New Low-Dimensional Organic–Inorganic Lead Halide Hybrid Systems Directed by Imidazo[1,5-a]pyridinium-Based Cation or Imines: Synthesis, Structures, Non-Covalent Interactions and Optical Properties
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and General Methods
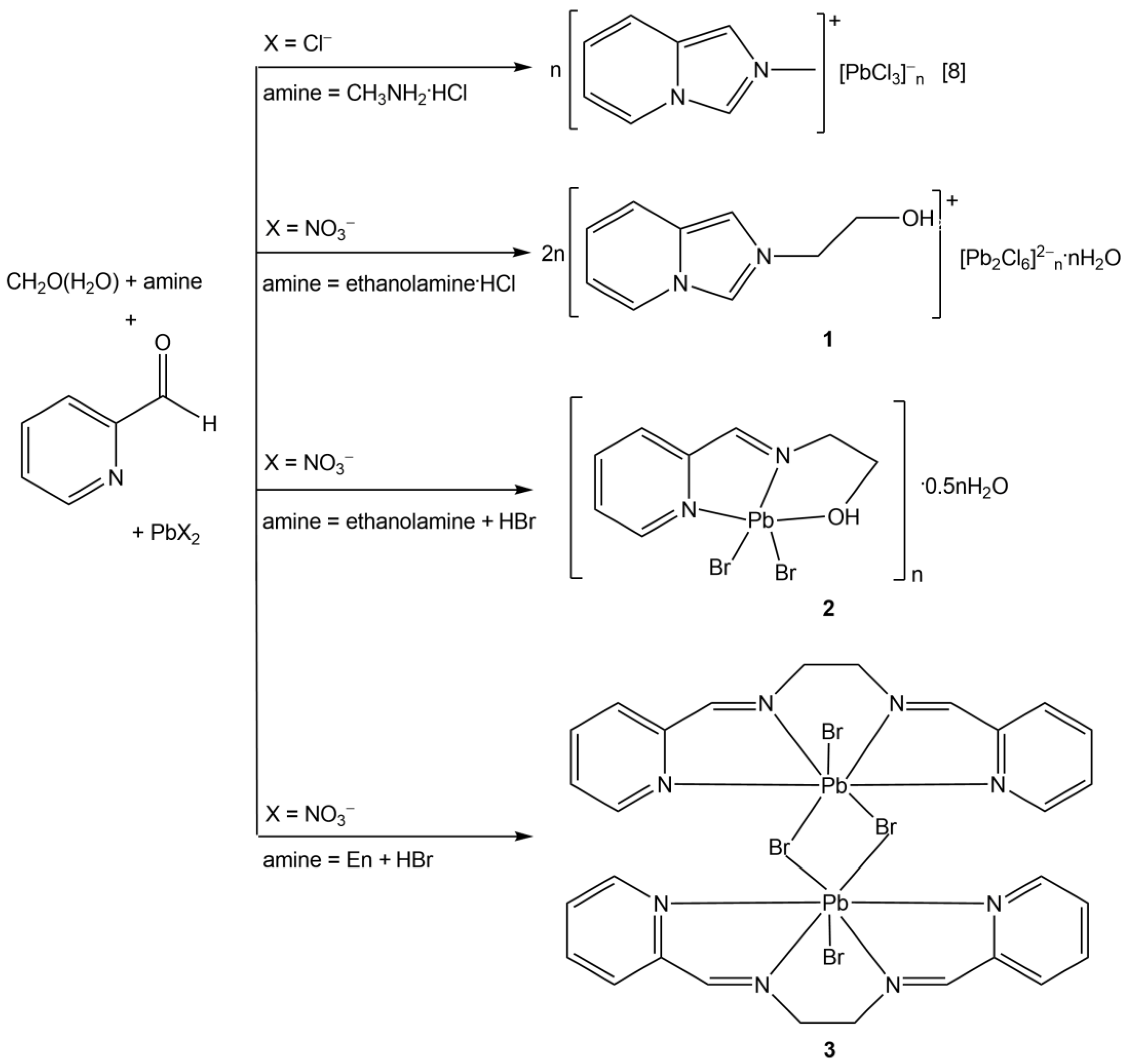
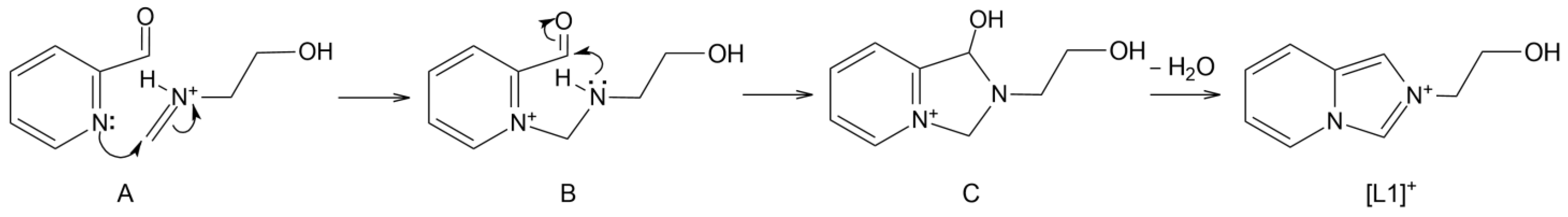
2.1.1. Synthesis of [L1]2n[Pb2Cl6]n∞·nH2O (1)
2.1.2. Synthesis of [PbBr2(L2)]n∞·0.5nH2O (2)
2.1.3. Synthesis of [PbBr2(L3)]2 (3)
2.2. Single Crystal Structure Determination of 1–3
2.3. Theoretical Calculations
3. Results and Discussion
3.1. Synthesis and Characterization
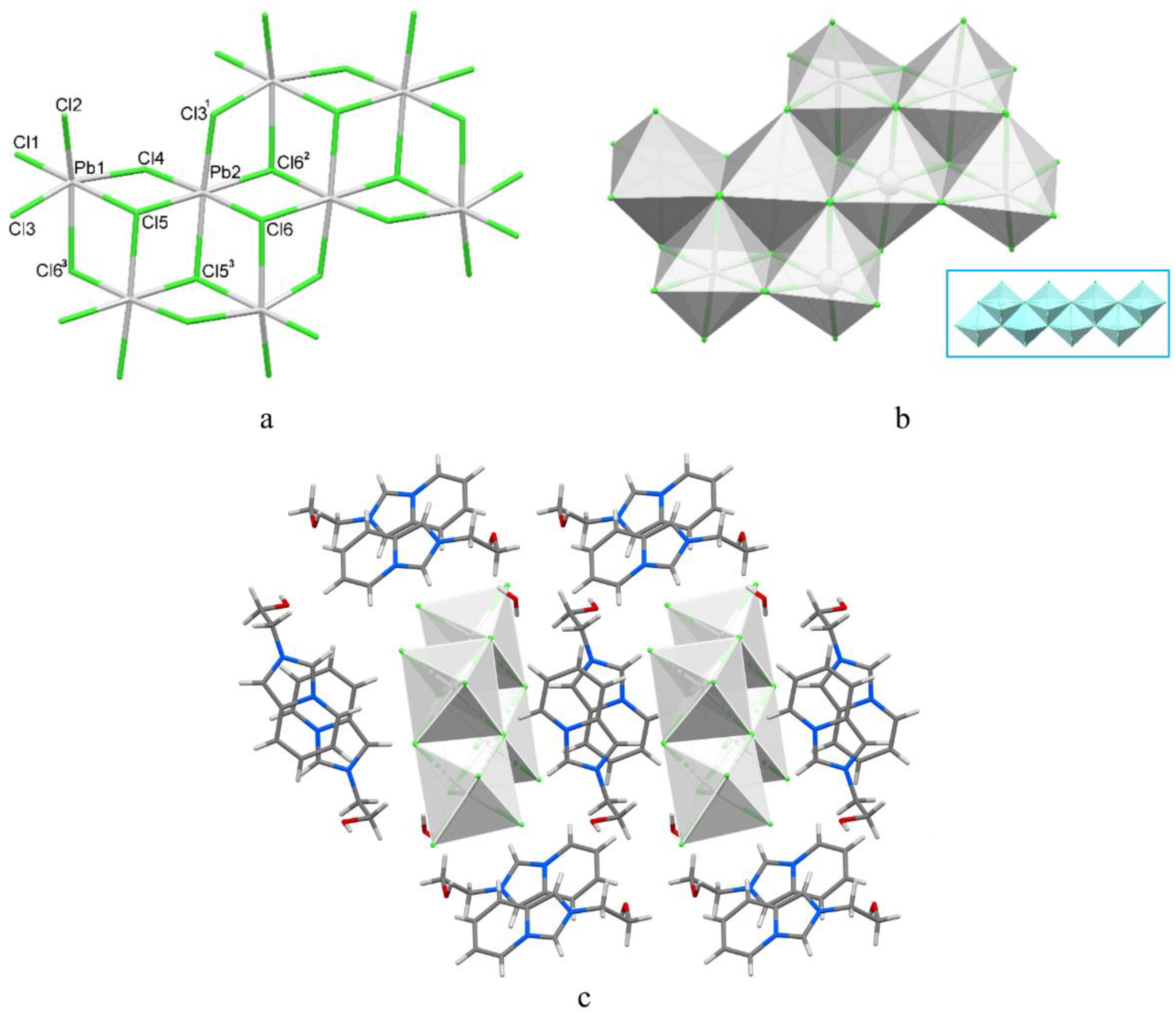
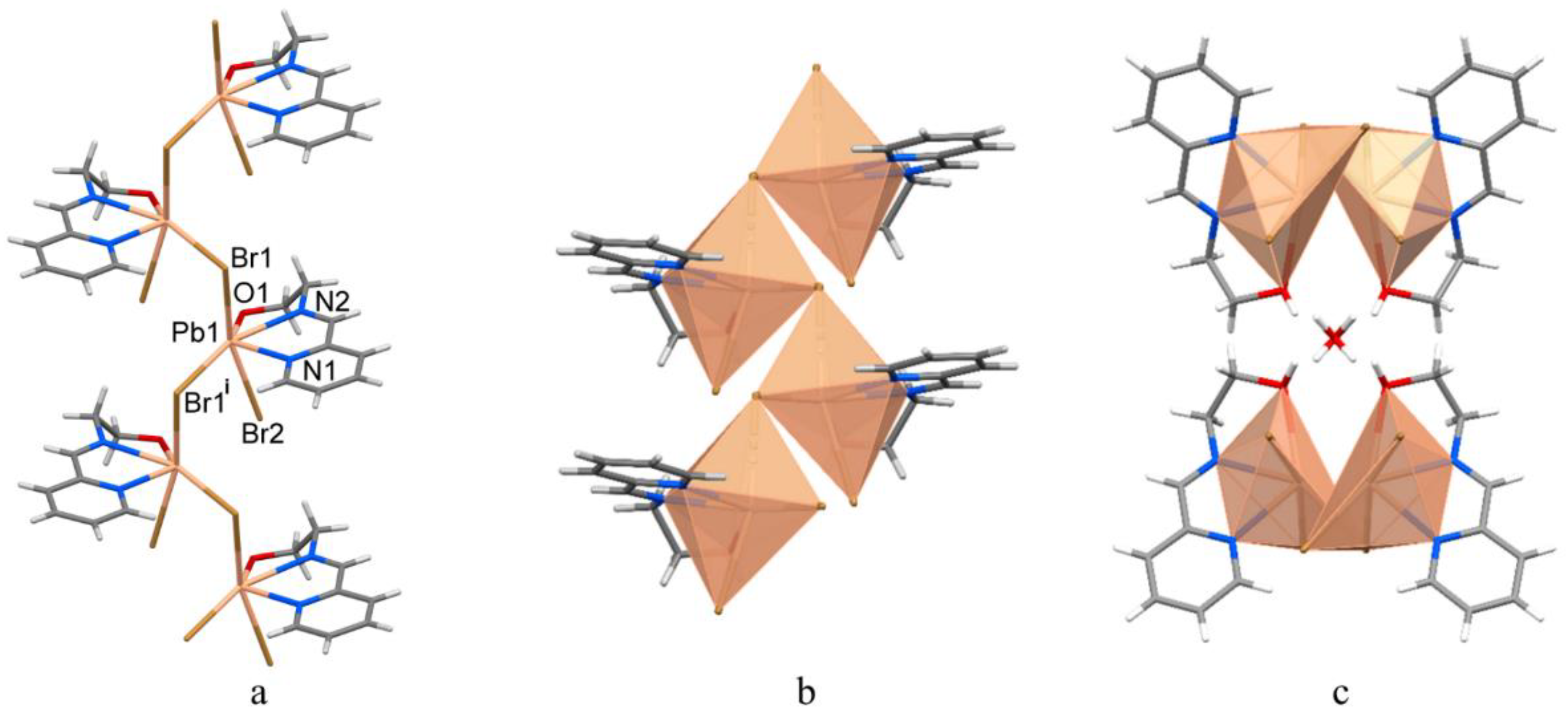
3.2. Structural Description of 1–3
| [L1]2n[Pb2Cl6]n∞·nH2O (1) | |||
|---|---|---|---|
| Pb1−Cl1 | 2.7587(16) | Pb2−Cl4 | 2.7924(15) |
| Pb1−Cl2 | 2.6509(14) | Pb2−Cl3 1 | 2.8177(14) |
| Pb1−Cl3 | 2.8223(13) | Pb2−Cl6 2 | 2.8730(13) |
| Pb1−Cl4 | 3.0750(14) | Pb2−Cl5 | 2.9355(13) |
| Pb1−Cl5 | 3.0472(14) | Pb2−Cl6 | 2.9613(14) |
| Pb1−Cl6 3 | 3.1516(14) | Pb2−Cl5 3 | 2.9957(14) |
| Cl2−Pb1−Cl1 | 93.17(5) | Cl4−Pb2−Cl3 1 | 94.36(5) |
| Cl2−Pb1−Cl3 | 86.32(4) | Cl4−Pb2−Cl6 2 | 96.36(4) |
| Cl1−Pb1−Cl3 | 94.13(5) | Cl3 1−Pb2−Cl6 3 | 82.39(4) |
| Cl2−Pb1−Cl5 | 90.30(4) | Cl4−Pb2−Cl5 | 87.03(4) |
| Cl1−Pb1−Cl5 | 175.07(4) | Cl3 1−Pb2−Cl5 | 94.79(4) |
| Cl3−Pb1−Cl5 | 89.58 (4) | Cl6 2−Pb2−Cl5 | 175.73(3) |
| Cl2−Pb1−Cl4 | 107.33(5) | Cl4−Pb2−Cl6 | 177.20(4) |
| Cl1−Pb1−Cl4 | 95.37(4) | Cl3 1−Pb2−Cl6 | 87.68(4) |
| Cl3−Pb1−Cl4 | 162.86(5) | Cl6 2−Pb2−Cl6 | 85.80(4) |
| Cl5−Pb1−Cl4 | 80.25(4) | Cl5−Pb2−Cl6 | 90.89(4) |
| Cl2−Pb1−Cl6 3 | 161.97(4) | Cl4−Pb2−Cl5 3 | 92.01(5) |
| Cl1−Pb1−Cl6 3 | 95.85(5) | Cl3 1−Pb2−Cl5 3 | 173.17(4) |
| Cl3−Pb1−Cl6 3 | 77.50(4) | Cl6 2−Pb2−Cl5 3 | 99.36(4) |
| Cl5−Pb1−Cl6 3 | 81.77(4) | Cl5−Pb2−Cl5 3 | 83.07(4) |
| Cl4−Pb1−Cl6 3 | 87.35(4) | Cl6−Pb2−Cl5 3 | 85.88(4) |
| [PbBr2(L2)]n∞·0.5nH2O (2) | |||
| Pb1−N1 | 2.584(7) | Pb1−Br2 | 2.9777(10) |
| Pb1−N2 | 2.562(7) | Pb1−Br1 4 | 3.0764(10) |
| Pb1−O1 | 2.839(7) | Pb1−Br1 | 3.0293(11) |
| N2−Pb1−N1 | 64.4(3) | O1−Pb1−Br1 4 | 146.36(15) |
