Abstract
Azobenzene derivatives are a unique class of photo-switch molecules with promising potential for nanoscale optical applications. We have studied the self-assembly and photo-induced mechanical switching of azobenzene derivatives on Au(111) at the single-molecule level by using scanning tunneling microscope (STM). 4,4′-Dibromo-azobenzene (Br-AB) molecules are assembled into two types of well-ordered structures on Au(111) surfaces in the trans-isomer configuration. Br-AB molecules experienced configurational changes from trans-to-cis photo-isomerization upon the exposure to the UV light. This photo-isomerization of Br-AB molecules was observed to occur at random sites of ordered structure, evidenced by the appearance of bright protrusions with the height increment. Our results may open up new routes to engineer nanoscale photo-switch molecular devices.
1. Introduction
On-surface intermolecular interactions by noncovalent bonding can result in two-dimensional (2D) supramolecular self-assembly [1]. These stabilized structures have shown promising applications in organic electronic and opto-electronic devices as active layers [2,3,4]. The 2D self-assembled molecular networks are poised by several types of interactions, e.g., hydrogen bonding [5], van der Waals interactions [6], halogen bonds [7,8], metal–organic coordination [9], etc. On the other hand, electrostatic interactions between molecules and substrate surfaces can provide additional pathways to direct self-assembly [10]. A number of molecular self-assembled structures based on intermolecular interactions have been investigated on the metal surfaces under UHV conditions. Additionally, molecule–substrate interactions can also be enhanced or obstructed by carefully selecting the substrate material [11]. These interactions significantly affect the structural and electronic properties of the resulting networks.
Previous studies have shown that surface properties can also be modified by providing surface passivation to decouple the molecules from the surface. The surface passivation could be performed by oxide [12], thiol, or halide layers [13] or by spacer groups [14]. This results in decreased reactivity and thus supports the formation of large-scale self-assembled ordered structures. Meanwhile, molecules chemisorbed on the bare substrate surfaces will result in disordered geometries [15]. Metal surfaces also provide catalytic properties to initiate Ullmann coupling reaction by the dehalogenation of organic molecules, as demonstrated by a large number of examples such as diiodo and dibromobenzene, as well as for large complex structured molecules [16]. On the other hand, photo-switching molecules provide additional dimensions to control the properties of the assembled networks. These molecules act as a photo mechanical switch at the single molecule scale [17]. It has been proposed that photo-switching properties would be retained in the self-assembled structures for their applications in photomechanical nanomachines and devices such as photoactive OFETs and optoelectronic switches [18]. This requires molecules with robust photo mechanical switching properties. Azobenzene is one such extensively studied photo-switch molecule. Previous STM studies on photo-switch azobenze molecules [19,20,21] on metal substrates have shown that photo-isomerization properties also depend upon the molecule–substrate coupling and inter-molecular interactions. Non-covalent inter-molecular interactions via halogen bonds derive self-assemblies with good crystalline properties and high charge mobility [22,23], which are beneficial for the organic photo-switch devices. However, there is a lack of studies on the photo-isomerization properties of photo-switch azobenzene-based supramolecular self-assemblies derived from halogen–halogen or halogen–hydrogen electrostatic interactions. Therefore, it is an important issue to study: how do the photo-switch halogenated molecules self-assemble on the catalytic substrate surface? Do photo-switching molecules retain their properties upon self-assembly for their use in photo-switch devices?
Here, we employ the scanning tunneling microscopy (STM) to spatially resolve the characteristics of photo-switchable azobenzene molecules on a gold surface. We observe that 4,4′-dibromo-azobenzene (Br-AB) molecules adsorbed on the Au(111) surface in the trans-isomer form self-assembles into two well-ordered structures. The grain boundaries of these two orientations are clearly resolved. Upon the exposure to UV light (365 nm), the adsorbed Br-AB molecules exhibit the configurational changes and appeared in an aggregated form. Such configurational changes are solely induced by the UV light exposure, in consistence with the expected photo-induced trans-to-cis isomerization for Br-AB molecules.
2. Experiment and Results
STM experiments were performed using variable temperature ultrahigh vacuum (UHV) STM (ScientaOmicron, Taunusstein, Germany) operating at base pressure (5 × 10−11 Torr). All STM experiments were performed at room temperature. The Au(111) surface was cleaned by repeated standard cycles of Ar-ion sputtering and annealing. The irradiation of UV light was performed using the 365 nm UV LED light source aligned at an external viewport directed towards the substrate surface. During the UV light irradiation, the sample was maintained in an UHV environment without exposure to the atmosphere. The whole experiment was performed multiple times to ensure the data reproducibility.
