Abstract
Heavy metals in wastewater represent one of the most serious concerns around the world. They cause significant harm to human health. Cockle shells have been considered a source of calcium carbonate (CaCO3), but their shells are disposed of as waste that pollutes the coastal environment. CaCO3 has attracted considerable attention as an adsorbent for heavy metals. To ensure the meaningful use of cockle shell (CS) waste and achieve a zero-waste production system, in this study, CaCO3 powder was synthesized from CS. It was characterized using XRD, TA/DTA, FESEM, and AAS. The XRD results illustrated that partial phase changes occur from aragonite (natural shell) to calcite (CaCO3), calcium hydroxide (Ca(OH)2), and calcium oxide (CaO) during heating. The calcined CS presented excellent adsorption performance for Pb and Cd. The Pb removal efficiency scores were about 97%, 96%, and 99% and the Cd removal efficiency scores were 100%, 98%, and 99% in a shorter time for calcined CS at 700 °C, 900 °C, and 950 °C, respectively. The results of this study show that the calcium carbonate from CS is an effective and low-cost adsorbent for the adsorption of Pb and Cd in aqueous solution.
1. Introduction
The world is dealing with the bioaccumulation of heavy metals in the environment, which is having an immediate impact on human health and the ecosystem’s food chain due to their toxicity. Therefore, it has become a primary global concern [1,2]. Heavy metals are emitted into the environment from various sources, such as the acceleration of industrialization, mining, smelting treatment, pesticide and fertilizer usage, and human activities [2,3,4]. Heavy metals in the form of free ions are highly toxic and carcinogenic, and their intake causes diseases and disorders since they are non-biodegradable [5,6]. Some of the harmful heavy metal ions at relatively low concentrations, such as cadmium (Cd), mercury (Hg), lead (Pb), chromium (Cr), silver (Ag), and arsenic (As), pose a potentially dangerous threat to human health, causing acute and chronic toxicities [6,7]. These heavy metal ions migrate easily within the water and remain in the environment, which can affect the quality of the water supply.
Hence, the development of an efficient technique to remove heavy metal ions from water systems is urgently required. Conventional methods for removing heavy metal ions from water and polluted aquatic environments include chemical precipitation, ion exchange, membrane methods, and electrochemical treatments. Nevertheless, there are limitations in terms of low removal efficiency, expensive equipment, and involvement with sludge production which creates treatment problems [8]. Therefore, a low-cost and environmentally friendly adsorbent is needed. Cockle is one of the most popular shellfish products in Thailand, with an overall production total of 5200 thousand tons. The volume of shells outweighs that of shellfish by more than 70%, so it is estimated that the amount of waste shells is at least 370 thousand tons annually [9]. They are dumped into coastal areas, causing potential environmental problems. CS can be considered a cheap source of CaCO3. They are made up of CaCO3, mainly in the mineral form of calcite or aragonite [10,11]. CaCO3 has attracted considerable attention as an adsorbent heavy metal [12]. CaCO3 is a key factor for absorbents because this biomaterial can be regenerated by low-cost HCl to desorb the heavy ions and maintain high adsorption capacities after multiple cycles [13]. Using CS as an adsorbent could represent an economic and environmental way forward, as the process is highly efficient and uses low-cost materials for the exploitation of aquaculture’s waste products to achieve a zero-waste production system. Many studies have used CaCO3 from shellfish to absorb heavy metals in wastewater [14,15,16]. Following calcination treatment above 700 °C, CaCO3 obtained from shells is transformed to CaO, and has been used in many applications, including as a renewable catalyst for biodiesel production [17], as a material to absorb CO2 for air-cleaning applications [18,19], and as an initial substrate to produce hydroxyapatite ceramic biomaterials [20]. However, the transformation of CaCO3 to calcium oxide (CaO) as a result of increasing calcination temperature has an impact on the absorption properties of heavy metals, as explored in a few studies. Therefore, the aim of this work is to study the influence of uncalcined and calcined shells on the phase transformations of CaCO3 and on the adsorption efficiency of heavy metals in water.
2. Materials and Methods
2.1. Cockle Shell (CS) Powder Preparation
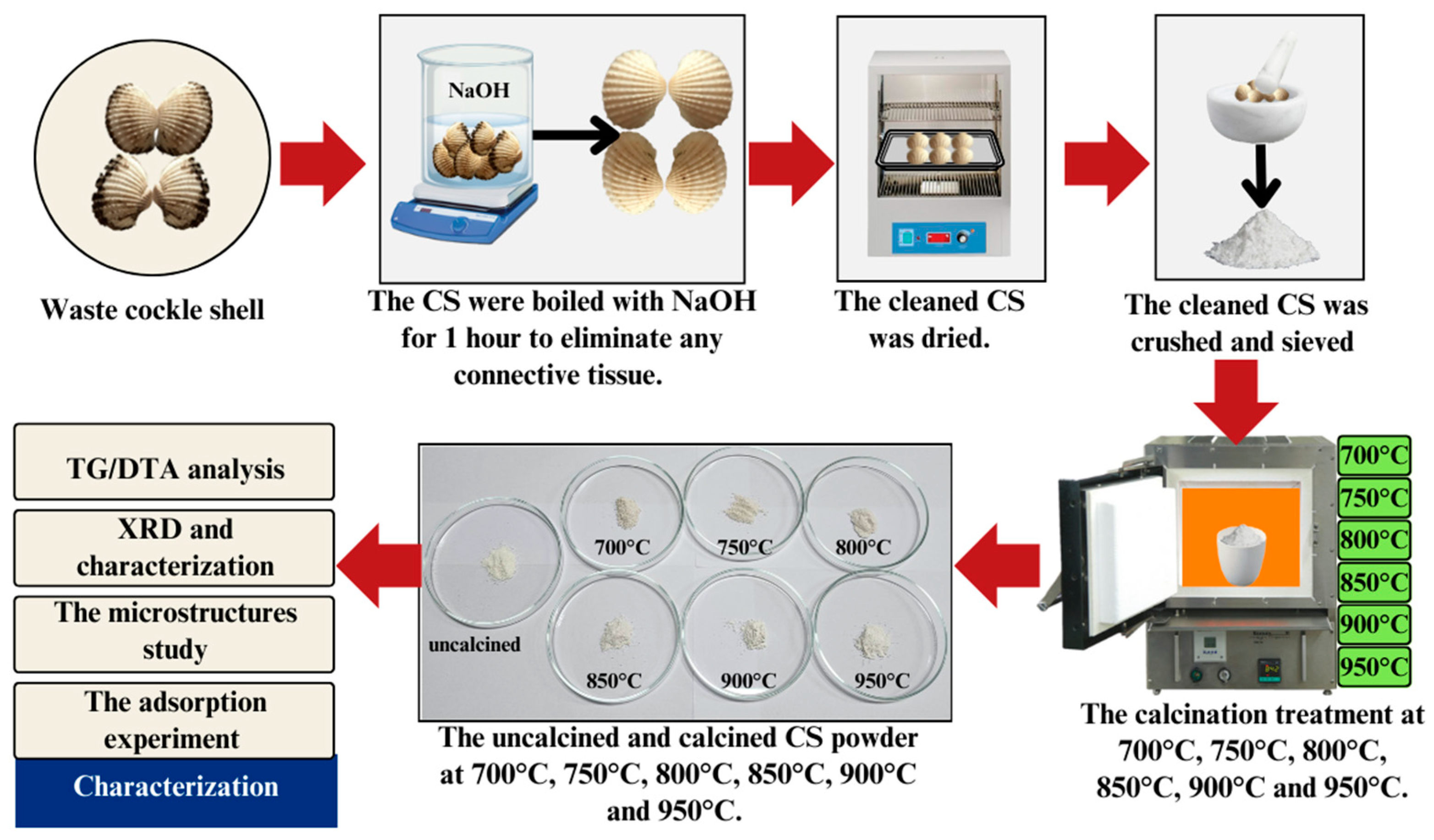
The waste CS was collected from a local supermarket. After being transported to the laboratory, the waste shells were scrubbed and cleaned thoroughly using a cellulose sponge to remove dirt. To eliminate any connective tissue and contaminated protein, the cockle shells were boiled with sodium hydroxide (NaOH) for 1 h. Then, the cleaned cockle shells were dried in an oven (Binder FED 400, GmbH Co, Germany) for 5–7 days at 80 °C. After that, the shells were crushed using a pestle and mortar (90 mm diameter Agate Top) and ground into powder. The shell powder was then sieved using a stainless-steel sieve with micro mesh (100 µm Retsch test sieve, Germany) and dried for 15 h in the oven. The shell powder was calcined at 700 °C, 750 °C, 800 °C, 850 °C, 900 °C, and 950 °C in an air atmosphere with a heating rate of 5 °C/min for 3 h and kept in an airtight polyethylene plastic bag (JP Packaging) until used to avoid a reaction with carbon dioxide (CO2) and humidity in the air. Figure 1 summarizes the CaCO3 preparation from the cockle shell and the testing method that was conducted for the X-ray diffraction (XRD) test, the microstructure test using a field emission scanning electron microscope (FESEM), and the heavy metal adsorption experiment.