| N2−Pb1−O1 | 61.8(2) | Br2−Pb1−Br1 4 | 89.35(3) |
| N1−Pb1−O1 | 123.3(2) | N2−Pb1−Br1 | 84.41(17) |
| N2−Pb1−Br2 | 85.72(18) | N1−Pb1−Br1 | 80.21(16) |
| N1−Pb1−Br2 | 80.57(16) | O1−Pb1−Br1 | 110.23(16) |
| O1−Pb1−Br2 | 79.24(16) | Br2−Pb1−Br1 | 160.70(3) |
| N2−Pb1−Br1 4 | 149.39(18) | Br1−Pb1−Br1 4 | 90.73(3) |
| N1−Pb1−Br1 4 | 85.02(18) | ||
| [PbBr2(L3)]2 (3) | |||
| Pb1−N1 | 2.745(6) | Pb1−Br1 | 3.0632(9) |
| Pb1−N2 | 2.625(5) | Pb1−Br2 | 2.9599(9) |
| Pb1−N3 | 2.621(6) | Pb1−Br1 3 | 3.2525(8) |
| Pb1−N4 | 2.808(6) | ||
| N2−Pb1−N1 | 61.15(17) | N2−Pb1−Br1 | 84.71(12) |
| N3−Pb1−N1 | 123.10(18) | N1−Pb1−Br1 | 93.02(11) |
| N3−Pb1−N2 | 63.60(19) | N4−Pb1−Br1 | 85.58(12) |
| N3−Pb1−N4 | 60.4(2) | Br2−Pb1−Br1 | 174.31(2) |
| N2−Pb1−N4 | 121.93(19) | N3−Pb1−Br1 3 | 149.42(14) |
| N1−Pb1−N4 | 176.39(16) | N2−Pb1−Br1 3 | 145.63(13) |
| N3−Pb1−Br2 | 81.33(13) | N1−Pb1−Br1 3 | 87.48(13) |
| N2−Pb1−Br2 | 89.91(12) | N4−Pb1−Br1 3 | 89.05(15) |
| N1−Pb1−Br2 | 85.97(12) | Br2−Pb1−Br1 3 | 102.31(3) |
| N4−Pb1−Br2 | 95.75(12) | Br1−Pb1−Br1 3 | 83.22(2) |
| N3−Pb1−Br1 | 94.60(13) |
3.3. Hirshfeld Surface and QTAIM Analyses
3.4. Optical Properties of 1–3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Green, M.A.; Hishikawa, Y.; Warta, W.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Ho-Baillie, A.W. Solar cell efficiency tables (version 50). Prog. Photovolt. Res. Appl. 2017, 25, 668–676. [Google Scholar] [CrossRef]
- Sutherland, B.R.; Sargent, E.H. Perovskite photonic sources. Nat. Photon. 2016, 10, 295–302. [Google Scholar] [CrossRef]
- Saparov, B.; Mitzi, D.B. Organic-Inorganic Perovskites: Structural Versatility for Functional Materials Design. Chem. Rev. 2016, 116, 4558–4596. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Jaffe, A.; Dohner, E.R.; Lindenberg, A.M.; Karunadasa, H.I. Structural origins of broadband emission from layered Pb–Br hybrid perovskites. Chem. Sci. 2017, 8, 4497–4504. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhou, C.; Tian, Y.; Shu, Y.; Messier, J.; Wang, J.C.; Van De Burgt, L.J.; Kountouriotis, K.; Xin, Y.; Holt, E.; et al. One-dimensional organic lead halide perovskites with efficient bluish white-light emission. Nat. Commun. 2017, 8, 14051. [Google Scholar] [CrossRef]