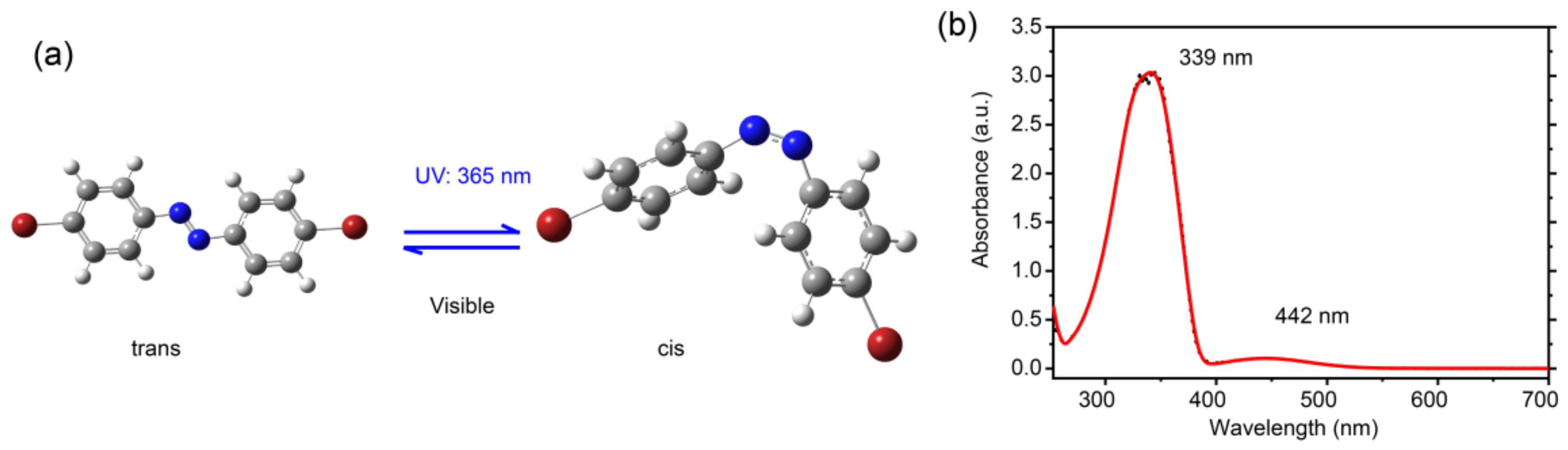
Figure 1a shows the chemical structure of the Br-AB molecule. Br-AB molecules were purchased from BLD PHARM. Azobenzene are aromatic compounds consisting of two phenyl rings which are interconnected by the -N=N- functional group. It has two isomeric states, namely the cis-isomer (the “Z” form) and trans-isomer (the “E” form). Compared to the bent cis-state, the trans-form is more thermodynamically stable and has an extended shape, as shown in Figure 1a. The trans-state can further convert to cis-isomer upon the exposure to UV light [24]. The cis-state is a higher energy state which can undergo thermal relaxation in the dark environment. The irradiation with longer wavelength in the visible range, can also transform it into the trans-state [24]. The isomerization process can be achieved for many cycles without inducing photobleaching [25]. The substitution on azobenzene molecules directly affects the isomerization properties, as directly evidenced by the observed shifting of the absorption wavelength [26]. For example, the substitution with an electro-negative group at both ortho and para positions results in the red shift of the absorption maxima of the trans-state [26,27,28]. In order to check the influence of highly electronegative bromine, we performed the UV–visible absorption spectroscopy. Figure 1b shows the absorption spectra of the dibromo-azobenzene solution in dichloromethane. It shows two peak maxima at 339 nm and 442 nm. A comparison with the literature shows that the absorption peak at 339 nm is corresponding to the trans-state due to π–π* electron transitions, while the absorption peak at 442 nm to the cis-isomer due to n–π* electron transitions [27]. On the other hand, a comparison with pristine azobenzene molecules’ absorption shows that a 339 nm peak associated with the trans isomer is red-shifted, while the 442 nm peak associated with the cis-isomer is blue-shifted [28,29]. Such results indicate that Br-AB molecules mainly adopt the trans-state in the solution, which is consistent with the fact that the trans-state is the energetically stable state.
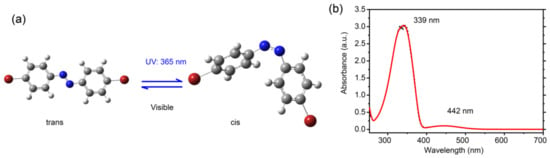
Figure 1.
(a) Chemical structure of 4,4′-dibromo-azobenzene (Br-AB). Upon UV light exposure, the trans-isomer transforms into cis-isomer; and (b) Absorption spectra of the Br-AB molecular solution in dichloromethane.
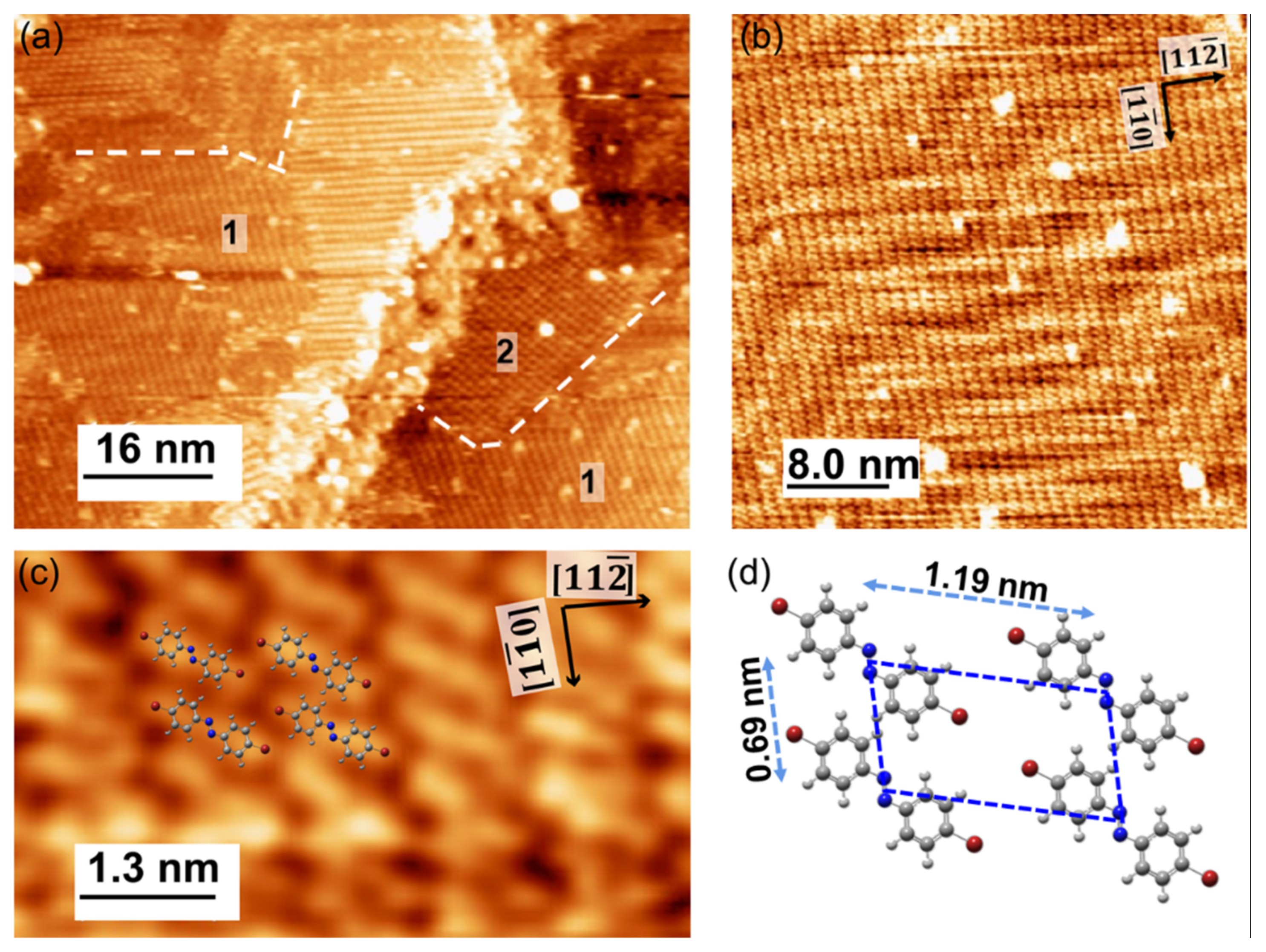
