Investigating the Electrochemical Properties of a Semiconductor Heterostructure Composite Based on WO3-CaFe2O4 Particles Planted on Porous Ni-Foam for Fuel Cell Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis Procedures
2.2. Characterizations Tools & Electrochemical Measurements
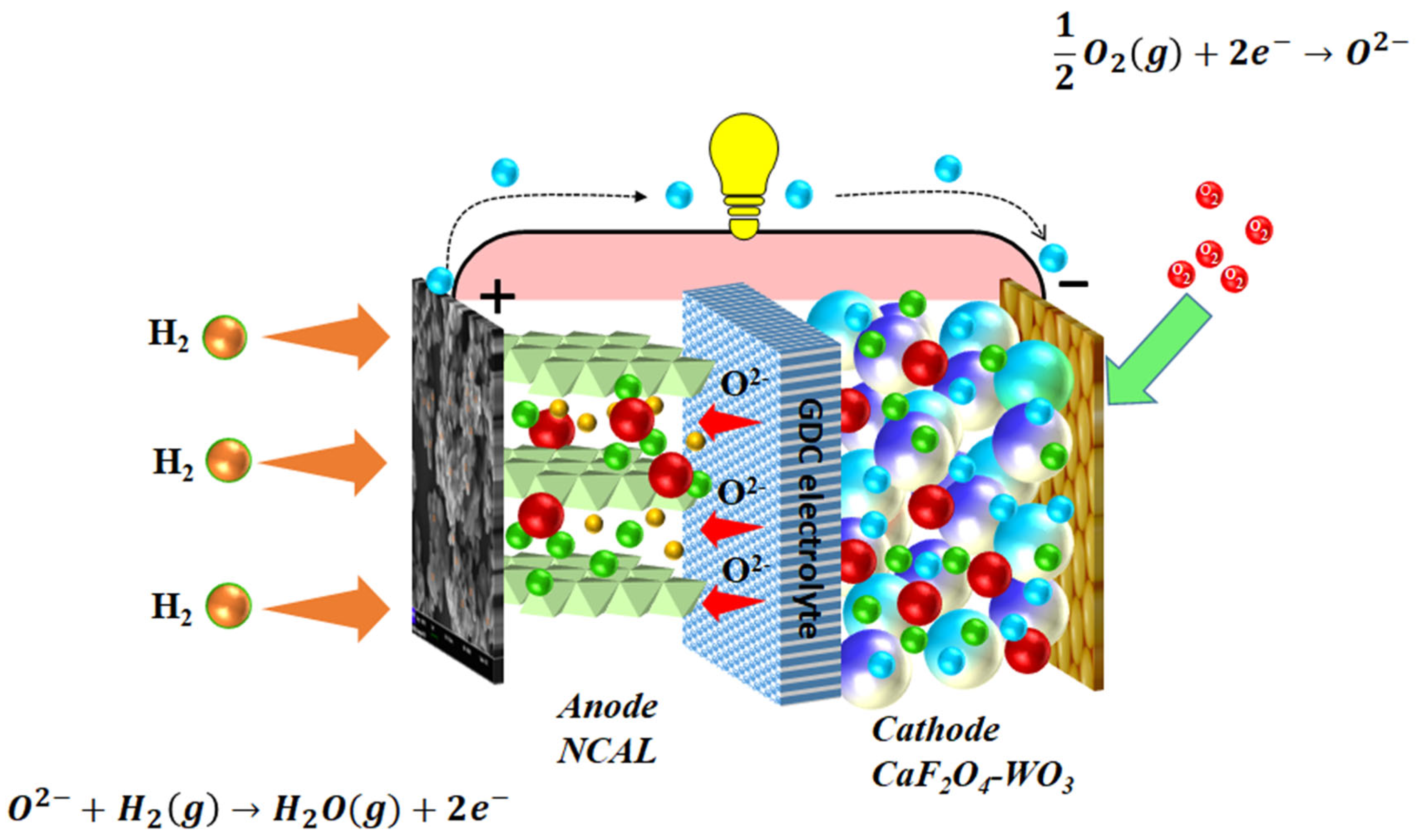
2.3. Complete Fabrication of Fuel Cells
3. Results
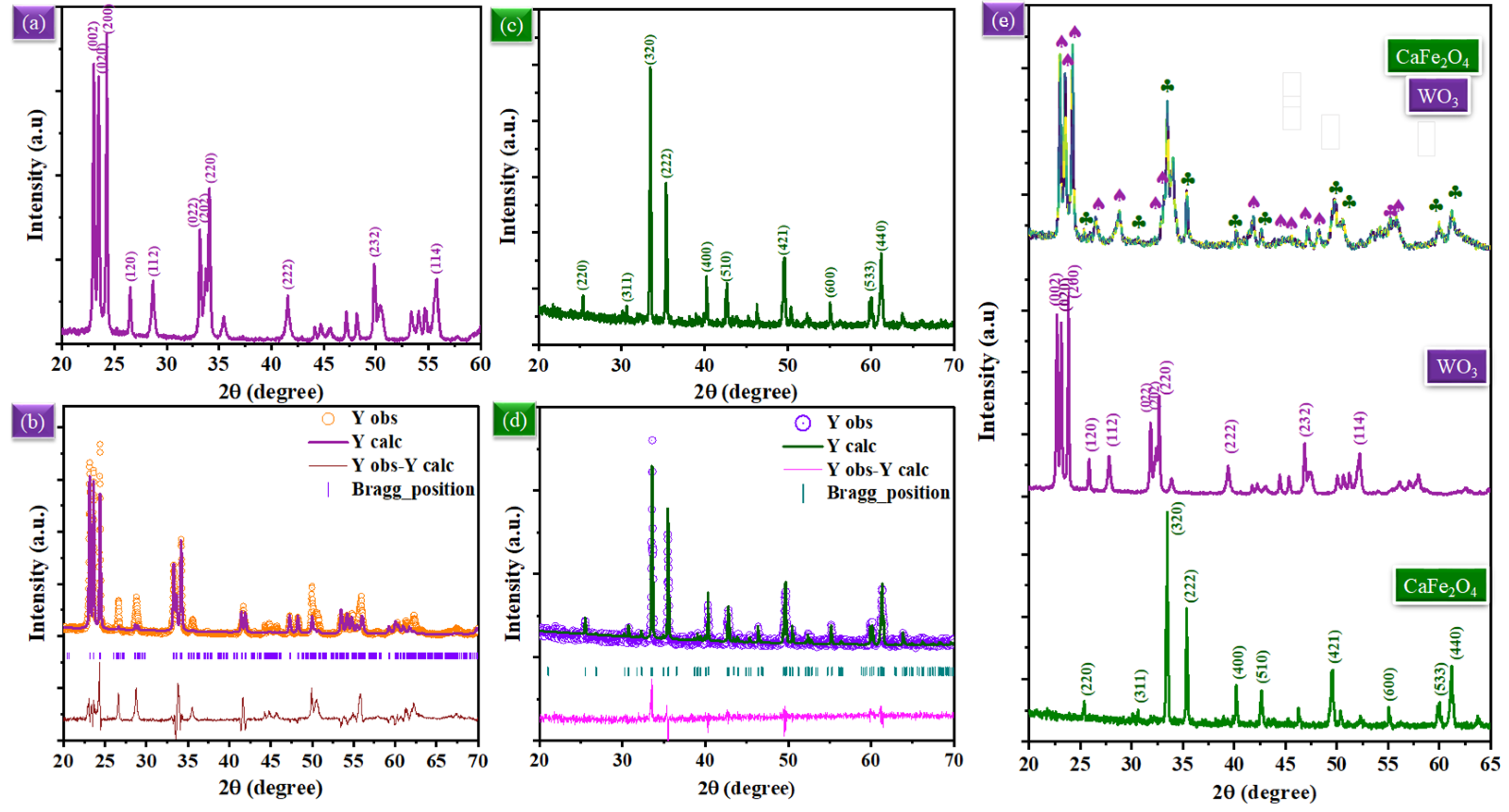
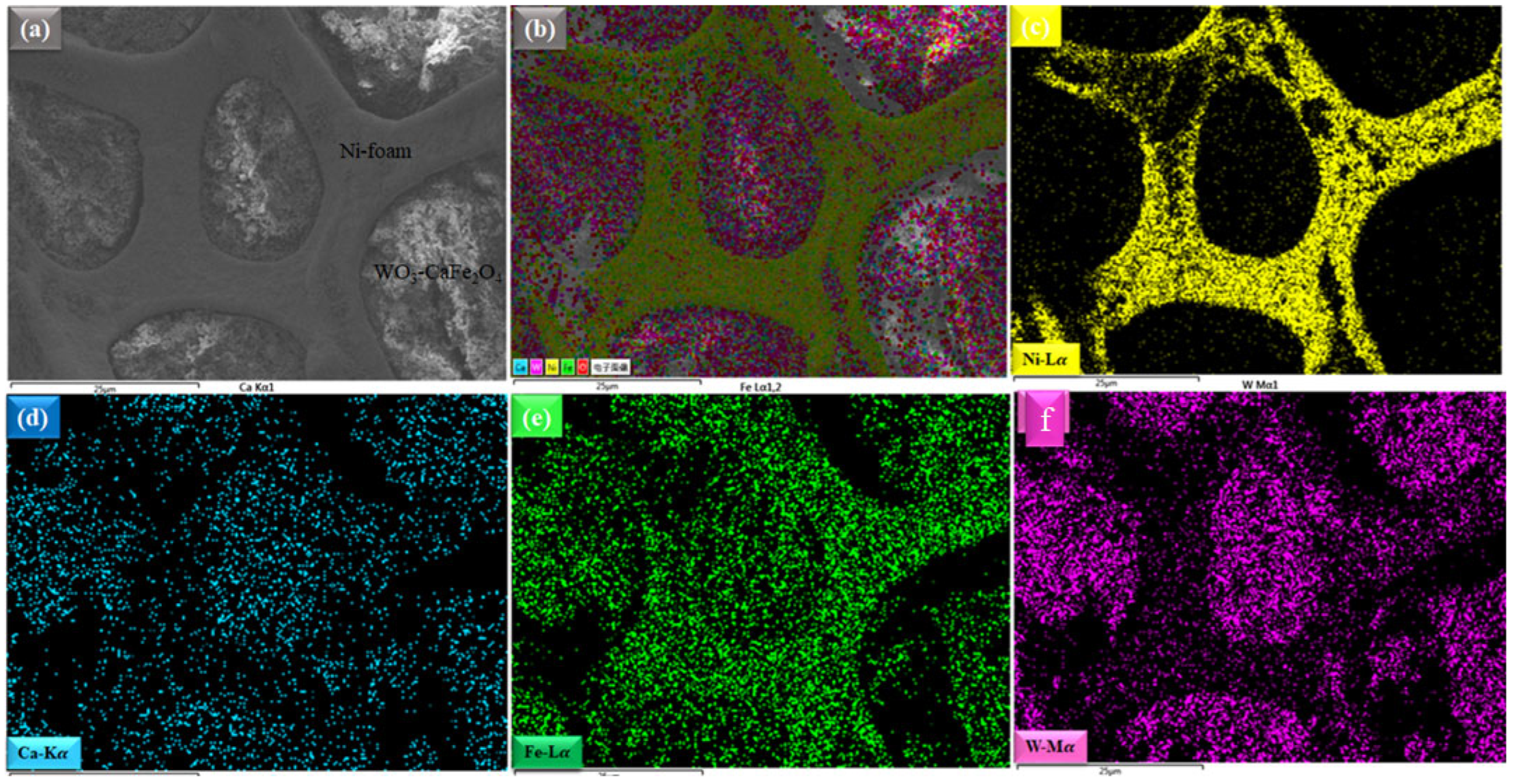
3.1. Structure & Composition Analysis
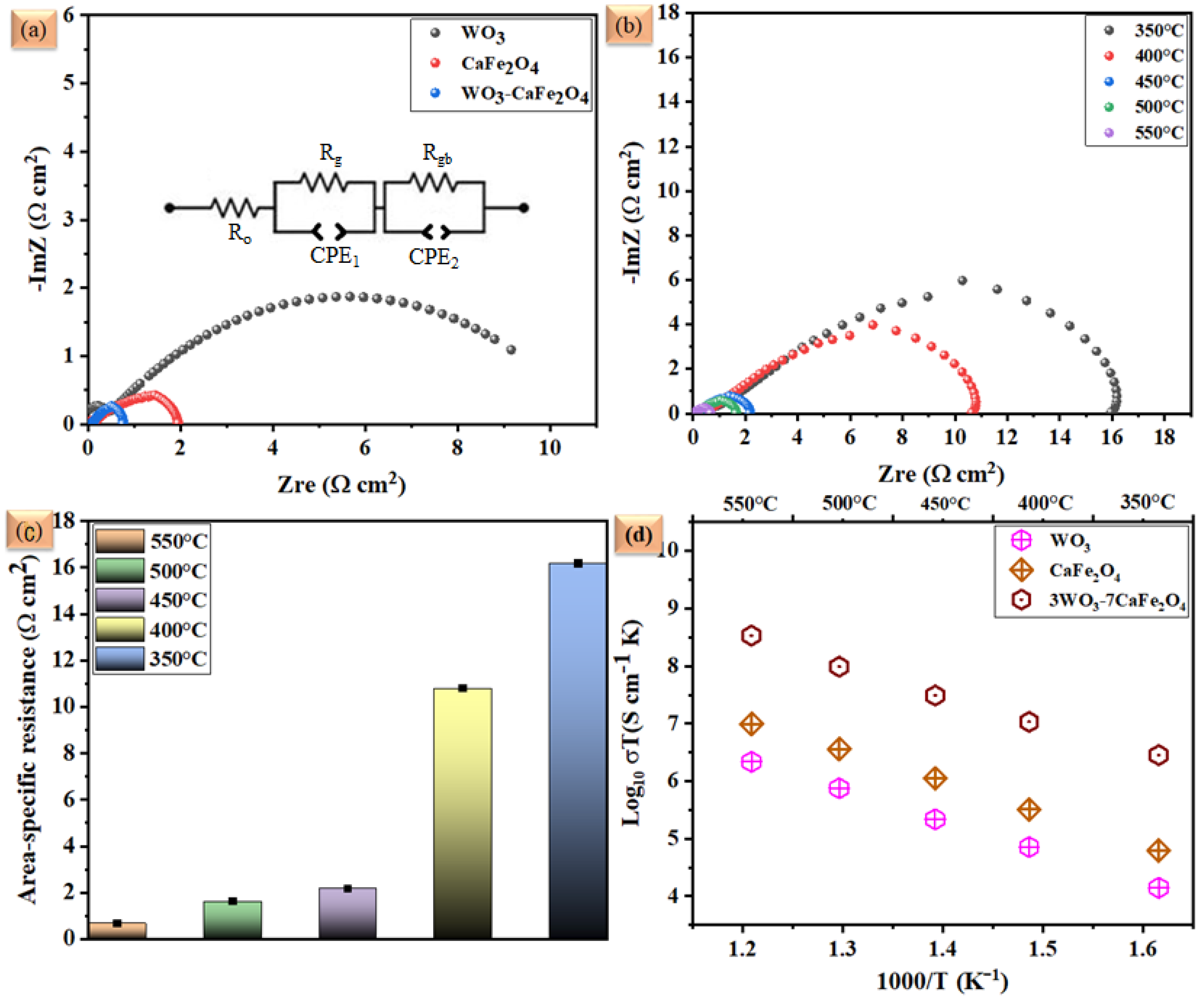
3.2. Electrochemical Impedance and Electrical Conductivity
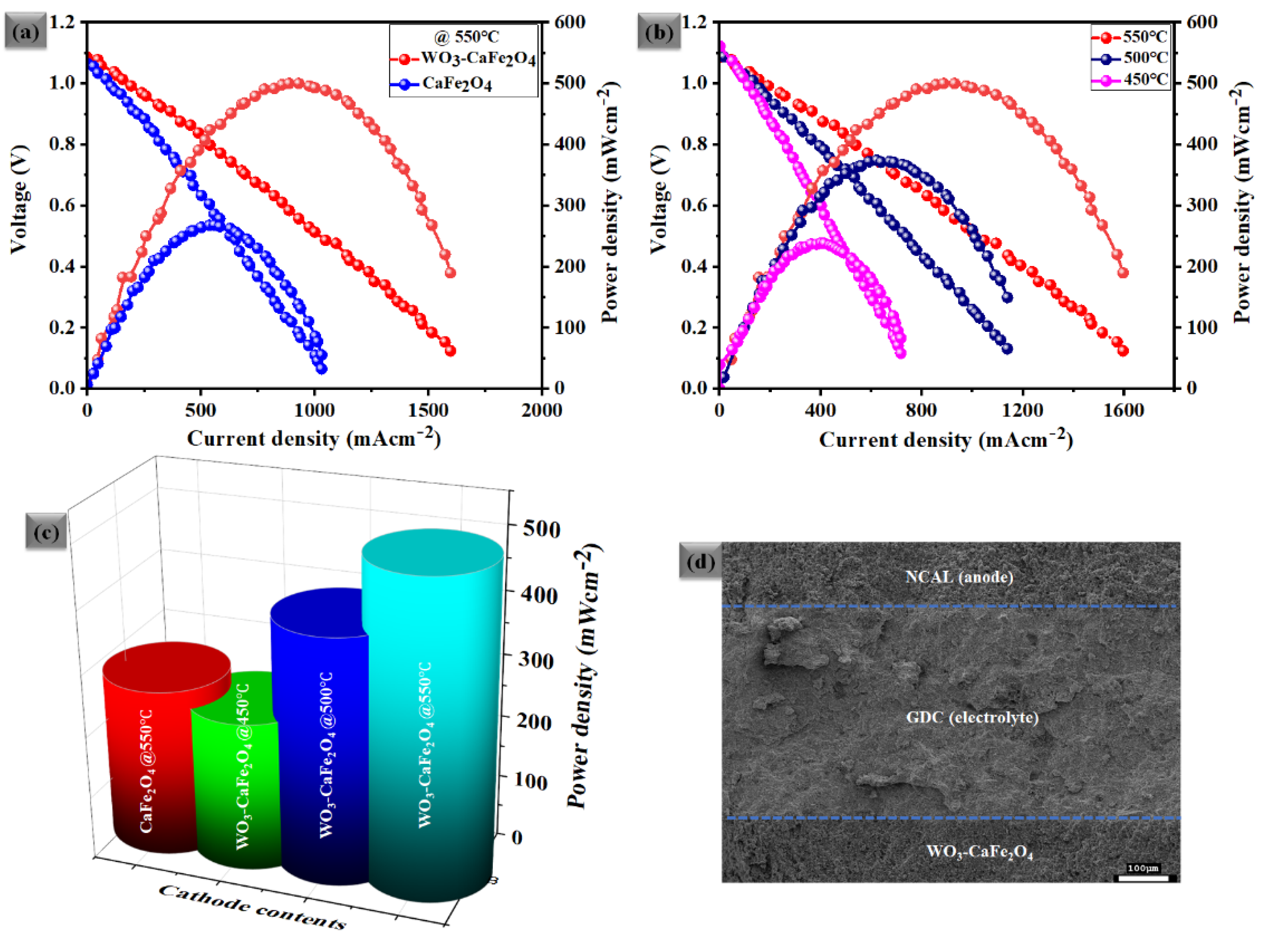
3.3. Electrochemical Performance Measurements
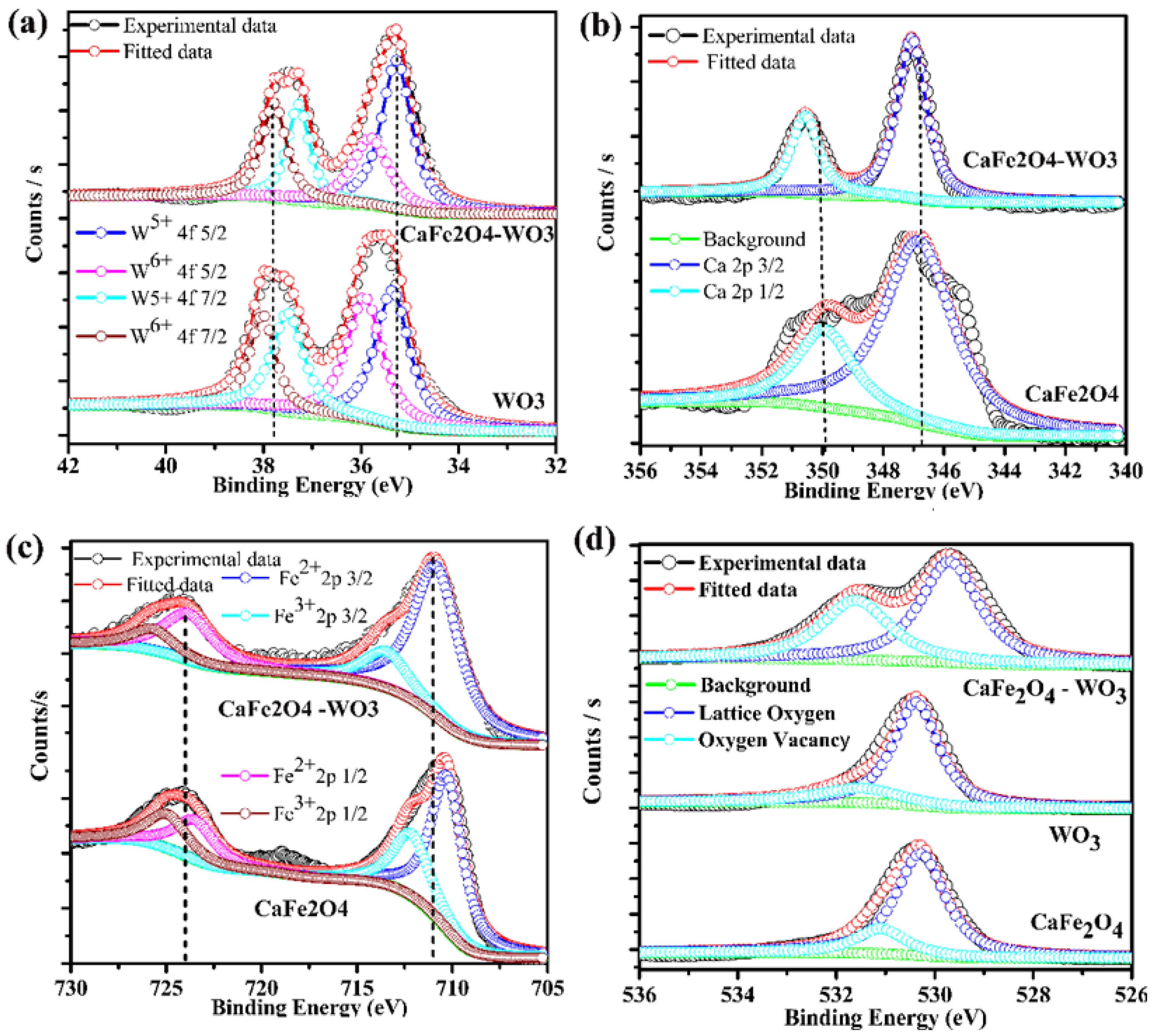
3.4. Spectroscopic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ormerod, R.M. Solid oxide fuel cells. Chem. Soc. Rev. 2003, 32, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.J. Materials for solid oxide fuel cells. Chem. Mater. 2010, 22, 660–674. [Google Scholar] [CrossRef]
- Wachsman, E.D.; Lee, K.T. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A. Progress in solid oxide fuel cell (SOFC) research. JOM 2019, 71, 88–89. [Google Scholar] [CrossRef]
- Zhang, C. Solid oxide fuel cells: Low temperature cathodes. Nat. Energy 2016, 1, 16200. [Google Scholar] [CrossRef]
