Synthesis, Crystal Structure and Properties of the New Laminar Quaternary Tellurides SrLnCuTe3 (Ln = Sm, Gd–Tm and Lu)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. X-ray Diffraction Analysis
2.4. Powder X-ray Diffraction
2.5. Electron-Beam Microprobe Analysis
2.6. Magnetic Measurements
3. Results
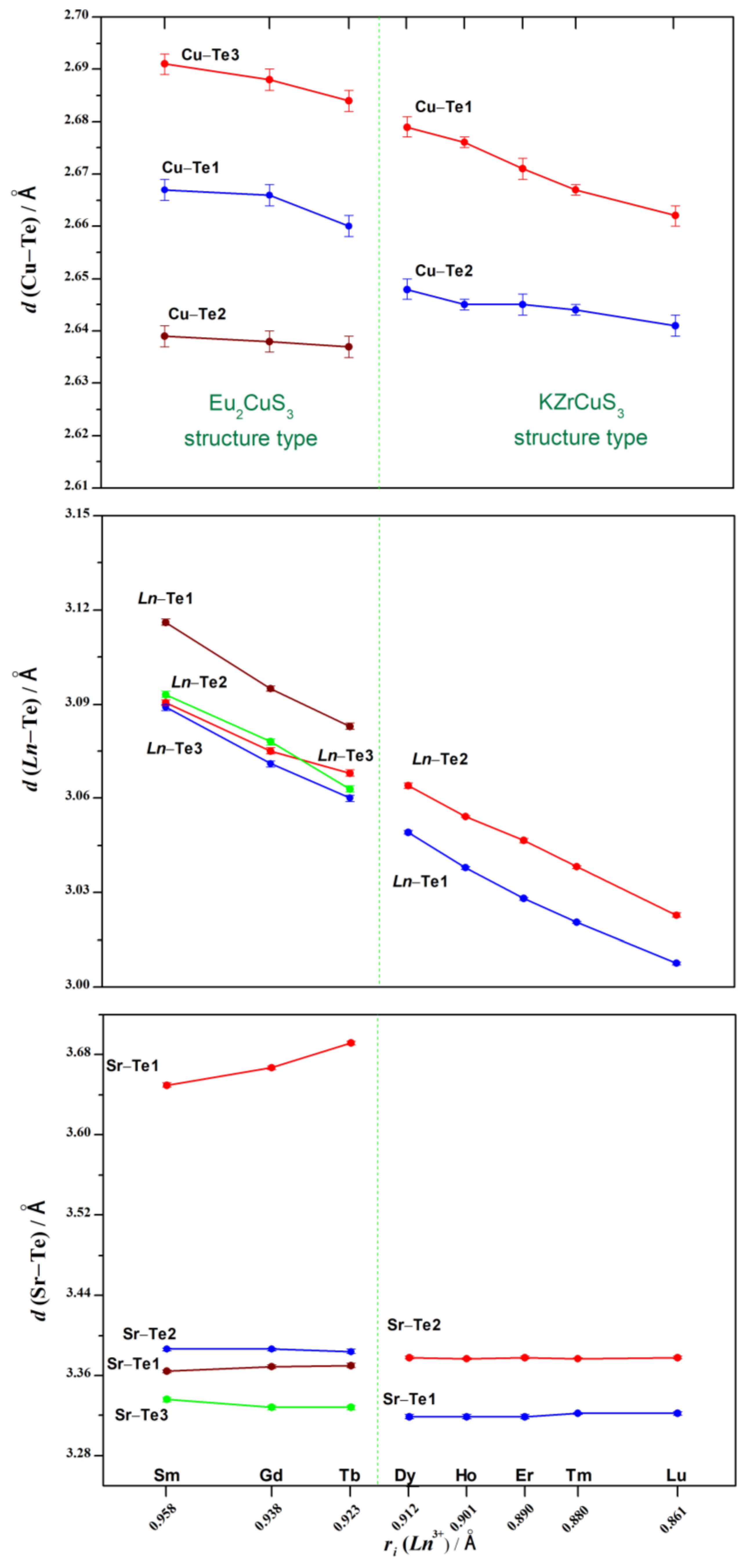
3.1. Crystal Structures of the SrLnCuTe3 Series
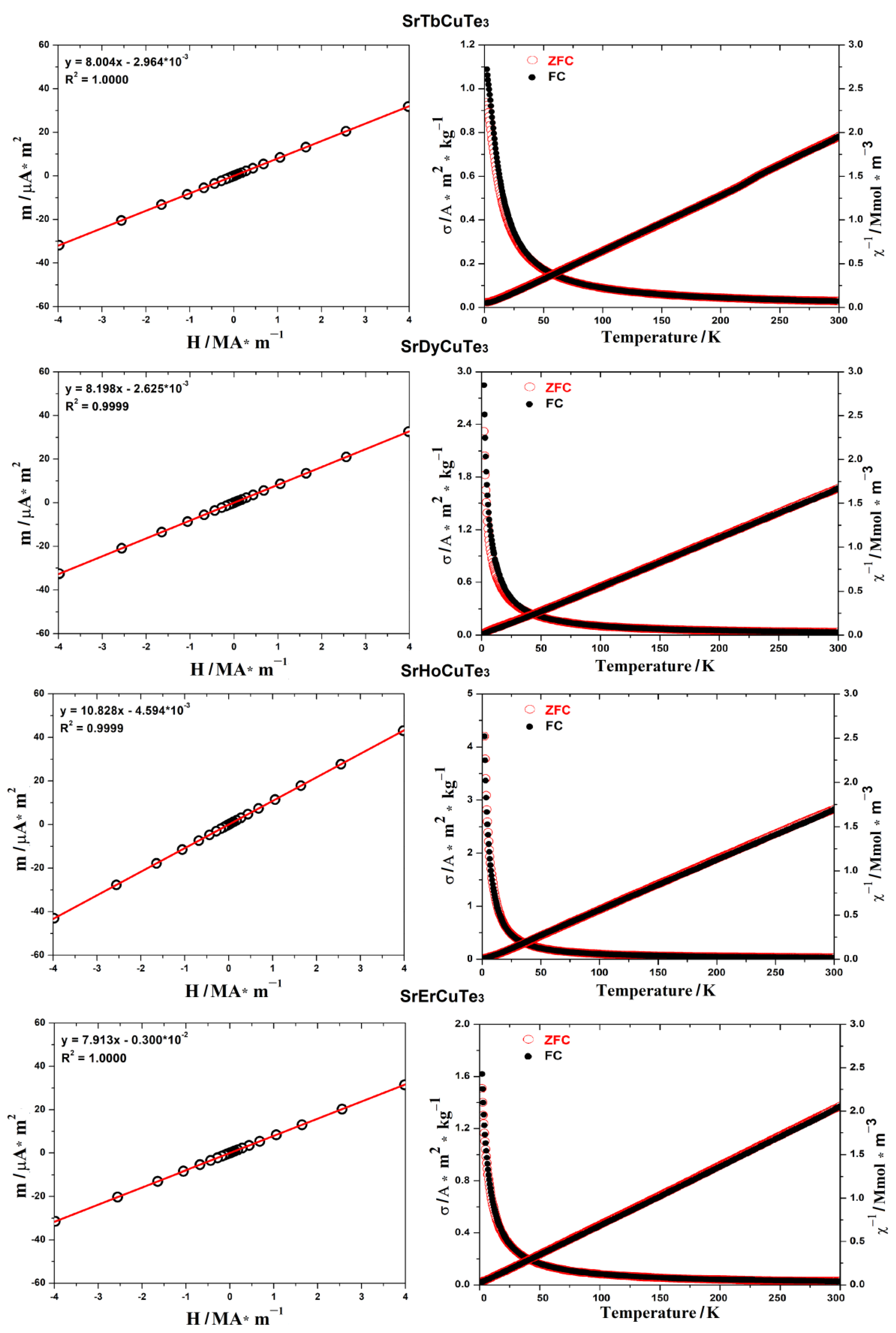
3.2. Magnetic Properties of the SrLnCuTe3 Series (Ln = Tb–Er)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, K.; Xia, Y.; Shen, J.; He, J.; Luo, Y.; Kanatzidis, M.G.; Wolverton, C. Accelerated discovery of a large family of quaternary chalcogenides with very low lattice thermal conductivity. Comput. Mater. 2021, 7, 82. [Google Scholar] [CrossRef]
- Ishtiyak, M.; Jana, S.; Karthikeyan, R.; Mamindla, R.; Tripathy, B.; Malladi, S.K.; Niranjan, M.; Prakash, J. Syntheses of five new layered quaternary chalcogenides SrScCuSe3, SrScCuTe3, BaScCuSe3, BaScCuTe3, and BaScAgTe3: Crystal structures, thermoelectric properties, and electronic structures. Inorg. Chem. Front. 2021, 8, 4086–4101. [Google Scholar] [CrossRef]
- Yang, Y.; Ibers, J.A. Synthesis and characterization of a series of quaternary chalcogenides BaLnMQ3 (Ln = rare earth, M = coinage metal, Q = Se or Te). J. Solid State Chem. 1999, 147, 366–371. [Google Scholar] [CrossRef]
- Mansuetto, M.F.; Keane, P.M.; Ibers, J.A. Synthesis and Structures of the New Group IV Chalcogenides NaCuTiS3 and NaCuZrQ3 (Q = S, Se, Te). J. Solid State Chem. 1993, 105, 580–587. [Google Scholar] [CrossRef]
- Mansuetto, M.F.; Keane, P.M.; Ibers, J.A. Synthesis, structure, and conductivity of the new group IV chalcogenides, KCuZrQ3 (Q = S, Se, Te). J. Solid State Chem. 1992, 101, 257–264. [Google Scholar] [CrossRef]
- Huang, F.Q.; Ibers, J.A. Syntheses, Structures, and Theoretical Study of LaCuSTe and SmCuSTe. Inorg Chem. 1999, 38, 5978–5983. [Google Scholar] [CrossRef]
- Babo, J.-M.; Albrecht-Schmitt, T.E. Ce2AgYb5/3Se6, La2CuErTe5, and Ce2CuTmTe5: Three new quaternary interlanthanide chalcogenides. J. Solid State Chem. 2013, 197, 414–419. [Google Scholar] [CrossRef]
- Patschke, R.; Brazis, P.; Kannewurf, C.R.; Kanatzidis, M.G. Cu0.66EuTe2, KCu2EuTe4, and Na0.2Ag2.8EuTe4: Compounds with modulated square Te nets. J. Mater. Chem. 1999, 9, 2293–2296. [Google Scholar] [CrossRef]