After the cycles of sputtering and annealing, the clean Au(111) surface was achieved and the cleanness of the surface can be checked by STM images. The surface was scanned by an electrochemically etched tungsten tip in constant current mode. Subsequently, the Br-AB molecules were thermally evaporated with the source temperature of 363 K on the Au(111) surface with the deposition rate of 0.05 ML/min at a substrate temperature of ~300 K. Figure 2a shows that the STM image of the Br-AB monolayer on Au(111). The scanned image shows the existence of two different molecular configurations on the Au(111) surface. The intact herringbone reconstructions of the Au(111) surface are observable, indicating a weak interaction between the Au substrate and molecules. Furthermore, different grain boundaries are also visible, representing different molecular orientations. Note that the orientation of herringbones is along the direction of Au(111) surface, as depicted in the figures. These molecular orientations probably arise from different intermolecular interactions. The Br-AB molecule has two bromine atoms which could dictate these interactions, such as Br-Br, Br-H, as well as H-H and N-H-like bonding on surface [5,7,8,9]. At high coverage in saturation regimes, where molecules are more closed-packed, the intermolecular interactions become dominant over the molecule substrate forces, and therefore do not affect the underlying herringbone reconstruction and lead to the observation of the underlying herringbones of Au(111); thus, the resulting phases do not arrange with the herringbone reconstruction [30]. The role of the substrate in different growth orientations could be excluded, as depicted by the weak interaction of the molecules with the substrate by the observation of a clear herringbone structure. Figure 2b shows the scan image of the Br-AB molecules with herringbone in the background. This further encourages the choice of substrate to reduce the hybridization of the azo group with the substrate surface in order to study the photo-isomerization reaction [31]. Figure 2c shows the close-up image of the Br-AB molecule on Au(111) surface. The proposed molecular arrangement is overlaid on the topographic image based on the accurate distance measurement. The proposed molecular structure model is consistent with the physical size of the molecules and features observed in the STM image [32]. Figure 2d shows a proposed molecular configuration based on the topographic image. The ball-and-stick model of Br-AB in the trans-state shows the arrangement of molecules seen in Figure 2c.
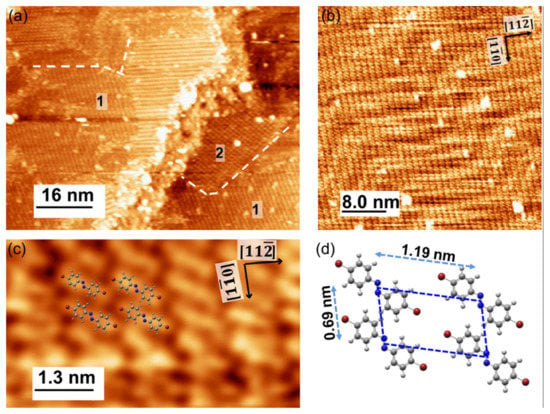
Figure 2.
(a) STM constant-current image of Br-AB molecules on the Au(111) surface (−1.2 V, 0.3 nA, 80 × 51 nm2); (b) Scan image of the Br-AB molecules with herringbone in the background (−1.2 V, 600 pA, 40 × 40 nm2); (c) Close-up image of Br-AB molecules on Au(111). The proposed molecular self-assembly structural model is overlaid on the image, (−1.2 V, 600 pA, 7 × 4 nm2); (d) Ball–stick model of the trans-isomer of the Br-AB molecule representing the arrangement as seen in (c). The red, grey, blue, and white balls correspond to the bromine, carbon, nitrogen, and hydrogen atoms, respectively.