- Peng, Y.; Yao, Y.; Li, L.; Wu, Z.; Wang, S.; Luo, J. White-light emission in a chiral one-dimensional organic–inorganic hybrid perovskite. J. Mater. Chem. C 2018, 6, 6033–6037. [Google Scholar] [CrossRef]
- Buvaylo, E.A.; Kokozay, V.N.; Linnik, R.P.; Vassilyeva, O.Y.; Skelton, B.W. Hybrid organic–inorganic chlorozincate and a molecular zinc complex involving the in situ formed imidazo [1,5-a]pyridinium cation: Serendipitous oxidative cyclization, structures and photophysical properties. Dalton Trans. 2015, 44, 13735–13744. [Google Scholar] [CrossRef]
- Vassilyeva, O.Y.; Buvaylo, E.A.; Linnik, R.P.; Nesterov, D.S.; Trachevsky, V.V.; Skelton, B.W. Synthetic strategy towards halometallates with imidazo [1,5-a]pyridinium-based counterions. CrystEngComm 2020, 22, 5096–5105. [Google Scholar] [CrossRef]
- Vassilyeva, O.Y.; Buvaylo, E.A.; Kokozay, V.N.; Skelton, B.W.; Sobolev, A.N. Crystal structures of an imidazo[1,5-a] pyridinium-based ligand and its (C13H12N3)2[CdI4] hybrid salt. Acta Cryst. 2019, 75, 1209–1214. [Google Scholar] [CrossRef]
- Vassilyeva, O.; Buvaylo, E.; Kokozay, V.; Skelton, B.W.; Rajnák, C.; Titis, J.; Boca, R. Long magnetic relaxation time of tetracoordinate Co2+ in imidazo [1,5-a]pyridinium-based (C13H12N3)2[CoCl4] hybrid salt and [Co(C13H12N3)Cl3] molecular complex. Dalton Trans. 2019, 48, 11278–11284. [Google Scholar] [CrossRef] [PubMed]
- Vassilyeva, O.Y.; Buvaylo, E.A.; Lobko, Y.V.; Linnik, R.P.; Kokozay, V.N.; Skelton, B.W. Organic–inorganic hybrid tetrachlorocadmates as promising fluorescent agents for cross-linked polyurethanes: Synthesis, crystal structures and extended performance analysis. RSC Adv. 2021, 11, 7713–7722. [Google Scholar] [CrossRef] [PubMed]
- Vassilyeva, O.Y.; Buvaylo, E.A.; Kokozay, V.N.; Skelton, B.W. Organic-inorganic hybrid mixed-halide ZnII and CdII tetrahalometallates with the 2-methylimidazo[1,5-a]pyridinium cation. Acta Cryst. 2022, 78, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Lyons, A.S.; Ingersoll, M.A.; Muniyan, S.; D’Cunha, N.; Robinson, T.; Hoelting, K.; Dwyer, J.G.; Biu, X.R.; Batra, S.K.; Lin, M.F. Novel imidazopyridine derivatives possess anti-tumor effect on human castration-resistant prostate cancer cells. PLoS ONE 2015, 10, e0131811. [Google Scholar]