- Rioja-Monllor, L.; Bernuy-Lopez, C.; Fontaine, M.-L.; Grande, T.; Einarsrud, M.-A. Processing of high performance composite cathodes for protonic ceramic fuel cells by exsolution. J. Mater. Chem. A 2019, 7, 8609–8619. [Google Scholar] [CrossRef]
- Han, G.D.; Bae, K.; Kang, E.H.; Choi, H.J.; Shim, J.H. Inkjet printing for manufacturing solid oxide fuel cells. ACS Energy Lett. 2020, 5, 1586–1592. [Google Scholar] [CrossRef]
- Leng, Y.; Chan, S.; Khor, K.; Jiang, S. Performance evaluation of anode-supported solid oxide fuel cells with thin film YSZ electrolyte. Int. J. Hydrogen Energy 2004, 29, 1025–1033. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Hagen, A.; Barfod, R.; Chen, M.; Wang, H.-J.; Poulsen, F.W.; Hendriksen, P.V. Microstructural studies on degradation of interface between LSM–YSZ cathode and YSZ electrolyte in SOFCs. Solid State Ion. 2009, 180, 1298–1304. [Google Scholar] [CrossRef]
- Miao, L.; Hou, J.; Dong, K.; Liu, W. A strategy for improving the sinterability and electrochemical properties of ceria-based LT-SOFCs using bismuth oxide additive. Int. J. Hydrogen Energy 2019, 44, 5447–5453. [Google Scholar] [CrossRef]
- Tihtih, M.; Ibrahim, J.E.F.; Basyooni, M.A.; Kurovics, E.; Belaid, W.; Hussainova, I.; Kocserha, I. Role of A-site (Sr), B-site (Y), and A, B sites (Sr, Y) substitution in lead-free BaTiO3 ceramic compounds: Structural, optical, microstructure, mechanical, and thermal conductivity properties. Ceram. Int. 2022, 49, 1947–1959. [Google Scholar] [CrossRef]