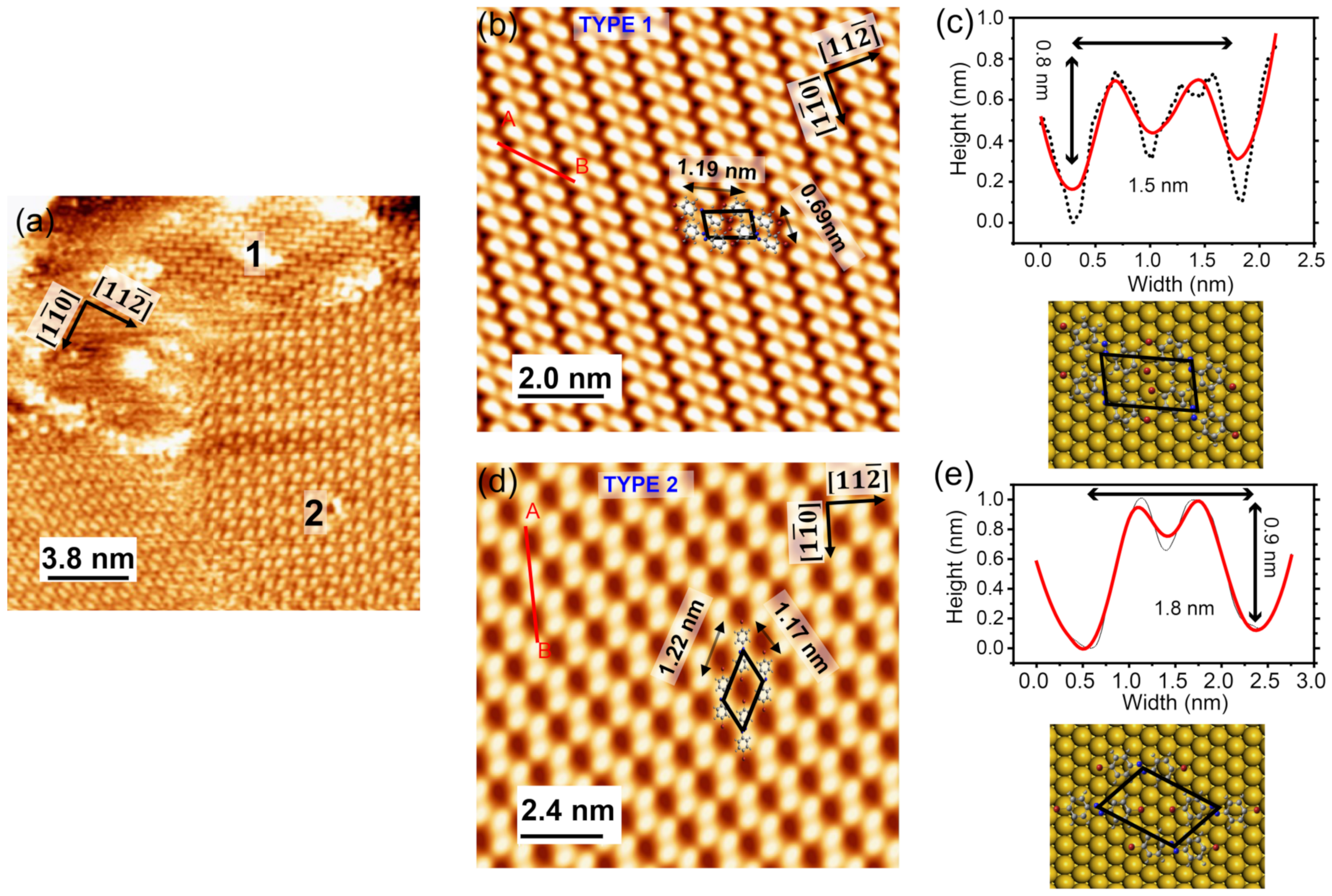
With the increasing molecular coverage, where the Au(111) surface is saturated by Br-AB molecules, two different molecular orderings were observed, as shown in Figure 3a. In order to study the different configurations of the adsorbed molecules, we zoomed in by scanning small regions. Figure 3b shows one of the areas named type 1. The STM image shows a typical two-lobe structure, similar to the reported images of the azobenzene molecules grown on the Au(111) surface [30]. Figure 3c shows the measured dimensions of such two-lobe structures, which is ~1.5 nm in width and an apparent height of 0.8 nm. A comparison of the measured dimension with the expected Br-AB size, suggests that the two-lobe structure corresponds to the target Br-AB molecule. This could be further confirmed by comparing the dimension with the TTB-AB [33] and azobenzene molecules [30]. Each lobe appeared in the STM topographic image, and corresponded to the position of one phenyl ring of Br-AB molecule. Furthermore, the two-lobe structure indicates that Br-AB molecules are adsorbed in the planar configuration on the Au(111). The orientation of Br-AB molecules with respect to the Au(111) surface atoms was determined by comparing the two-lobe molecular structure with the orientations of underlying herringbones, and the atomic row configuration of the Au(111) surface is shown in Figure 3c (bottom panel). The molecular lattice can be defined by the lattice vectors aligned with the crystallographic directions of Au(111). The lattice vector lengths for this periodic molecular structure type 1 were found to be 1.19 ± 0.1 nm and 0.69 ± 0.1 nm, as averaged over many molecular sites. The dihedral angle α = 101 ± 1°. This periodic arrangement of phase 1 is a closed-packed structure, such that the phenyl lobes of Br-AB lie on the slightly distorted hcp lattice [30].
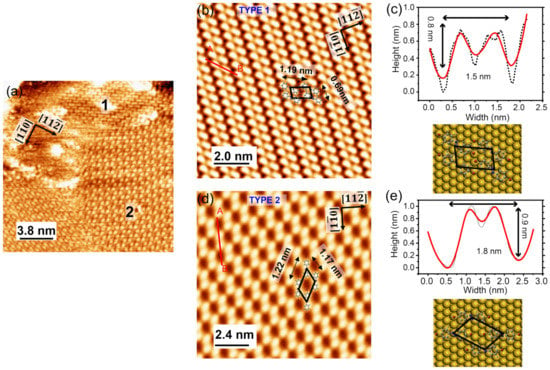
Figure 3.