- Song, G.J.; Bai, S.Y.; Dai, X.; Cao, X.Q.; Zhao, B.X. A ratiometric lysosomal pH probe based on the imidazo[1,5-a]pyridine–rhodamine FRET and ICT system. RSC Adv. 2016, 6, 41317–41322. [Google Scholar] [CrossRef]
- Volpi, G.; Garino, C.; Fresta, E.; Casamassa, E.; Giordano, M.; Barolo, C.; Viscardi, G. Strategies to increase the quantum yield: Luminescent methoxylated imidazo[1,5-a]pyridines. Dyes Pigment. 2021, 192, 109455. [Google Scholar] [CrossRef]
- Yagishita, F.; Nii, C.; Tezuka, Y.; Tabata, A.; Nagamune, H.; Uemura, N.; Yoshida, Y.; Mino, T.; Sakamoto, M.; Kawamura, Y. Fluorescent N-heteroarenes having large stokes shift and water solubility suitable for bioimaging. Asian J. Org. Chem. 2018, 7, 1614–1619. [Google Scholar] [CrossRef]
- Kortüm, G.; Braun, W.; Herzog, G. Principles and techniques of diffuse-reflectance spectroscopy. Angew. Chem. Int. Ed. 1963, 2, 333–341. [Google Scholar] [CrossRef]
- Landi, S., Jr.; Segundo, I.R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C.J. Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements. Solid State Commun. 2022, 341, 114573. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Neese, F. Software update: The ORCA program system—Version 5.0. Wil. Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wil. Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Mardirossian, N.; Head-Gordon, M. ωB97M-V: A combinatorially optimized, range-separated hybrid, meta-GGA density functional with VV10 nonlocal correlation. J. Chem. Phys. 2016, 144, 214110. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Pantazis, D.A.; Neese, F. All-electron scalar relativistic basis sets for the 6p elements. Theor. Chem. Acc. 2012, 131, 1292. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Stoychev, G.L.; Auer, A.A.; Neese, F. Automatic Generation of Auxiliary Basis Sets. J. Chem. Theor. Comput. 2017, 13, 554–562. [Google Scholar] [CrossRef]