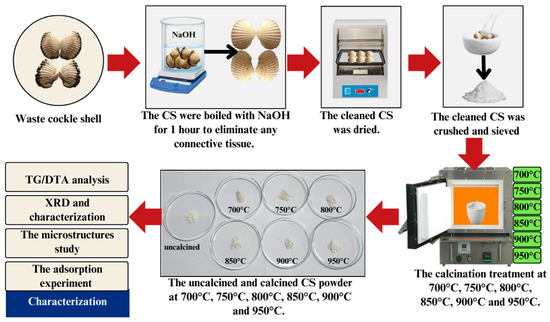
Figure 1.
Schematic representation of the experimental approach.
2.2. CS powder Characterization
2.2.1. Thermogravimetric Analysis (TG) and Differential Thermal Analysis (DTA)
Thermogravimetric and differential thermal analyses were performed on the uncalcined (natural) CS powder from the waste cockle shell to understand the weight loss and thermal behavior of natural cockle shell powders. They were carried out using a simultaneous thermal analyzer (NETZSCH STA 449 F3 Jupiter, NETZSCH, Gerätebau GmbH, Germany) at a heating rate of 20 °C/min in an inert (Nitrogen) atmosphere in the temperature range of 30 °C–1100 °C.
2.2.2. The X-ray Diffraction (XRD) and Rietveld Refinement Characterization
To identify the crystal structure and phase of uncalcined and calcined CS powder, the X-ray diffraction (XRD) characterization was performed on an X-ray diffractometer (XRD, Philip PW 3040/60 X′Pert Pro, Eindhoven, Netherlands) using Cu Kα radiation over a 2θ range from 10° to 80° with a step size of 0.04° at a scanning speed of 3 °/min. The XRD data were fitted with the Rietveld refinement procedure by employing the FullProf program.
2.2.3. The Microstructures Study
The uncalcined and calcined CS powder’s microstructures were observed using a field emission scanning electron microscope (FESEM) (FESEM Apreo S, Thermo Fisher Scientific Inc., Waltham, MA, USA). The calcined shell powder was mounted on a metal stub using a silver tape-style adhesive which increases conductivity. The microscope operated at 10 kV in high-vacuum (HV) mode with an ETD detector beam current set to 0.05 nA. The FESEM morphological examination at 3000 magnification was analyzed.
2.2.4. Determination of Lead (Pb) and Cadmium (Cd) Absorption
The Pb and Cd adsorption experiments were studied as a function of the contact time by varying contact time while keeping other parameters (pH, adsorbent dose, initial concentration, and agitation speed) constant. The adsorption experiments have been presented in detail in a previous study [21]. The adsorption experiments were conducted by varying the contact time from 5 to 100 min at pH 6.0, with initial Pb and Cd concentrations of 3 mg/L. The Cd and Pb solutions were tested separately with an adsorbent dose of 1 g/L. All experiments were run in 100 mL Erlenmeyer flasks at a 200 rpm speed and at room temperature. The mixture was filtered through filter paper and the Pb and Cd concentrations in the supernatant were analyzed by atomic absorption spectrophotometry (AAS) (Perkin Elmer: PinAAcle 900F AA spectrometer, USA). The percentage removal efficiency (%E) was determined as follows:
where and are the initial and equilibrium concentrations of Pb and Cd (mg/L), respectively [5,21].
2.2.5. Statistical Analysis
Statistical analysis was performed using OriginPro version 9 (OriginLab, Northampton, MA, USA). All the experiments were carried out in triplicate and all data are presented as mean values ± standard deviation (SD). One-way ANOVA with post hoc comparisons and the Bonferroni test were used to analyze the statistical variation between uncalcined and calcined CS powder at 700 °C, 900 °C, and 950 °C.