- Patschke, R.; Heising, J.; Kanatzidis, M.; Brazis, P.; Kannewurf, C.R. KCuCeTe4: A New Intergrowth Rare Earth Telluride with an Incommensurate Superstructure Associated with a Distorted Square Net of Tellurium. Chem Mater. 1998, 10, 695–697. [Google Scholar] [CrossRef]
- Babo, J.-M.; Choi, E.S.; Albrecht-Schmitt, T.E. Synthesis, structure, magnetism, and optical properties of Cs2Cu3DyTe4. Inorg Chem. 2012, 51, 11730–11735. [Google Scholar] [CrossRef]
- Malliakas, C.D.; Kanatzidis, M.G. Charge density waves in the square nets of tellurium of AMRETe4 (A = K, Na; M = Cu, Ag; RE = La, Ce). J. Am. Chem. Soc. 2007, 129, 10675–10677. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Xia, Y.; He, J.; Wolverton, C. High thermoelectric performance in BaAgYTe3 via low lattice thermal conductivity induced by bonding heterogeneity. Phys. Rev. Mater. 2019, 3, 085402. [Google Scholar] [CrossRef]
- Pal, K.; Hua, X.; Xia, Y.; Wolverton, C. Unraveling the structure-valence-property relationships in AMM′Q3 chalcogenides with promising thermoelectric performance. ACS Appl. Energy Mater. 2019, 3, 2110–2119. [Google Scholar] [CrossRef]
- Cody, J.A.; Ibers, J.A. Uranium Tellurides: New One- and Two-Dimensional Compounds CsUTe6, CsTiUTe5, Cs8Hf5UTe30.6, and CsCuUTe3. Inorg. Chem. 1995, 34, 3165–3172. [Google Scholar] [CrossRef]
- Babo, J.-M.; Strobel, S.; Schleid, T. Syntheses and Crystal Structures of CsCuNd2Se4 and CsCuGd2Te4: Two Non-Isotypical Cesium Copper Lanthanide Chalcogenides with Chains of Vertex-Shared [CuCh4]7– Tetrahedra. Z. Anorg. Allg. Chem. 2010, 636, 349–355. [Google Scholar] [CrossRef]
- Babo, J.-M.; Schleid, T. CsCu2Sc3Te6 and CsCuY2Te4: Two new quaternary cesium copper rare-earth metal tellurides. Solid State Sci. 2010, 12, 238–245. [Google Scholar] [CrossRef]
- Huang, F.Q.; Ibers, J.A. Syntheses and Structures of the Quaternary Copper Tellurides K3Ln4Cu5Te10 (Ln = Sm, Gd, Er), Rb3Ln4Cu5Te10 (Ln = Nd, Gd), and Cs3Gd4Cu5Te10. J. Solid State Chem. 2001, 160, 409–414. [Google Scholar] [CrossRef]
- Huang, F.Q.; Choe, W.; Lee, S.; Chu, J.S. Syntheses and Crystal Structures of Three Copper Tellurides: BaDyCuTe3, K1.5Dy2Cu2.5Te5, and Acentric K0.5Ba0.5DyCu1.5Te3. Chem. Mater. 1998, 10, 1320–1326. [Google Scholar] [CrossRef]