- van Wüllen, C. Molecular density functional calculations in the regular relativistic approximation: Method, application to coinage metal diatomics, hydrides, fluorides and chlorides, and comparison with first-order relativistic calculations. J. Chem. Phys. 1998, 109, 392. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, A.J.; Yang, W.T. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Dobrzycki, L.; Wozniak, K. Inorganic–organic hybrid salts of diaminobenzenes and related cations. CrystEngComm 2008, 10, 577–589. [Google Scholar] [CrossRef]
- Barkaoui, H.; Abid, H.; Yangui, A.; Triki, S.; Boukheddaden, K.; Abid, Y. Yellowish white-light emission involving resonant energy transfer in a new one-dimensional hybrid material: (C9H10N2)PbCl4. J. Phys. Chem. C 2018, 122, 24253–24261. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Wu, G.; Zhou, C.; Ming, W.; Han, D.; Chen, S.; Yang, D.; Besara, T.; Neu, J.; Siegrist, T.; Du, M.H.; et al. A one-dimensional organic lead chloride hybrid with excitation-dependent broadband emissions. ACS Energy Lett. 2018, 3, 1443–1449. [Google Scholar] [CrossRef]
- Martinez, C.R.; Iverson, B.L. Rethinking the term “pi-stacking”. Chem. Sci. 2012, 3, 2191–2201. [Google Scholar] [CrossRef]
- Sun, X.Y.; Yue, M.; Jiang, Y.X.; Zhao, C.H.; Liao, Y.Y.; Lei, X.W.; Yue, C.Y. Combining dual-light emissions to achieve efficient broadband yellowish-green luminescence in one-dimensional hybrid lead halides. Inorg. Chem. 2021, 60, 1491–1498. [Google Scholar] [CrossRef]
- Mao, L.; Guo, P.; Kepenekian, M.; Hadar, I.; Katan, C.; Even, J.; Schaller, R.D.; Stoumpos, C.C.; Kanatzidis, M.G. Structural diversity in white-light-emitting hybrid lead bromide perovskites. JACS 2018, 140, 13078–13088. [Google Scholar] [CrossRef]
- Roy, S.; Choubey, S.; Bhar, K.; Khan, S.; Mitra, P.; Ghosh, B.K. Syntheses, structures, and luminescence behavior of terephthalate bridged lead(II) complexes with tetradentate N-donor Schiff bases. J. Mol. Struct. 2013, 1051, 328–335. [Google Scholar] [CrossRef]