3. Results and Discussion
3.1. The Weight Loss and Thermal Behaviors of CS Powder
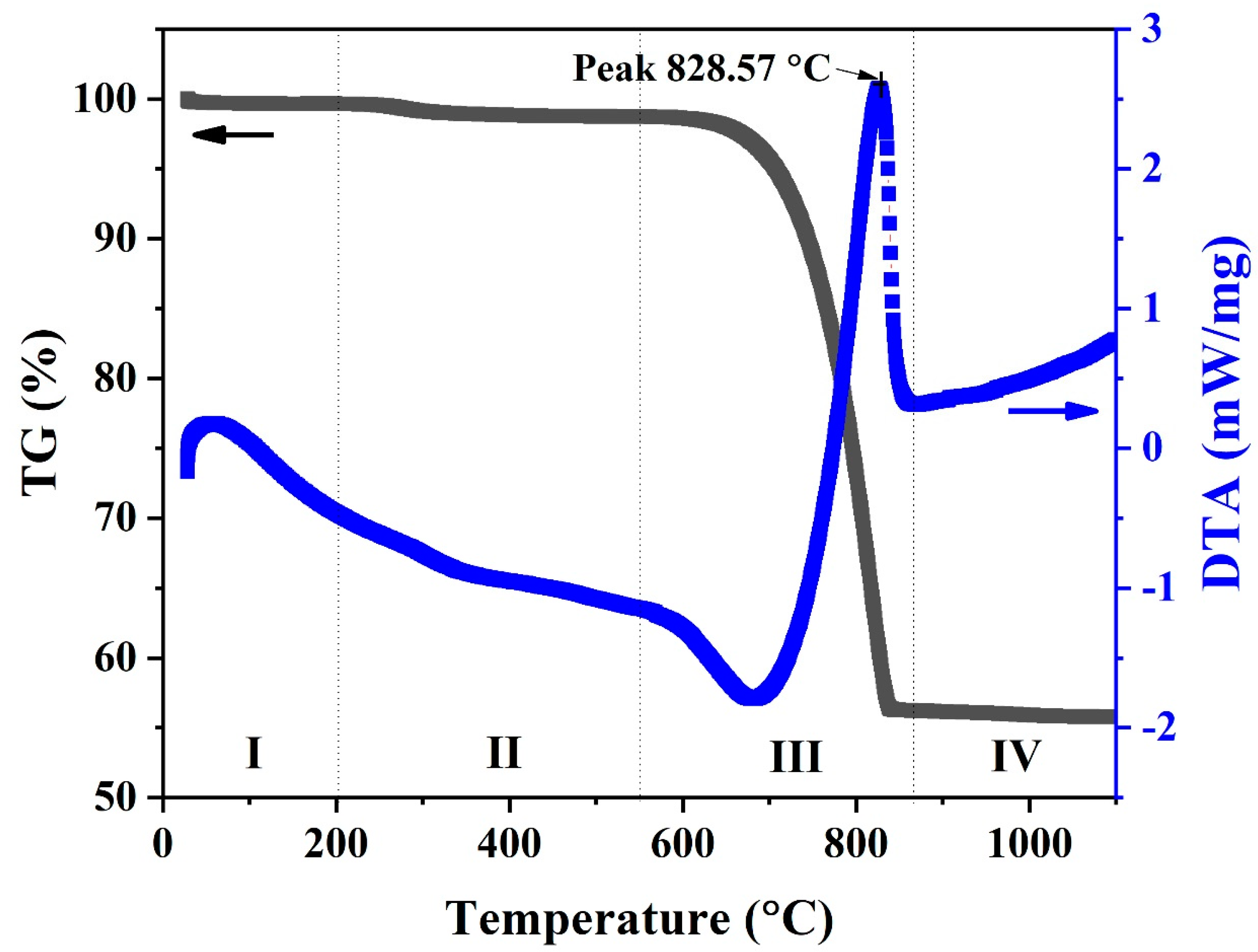
TG and DTA testing were conducted for samples taken from the uncalcined (natural) powder from the waste cockle shells. The TG/DTA curve for the uncalcined CS powder is shown in Figure 2. The expected thermal behavior was clearly visible, with four distinct stages of weight loss. There was a clear difference in the TG curves. In the first stage (I), small weight loss was observed at temperatures below 200 °C. This can be ascribed to the sample’s moisture content and corresponds to a broad endothermic peak in the DTA result. In the second stage (II), at temperatures between 200 °C and 550 °C, the weight of samples decreased slightly by 1–2%, which is related to the dehydration of water from the carbonate lattice and transformation of aragonite to calcite phases [22,23,24,25]. The third state (III) was observed between 550 °C and 850 °C. The weight decreased rapidly until the samples had lost 43% of their weight and were subjected to differential thermal analysis at 828.57 °C. This state is a release process of carbon dioxide (CO2) as a result of calcium carbonate decomposing a fraction of the sample into calcium oxide [24,25]. The final state (IV) was observed at a temperature above 850 °C. The weight of the sample remained almost constant.
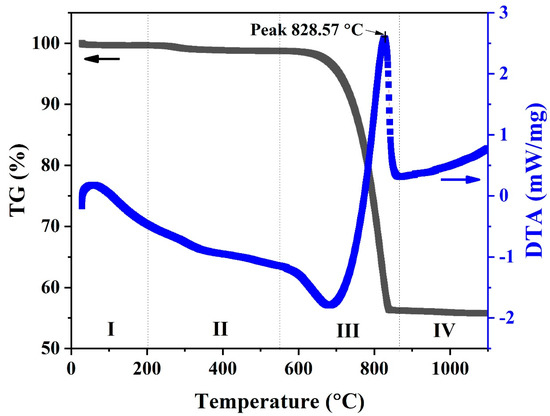
Figure 2.
TG (Black line) and DTA (Blue line) curves of uncalcined (natural) powders from cockle shell.
3.2. XRD and Rietveld Refinement on X-ray Diffraction Patterns Analysis
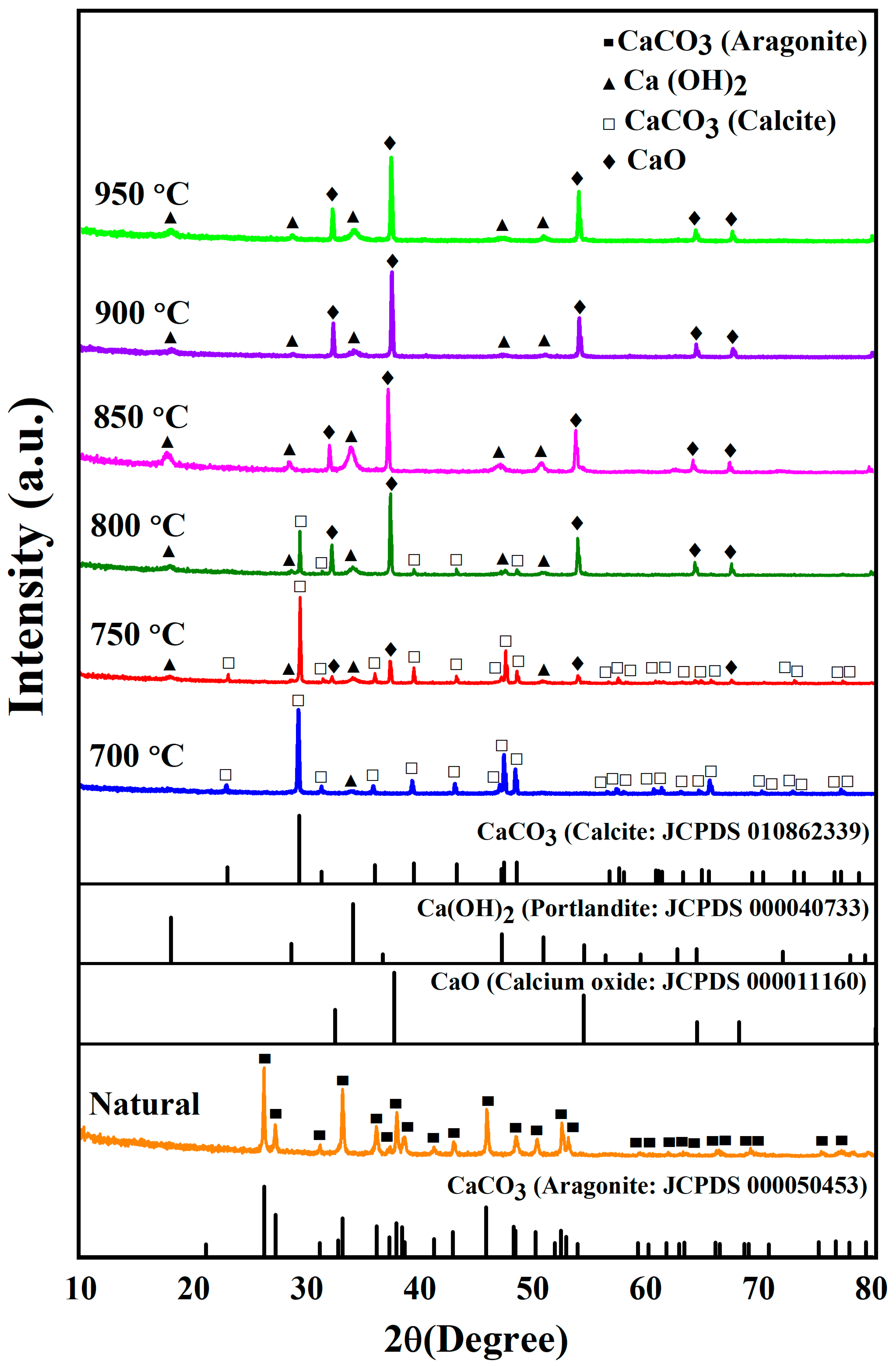
Figure 3 shows the XRD diffraction patterns of uncalcined (natural) and calcined CS powders between 700 and 950 °C. The XRD diffraction pattern of uncalcined CS powder is characteristic of calcium carbonate in the aragonite phase (JCPDS file no. 000050453) and corresponds with previous studies [25]. XRD patterns of calcined cockle shells present a complete transformation of CaCO3 to calcium oxide (CaO). At 700 °C, the samples were characteristic of calcium carbonate in the calcite phase (JCPDS file no. 010862339) as the main peak. They also presented a small peak of calcium hydroxide Ca(OH)2 corresponding with JCPDS file no. 000040733. At 750 °C, the samples still contained calcium carbonate in the calcite phase of the main peak and also presented a small peak of Ca(OH)2 and CaO. At 800 °C, the samples provided the main peak of CaO, corresponding to JCPDS file no. 000011160, and small peaks of CaCO3 and Ca(OH)2. As the calcination temperature increased from 850 °C to 950 °C, the samples contained CaO in the main peak and a small peak of Ca(OH)2. These temperatures completely removed the calcium carbonate in the calcite phase. Thus, this indicates that for the thermal decomposition of CS, CaCO3 converts into CaO.