- Tihtih, M.; Ibrahim, J.E.F.M.; Basyooni, M.A.; Belaid, W.; Gömze, L.A.; Kocserha, I. Structural, optical, and electronic properties of barium titanate: Experiment characterisation and first-principles study. Mater. Technol. 2022, 37, 2995–3005. [Google Scholar] [CrossRef]
- Mushtaq, N.; Lu, Y.; Xia, C.; Dong, W.; Wang, B.; Shah, M.Y.; Rauf, S.; Akbar, M.; Hu, E.; Raza, R. Promoted electrocatalytic activity and ionic transport simultaneously in dual functional Ba0.5Sr0.5Fe0.8Sb0.2O3-δ-Sm0.2Ce0.8O2-δ heterostructure. Appl. Catal. B Environ. 2021, 298, 120503. [Google Scholar] [CrossRef]
- Zhou, X.-D. Kill Two Problems with One Dual-Ion Cell. Joule 2019, 3, 2595–2597. [Google Scholar] [CrossRef]
- Shah, M.Y.; Lu, Y.; Mushtaq, N.; Rauf, S.; Yousaf, M.; Asghar, M.I.; Lund, P.D.; Zhu, B. Demonstrating the potential of iron-doped strontium titanate electrolyte with high-performance for low temperature ceramic fuel cells. Renew. Energy 2022, 196, 901–911. [Google Scholar] [CrossRef]
- Choi, S.; Kucharczyk, C.J.; Liang, Y.; Zhang, X.; Takeuchi, I.; Ji, H.-I.; Haile, S.M. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 2018, 3, 202–210. [Google Scholar] [CrossRef]