- Meng, C.-Y.; Chen, H.; Wan, P.; Chen, L. Syntheses, Structures, and Magnetic and Thermoelectric Properties of Double-Tunnel Tellurides: AxRE2Cu6–xTe6 (A = K–Cs; RE = La–Nd). Chem. Mater. 2011, 23, 4910–4919. [Google Scholar] [CrossRef]
- Koscielski, L.A.; Ibers, J.A. The structural chemistry of quaternary chalcogenides of the type AMM‘Q3. Z. Anorg. Allg. Chem. 2012, 638, 2585–2593. [Google Scholar] [CrossRef]
- Eickmeier, K.; Poschkamp, R.; Dronskowski, R.; Steinberg, S. Exploring the impact of lone pairs on the structural features of alkaline-earth (A) transition-metal (M,M′) chalcogenides (Q) AMM’Q3. Eur. J. Inorg. Chem. 2022, 28, e202200360. [Google Scholar] [CrossRef]
- Patschke, R.; Brazis, P.; Kannewurf, C.R.; Kanatzidis, M. Rb2Cu3CeTe5: A quaternary semiconducting compound with a two-dimensional polytelluride framework. J. Mater. Chem. 1998, 8, 2587–2589. [Google Scholar] [CrossRef]
- Pell, M.A.; Ibers, J.A. Synthesis and structure of TlCuTiTe3. J. Alloys Compd. 1996, 240, 37–41. [Google Scholar] [CrossRef]
- Wu, P.; Christuk, A.E.; Ibers, J.A. New quaternary chalcogenides BaLnMQ3 (Ln = Rare Earth or Sc; M = Cu, Ag; Q = S, Se). Structure and property variation vs. rare-earth element. J. Solid State Chem. 1994, 110, 337–344. [Google Scholar] [CrossRef]
- Huang, F.Q.; Mitchell, K.; Ibers, J.A. New layered materials: Syntheses, structures, and optical and magnetic properties of CsGdZnSe3, CsZrCuSe3, CsUCuSe3, and BaGdCuSe3. Inorg. Chem. 2001, 40, 5123–5126. [Google Scholar] [CrossRef] [PubMed]
- Ruseikina, A.V.; Molokeev, M.S.; Chernyshev, V.A.; Aleksandrovsky, A.S.; Krylov, A.S.; Krylova, S.N.; Velikanov, D.A.; Grigoriev, M.V.; Maximov, N.G.; Shestakov, N.P.; et al. Synthesis, structure, and properties of EuScCuS3 and SrScCuS3. J. Solid State Chem. 2021, 296, 121926. [Google Scholar] [CrossRef]
- Eberle, M.A.; Schleid, T. Expanding the SrCuRES3 Series with the Rare-Earth Metals Scandium and Yttrium. Z. Kristallogr. 2016, S36, 71. [Google Scholar]
- Ruseikina, A.V.; Solov’ev, L.A. Crystal structures of a- and b-SrCeCuS3. Russ. J. Inorg. Chem. 2016, 61, 482–487. [Google Scholar] [CrossRef]
