziruddin, A.R.; Cebrián, C.; Iordache, A.; Daniliuc, C.G.; De Cola, L.; Strassert, C.A. Correlating the Structural and Photo physical Features of Pincer Luminophores and Monodentate Ancillary Ligands in Pt II Phosphors. Eur. J. Inorg. Chem. 2015, 2015, 5822–5831. [Google Scholar] [CrossRef]
- Pappenfus, T.M.; Burney, J.R.; McGee, K.A.; Lee, G.G.; Jarvis, L.R.; Ekerholm, D.P.; Farah, M.; Smith, L.I.; Hinkle, L.M.; Mann, K.R. Alternative syntheses and reactivity of platinum(II) terpyridyl acetonitrile complexes. Inorg. Chim. Acta 2010, 363, 3214–3221. [Google Scholar] [CrossRef]
- Ho, Y.-M.; Koo, C.-K.; Wong, K.-L.; Kong, H.-K.; Chan, C.T.-L.; Kwok, W.-M.; Chow, C.-F.; Lam, M.H.-W.; Wong, W.-Y. The synthesis and photophysical studies of cyclometalated Pt(ii) complexes with C,N,N-ligands containing imidazolyl donors. Dalton Trans. 2012, 41, 1792–1800. [Google Scholar] [CrossRef]
- Koo, C.-K.; Ho, Y.-M.; Chow, C.-F.; Lam, M.H.-W.; Lau, T.-C.; Wong, W.-Y. Synthesis and Spectroscopic Studies of Cyclometalated Pt(II) Complexes Containing a Functionalized Cyclometalating Ligand, 2-Phenyl-6-(1H-pyrazol-3-yl)-pyridine. Inorg. Chem. 2007, 46, 3603–3612. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-Q.; Wang, D.-H.; Chi, S.-M.; Gan, X.; Fu, W.-F. Synthesis, structures and spectroscopic properties of platinum(II), copper(I) and zinc(II) complexes bearing 4-(p-dimethylaminophenyl)-6-phenyl-2,2′-bipyridine ligand. Inorg. Chim. Acta 2009, 362, 2529–2536. [Google Scholar] [CrossRef]
- Kui, S.C.F.; Sham, I.H.T.; Cheung, C.C.C.; Ma, C.-W.; Yan, B.; Zhu, N.; Che, C.-M.; Fu, W.-F. Platinum(II) Complexes with π-Conjugated, Naphthyl-Substituted, Cyclometalated Ligands (RC^N^N): Structures and Photo- and Electroluminescence. Chem. A Eur. J. 2007, 13, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.A.; Clarkson, G.J.; Rourke, J.P. Oxidation of an o-tolyl phosphine complex of platinum: C-H activation and transcyclometallation. J. Organomet. Chem. 2017, 851, 115–121. [Google Scholar] [CrossRef]
- Shaw, P.A.; Clarkson, G.J.; Rourke, J.P. Long-Lived Five-Coordinate Platinum(IV) Intermediates: Regiospecific C–C Coupling. Organometallics 2016, 35, 3751–3762. [Google Scholar] [CrossRef]
- Fuertes, S.; Brayshaw, S.K.; Raithby, P.R.; Schiffers, S.; Warren, M.R. New C∧N∧C Bis-Cyclometalated Platinum(II) Complexes: Synthesis, Structures, and Photophysical Properties. Organometallics 2012, 31, 105–119. [Google Scholar] [CrossRef]
- Shaw, P.A.; Clarkson, G.J.; Rourke, J.P. Reversible C–C bond formation at a triply cyclometallated platinum(iv) centre. Chem. Sci. 2017, 8, 5547–5558. [Google Scholar] [CrossRef]
- Shaw, P.A.; Phillips, J.M.; Newman, C.P.; Clarkson, G.J.; Rourke, J.P. Intramolecular transcyclometallation: The exchange of an aryl–Pt bond for an alkyl–Pt bond via an agostic intermediate. Chem. Commun. 2015, 51, 8365–8368. [Google Scholar] [CrossRef]
- Baya, M.; Belío, U.; Fernández, I.; Fuertes, S.; Martín, A. Unusual Metal-Metal Bonding in a Dinuclear Pt-Au Complex: Snapshot of a Transmetalation Process. Angew. Chem. Int. Ed. 2016, 55, 6978–6982. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(i) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Melník, M. Structural isomerism of copper(II) compounds. Coord. Chem. Rev. 1982, 47, 239–261. [Google Scholar] [CrossRef]
- Melnik, M.; Holloway, C.E. Stereochemistry of platinum coordination compounds. Coord. Chem. Rev. 2006, 250, 2261–2270. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).