- Song, X.; Guo, W.; Guo, Y.; Mushtaq, N.; Shah, M.Y.; Irshad, M.S.; Lund, P.D.; Asghar, M.I. Nanocrystalline surface layer of WO3 for enhanced proton transport during fuel cell operation. Crystals 2021, 11, 1595. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.; Mushtaq, N.; Shah, M.Y.; Irshad, S.; Rauf, S.; Motola, M.; Yan, S.; Zhu, B. Excellent oxygen reduction electrocatalytic activity of nanostructured CaFe2O4 particles embedded microporous Ni-Foam. Int. J. Hydrogen Energy 2022, 47, 10331–10340. [Google Scholar] [CrossRef]
- Zhou, X.; Hou, N.; Gan, T.; Fan, L.; Zhang, Y.; Li, J.; Gao, G.; Zhao, Y.; Li, Y. Enhanced oxygen reduction reaction activity of BaCe0.2Fe0.8O3-δ cathode for proton-conducting solid oxide fuel cells via Pr-doping. J. Power Sources 2021, 495, 229776. [Google Scholar] [CrossRef]
- Tarutina, L.R.; Lyagaeva, J.G.; Farlenkov, A.S.; Vylkov, A.I.; Vdovin, G.K.; Murashkina, A.A.; Demin, A.K.; Medvedev, D.A. Doped (Nd, Ba) FeO3 oxides as potential electrodes for symmetrically designed protonic ceramic electrochemical cells. J. Solid State Electrochem. 2020, 24, 1453–1462. [Google Scholar] [CrossRef]
- Duan, C.; Tong, J.; Shang, M.; Nikodemski, S.; Sanders, M.; Ricote, S.; Almansoori, A.; O’Hayre, R. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 2015, 349, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, N.; Xia, C.; Dong, W.; Wang, B.; Raza, R.; Ali, A.; Afzal, M.; Zhu, B. Tuning the energy band structure at interfaces of the SrFe0.75Ti0.25O3−δ–Sm0.25Ce0.75O2−δ heterostructure for fast ionic transport. ACS Appl. Mater. Interfaces 2019, 11, 38737–38745. [Google Scholar] [CrossRef]