- Maier, S.; Prakash, J.; Berthebaud, D.; Perez, O.; Bobev, S.; Gascoin, F. Crystal structures of the four new quaternary copper(I) selenides A0.5CuZrSe3 and ACuYSe3 (A = Sr, Ba). J. Solid State Chem. 2016, 242, 14–20. [Google Scholar] [CrossRef]
- Ruseikina, A.V.; Solovyov, L.A.; Grigoriev, M.V.; Andreev, O.V. Crystal structure variations in the series SrLnCuS3 (Ln = La, Pr, Sm, Gd, Er and Lu). Acta Crystallogr. 2019, 75, 584–588. [Google Scholar] [CrossRef]
- Sikerina, N.V. Regularities of Phase Equilibria in SrS–Ln2S3–Cu2S Systems, Preparation and Structure of SrLnCuS3 Compounds. Ph.D. Thesis, University of Tyumen, Tyumen, Russia, 2005. [Google Scholar]
- Ruseikina, A.V.; Demchuk, Z.A. Crystal structure and properties of AHoCuS3 (A= Sr or Eu). Russ. J. Inorg. Chem. 2017, 62, 27–32. [Google Scholar] [CrossRef]
- Eberle, M.A. Darstellung und Charakterisierung quaternärer Seltenerdmetall-Verbindungen in Kombination mit Kupfer und Schwefel. Ph.D. Thesis, Stuttgart University, Stuttgart, Germany, 16 December 2016. [Google Scholar]
- Ruseikina, A.V.; Grigoriev, M.V.; Solovyov, L.A.; Chernyshev, V.A.; Aleksandrovsky, A.S.; Krylov, A.S.; Krylova, S.N.; Shestakov, N.P.; Velikanov, D.A.; Garmonov, A.A.; et al. A Challenge toward Novel Quaternary Sulfides SrLnCuS3 (Ln = La, Nd, Tm): Unraveling Synthetic Pathways, Structures and Properties. Int. J. Mol. Sci. 2022, 23, 12438. [Google Scholar] [CrossRef] [PubMed]
- Strobel, S.; Schleid, T. Three structure types for strontium copper(I) lanthanide(III) selenides SrCuMSe3 (M = La, Gd, Lu). J. Alloys Compd. 2006, 418, 80–85. [Google Scholar] [CrossRef]
- Strobel, S.; Schleid, T. Quaternary Strontium Copper(I) Lanthanoid(III) Selenides with Cerium and Praseodymium: SrCuCeSe3 and SrCuPrSe3, Unequal Brother and Sister. Z. Naturforsch. 2004, 59b, 985–991. [Google Scholar] [CrossRef]
- Eberle, M.A.; Strobel, S.; Schleid, T. SrCuNdS3: A new Compound with two Different Crystal Structures. Z. Kristallogr. 2014, S34, 139. [Google Scholar]
- Ruseikina, A.V.; Solov’ev, L.A.; Galenko, E.O.; Grigor’ev, M.V. Refined Crystal Structures of SrLnCuS3 (Ln = Er, Yb). Russ. J. Inorg. Chem. 2018, 63, 1225–1231. [Google Scholar] [CrossRef]
- Ruseikina, A.V.; Andreev, O.V.; Galenko, E.O.; Koltsov, S.I. Trends in thermodynamic parameters of phase transitions of lanthanide sulfides SrLnCuS3 (Ln = La–Lu). J. Therm. Anal. Calorim. 2017, 128, 993–999. [Google Scholar] [CrossRef]