(a) STM topographic image of Br-AB molecules on Au(111) exhibits domains with different molecular orientations (−0.5 V, 0.7 nA, 19 × 19 nm2). (b) Two-lobe structure of Br-AB type 1 molecules on the Au(111) substrate surface (−0.5 V, 600 pA, 10 × 10 nm2). (c) Height profile of the dibromo-azobenzene molecule on Au(111) in the type 1 phase, measured along the red line (A–B in the panel) (b). The bottom panel shows the ball-and-stick model of the Br-AB molecule representing the arrangement, as seen in (b). (d) The STM topographic image of the type 2 configuration of the Br-AB molecules (−1.2 V, 0.6 nA, 12 × 8 nm2). (e) Height profile of the dibromo-azobenzene molecule on Au(111) in the type 2 phase, measured along the red A–B line in panel (d). Bottom panel shows ball-and-stick model of Br-AB molecule representing the arrangement as seen in (d).
Our STM topographic imaging shows another molecular arrangement, here named as type 2 and shown in Figure 3d. In this molecular lattice, the molecules are arranged in the honeycomb-type structure. This phase has a lower packing fraction compared to the type 1 phase. The lattice vector lengths of the type 2 periodic molecular structures were found to be 1.22 ± 0.1 nm and 1.17 ± 0.1 nm. The dihedral angle β = 45 ± 1°. These vector lengths are slightly bigger than type 1. On the other hand, the dimensions of one of the Br-AB molecules in type 2 phase are 1.8 nm width and 0.9 nm height. Figure 3e shows the measured dimensions of the single molecule in type 2 configuration. A comparison of the dimensions and lattice vector lengths suggests that both type 1 and type 2 superstructures experience different interactions. These interactions might be assigned to the H-H and N-H bonding of Br-AB molecules and to the interaction of Br atoms with hydrogen atoms attached to the benzene ring, producing Br-H bonds, respectively [34,35].
Since azobenzene is an isomeric molecule, it shows two configuration states upon photoexcitation. The topographic images clearly show that these molecules lie flat on the surface after deposition, which reflects a thermodynamically stable trans-state. We do not observe any of the molecules in the standing configuration representing the cis-isomeric state. This is further confirmed by comparing the height profile as well as the adsorption behavior with the previous literature [17,33,36].
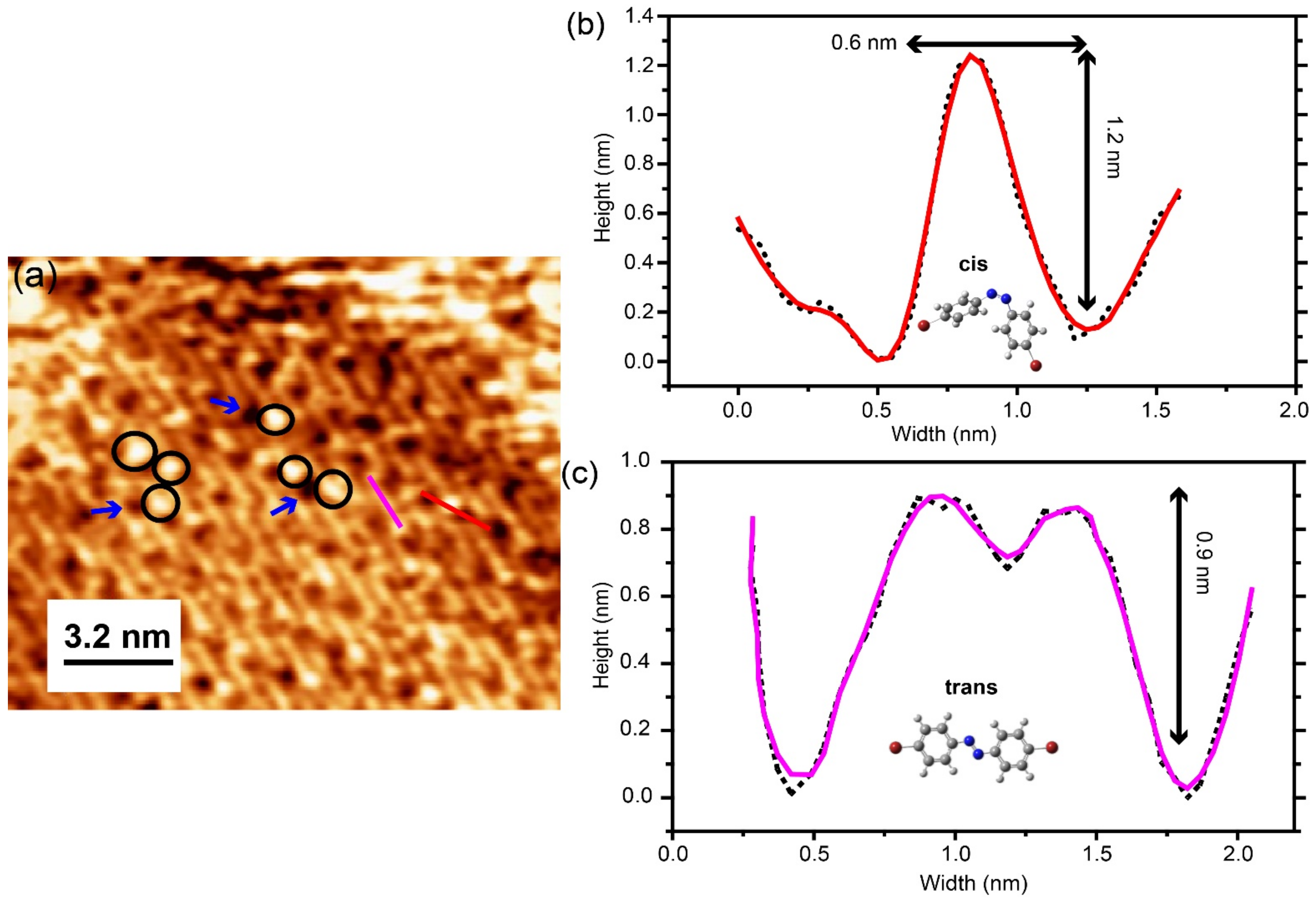
In order to study the isomerization process of different assemblies of the Br-AB molecules, we performed photo-switching experiments. For this purpose, we shined the UV light of a wavelength of 365 nm with a power of 2 Watt for a 15 min exposure on the as-prepared Br-AB molecules adsorbed on the Au(111) surface. The initial stage of the molecules was in the trans-isomer lying flat on the surface and all molecules appeared to be the same before the UV light exposure regardless of phase. After exposure to UV light, the molecular aggregates were observed along the domain boundary edges as shown in Figure 