Crystal Growth of the R2SiO5 Compounds (R = Dy, Ho, and Er) by the Floating Zone Method Using a Laser-Diode-Heated Furnace
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Floating Zone Crystal Growth Using a Conventional Floating Zone Furnace
3.2. Floating Zone Crystal Growth Using a Laser-Diode-Heated Floating Zone Furnace
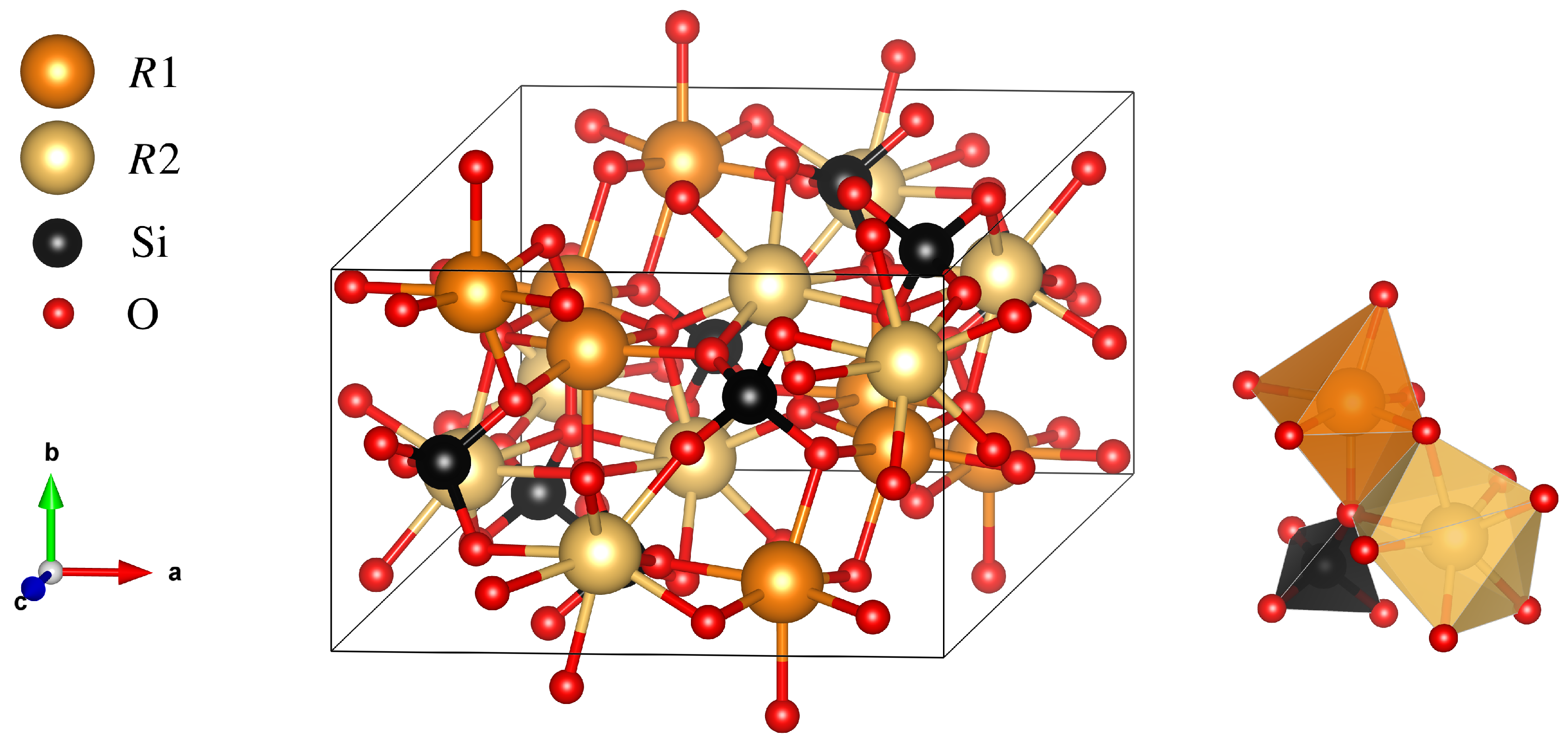
3.3. Characterization of the Crystal Boules Grown by the Floating Zone Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| TBC | Thermal barrier coating |
| EBC | Environmental barrier coating |
| FZ | Floating zone |
| LDFZ | Laser-diode heated floating zone |
| PXRD | Powder X-ray diffraction |
| PHD | Pulse height distribution |
| SCXRD | Single crystal X-ray diffraction |
References
- Toropov, N.A.; Bondar, I.A. Rare-earth silicates Communication 1. Phase diagram of the system La2O3-SiO2. Russ. Chem. Bull. 1960, 9, 145–148. [Google Scholar] [CrossRef]
- Leonov, A.I.; Rudenko, V.S.; Keler, É.K. Reactions between Ce2O3 and SiO2 at high temperatures. Russ. Chem. Bull. 1961, 10, 1797–1804. [Google Scholar] [CrossRef]
- Toropov, N.A.; Bondar, I.A. Lanthanum silicate 2La2O3·3SiO2. Russ. Chem. Bull. 1959, 8, 528–530. [Google Scholar] [CrossRef]
- Toropov, N.A.; Galakhov, F.Y.; Konovalova, S.F. Silicates of the rare earth elements Communication 2. Phase diagram of the binary system gadolinium oxide-silica. Russ. Chem. Bull. 1961, 10, 497–501. [Google Scholar] [CrossRef]
- Toropov, N.A.; Bondar, I.A. Silicates of the rare earth elements Communication 3. Phase diagram of the binary system yttrium oxide-silica. Russ. Chem. Bull. 1961, 10, 502–508. [Google Scholar] [CrossRef]
- Toropov, N.A.; Bondar, I.A. Silicates of rare-earth elements Comunication 4. New silicates in the system La2O3-SiO2. Russ. Chem. Bull. 1961, 10, 682–687. [Google Scholar] [CrossRef]
- Toropov, N.A.; Galakhov, F.Y.; Konovalova, S.F. Silicates of the rare earth elements Communication 5. phase diagrams of the Dy2O3-SiO2 and Er2O3-SiO2 systems. Russ. Chem. Bull. 1961, 10, 1271–1277. [Google Scholar] [CrossRef]
- Toropov, N.A.; Bondar, I.A. Silicates of the rare earth elements Communication 6. Phase diagrams of the binary systems Sm2O3-SiO2 and Yb2O3-SiO2, and comparison of these silicates with the other rare earth element silicates which have been studied. Russ. Chem. Bull. 1961, 10, 1278–1285. [Google Scholar] [CrossRef]
- Lazarev, A.N.; Tenisheva, T.F. The vibration spectra and structures of some rare earth element silicates. Russ. Chem. Bull. 1961, 10, 894–901. [Google Scholar] [CrossRef]