- Ruseikina, A.V.; Solov’ev, L.A.; Andreev, O.V. Crystal Structures and Properties of SrLnCuS3 (Ln = La, Pr). Russ. J. Inorg. Chem. 2014, 59, 196–201. [Google Scholar] [CrossRef]
- Grigoriev, M.V.; Solovyov, L.A.; Ruseikina, A.V.; Aleksandrovsky, A.S.; Chernyshev, V.A.; Velikanov, D.A.; Garmonov, A.A.; Molokeev, M.S.; Oreshonkov, A.S.; Shestakov, N.P.; et al. Quaternary Selenides EuLnCuSe3: Synthesis, Structures, Properties and In Silico Studies. Int. J. Mol. Sci. 2022, 23, 1503. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar]
- PLATON. A Multipurpose Crystallographic Tool; Utrecht University: Utrecht, The Netherlands, 2008. [Google Scholar]
- Wakeshima, M.; Furuuchi, F.; Hinatsu, Y. Crystal structures and magnetic properties of novel rare-earth copper sulfides, EuRCuS3 (R = Y, Gd–Lu). J. Phys. Condens. Matter. 2004, 16, 5503–5518. [Google Scholar] [CrossRef]
- Ruseikina, A.V.; Chernyshev, V.A.; Velikanov, D.A.; Aleksandrovsky, A.S.; Shestakov, N.P.; Molokeev, M.S.; Grigoriev, M.V.; Andreev, O.V.; Garmonov, A.A.; Matigorov, A.V.; et al. Regularities of the property changes in the compounds EuLnCuS3 (Ln = La–Lu). J. Alloys Compd. 2021, 874, 159968. [Google Scholar] [CrossRef]
- Ruseikina, A.V.; Andreev, O.V. Regularities of change in the structural parameters of EuLnCuS3 (Ln = La–Nd, Sm, Gd, Ho). Russ. J. Inorg. Chem. 2017, 62, 160–167. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Baur, W. The geometry of polyhedral distortions. Predictive relationships for the phosphate group. Acta Crystallogr. 1974, B30, 1195–1215. [Google Scholar] [CrossRef]
- Mikheykin, A.S.; Chernyshov, D.Y.; Bush, A.A.; Prokhorov, A.S.; Yuzyuk, Y.I.; Dmitriev, V.P. Features of the Jahn-Teller transition in Ni1–xCoxCr2O4 solid solutions. Phys. Solid State 2014, 56, 785–791. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef]
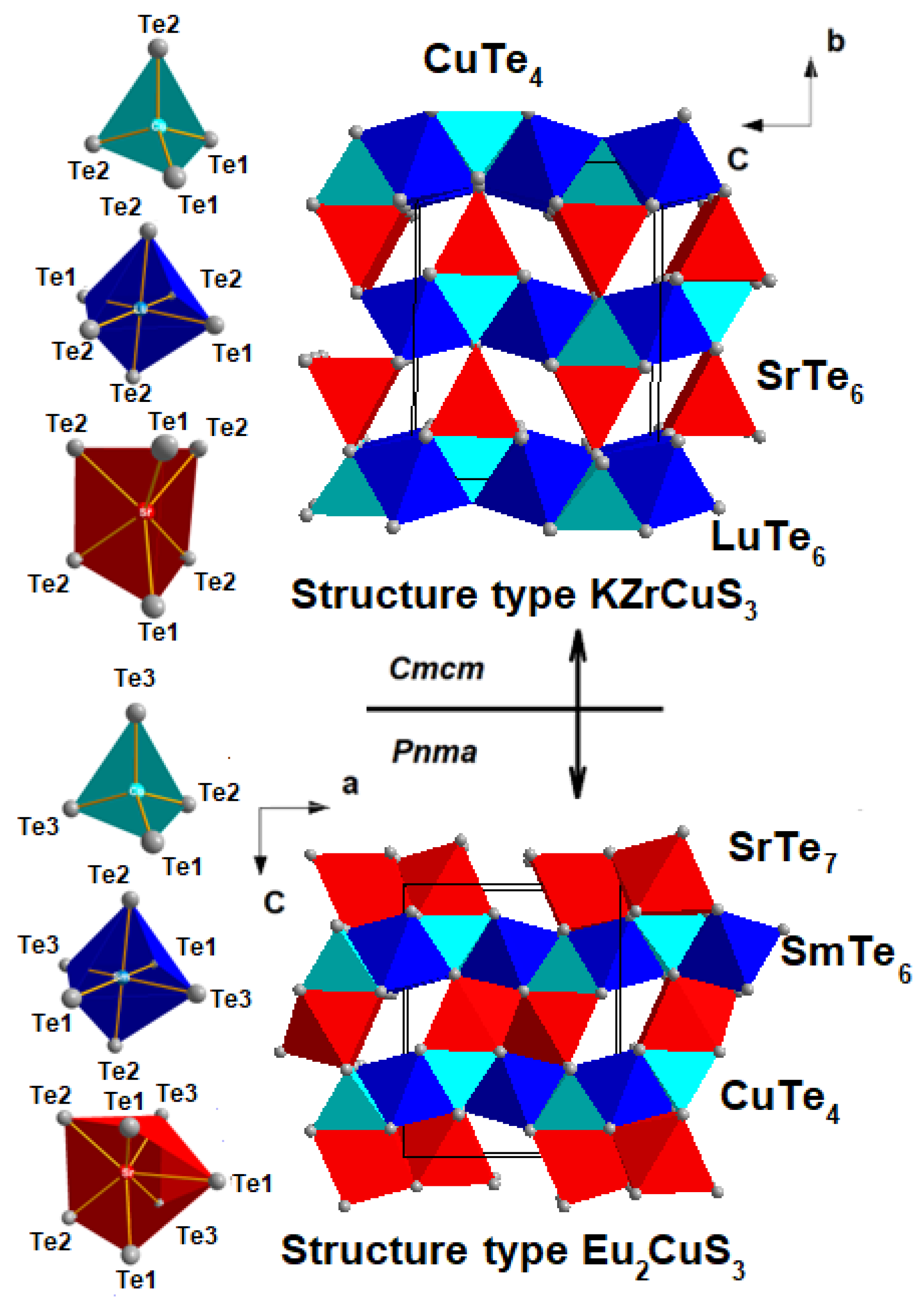



| SrSmCuTe3 | SrGdCuTe3 | SrTbCuTe3 | |
|---|---|---|---|
| Molecular weight | 684.31 | 691.21 | 692.88 |
| Space group | Pnma | ||
| Structure type | Eu2CuS3 | ||
| Z | 4 | ||
| a (Å) | 11.4592(7) | 11.3886(7) | 11.3418(7) |
| b (Å) | 4.3706(3) | 4.3534(3) | 4.3491(3) |
| c (Å) | 14.4425(9) | 14.4522(9) | 14.4326(9) |
| V (Å3) | 723.33(5) | 716.53(5) | 711.91(6) |