- Hashemi, L.; Morsali, A. Sonochemical synthesis of nano-structured lead(II) complex: Precursor for the preparation of PbO nano-structures. J. Coord. Chem. 2011, 64, 4088–4097. [Google Scholar] [CrossRef]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring Nature and Predicting Strength of Hydrogen Bonds: A Correlation Analysis between Atoms-in-Molecules Descriptors, Binding Energies, and Energy Components of Symmetry-Adapted Perturbation Theory. J. Comput. Chem. 2019, 40, 2868–2881. [Google Scholar] [CrossRef] [PubMed]
- Contreras-García, J.; Yang, W.; Johnson, E.R. Analysis of Hydrogen-Bond Interaction Potentials from the Electron Density: Integration of Noncovalent Interaction Regions. J. Phys. Chem. A 2011, 115, 12983–12990. [Google Scholar] [CrossRef] [PubMed]
- Kamminga, M.E.; de Wijs, G.A.; Havenith, R.W.; Blake, G.R.; Palstra, T.T. The role of connectivity on electronic properties of lead iodide perovskite-derived compounds. Inorg. Chem. 2017, 56, 8408–8414. [Google Scholar] [CrossRef] [PubMed]

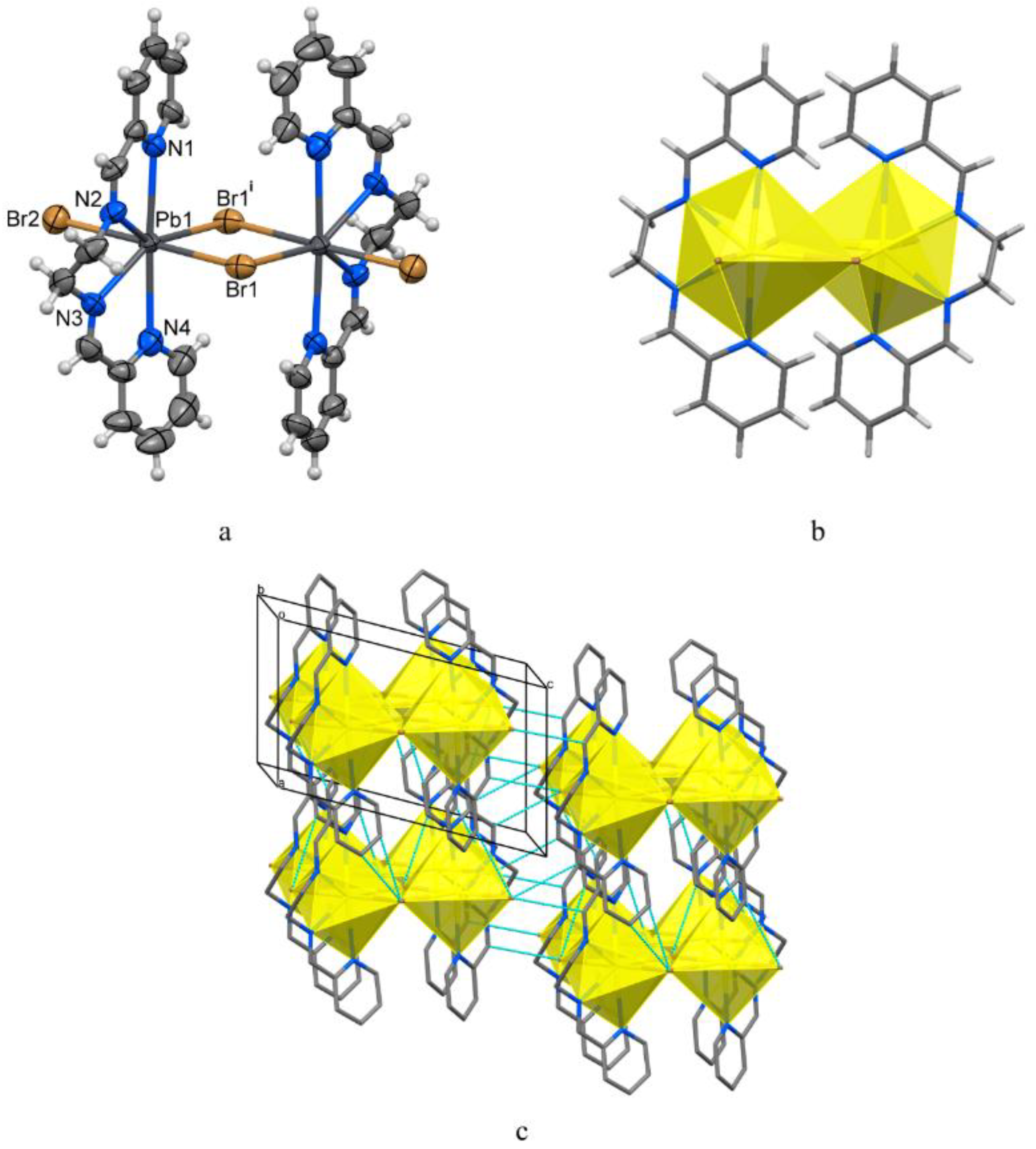
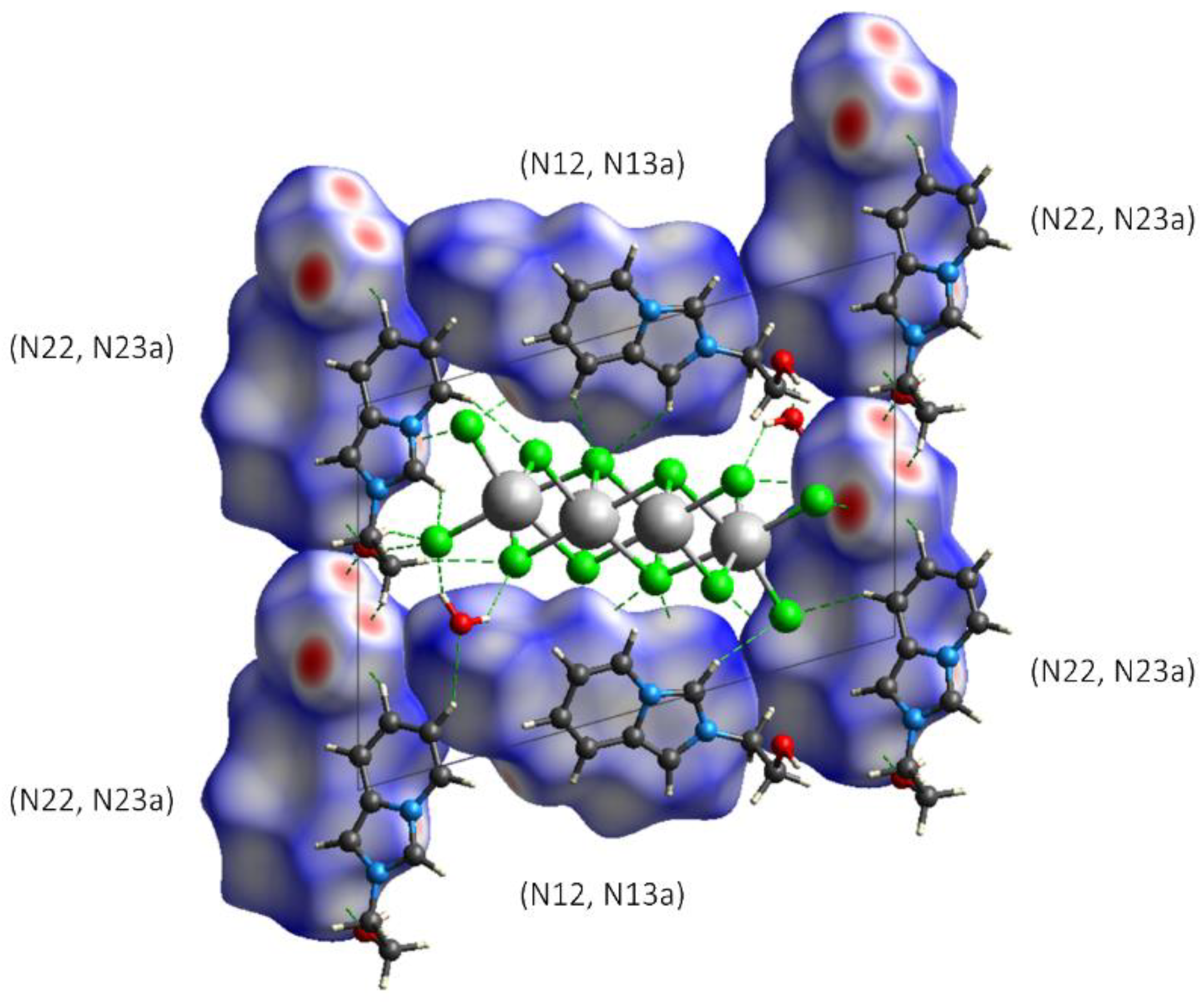
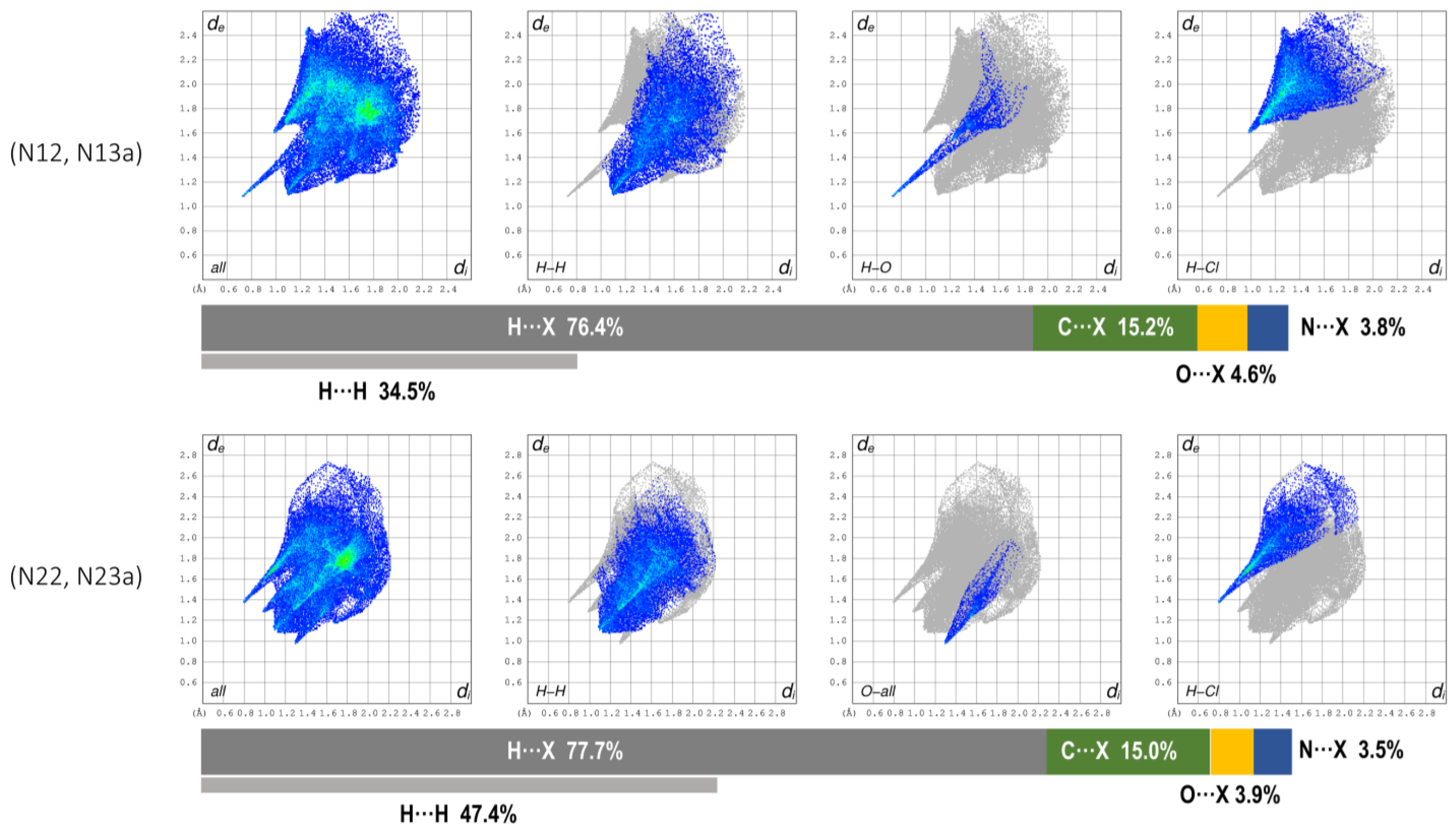

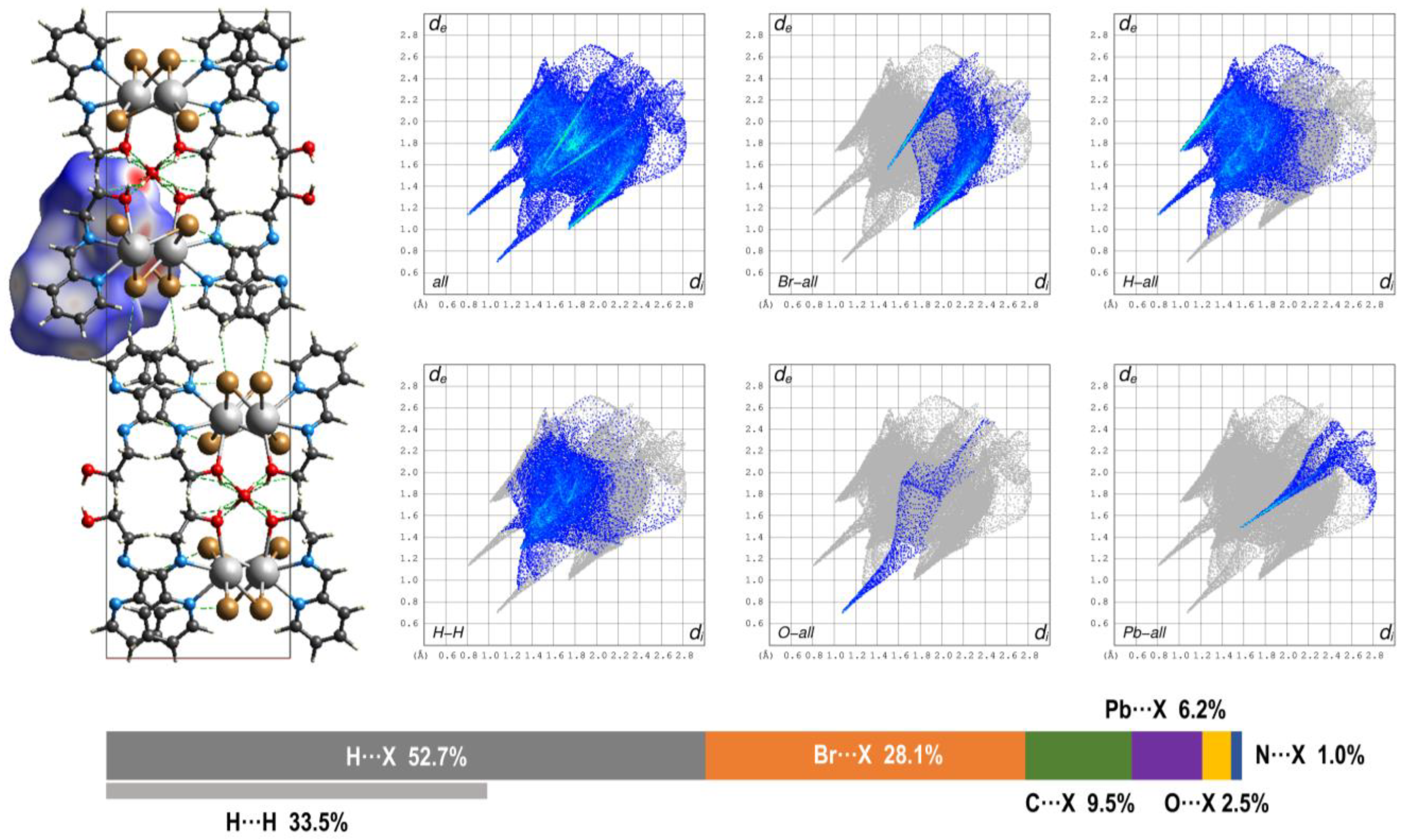
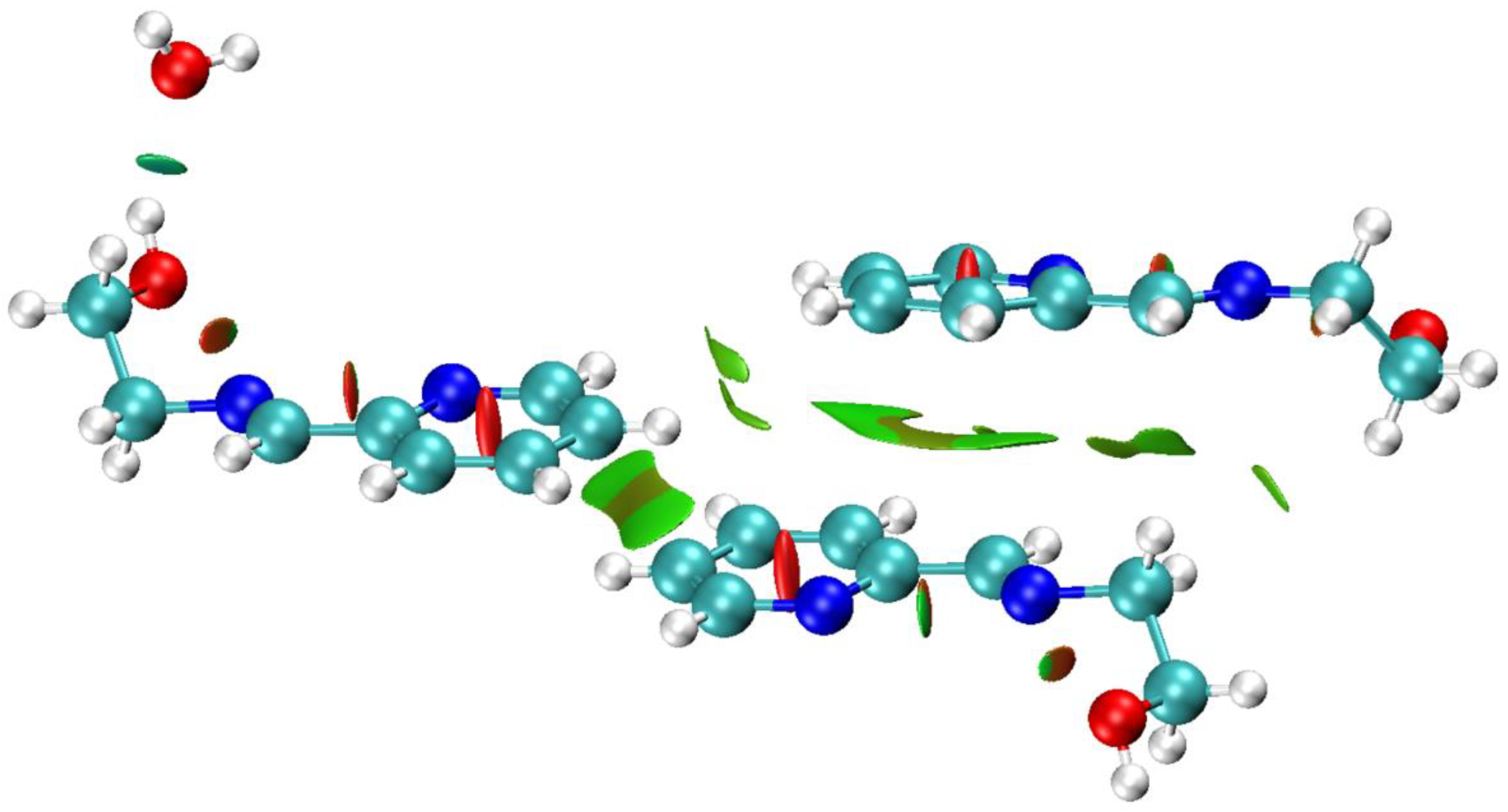
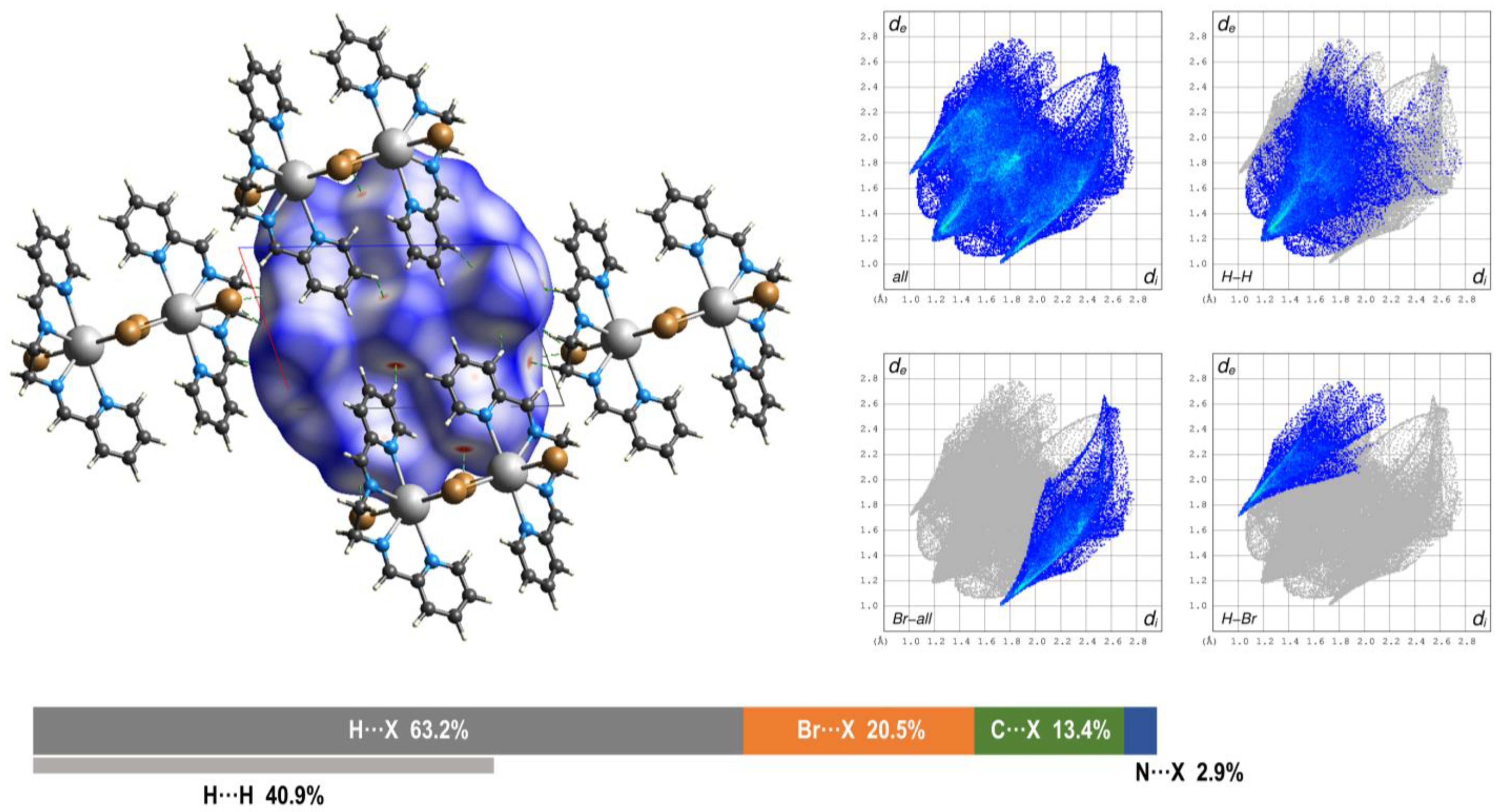
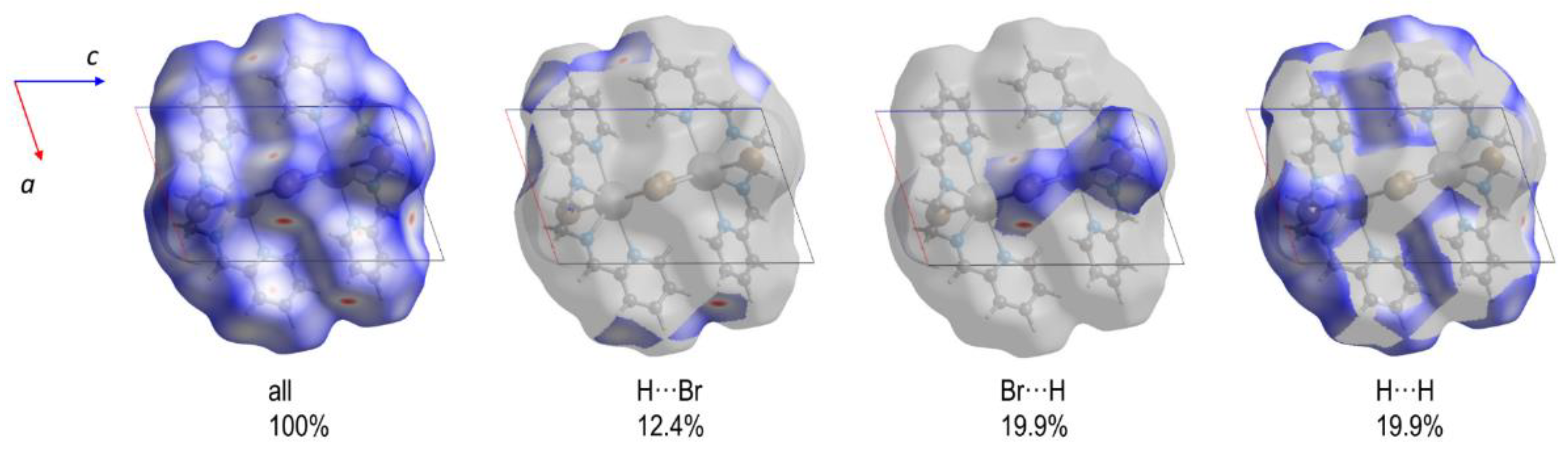
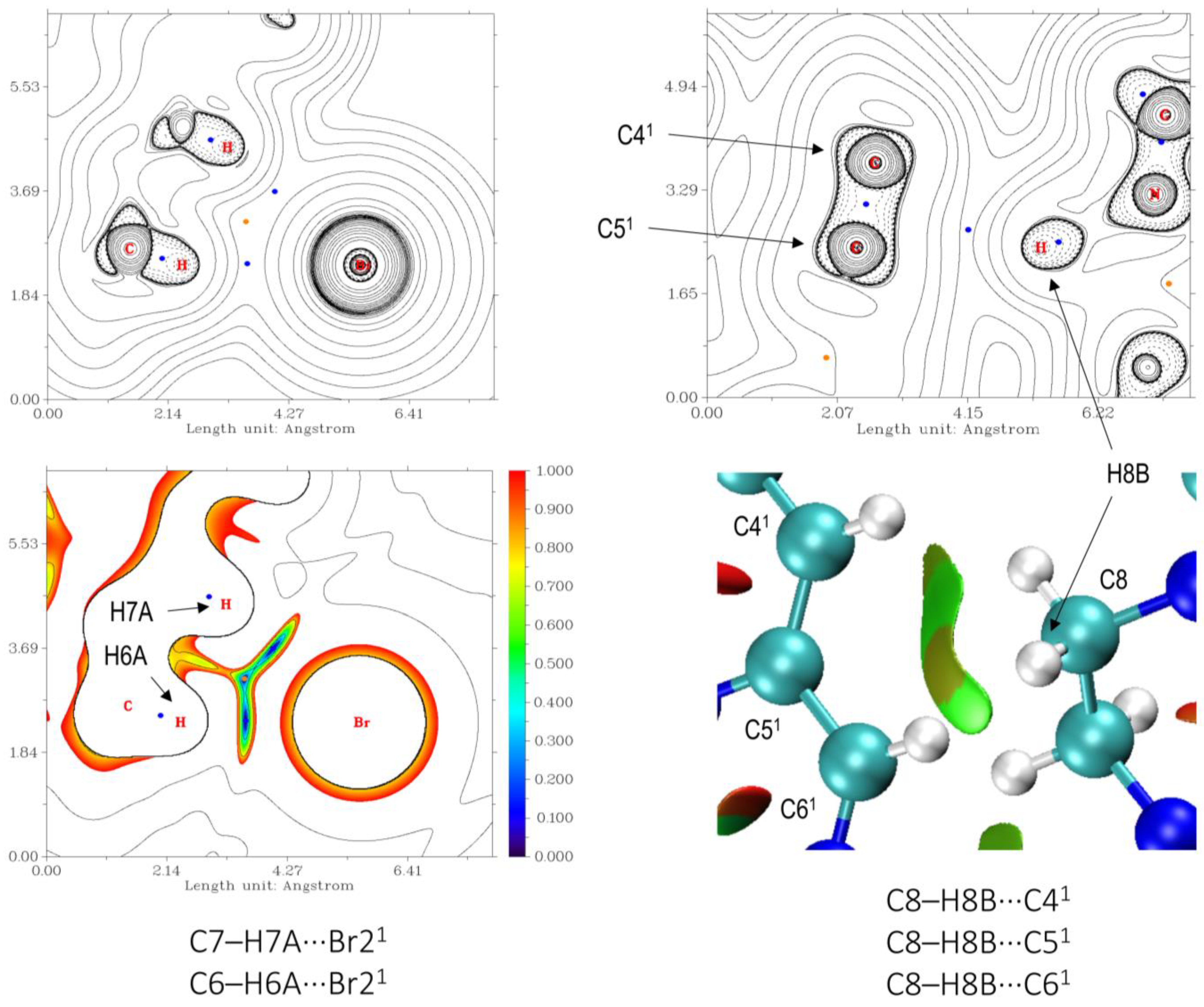

| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C18H24Cl6N4O3Pb2 | C16H22Br4N4O3Pb2 | C28H28Br4N8Pb2 |
| Formula weight | 971.49 | 1052.39 | 1210.60 |
| Temperature/K | 100(2) | 296(2) | 296(2) |
| Crystal system | Triclinic | Orthorhombic | Triclinic |
| Space group | P (No. 2) | Pccn (No. 56) | P (No. 2) |
| a/Å | 7.5232(3) | 9.5345(14) | 8.9423(5) |
| b/Å | 11.3164(5) | 33.493(5) | 9.3263(8) |
| c/Å | 16.5513(6) | 7.9626(12) | 12.3991(7) |
| α/° | 105.748(3) | 90 | 82.812(3) |
| β/° | 96.367(3) | 90 | 70.156(2) |
| γ/° | 90.885(3) | 90 | 61.408(2) |
| V/Å3 | 1346.32(10) | 2542.7(6) | 853.18(10) |
| Z | 2 | 4 | 1 |
| Calculated density Dc/g cm−3 | 2.396 | 2.749 | 2.356 |
| Absorption coefficient μ/mm−1 | 13.112 | 19.534 | 14.569 |
| F000 | 900 | 1896 | 556 |
| θ range for data collection/° | 2.547 to 26.372 | 2.809 to 28.337 | 2.975 to 29.994 |
| Completeness to θmax | 0.999 | 0.996 | 0.997 |