- Keler, É.K.; Godina, N.A.; Savchenko, E.P. Reactions in the solid phase of silica with oxides of rare earth elements (La2O3, Nd2O3, Gd2O3). Russ. Chem. Bull. 1961, 10, 1613–1618. [Google Scholar] [CrossRef]
- Keler, É.K.; Godina, N.A.; Savchenko, E.P. Reactions in the solid phase between silica and praeseodymium oxide. Russ. Chem. Bull. 1961, 10, 1619–1623. [Google Scholar] [CrossRef]
- Bondar, I.A.; Galakhov, F.Y.; Toropov, N.A. Silicates of the rare earths Part 7. Solid solutions between silicates of lanthanum and samarium, gadolinium, and dysprosium. Russ. Chem. Bull. 1962, 11, 351–355. [Google Scholar] [CrossRef]
- Bondar, I.A. Silicates of the rare earths Part 8. Solid solutions between silicates of lanthanum and ytterbium. Russ. Chem. Bull. 1962, 11, 356–360. [Google Scholar] [CrossRef]
- Toropov, N.A.; Galakhov, F.Y.; Konovalova, S.F. Silicates of the rare earths Part 9. Solid solutions between silicates of yttrium and erbium. Russ. Chem. Bull. 1962, 11, 689–693. [Google Scholar] [CrossRef]
- Leonov, A.I. The valence of cerium in synthetic and natural cerium aluminates and silicates. Part 2. Cerium silicates. Russ. Chem. Bull. 1963, 12, 1922–1926. [Google Scholar] [CrossRef]
- Felsche, J. A new cerium(III) orthosilicate with the apatite structure. Naturwissenschaften 1969, 56, 325–326. [Google Scholar] [CrossRef]
- Felsche, J. A new silicate structure containing linear [Si3O10] groups. Naturwissenschaften 1972, 59, 35–36. [Google Scholar] [CrossRef]
- Felsche, J. Rare earth silicates with the apatite structure. J. Solid State Chem. 1972, 5, 266–275. [Google Scholar] [CrossRef]
- Felsche, J. The crystal chemistry of the rare-earth silicates. In Proceedings of the Rare Earths; Springer: Berlin/Heidelberg, Germany, 1973; pp. 99–197. [Google Scholar]
- Bondar, I. Rare-earth silicates. Ceram. Int. 1982, 8, 83–89. [Google Scholar] [CrossRef]
- Zhang, F.X.; Xiao, H.Y.; Lang, M.; Zhang, J.M.; Zhang, Y.; Weber, W.J.; Ewing, R.C. Structure and properties of rare earth silicates with the apatite structure at high pressure. Phys. Chem. Miner. 2013, 40, 817–825. [Google Scholar] [CrossRef]
- Kobayashi, K.; Sakka, Y. Rudimental research progress of rare-earth silicate oxyapatites: Their identification as a new compound until discovery of their oxygen ion conductivity. J. Ceram. Soc. Jpn. 2014, 122, 649–663. [Google Scholar] [CrossRef]