| ρcal (g/cm3) | 6.284 | 6.407 | 6.465 |
| μ (mm–1) | 30.006 | 31.351 | 32.173 |
| Reflections measured | 12811 | 11880 | 12049 |
| Reflections independent | 939 | 930 | 929 |
| Reflections with Fo > 4σ(Fo) | 779 | 756 | 711 |
| 2θmax (°) | 54.97 | 54.93 | 55.01 |
| h, k, l limits | –14 ≤ h ≤ 14; –5 ≤ k ≤ 5; –18 ≤ l ≤ 18 | ||
| Rint | 0.106 | 0.081 | 0.092 |
| Refinement results | |||
| Number of refinement parameters | 38 | ||
| R1 with Fo > 4σ(Fo) | 0.032 | 0.029 | 0.034 |
| wR2 | 0.082 | 0.068 | 0.078 |
| Goof | 1.108 | 1.050 | 1.002 |
| ∆ρmax(e/Å3) | 1.756 | 1.207 | 1.692 |
| ∆ρmin(e/Å3) | –1.809 | –1.263 | –1.616 |
| Extinction coefficient, ε | 0.0026(2) | 0.0022(2) | 0.0036(2) |
| CSD-number | 2232068 | 2232064 | 2232069 |
| SrDyCuTe3 | SrHoCuTe3 | SrErCuTe3 | SrTmCuTe3 | SrLuCuTe3 | |
|---|---|---|---|---|---|
| Molecular weight | 696.46 | 698.89 | 701.22 | 702.89 | 708.93 |
| Space group | Cmcm | ||||
| Structure type | KZrCuS3 | ||||
| Z | 4 | ||||
| a (Å) | 4.3405(3) | 4.3314(3) | 4.3258(3) | 4.3198(3) | 4.3064(3) |
| b (Å) | 14.4298(9) | 14.4179(9) | 14.4123(9) | 14.4037(9) | 14.3879(9) |
| c (Å) | 11.2972(7) | 11.2532(7) | 11.2176(7) | 11.1902(7) | 11.1408(7) |
| V (Å3) | 707.54(5) | 702.85(4) | 699.36(6) | 696.28(4) | 690.28(6) |
| ρcal (g/cm3) | 6.538 | 6.605 | 6.660 | 6.705 | 6.822 |
| μ (mm–1) | 32.936 | 33.783 | 34.637 | 35.480 | 37.237 |
| Reflections measured | 7403 | 6637 | 6693 | 9931 | 6818 |
| Reflections independent | 484 | 484 | 483 | 482 | 480 |
| Reflections with Fo > 4σ(Fo) | 420 | 462 | 444 | 446 | 429 |
| 2θmax (°) | 54.878 | 54.934 | 54.922 | 54.938 | 55.016 |
| h, k, l limits | –5 ≤ h ≤5; –18 ≤ k ≤ 18; –14 ≤ l ≤ 14 | ||||
| Rint | 0.074 | 0.044 | 0.062 | 0.070 | 0.073 |
| Refinement results | |||||
| Number of refinement parameters | 24 | ||||
| R1 with Fo > 4σ(Fo) | 0.026 | 0.018 | 0.023 | 0.018 | 0.025 |
| wR2 | 0.059 | 0.040 | 0.054 | 0.043 | 0.045 |
| Goof | 1.055 | 1.109 | 1.102 | 1.079 | 1.088 |
| ∆ρmax (e/Å3) | 1.413 | 1.244 | 1.604 | 0.859 | 1.781 |
| ∆ρmin (e/Å3) | –1.472 | –1.293 | –1.638 | –0.906 | –1.853 |
| Extinction coefficient, ε | 0.0011(1) | 0.00184(9) | 0.0008(1) | 0.00125(9) | 0.00082(7) |
| CSD-number | 2232062 | 2232065 | 2232063 | 2223070 | 2232066 |
| Atom | x/a | y/b | z/c | Uiso*/Ueq (Å2) |