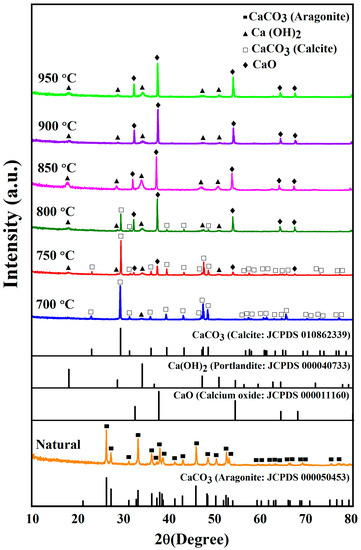
Figure 3.
XRD patterns of uncalcined (natural) and calcined CS powders between 700 °C and 950 °C.
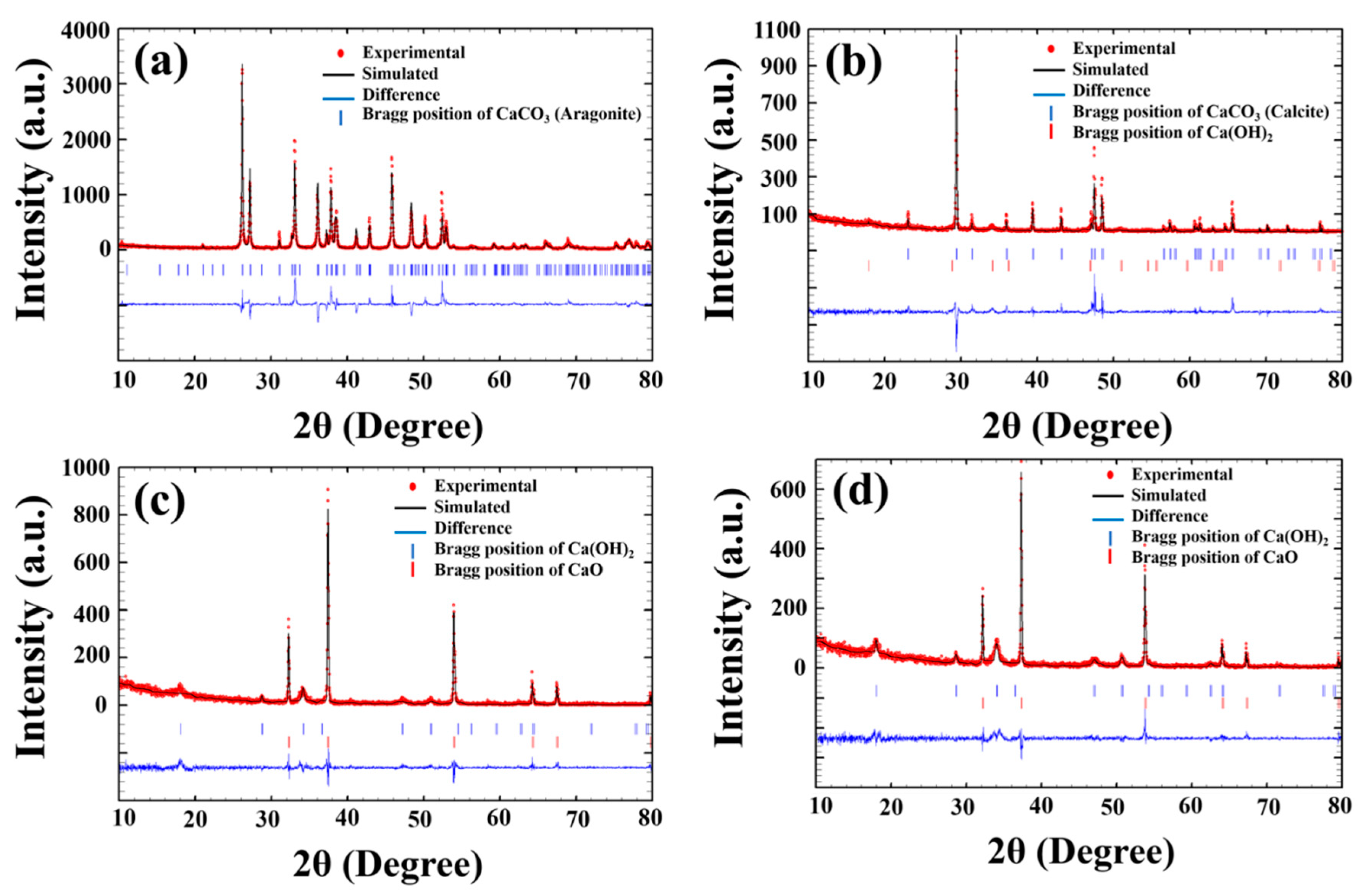
To confirm the effect of calcination temperature on the phase structure, the Rietveld refinement technique was used by fitting the XRD data with the Fullprof software, as shown in Figure 4. For simulation modeling, the cell parameter, lattice constants, space group, and atom functional positions can refer to the Crystallography Open Database (COD). In addition, the model’s symmetry was changed whenever the chi-square test (χ2) and goodness of fit factor became substantially lower than in the model with a higher crystallographic symmetry [26].
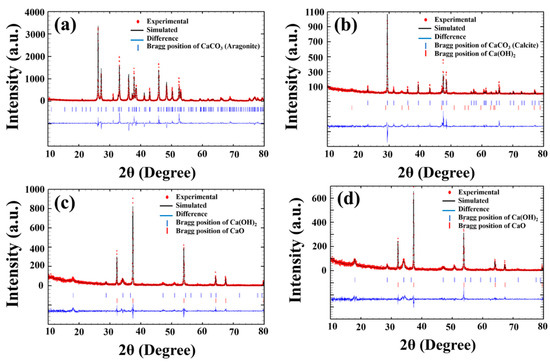
Figure 4.
Fitted XRD patterns of the CS: (a) uncalcined and calcined CS powder at (b) 700 °C, (c) 900 °C and (d) 950 °C by the Rietveld refinement technique.