- Shah, M.Y.; Lu, Y.; Mushtaq, N.; Yousaf, M.; Rauf, S.; Asghar, M.I.; Lund, P.D.; Zhu, B. Perovskite Al-SrTiO3 semiconductor electrolyte with superionic conduction in ceramic fuel cells. Sustain. Energy Fuels 2022, 6, 3794–3805. [Google Scholar] [CrossRef]
- Mueller, D.N.; De Souza, R.A.; Yoo, H.-I.; Martin, M. Phase stability and oxygen nonstoichiometry of highly oxygen-deficient perovskite-type oxides: A case study of (Ba, Sr)(Co, Fe)O3−δ. Chem. Mater. 2012, 24, 269–274. [Google Scholar] [CrossRef]
- Chandramohan, P.; Srinivasan, M.; Velmurugan, S.; Narasimhan, S. Cation distribution and particle size effect on Raman spectrum of CoFe2O4. J. Solid State Chem. 2011, 184, 89–96. [Google Scholar] [CrossRef]
- Singh, S.; Khare, N. Defects/strain influenced magnetic properties and inverse of surface spin canting effect in single domain CoFe2O4 nanoparticles. Appl. Surf. Sci. 2016, 364, 783–788. [Google Scholar] [CrossRef]
- Kotomin, E.A.; Mastrikov, Y.A.; Merkle, R.; Maier, J. First principles calculations of oxygen reduction reaction at fuel cell cathodes. Curr. Opin. Electrochem. 2020, 19, 122–128. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Bououdina, M.; Vijaya, J.J.; Kennedy, L.J. Spinel ferrite nanoparticles: Synthesis, crystal structure, properties, and perspective applications. In Proceedings of the International Conference on Nanotechnology and Nanomaterials, Lviv, Ukraine, 24–27 August 2016; Springer International Publishing: Berlin/Heidelberg, Germany; pp. 305–325. [Google Scholar]
- Zuo, F.; Wang, L.; Wu, T.; Zhang, Z.; Borchardt, D.; Feng, P. Self-doped Ti3+ enhanced photocatalyst for hydrogen production under visible light. J. Am. Chem. Soc. 2010, 132, 11856–11857. [Google Scholar] [CrossRef]
- Liu, M.; Lynch, M.E.; Blinn, K.; Alamgir, F.M.; Choi, Y. Rational SOFC material design: New advances and tools. Mater. Today 2011, 14, 534–546. [Google Scholar] [CrossRef]
- Vøllestad, E.; Strandbakke, R.; Tarach, M.; Catalán-Martínez, D.; Fontaine, M.-L.; Beeaff, D.; Clark, D.R.; Serra, J.M.; Norby, T. Mixed proton and electron conducting double perovskite anodes for stable and efficient tubular proton ceramic electrolysers. Nat. Mater. 2019, 18, 752–759. [Google Scholar] [CrossRef]
- An, H.; Lee, H.-W.; Kim, B.-K.; Son, J.-W.; Yoon, K.J.; Kim, H.; Shin, D.; Ji, H.-I.; Lee, J.-H. A 5 × 5 cm2 protonic ceramic fuel cell with a power density of 1.3 W cm−2 at 600 °C. Nat. Energy 2018, 3, 870–875. [Google Scholar] [CrossRef]
- Campbell, C.T.; Peden, C.H. Oxygen vacancies and catalysis on ceria surfaces. Science 2005, 309, 713–714. [Google Scholar] [CrossRef]