4a. Additionally, molecular-sized bright protrusions appeared on the islands marked by black circles. Most of these optically induced structures tended to have a similar apparent height of 1.2 nm. Figure 4b shows one such height profile measured from bright protrusions. This apparent height is different in comparison to the 0.9 nm height of the flat-lying trans molecules imaged at the same bias voltage [20,37,38] of −1.2 V, as shown in Figure 3e and Figure 4c. Note that these molecule heights are measured by STM, taken at identical scanning parameters in order to minimize the effect of the electron distribution function. These newly emerged structures with a similar apparent height suggest that they represent the single molecules in the new cis-state, as reported for cis-azobenzene isomers on metals [33]. The photo-switched single cis-molecules typically appeared near the dark spots marked by arrows at random positions, which is similar to the case of TTB-azobenzene on Au(111) [33,39]. By comparing the STM images with reference [33], in which the dark strips appear along with bright protrusions upon photo-isomerization, we refer to these dark spots as vacancies, possibly originated from either the change in configuration of planar trans-isomer on the surface or the rearrangement of self-assembly due to the heat generated by UV light. Although the exact mechanism of such observation is still unclear. The observation of the cis-isomer clusters and vacancies after the exposure of UV light suggests that molecules in the cis-isomer state tend to aggregate, which is different from the previous reports [17,33,39].
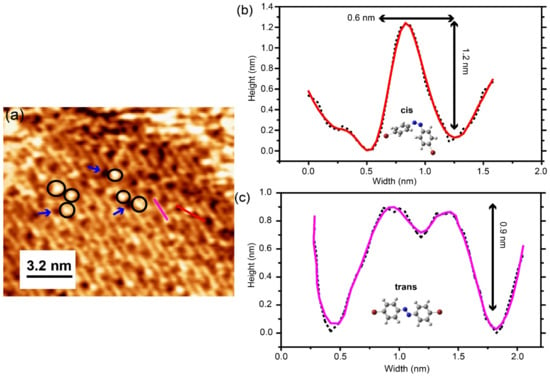
Figure 4.
(a) STM topographic image of the photo-isomerization reaction of azo/Au(111) after the exposure to 365 nm UV light. Areas highlighted areas in circles show the cis-isomer states. Arrows show the vacancies. (b) Height profile of cis-isomers, measured along the red line in (a). Inset shows the model of Br-AB molecule in the cis-isomer state. (c) Height profile of trans-isomers, measured along the purple line in (a). The inset shows the model of Br-AB molecule in the trans-isomer state. Both the height profile measurements were performed under imaging conditions: (−1.2 V, 0.3 nA, 16 × 12 nm2). Black dots represent the raw data, red and purple solid lines represent the smoothed data.
3. Conclusions
In conclusion, we studied the self-assembly properties of the photo-switch molecules on the Au(111) surface. At high coverages, molecular assemblies are derived by inter-molecular interactions as dominating forces. We experimentally observed the photo-induced trans-to-cis isomerization for Br-AB molecules at a metal surface. The photo-switching of the molecules occurs at random sites. These results show important aspects for future photomechanical device engineering and open new opportunities for nanometer-size electronic devices such as light-induced functionalities in molecular machines, photoactive OFETs, and optoelectronic switches.