This result shows that there was also aragonite with space group P1 in the database for CaCO3, which perfectly fits the uncalcined (natural) cockle shell powder, showing low values of χ2 = 3.28, Rp = 24.7, Rwp = 26.6, and Rexp = 14.70, and that the phase percentage was 100%, as listed in Table 1. For the sample calcined at 700 °C, the best refinement was obtained using a mixing phase between calcite (CaCO3) with space group R3C and calcium hydroxide (Ca(OH)2) with space group P3m1. The phase percentages between R3C and P3m1 were 99.63 and 0.37. For the 750 °C and 800 °C samples, the triple phase of calcite (CaCO3) with space group R3C, calcium hydroxide (Ca(OH)2) with space group P3m1, and calcium oxide (CaO) with space group Fm3m was carried out until the best fit was obtained. The phase percentages were CaCO3 = 82.50, Ca(OH)2 = 7.40, and CaO = 10.10 for the samples calcined at 750 °C and CaCO3 = 29.89, Ca(OH)2 = 8.39, and CaO = 61.72 for the samples calcined at 800 °C. When calcination temperature increased from 850 °C to 950 °C, the P3m1 (Ca(OH)2) and Fm3m (CaO) space groups were used as initial models for the refinement, which show the phase percentages of CaO = 88.15 and Ca(OH)2 = 11.85 for calcination at 850 °C, CaO = 91.82 and Ca(OH)2 = 8.18 for calcination at 900 °C, and CaO = 95.20 and CaO = 4.80 for calcination at 950 °C (as listed in Table 1). This indicates that calcination temperature affects the decomposition process of CaCO3 from the cockle shell. CaCO3 can be converted into CaO following Equation (2), which is used in industry and daily practices such as wastewater and sewage treatment, glass production, construction material, agriculture, and more [24]. CaO has also been employed as the base sorbent for carbon dioxide (CO2) capture [27,28]. The existing materials of CO2 adsorbents, such as amine-based adsorbents, activated carbon, and molecular sieves, are able to withstand low-temperature processes (40 °C–160 °C) [24,27].

Table 1.
Rietveld structural refinement results for uncalcined (natural) and calcined CS powders between 700 °C and 950 °C.
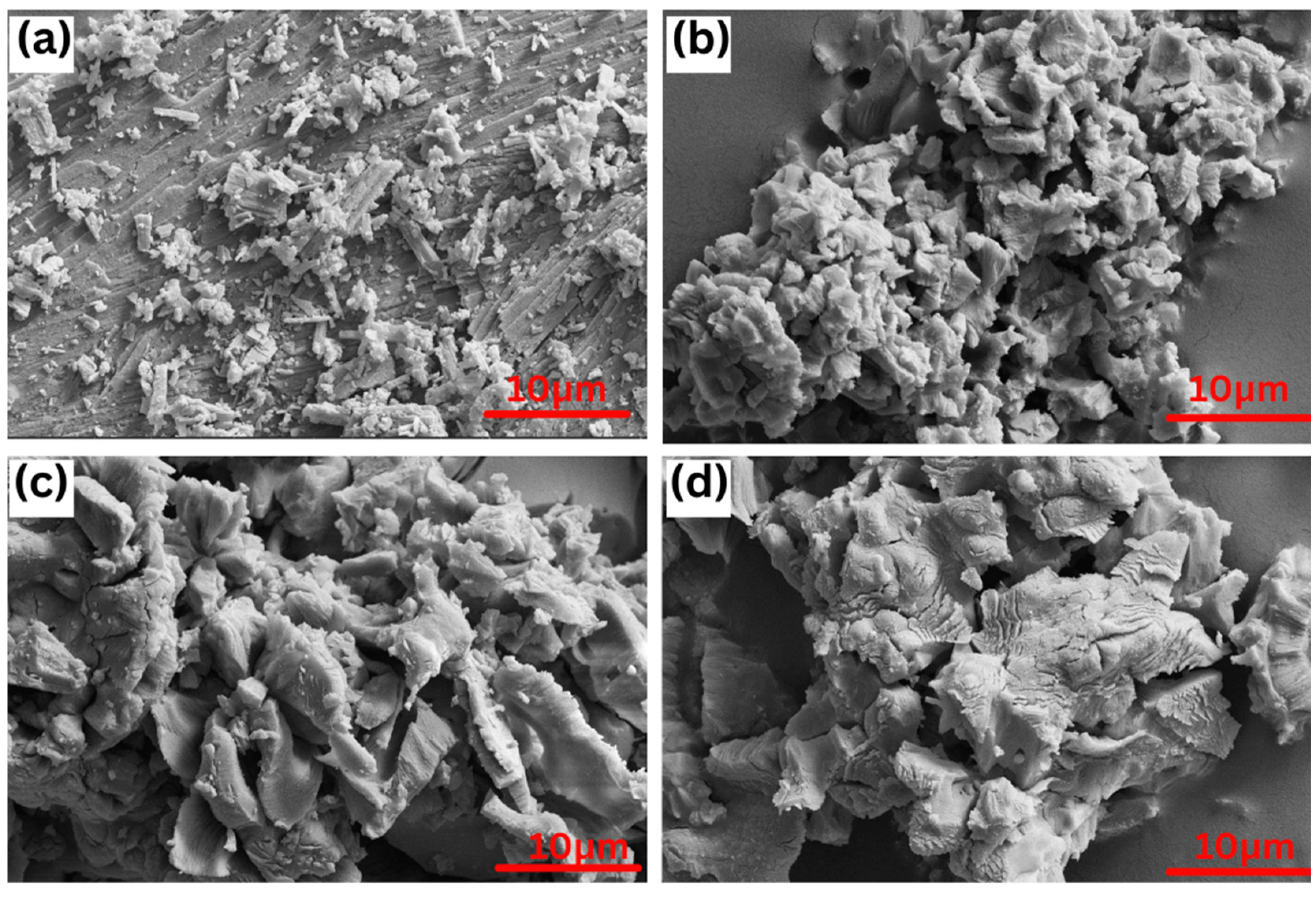
3.3. The Microstructures of Uncalcined and Calcined CS Powder
The surface morphologies of uncalcined and calcined CS powder at 700 °C, 900 °C, and 950 °C are listed in Figure 5. All the samples showed a non-uniform irregular with a length of up to 20 μm. The uncalcined sample showed a plate shape with parallel cleavages, as seen in Figure 5a. After calcination at 700 °C–950 °C, the surface morphologies of the sample types changed quite noticeably. The surface of calcined CS powder exhibited an agglomerated structure and a matrix phase behavior in liquid phase formation due to the removal of water and organic material from the raw shells, which is consistent with the results of Laonapakul et al. [29]. Moreover, the surface morphology increased with increasing calcination temperature, as seen in Figure 5b–d.
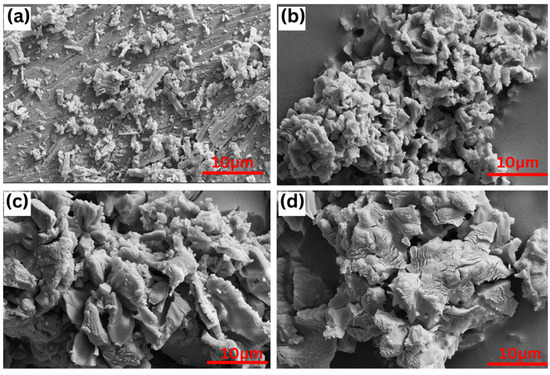
Figure 5.