- Ptáček, P. Rare-earth Element-bearing Apatites and Oxyapatites. In Apatites and Their Synthetic Analogues; IntechOpen: Rijeka, Croatia, 2016; Chapter 5. [Google Scholar] [CrossRef]
- Lazarev, A.N.; Tenisheva, T.F.; Bondar, I.A.; Koroleva, L.N. The structure of rare earth pyrosilicates. Russ. Chem. Bull. 1962, 11, 514–516. [Google Scholar] [CrossRef]
- Felsche, J.; Hirsiger, W. The polymorphs of the rare-earth pyrosilicates R.E.2Si2O7, [R.E.: La, Ce, Pr, Nd, Sm]. J. Less-Common Met. 1969, 18, 131–137. [Google Scholar] [CrossRef]
- Smolin, Y.I.; Shepelev, Y.F. The crystal structures of the rare earth pyrosilicates. Acta Cryst. 1970, B26, 484–492. [Google Scholar] [CrossRef]
- Felsche, J. Polymorphism and crystal data of the rare-earth disilicates of type R.E.2Si2O7. J. Less-Common Met. 1970, 21, 1–14. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, Y.; He, J.; Jiang, W.; Li, J.; Ni, H.; Zhang, Q.; Lin, L. On the Crystal Chemistry of RE2Si2O7: Revisited Structures, Group–Subgroup Relationship, and Insights of Ce3+-Activated Radioluminescence. Chem. Mater. 2023, 35, 2635–2646. [Google Scholar] [CrossRef]
- Müller-Bunz, H.; Schleid, T. Über die Oxidsilicate M2O[SiO4] der schweren Lanthanoide (M = Dy–Lu) im A-Typ. Z. Anorg. Allg. Chem. 1999, 625, 613–618. [Google Scholar] [CrossRef]
- Wang, J.; Tian, S.; Li, G.; Liao, F.; Jing, X. Preparation and X-ray characterization of low-temperature phases of R2SiO5 (R = rare earth elements). Mater. Res. Bull. 2001, 36, 1855–1861. [Google Scholar] [CrossRef]
- Rey-García, F.; Costa, F.M.; Zaldo, C. Laser floating zone growth of Yb, or Nd, doped (Lu0.3Gd0.7)2SiO5 oxyorthosilicate single-crystal rods with efficient laser performance. J. Mater. Chem. C 2020, 8, 2065–2073. [Google Scholar] [CrossRef]
- Phanon, D.; Černý, R. Crystal Structure of the B-type Dierbium Oxide ortho-Oxosilicate Er2O[SiO4]. Z. Anorg. Allg. Chem. 2008, 634, 1833–1835. [Google Scholar] [CrossRef]
- Ryba-Romanowski, W.; Strzęp, A.; Lisiecki, R.; Berkowski, M.; Rodriguez-Rodriguez, H.; Martin, I. Effect of substitution of lutetium by gadolinium on emission characteristics of (LuxGd1-x)2SiO5: Sm3+ single crystals. Opt. Mater. Express 2014, 4, 739–752. [Google Scholar] [CrossRef]
- Seminko, V.; Maksimchuk, P.; Bespalova, I.; Malyukin, Y. Different Roles of Ce3+ Optical Centers in Oxyorthosilicate Nanocrystals at X-ray and UV Excitation. Crystals 2019, 9, 114. [Google Scholar] [CrossRef]