Author Contributions
Conceptualization, T.I.; X.Z. and M.P.; methodology, T.I., M.P. and J.G.; software, T.I., M.L., H.L., S.F. and X.C.; validation, T.I., M.P. and J.G.; formal analysis, T.I., X.Z., M.P. and F.L.; investigation, T.I. and X.Z.; resources, J.G., F.L. and M.P.; data curation, T.I. and X.Z.; writing—original draft preparation, T.I., M.P. and X.X.; writing—review and editing, T.I., M.P., F.L. and M.L.; visualization, T.I.; supervision, M.P. and J.G.; project administration, M.P. and J.G.; funding acquisition, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (Project Codes 21972083 and 22102129), the Fundamental Research Funds for the Central Universities (GK202102008), the Support Program for top-notch young talents in Shaanxi Province (1511000066), and the China Postdoctoral Science Foundation (2021M692615 and 2022T150528).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- EllGarah, M.; Lipton-Duffin, J.; MacLeod, J.M.; Gutzler, R.; Palmino, F.; Luzet, V.; Chérioux, F.; Rosei, F. Self-Assembly of a Halogenated Molecule on Oxide-Passivated Cu(110). Chem. Asian J. 2013, 8, 1813–1817. [Google Scholar] [CrossRef] [PubMed]
- Barth, J.V.; Costantini, G.; Kern, K. Engineering atomic and molecular nanostructures at surfaces. Nature 2005, 437, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Green, J.E.; Wook Choi, J.; Boukai, A.; Bunimovich, Y.; Johnston-Halperin, E.; DeIonno, E.; Luo, Y.; Sheriff, B.A.; Xu, K.; Shik Shin, Y.; et al. A 160-kilobit molecular electronic memory patterned at 1011 bits per square centimetre. Nature 2007, 445, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Rosei, F.; Schunack, M.; Naitoh, Y.; Jiang, P.; Gourdon, A.; Laegsgaard, E.; Stensgaard, I.; Joachim, C.; Besenbacher, F. Properties of large organic molecules on metal surfaces. Prog. Surf. Sci. 2003, 71, 95–146. [Google Scholar] [CrossRef]
- Fan, Z.; Zou, Y.; Liu, C.; Xiang, S.; Zhang, Z. Hydrogen-Bonded Organic Frameworks: Functionalized Construction Strategy by Nitrogen-Containing Functional Group. Chem. A Eur. J. 2022, 28, e202200422. [Google Scholar] [CrossRef]
- Shen, B.; Chen, X.; Wang, H.; Xiong, H.; Bosch, E.G.T.; Lazić, I.; Cai, D.; Qian, W.; Jin, S.; Liu, X.; et al. A single-molecule van der Waals compass. Nature 2021, 592, 541–544. [Google Scholar] [CrossRef]
- Szell, P.M.J.; Zablotny, S.; Bryce, D.L. Halogen bonding as a supramolecular dynamics catalyst. Nat. Commun. 2019, 10, 916. [Google Scholar] [CrossRef]
- Gutzler, R.; Fu, C.; Dadvand, A.; Hua, Y.; MacLeod, J.M.; Rosei, F.; Perepichka, D.F. Halogen bonds in 2D supramolecular self-assembly of organic semiconductors. Nanoscale 2012, 4, 5965. [Google Scholar] [CrossRef]
- Liu, J.; Abel, M.; Lin, N. On-Surface Synthesis: A New Route Realizing Single-Layer Conjugated Metal–Organic Structures. J. Phys. Chem. Lett. 2022, 13, 1356–1365. [Google Scholar] [CrossRef]
- El Garah, M.; Makoudi, Y.; Duverger, É.; Palmino, F.; Rochefort, A.; Chérioux, F. Large-Scale Patterning of Zwitterionic Molecules on a Si(111)-7 × 7 Surface. ACS Nano 2011, 5, 424–428. [Google Scholar] [CrossRef]
- Bartels, L. Tailoring molecular layers at metal surfaces. Nat. Chem. 2010, 2, 87–95. [Google Scholar] [CrossRef]
- Qiu, X.H. Vibrationally Resolved Fluorescence Excited with Submolecular Precision. Science 2003, 299, 542–546. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, Y.; Zhang, Y.; Yu, Y.-J.; Kuang, Y.-M.; Zhang, L.; Meng, Q.-S.; Luo, Y.; Yang, J.-L.; Dong, Z.-C.; et al. Visualizing coherent intermolecular dipole–dipole coupling in real space. Nature 2016, 531, 623–627. [Google Scholar] [CrossRef]
- Ijaz, T.; Yang, B.; Wang, R.; Zhu, J.; Farrukh, A.; Chen, G.; Franc, G.; Zhang, Y.; Gourdon, A.; Dong, Z. Self-decoupled tetrapodal perylene molecules for luminescence studies of isolated emitters on Au(111). Appl. Phys. Lett. 2019, 115, 173101. [Google Scholar] [CrossRef]
- Miwa, J.A.; Cicoira, F.; Lipton-Duffin, J.; Perepichka, D.F.; Santato, C.; Rosei, F. Self-assembly of rubrene on Cu(111). Nanotechnology 2008, 19, 424021. [Google Scholar] [CrossRef]
- Hu, J.; Hu, J.; Wang, H.; Shen, K.; Zhang, H.; Huang, C.; Xie, L.; Tian, Q.; Huang, H.; Jiang, Z.; et al. Initiating Ullmann-like coupling of Br2Py by a semimetal surface. Sci. Rep. 2021, 11, 3414. [Google Scholar] [CrossRef]
- Comstock, M.J.; Levy, N.; Kirakosian, A.; Cho, J.; Lauterwasser, F.; Harvey, J.H.; Strubbe, D.A.; Fréchet, J.M.J.; Trauner, D.; Louie, S.G.; et al. Reversible Photomechanical Switching of Individual Engineered Molecules at a Metallic Surface. Phys. Rev. Lett. 2007, 99, 038301. [Google Scholar] [CrossRef]
- Wakayama, Y.; Hayakawa, R.; Seo, H.-S. Recent progress in photoactive organic field-effect transistors. Sci. Technol. Adv. Mater. 2014, 15, 024202. [Google Scholar] [CrossRef]