Representative SEM image of the CS powder: (a) uncalcined and calcined at (b) 700 °C, (c) 900 °C, and (d) 950 °C with magnification 3000.
3.4. The Pb and Cd Adsorption Capacity
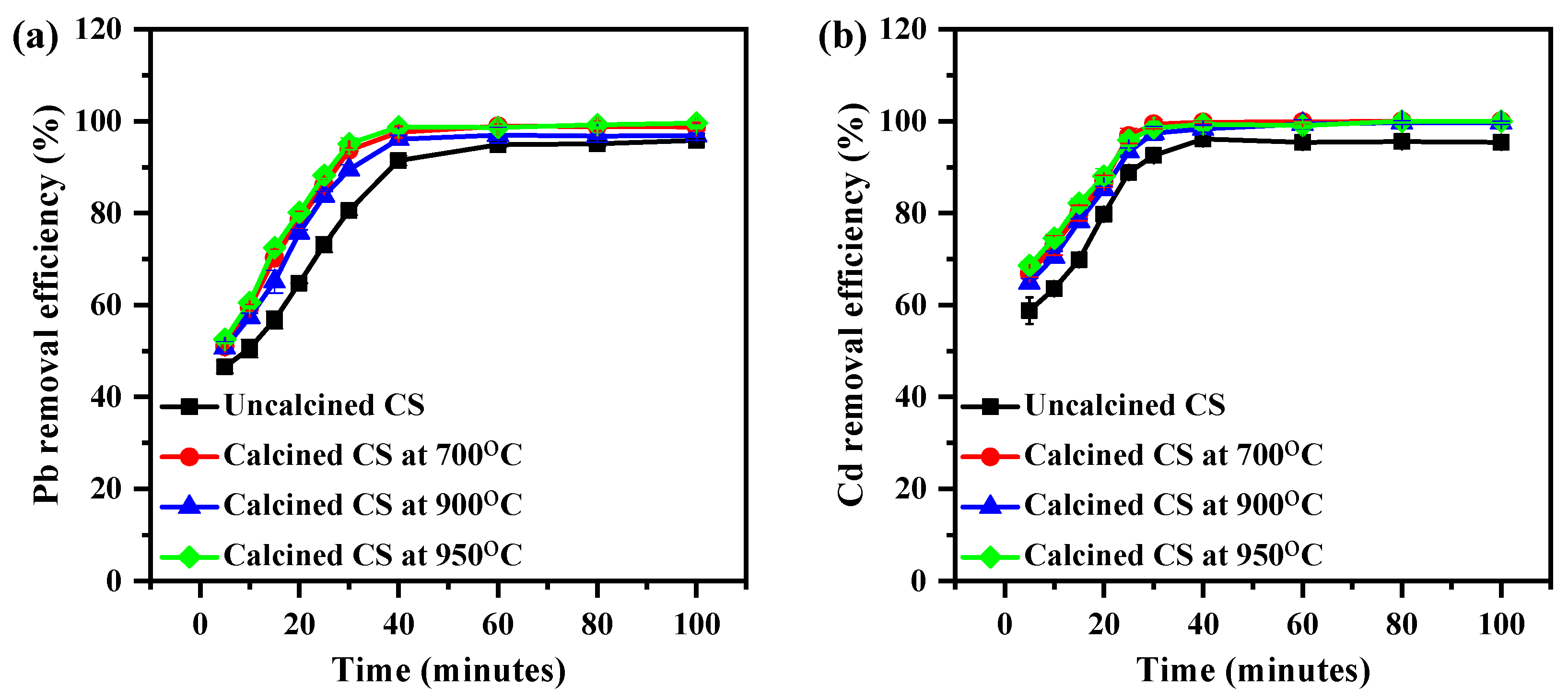
The Pb and Cd adsorption levels of CS were also investigated as a function of time. Hence, the other parameters, e.g., room temperature with a 200 rpm shaking condition, the initial concentration (3 ppm), and the dosage (1 g of CS), were kept constant. Using Equation (1), the percentage removal efficiency (%E) was calculated, as illustrated in Figure 6. As shown in Figure 6a, it was observed that the Pb removal process of uncalcined and calcined CS encompasses two main stages. In the first stage, within 30 min, the Pb removal efficiency increased rapidly with an increase in the contact time. In the second stage, which took place after 30 min, the Pb removal efficiency tended to become constant. The Pb removal percentage increased over 30 min from 46.5 ± 1.4% to 91.43 ± 1.0%, 50.9 ± 0.2% to 97.6 ± 1.2%, 50.7 ± 1.2% to 96.0 ± 0.8%, and 52.5 ± 0.7% to 98.75 ± 0.7% for uncalcined and calcined CS powder at 700, 900, and 950 °C, respectively.
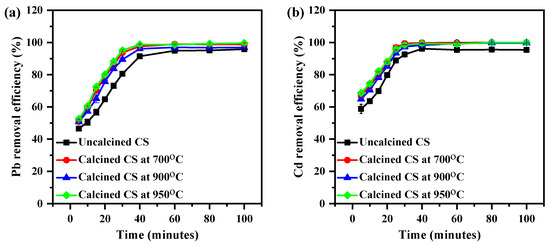
Figure 6.
The effect of time on the removal efficiency of (a) Pb and (b) Cd on CS from aqueous media using the CS at various calcined temperatures (3 ppm of Cd; 1 g of CS, mixture rate of 200 rpm).
Figure 6b shows a similar trend for the Cd removal percentage of CS. It was noticed that when the time was increased from 5 to 30 min, the Cd removal rate increased from 58.8 ± 2.8% to 96.1 ± 0.5%, 66.9 ± 0.8% to 99.8 ± 0.3%, 64.8 ± 0.9% to 98.3 ± 0.7%, and 68.6 ± 0.8% to 99.3 ± 0.9% for uncalcined and calcined CS at 700, 900, and 950 °C, respectively. An explanation for this behavior is that all the sites on the surface of the calcined CS are vacant at the initial stage. However, with increasing contact time, there was a progressive increase in the number of bonds in active sites on the calcined CS surface which underwent an adsorption interaction with metal ions. The heavy metal became less efficient [30,31,32].