- Chen, M.; Paulson, S.; Kan, W.H.; Thangadurai, V.; Birss, V. Surface and bulk study of strontium-rich chromium ferrite oxide as a robust solid oxide fuel cell cathode. J. Mater. Chem. A 2015, 3, 22614–22626. [Google Scholar] [CrossRef]
- Mantzavinos, D.; Hartley, A.; Metcalfe, I.S.; Sahibzada, M. Oxygen stoichiometries in La1−xSrxCo1−yFeyO3−δ perovskites at reduced oxygen partial pressures. Solid State Ion. 2000, 134, 103–109. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, H.; Li, X.; Li, Q.; Wang, J.; Zhu, X.; Yang, W. Oxygen evolution reaction over Fe site of BaZrxFe1−xO3−δ perovskite oxides. Electrochim. Acta 2017, 241, 433–439. [Google Scholar] [CrossRef]
- Wang, Z.; You, Y.; Yuan, J.; Yin, Y.-X.; Li, Y.-T.; Xin, S.; Zhang, D. Nickel-Doped La0.8Sr0.2Mn1−xNixO3 Nanoparticles Containing Abundant Oxygen Vacancies as an Optimized Bifunctional Catalyst for Oxygen Cathode in Rechargeable Lithium–Air Batteries. ACS Appl. Mater. Interfaces 2016, 8, 6520–6528. [Google Scholar] [CrossRef]
- Oh, N.K.; Kim, C.; Lee, J.; Kwon, O.; Choi, Y.; Jung, G.Y.; Lim, H.Y.; Kwak, S.K.; Kim, G.; Park, H. In-situ local phase-transitioned MoSe2 in La0.5Sr0.5CoO3-δ heterostructure and stable overall water electrolysis over 1000 hours. Nat. Commun. 2019, 10, 1723. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Qiu, F.; Alomar, M.; Alqarni, A.S.; Mushtaq, N.; Yousaf Shah, M.A.K.; Qi, F.; Yan, S.; Lu, Y. Investigating the Electrochemical Properties of a Semiconductor Heterostructure Composite Based on WO3-CaFe2O4 Particles Planted on Porous Ni-Foam for Fuel Cell Applications. Crystals 2023, 13, 444. https://doi.org/10.3390/cryst13030444
Li J, Qiu F, Alomar M, Alqarni AS, Mushtaq N, Yousaf Shah MAK, Qi F, Yan S, Lu Y. Investigating the Electrochemical Properties of a Semiconductor Heterostructure Composite Based on WO3-CaFe2O4 Particles Planted on Porous Ni-Foam for Fuel Cell Applications. Crystals. 2023; 13(3):444. https://doi.org/10.3390/cryst13030444
Chicago/Turabian StyleLi, Junjiao, Fei Qiu, Muneerah Alomar, Areej S. Alqarni, Naveed Mushtaq, M. A. K. Yousaf Shah, Fenghua Qi, Senlin Yan, and Yuzheng Lu. 2023. "Investigating the Electrochemical Properties of a Semiconductor Heterostructure Composite Based on WO3-CaFe2O4 Particles Planted on Porous Ni-Foam for Fuel Cell Applications" Crystals 13, no. 3: 444. https://doi.org/10.3390/cryst13030444
APA StyleLi, J., Qiu, F., Alomar, M., Alqarni, A. S., Mushtaq, N., Yousaf Shah, M. A. K., Qi, F., Yan, S., & Lu, Y. (2023). Investigating the Electrochemical Properties of a Semiconductor Heterostructure Composite Based on WO3-CaFe2O4 Particles Planted on Porous Ni-Foam for Fuel Cell Applications. Crystals, 13(3), 444. https://doi.org/10.3390/cryst13030444