| Reflections collected | 11,257 | 77,380 | 44,646 |
| Independent reflections | 5508 [Rint = 0.0330] | 3175 [Rint = 0.0690] | 5257 [Rint = 0.0844] |
| Min. and max. transmission | 0.260 and 1.0 | 0.3359 and 0.7457 | 0.3640 and 0.7461 |
| Data/restraints/parameters | 5508/237/341 | 3175/3/138 | 5257/0/190 |
| Reflections (I > 2σ(I)) | 4556 | 2783 | 4455 |
| GoF | 1.000 | 1.264 | 1.052 |
| R1, wR2 [I > 2σ(I)] | 0.0278, 0.0536 | 0.0469, 0.1013 | 0.0446, 0.1158 |
| R1, wR2 (all data) | 0.0382, 0.0581 | 0.0547, 0.1044 | 0.0550, 0.1245 |
| Largest diff. peak and hole/e Å−3 | 1.498 and −1.159 | 1.420 and −2.146 | 2.430 and −1.690 |
| CCDC no. | 2217143 | 2223148 | 2223147 |
| D–H∙∙∙A | d(D–H) | d(H∙∙∙A) | d(D∙∙∙A) | ∠(DHA) |
|---|---|---|---|---|
| [L1]2n[Pb2Cl6]n∞·nH2O (1) [b] | ||||
| C11–H11···Cl5 | 0.95 | 2.90 | 3.627(5) | 134 |
| C12–H12B···Cl2 1 | 0.99 | 2.96 | 3.862(6) | 152 |
| C13–H13···Cl2 1 | 0.95 | 2.71 | 3.558(6) | 148 |
| C14–H14···Cl6 2 | 0.95 | 2.86 | 3.506(5) | 126 |
| C15–H15···Cl6 2 | 0.95 | 2.86 | 3.509(5) | 127 |
| C17–H17···Cl3 3 | 0.95 | 2.96 | 3.529(5) | 120 |
| C17–H17···Cl5 | 0.95 | 2.88 | 3.649(5) | 139 |
| C22–H22B···Cl1 3 | 0.99 | 2.81 | 3.705(11) | 150 |
| C23–H23···Cl1 3 | 0.95 | 2.84 | 3.481(10) | 126 |
| C24–H24···Cl3 3 | 0.95 | 2.75 | 3.597(12) | 149 |
| C25–H25···O01W 2 | 0.95 | 2.48 | 3.325(12) | 148 |
| C27–H27···Cl2 4 | 0.95 | 2.85 | 3.716(14) | 153 |
| C221–H22C···O221 5 | 0.99 | 2.39 | 3.105(13) | 128 |
| C221–H22D···Cl4 | 0.99 | 2.80 | 3.753(12) | 161 |
| O01W–H01A···Cl1 6 | 0.85 | 2.34 | 3.179(7) | 172 |
| O01W–H01B···Cl4 7 | 0.85 | 2.37 | 3.168(6) | 155 |
| O121–H121···O01W | 0.84 | 1.94 | 2.710(8) | 151 |
| O221–H221···Cl1 | 0.84 | 2.24 | 2.967(8) | 145 |
| [PbBr2(L2)]n∞·0.5nH2O (2) | ||||
| O1–H1···O1W | 0.84(2) | 2.06(7) | 2.829(10) | 152(13) |
| O1W–H1W···O1 8 | 0.84(2) | 1.92(3) | 2.740(9) | 164(4) |
| C1–H1A···Br1 9 | 0.93 | 2.90 | 3.657(13) | 139.3 |
| C2–H2A···Br1 10 | 0.93 | 2.94 | 3.845(13) | 164.3 |
| C4–H4A···Br1 11 | 0.93 | 2.90 | 3.631(11) | 136.3 |
| C6–H6A···Br2 12 | 0.93 | 2.89 | 3.819(9) | 172.8 |
| C8–H8A···Br2 13 | 0.97 | 3.14 | 3.881(10) | 134.4 |
| [PbBr2(L3)]2 (3) | ||||