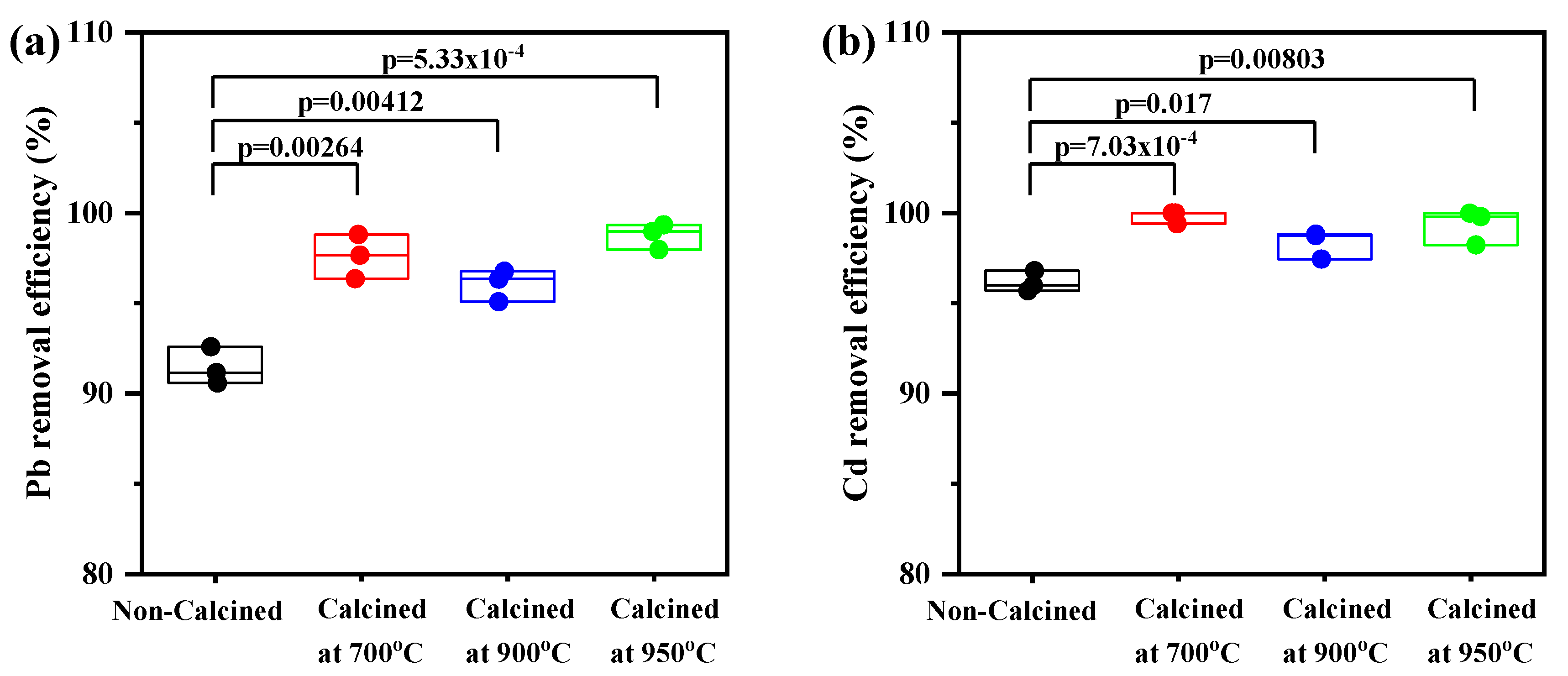
The calcination treatment’s effect on the adsorption of Pb and Cd is presented in Figure 7. There were no statistically significant differences in the Pb removal efficiency when comparing the uncalcined CS at different temperatures; however, significant differences were observed between the uncalcined and calcined CS at 700 °C (p = 0.00264), (c) 900 °C (p = 0.00412), and (d) 950 °C (p = 5.33 × 10−4) (Figure 7a).
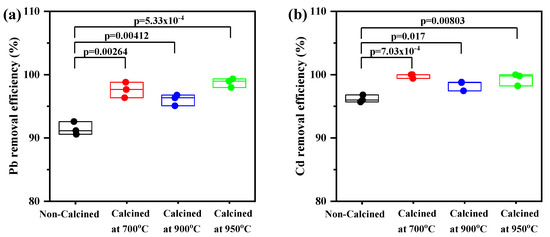
Figure 7.
Box–whisker plots presented for the heavy metal removal efficiency (%) of uncalcined and calcined CS at 700, 900, and 950°C: (a) Pb and (b) Cd. All values are shown with boxes representing the 25th and 75th percentiles of data. Whiskers represent the 5th and 95th percentiles of data.
Similarly, there was a significant difference between uncalcined and calcined CS. The mean value of Cd removal percentage for uncalcined CS was lower than calcined CS at 700 °C (p = 7.03 × 10−4), (c) 900 °C (p = 0.0017), and (d) 950 °C (p = 0.00803) (Figure 7b). It can be explained by, as indicated in Figure 3, the XRD analysis, which showed that the crystal structure of CS is aragonite. Moreover, the surface morphology of CS presented lamellar aragonite structure before calcination, as shown in Figure 5a and as reported by Gopi et al. in 2013, who explored morphological and polymorphic transformations during the crystallization of CaCO3 in the presence of DTPA [33,34]. When the CS powder was heated, the amorphous CaCO3 (aragonite) was completely crystalline, and CaCO3 (calcite) and CaO were found at 700 °C and 900 °C, respectively. The surface of the CS powder exhibited roughness and numerous pores appeared (Figure 5d). The phase transformation during calcination occurs as a result, and the number of small pores formed by the release of CO2 will increase. The diffusion of Pb and Cd ions through the small pores is quick and can proceed to a larger extent [26,27,28].
4. Conclusions
To achieve a zero-waste production system and create wealth from waste, discarded CS was used as an effective and low-cost adsorbent for removing heavy metal ions from wastewater. Our study synthesized and investigated CaCO3 from discarded CS. Based on XRD data, the characterization of discarded CS powder during calcination treatment exhibited a change in the crystal structure. Before the CS powder’s calcination process, a form of calcium carbonate was present in the CS during the aragonite phase. During heating to a calcination temperature, there was a change from CaCO3 to CaO and a porous surface structure formed for the release of CO2, as confirmed by SEM images. Therefore, the calcination temperature played a significant role in the crystal phase transformation because organic components in the CS shells can be removed to form CaCO3 with pore channels on the surface, which is a critical factor for absorbents. The crystal phase transformation further affected the Pb and Cd removal percentage. The adsorption study showed that the calcined CS achieved higher adsorption performance than uncalcined CS. The Pb removal efficiency was about 97%, 96%, and 99% and the Cd removal efficiency was 100%, 98%, and 99% in a shorter time for calcined CS at 700, 900, and 950 °C, respectively. Our reported results will be helpful for understanding the crystal structure of CaCO3 from CS during the calcination process for a bio-adsorption application. In addition, the high removal efficiency also supports the notion that discarded CS is a successful bio-sorbent with low cost and high effectiveness.