- Alemani, M.; Selvanathan, S.; Ample, F.; Peters, M.V.; Rieder, K.-H.; Moresco, F.; Joachim, C.; Hecht, S.; Grill, L. Adsorption and Switching Properties of Azobenzene Derivatives on Different Noble Metal Surfaces: Au(111), Cu(111), and Au(100). J. Phys. Chem. C 2008, 112, 10509–10514. [Google Scholar] [CrossRef]
- Kumar, A.S.; Ye, T.; Takami, T.; Yu, B.-C.; Flatt, A.K.; Tour, J.M.; Weiss, P.S. Reversible Photo-Switching of Single Azobenzene Molecules in Controlled Nanoscale Environments. Nano Lett. 2008, 8, 1644–1648. [Google Scholar] [CrossRef]
- Henzl, J.; Mehlhorn, M.; Gawronski, H.; Rieder, K.-H.; Morgenstern, K. Reversiblecis-trans Isomerization of a Single Azobenzene Molecule. Angew. Chem. Int. Ed. 2006, 45, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, C.; Lai, W.; Zhang, A.; Huang, H.; Li, W. Halogenated conjugated molecules for ambipolar field-effect transistors and non-fullerene organic solar cells. Mater. Chem. Front. 2017, 1, 1389–1395. [Google Scholar] [CrossRef]
- Han, Z.; Czap, G.; Chiang, C.; Xu, C.; Wagner, P.J.; Wei, X.; Zhang, Y.; Wu, R.; Ho, W. Imaging the halogen bond in self-assembled halogenbenzenes on silver. Science 2017, 358, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Muždalo, A.; Saalfrank, P.; Vreede, J.; Santer, M. Cis -to- Trans Isomerization of Azobenzene Derivatives Studied with Transition Path Sampling and Quantum Mechanical/Molecular Mechanical Molecular Dynamics. J. Chem. Theory Comput. 2018, 14, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.R.; Minas Da Piedade, M.E.; Martinho Simões, J.A.; Simoni, J.A.; Teixeira, C.; Diogo, H.P.; Meng-Yan, Y.; Pilcher, G. Enthalpies of formation of cis-azobenzene and trans-azobenzene. J. Chem. Thermodyn. 1992, 24, 439–447. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef]
- Zhen, R.; Lu, W.; Zhou, Y.; Feng, M.; Wang, Y. Synthesis and Characterization of 4,4′-Dibromoazobenzene. Org. Polym. Mater. Res. 2020, 1, 6–8. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Gan, L.; Wang, G. Experimental and DFT study of UV–vis absorption spectra of azobenzene containing ester groups. Comput. Theor. Chem. 2021, 1200, 113244. [Google Scholar] [CrossRef]
- Cho, E.N.; Zhitomirsky, D.; Han, G.G.D.; Liu, Y.; Grossman, J.C. Molecularly Engineered Azobenzene Derivatives for High Energy Density Solid-State Solar Thermal Fuels. ACS Appl. Mater. Interfaces 2017, 9, 8679–8687. [Google Scholar] [CrossRef]
- Kirakosian, A.; Comstock, M.J.; Cho, J.; Crommie, M.F. Molecular commensurability with a surface reconstruction: STM study of azobenzene on Au(111). Phys. Rev. B 2005, 71, 113409. [Google Scholar] [CrossRef]
- McNellis, E.R.; Meyer, J.; Reuter, K. Azobenzene at coinage metal surfaces: Role of dispersive van der Waals interactions. Phys. Rev. B 2009, 80, 205414. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Q.; Ding, H.; Chen, X.; Yang, H.; Li, B.; Liu, X.; Lin, H.; Li, Q.; Gao, J.; et al. On-Surface Synthesis of Thiophene-Containing Large-Sized Organometallic Macrocycles on the Ag(111) Surface. J. Phys. Chem. C 2021, 125, 11454–11461. [Google Scholar] [CrossRef]
- Pechenezhskiy, I.V.; Cho, J.; Nguyen, G.D.; Berbil-Bautista, L.; Giles, B.L.; Poulsen, D.A.; Fréchet, J.M.J.; Crommie, M.F. Self-Assembly and Photomechanical Switching of an Azobenzene Derivative on GaAs(110): Scanning Tunneling Microscopy Study. J. Phys. Chem. C 2012, 116, 1052–1055. [Google Scholar] [CrossRef]
- Weigelt, S.; Busse, C.; Petersen, L.; Rauls, E.; Hammer, B.; Gothelf, K.V.; Besenbacher, F.; Linderoth, T.R. Chiral switching by spontaneous conformational change in adsorbed organic molecules. Nat. Mater. 2006, 5, 112–117. [Google Scholar] [CrossRef]
- Henzl, J.; Morgenstern, K. The frontier orbitals of a push-pull azobenzene adsorbed on a metal surface in different bonding geometries investigated by scanning tunneling spectroscopy and spectroscopy mapping. J. Chem. Phys. 2011, 135, 094702. [Google Scholar] [CrossRef]
- Choi, B.-Y.; Kahng, S.-J.; Kim, S.; Kim, H.; Kim, H.W.; Song, Y.J.; Ihm, J.; Kuk, Y. Conformational Molecular Switch of the Azobenzene Molecule: A Scanning Tunneling Microscopy Study. Phys. Rev. Lett. 2006, 96, 156106. [Google Scholar] [CrossRef]
- Qiu, X.H.; Nazin, G.V.; Ho, W. Mechanisms of Reversible Conformational Transitions in a Single Molecule. Phys. Rev. Lett. 2004, 93, 196806. [Google Scholar] [CrossRef]
- Alemani, M.; Peters, M.V.; Hecht, S.; Rieder, K.-H.; Moresco, F.; Grill, L. Electric Field-Induced Isomerization of Azobenzene by STM. J. Am. Chem. Soc. 2006, 128, 14446–14447. [Google Scholar] [CrossRef]
- Levy, N.; Comstock, M.J.; Cho, J.; Berbil-Bautista, L.; Kirakosian, A.; Lauterwasser, F.; Poulsen, D.A.; Fréchet, J.M.J.; Crommie, M.F. Self-Patterned Molecular Photoswitching in Nanoscale Surface Assemblies. Nano Lett. 2009, 9, 935–939. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).