| C2–H2A···Br1 14 | 0.93 | 2.88 | 3.690(8) | 147.0 |
| C4–H4B···Br2 3 | 0.93 | 3.09 | 3.890(9) | 145.2 |
| C6–H6A···Br2 5 | 0.93 | 3.15 | 3.977(7) | 149.7 |
| C7–H7A···Br2 5 | 0.97 | 3.16 | 4.095(8) | 161.4 |
| C11–H11A···Br2 15 | 0.93 | 3.07 | 3.869(10) | 145.1 |
| C13–H13A···Br1 13 | 0.93 | 2.97 | 3.684(9) | 134.9 |
| Contact | ρBCP(r), a.u. | Binding energy, kJ mol−1 |
|---|---|---|
| C2–H2A···Br1 14 [b] | 8.79 × 10−3 | –5.10 |
| C4–H4B···Br2 3 | 6.01 × 10−3 | –2.50 |
| C6–H6A···Br2 −5 | 5.32 × 10−3 | –1.86 |
| C7–H7A···Br2 −5 | 5.38 × 10−3 | –1.92 |
| C11–H11A···Br2 15 | 6.19 × 10−3 | –2.67 |
| C13–H13A···Br1 13 | 7.71 × 10−3 | –4.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassilyeva, O.Y.; Buvaylo, E.A.; Nesterova, O.V.; Sobolev, A.N.; Nesterov, D.S. New Low-Dimensional Organic–Inorganic Lead Halide Hybrid Systems Directed by Imidazo[1,5-a]pyridinium-Based Cation or Imines: Synthesis, Structures, Non-Covalent Interactions and Optical Properties. Crystals 2023, 13, 307. https://doi.org/10.3390/cryst13020307
Vassilyeva OY, Buvaylo EA, Nesterova OV, Sobolev AN, Nesterov DS. New Low-Dimensional Organic–Inorganic Lead Halide Hybrid Systems Directed by Imidazo[1,5-a]pyridinium-Based Cation or Imines: Synthesis, Structures, Non-Covalent Interactions and Optical Properties. Crystals. 2023; 13(2):307. https://doi.org/10.3390/cryst13020307
Chicago/Turabian StyleVassilyeva, Olga Yu., Elena A. Buvaylo, Oksana V. Nesterova, Alexandre N. Sobolev, and Dmytro S. Nesterov. 2023. "New Low-Dimensional Organic–Inorganic Lead Halide Hybrid Systems Directed by Imidazo[1,5-a]pyridinium-Based Cation or Imines: Synthesis, Structures, Non-Covalent Interactions and Optical Properties" Crystals 13, no. 2: 307. https://doi.org/10.3390/cryst13020307
APA StyleVassilyeva, O. Y., Buvaylo, E. A., Nesterova, O. V., Sobolev, A. N., & Nesterov, D. S. (2023). New Low-Dimensional Organic–Inorganic Lead Halide Hybrid Systems Directed by Imidazo[1,5-a]pyridinium-Based Cation or Imines: Synthesis, Structures, Non-Covalent Interactions and Optical Properties. Crystals, 13(2), 307. https://doi.org/10.3390/cryst13020307