Author Contributions
Conceptualization, R.S., N.V. and P.P.; methodology, R.S. and P.P.; validation, R.S., N.V. and P.P.; formal analysis, R.S. and P.P.; investigation, R.S. and P.P; writing—original draft preparation, N.V.; writing—review and editing, N.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Pibulsongkram Rajabhat University, grant number RDI-2-65-24.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The author confirms that the data supporting the reported results can be found within the article. Raw data that supports the findings of this work can be inquired to the corresponding authors, upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Han, R.; Zhou, B.; Huang, Y.; Lu, X.; Li, S.; Li, N. Bibliometric overview of research trends on heavy metal health risks and impacts in 1989–2018. J. Clean. Prod. 2020, 276, 123249. [Google Scholar] [CrossRef]
- Devi, R.V.; Nair, V.V.; Sathyamoorthy, P.; Doble, M. Mixture of CaCO3 Polymorphs Serves as Best Adsorbent of Heavy Metals in Quadruple System. J. Hazard. Toxic Radioact. Waste 2022, 26, 04021043. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Bhunia, P. Environmental Toxicants and Hazardous Contaminants: Recent Advances in Technologies for Sustainable Development. J. Hazardous, Toxic, Radioact. Waste 2017, 21, 02017001. [Google Scholar] [CrossRef]
- Dubey, S.P.; Gopal, K. Adsorption of chromium(VI) on low cost adsorbents derived from agricultural waste material: A comparative study. J. Hazard. Mater. 2007, 145, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Wei, D.; Guan, X.; Liang, Z.; Lan, L.; Lan, X.; Liu, P.; Mo, H.; Lan, P. Adsorption of copper (II) and cadmium (II) ions by in situ doped nano-calcium carbonate high-intensity chitin hydrogels. J. Hazard. Mater. 2021, 423, 127137. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. In Poisoning in the Modern World-New Tricks for an Old Dog? IntechOpen: London, UK, 2019; Volume 10, pp. 77–100. [Google Scholar] [CrossRef]
- Chalermwat, K.; Szuster, B.W.; Flaherty, M. Shellfish aquaculture in Thailand. Aquac. Econ. Manag. 2003, 7, 249–261. [Google Scholar] [CrossRef]
- Xu, Z.; Valeo, C.; Chu, A.; Zhao, Y. The Efficacy of Whole Oyster Shells for Removing Copper, Zinc, Chromium, and Cadmium Heavy Metal Ions from Stormwater. Sustainability 2021, 13, 4184. [Google Scholar] [CrossRef]
- Suzuki, M.; Nagasawa, H. Mollusk shell structures and their formation mechanism. Can. J. Zool. 2013, 91, 349–366. [Google Scholar] [CrossRef]
- Zeng, C.; Hu, H.; Feng, X.; Wang, K.; Zhang, Q. Activating CaCO3 to enhance lead removal from lead-zinc solution to serve as green technology for the purification of mine tailings. Chemosphere 2020, 249, 126227. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qian, Z.-J.; Zhou, C.; Su, W.; Hong, P.; Liu, S.; He, L.; Chen, Z.; Ji, H. Mussel-inspired synthesis of polydopamine-functionalized calcium carbonate as reusable adsorbents for heavy metal ions. RSC Adv. 2014, 4, 47848–47852. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Wu, H.-M.; Hsieh, S.-L.; Li, J.-S.; Dong, C.; Chen, C.-W.; Hsieh, S. Preparation of vaterite calcium carbonate granules from discarded oyster shells as an adsorbent for heavy metal ions removal. Chemosphere 2020, 254, 126903. [Google Scholar] [CrossRef]
- Yavuz, Ö.; Guzel, R.; Aydin, F.; Tegin, I.; Ziyadanogullari, R. Removal of Cadmium and Lead from Aqueous Solution by Calcite. Pol. J. Environ. Stud. 2007, 16, 467–471. [Google Scholar]
- Vu, X.H.; Nguyen, L.H.; Van, H.T.; Nguyen, D.V.; Nguyen, T.H.; Nguyen, Q.T.; Ha, L.T. Adsorption of chromium (VI) onto freshwater snail shell-derived biosorbent from aqueous solutions: Equilibrium, kinetics, and thermodynamics. J. Chem. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Viriya-empikul, N.; Krasae, P.; Puttasawat, B.; Yoosuk, B.; Chollacoop, N.; Faungnawakij, K. Waste shells of mollusk and egg as biodiesel production catalysts. Bioresour. Technol. 2010, 101, 3765–3767. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhao, C.S.; Chen, H.C.; Duan, L.B.; Chen, X.P. CO2 capture behavior of shell during calcination/carbonation cycles. Chem. Eng. Technol. 2009, 32, 1176–1182. [Google Scholar] [CrossRef]
- Ma, K.W.; Teng, H. CaO powders from oyster shells for efficient CO2 capture in multiple carbonation cycles. J. Am. Ceram. Soc. 2010, 93, 221–227. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, M.; Zhang, M.; Hou, S.; Kong, S.; Yang, L.; Deng, L. Preparation of Chinese mystery snail shells derived hydroxyapatite with different morphology using condensed phosphate sources. Ceram. Int. 2016, 42, 16671–16676. [Google Scholar] [CrossRef]
- Fernández Pérez, B.; Ayala Espina, J.; Fernández González, M.D.L.Á. Adsorption of Heavy Metals Ions from Mining Metallurgical Tailings Leachate Using a Shell-Based Adsorbent: Characterization, Kinetics and Isotherm Studies. Materials 2022, 15, 5315. [Google Scholar] [CrossRef]
- Asmi, D.; Zulfia, A. Blood Cockle Shells Waste as Renewable Source for the Production of Biogenic CaCO3 and Its Characterisation. IOP Conf. Ser. Earth Environ. Sci. 2017, 94, 012049. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Mohamed, M.; Yusup, S. A study of calcination and carbonation of cockle shell. World Acad. Eng. Technol. 2011, 60, 818–823. [Google Scholar]
- Mohamed, M.; Yusup, S.; Maitra, S. Decomposition study of calcium carbonate in cockle shell. Eng. Sci. Technol. 2012, 7, 1–10. [Google Scholar]
- Kamba, A.S.; Ismail, M.; Ibrahim, T.A.T.; Zakaria, Z.A.B. Synthesis and Characterisation of Calcium Carbonate Aragonite Nanocrystals from Cockle Shell Powder (Anadara granosa). J. Nanomater. 2013, 2013, 398357. [Google Scholar] [CrossRef]
- Gražulis, S.; Merkys, A.; Vaitkus, A.; Chateigner, D.; Lutterotti, L.; Moeck, P.; Quiros, M.; Downs, R.T.; Kaminsky, W.; Le Bail, A. Crystallography Open Database: History, Development, and Perspectives. Mat. Inf. 2019, 10, 1–39. [Google Scholar] [CrossRef]
- Lu, H.P.; Smirniotis, G.; Ernst, F.O.; Pratsinis, S.E. Nano-structured Ca-based sorbents with high CO2 uptake efficiency. Chem. Eng. Sci. 2009, 64, 1936–1943. [Google Scholar] [CrossRef]
- Dennis, J.S.; Pacciani, R. The rate and extent of uptake of CO2 by a synthetic CaO-containing sorbent. Chem. Eng. Sci. 2009, 64, 2147–2157. [Google Scholar]
- Laonapakula, T.; Sutthia, R.; Chaikoolb, P.; Mutohc, Y.; Chindaprasirt, P. Optimum conditions for preparation of bio-calcium from blood cockle and golden apple snail shells and characterization. Sci. Asia 2019, 45, 10–20. [Google Scholar] [CrossRef]
- Ahmadi, F.; Esmaeili, H. Chemically modified bentonite/Fe3O4 nanocomposite for Pb (II), Cd (II), and Ni (II) removal from synthetic wastewater. Desalin. Water. Treat. 2018, 110, 154–167. [Google Scholar] [CrossRef]
- Heidari, B.; Bakhtiari, A.R.; Shirneshan, G. Concentrations of Cd, Cu, Pb and Zn in soft tissue of oyster (Saccostrea cucullata) collected from the Lengeh Port coast, Persian Gulf, Iran: A comparison with the permissible limits for public health. Food Chem. 2013, 141, 3014–3019. [Google Scholar] [CrossRef]
- Esmaeili, H.; Tamjidi, S.; Abed, M. Removal of Cu(II), Co(II) and Pb(II) from synthetic and real wastewater using calcified Solamen Vaillanti snail shell. Desalin. Water Treat. 2020, 174, 324–335. [Google Scholar] [CrossRef]
- Gopi, S.; Subramanian, V.K.; Palanisamy, K. Aragonite–calcite–vaterite: A temperature influenced sequential polymorphic transformation of CaCO3 in the presence of DTPA. Mater. Res. Bull. 2013, 48, 1906–1912. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, F.; Ouyang, X.K.; Yang, L.Y.; Wang, Y. Adsorption of Pb (II) from aqueous solution by mussel shell-based adsorbent: Preparation, characterization, and adsorption performance. Materials 2021, 14, 741. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).