|---|---|---|---|---|
| SrSmCuTe3 | ||||
| Sr | 0.26969(11) | 1/4 | 0.50279(9) | 0.0267(3) |
| Sm | 0.01491(5) | 1/4 | 0.74492(4) | 0.0215(2) |
| Cu | 0.24071(13) | 1/4 | 0.22143(12) | 0.0312(4) |
| Te1 | 0.05395(7) | 1/4 | 0.11132(6) | 0.0217(2) |
| Te2 | 0.41676(7) | 1/4 | 0.10364(6) | 0.0226(2) |
| Te3 | 0.26215(7) | 1/4 | 0.83011(6) | 0.0212(2) |
| SrGdCuTe3 | ||||
| Sr | 0.26663(9) | 1/4 | 0.50313(8) | 0.0243(3) |
| Gd | 0.01350(4) | 1/4 | 0.74611(3) | 0.0188(2) |
| Cu | 0.24205(12) | 1/4 | 0.22125(11) | 0.0288(4) |
| Te1 | 0.05418(6) | 1/4 | 0.11125(5) | 0.0190(2) |
| Te2 | 0.42077(6) | 1/4 | 0.10495(5) | 0.0196(2) |
| Te3 | 0.26121(6) | 1/4 | 0.83009(5) | 0.0186(2) |
| SrTbCuTe3 | ||||
| Sr | 0.26394(11) | 1/4 | 0.50354(9) | 0.0247(3) |
| Tb | 0.01132(5) | 1/4 | 0.74680(4) | 0.0200(2) |
| Cu | 0.24340(14) | 1/4 | 0.22102(12) | 0.0281(4) |
| Te1 | 0.05508(7) | 1/4 | 0.11105(6) | 0.0190(3) |
| Te2 | 0.42398(7) | 1/4 | 0.10569(6) | 0.0196(3) |
| Te3 | 0.25953(7) | 1/4 | 0.82978(6) | 0.0183(2) |
| SrDyCuTe3 | ||||
| Sr | 0 | 0.75369(9) | 1/4 | 0.0313(4) |
| Dy | 0 | 0 | 0 | 0.0245(2) |
| Cu | 0 | 0.47094(13) | 1/4 | 0.0301(4) |
| Te1 | 0 | 0.07951(6) | 1/4 | 0.0217(3) |
| Te2 | 0 | 0.35881(4) | 0.06439(5) | 0.0253(2) |
| SrHoCuTe3 | ||||
| Sr | 0 | 0.75402(7) | 1/4 | 0.0258(2) |
| Ho | 0 | 0 | 0 | 0.0194(2) |
| Cu | 0 | 0.47067(9) | 1/4 | 0.0269(3) |
| Te1 | 0 | 0.07945(4) | 1/4 | 0.0179(2) |
| Te2 | 0 | 0.35906(3) | 0.06332(4) | 0.0192(2) |
| SrErCuTe3 | ||||
| Sr | 0 | 0.75401(9) | 1/4 | 0.0248(3) |
| Er | 0 | 0 | 0 | 0.0193(2) |
| Cu | 0 | 0.47055(12) | 1/4 | 0.0273(4) |
| Te1 | 0 | 0.07917(5) | 1/4 | 0.0183(2) |
| Te2 | 0 | 0.35928(4) | 0.06242(5) | 0.0190(2) |
| SrTmCuTe3 | ||||
| Sr | 0 | 0.75437(7) | 1/4 | 0.0230(3) |
| Tm | 0 | 0 | 0 | 0.0176(2) |
| Cu | 0 | 0.47054(9) | 1/4 | 0.0254(3) |
| Te1 | 0 | 0.07905(4) | 1/4 | 0.0164(2) |
| Te2 | 0 | 0.35962(3) | 0.06173(4) | 0.0172(2) |
| SrLuCuTe3 | ||||
| Sr | 0 | 0.75465(9) | 1/4 | 0.0234(3) |
| Lu | 0 | 0 | 0 | 0.0173(2) |
| Cu | 0 | 0.47013(12) | 1/4 | 0.0261(4) |
| Te1 | 0 | 0.07884(6) | 1/4 | 0.0169(2) |
| Te2 | 0 | 0.36010(4) | 0.06020(5) | 0.0177(2) |
| Compound | Structure Type | DI(Te—Cu—Te) | DI(Cu—Te) | DI(Te···Te) | τ4 |
|---|---|---|---|---|---|
| SrSmCuTe3 | Eu2CuS3 | 0.0214 | 0.0491 | 0.0157 | 0.955 |
| SrGdCuTe3 | Eu2CuS3 | 0.0208 | 0.0499 | 0.0155 | 0.957 |
| SrTbCuTe3 | Eu2CuS3 | 0.0192 | 0.0512 | 0.0152 | 0.961 |
| SrDyCuTe3 | KZrCuS3 | 0.0182 | 0.0521 | 0.0161 | 0.979 |
| SrHoCuTe3 | KZrCuS3 | 0.0174 | 0.0533 | 0.0173 | 0.980 |
| SrErCuTe3 | KZrCuS3 | 0.0164 | 0.0541 | 0.0181 | 0.981 |
| SrTmCuTe3 | KZrCuS3 | 0.0155 | 0.0550 | 0.0191 | 0.982 |
| SrLuCuTe3 | KZrCuS3 | 0.0140 | 0.0564 | 0.0205 | 0.984 |
| SrTbCuTe3 | SrDyCuTe3 | SrHoCuTe3 | SrErCuTe3 | |
|---|---|---|---|---|
| Space group | Pnma | Cmcm | ||
| Structure type | Eu2CuS3 | KZrCuS3 | ||
| Experimental μ 300 K (μB) | 9.57 | 10.34 | 10.49 | 9.33 |
| Experimental μ2–300 K (μB) | 9.80 | 10.63 | 10,59 | 9.64 |
| Calculated μ (μB) | 9.721 | 10.646 | 10.607 | 9.581 |
| Experimental C300 K (K·m3·kmol–1) | 0.144 | 0.168 | 0.173 | 0.137 |
| Experimental C2–300 K (K·m3·kmol−1) | 0.151 | 0.178 | 0.176 | 0.146 |
| Calculated C (K·m3·kmol−1) | 0.149 | 0.178 | 0.177 | 0.144 |
| θp (K) | 5.2 | 2.9 | 1.1 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruseikina, A.V.; Grigoriev, M.V.; Molokeev, M.S.; Garmonov, A.A.; Elyshev, A.V.; Locke, R.J.C.; Schleid, T. Synthesis, Crystal Structure and Properties of the New Laminar Quaternary Tellurides SrLnCuTe3 (Ln = Sm, Gd–Tm and Lu). Crystals 2023, 13, 291. https://doi.org/10.3390/cryst13020291
Ruseikina AV, Grigoriev MV, Molokeev MS, Garmonov AA, Elyshev AV, Locke RJC, Schleid T. Synthesis, Crystal Structure and Properties of the New Laminar Quaternary Tellurides SrLnCuTe3 (Ln = Sm, Gd–Tm and Lu). Crystals. 2023; 13(2):291. https://doi.org/10.3390/cryst13020291
Chicago/Turabian StyleRuseikina, Anna V., Maxim V. Grigoriev, Maxim S. Molokeev, Alexander A. Garmonov, Andrey V. Elyshev, Ralf J. C. Locke, and Thomas Schleid. 2023. "Synthesis, Crystal Structure and Properties of the New Laminar Quaternary Tellurides SrLnCuTe3 (Ln = Sm, Gd–Tm and Lu)" Crystals 13, no. 2: 291. https://doi.org/10.3390/cryst13020291
APA StyleRuseikina, A. V., Grigoriev, M. V., Molokeev, M. S., Garmonov, A. A., Elyshev, A. V., Locke, R. J. C., & Schleid, T. (2023). Synthesis, Crystal Structure and Properties of the New Laminar Quaternary Tellurides SrLnCuTe3 (Ln = Sm, Gd–Tm and Lu). Crystals, 13(2), 291. https://doi.org/10.3390/cryst13020291