- Ren, X.; Tian, Z.; Zhang, J.; Wang, J. Equiatomic quaternary (Y1/4Ho1/4Er1/4Yb1/4)2SiO5 silicate: A perspective multifunctional thermal and environmental barrier coating material. Scr. Mater. 2019, 168, 47–50. [Google Scholar] [CrossRef]
- Sukhanov, A.; Likerov, R.; Eremina, R.; Yatsyk, I.; Gavrilova, T.; Tarasov, V.; Zavartsev, Y.; Kutovoi, S. Crystal environment of impurity Nd3+ ion in yttrium and scandium orthosilicate crystals. J. Magn. Reson. 2018, 295, 12–16. [Google Scholar] [CrossRef]
- Sun, Z.; Li, M.; Zhou, Y. Thermal properties of single-phase Y2SiO5. J. Eur. Ceram. Soc. 2009, 29, 551–557. [Google Scholar] [CrossRef]
- Belt, R.F.; Catalano, J.A. Single Crystal Growth of Cerium Doped Rare Earth Silicates. In Advanced Solid State Lasers; Optica Publishing Group: Washington, DC, USA, 1986; p. ThA2. [Google Scholar] [CrossRef]
- Utsu, T.; Akiyama, S. Growth and applications of Gd2SiO5: Ce scintillators. J. Cryst. Growth 1991, 109, 385–391. [Google Scholar] [CrossRef]
- Tanaka, M.; Hara, K.; Kim, S.; Kondo, K.; Takano, H.; Kobayashi, M.; Ishibashi, H.; Kurashige, K.; Susa, K.; Ishii, M. Applications of cerium-doped gadolinium silicate Gd2SiO5:Ce scintillator to calorimeters in high-radiation environment. Nucl. Instrum. Methods 1998, 404, 283–294. [Google Scholar] [CrossRef]
- Leonyuk, N.I.; Belokoneva, E.L.; Bocelli, G.; Righi, L.; Shvanskii, E.V.; Henrykhson, R.V.; Kulman, N.V.; Kozhbakhteeva, D.E. Crystal Growth and Structural Refinements of the Y2SiO5, Y2Si2O7 and LaBSiO5 Single Crystals. Cryst. Res. Technol. 1999, 34, 1175–1182. [Google Scholar] [CrossRef]
- Campos, S.; Petit, J.; Viana, B.; Jandl, S.; Vivien, D.; Ferrand, B. Spectroscopic investigation of the laser materials Yb3+:RE2SiO5, RE = Y, Sc, Lu. In Solid State Lasers and Amplifiers; Sennaroglu, A., Fujimoto, J.G., Pollock, C.R., Eds.; International Society for Optics and Photonics, SPIE: Bellingham, WA, USA, 2004; Volume 5460, pp. 335–343. [Google Scholar] [CrossRef]
- Lee, W.G.; Lee, D.H.; Kim, Y.K.; Kim, J.K.; Park, J.W. Growth and Characteristics of Gd2SiO5 Crystal Doped Ce3+. J. Nucl. Sci. Technol. 2008, 45, 572–574. [Google Scholar] [CrossRef]
- Xu, X.; Xu, W.; Cheng, Y.; Li, D.; Cheng, S.; Wu, F.; Zhao, Z.; Zhou, G.; Xu, J. Investigation on upconversion luminescence in Yb3+/Er3+-codoped Gd2SiO5 single crystal. Phys. Status Solidi A 2010, 207, 432–435. [Google Scholar] [CrossRef]
- Liu, L.; Wang, C.; Zhang, D.; Zhang, Q.; Ning, K.; Wang, J.; Sun, X. Dielectric Relaxations and Phase Transition in Laser Crystals Gd2SiO5 and Yb-Doped Gd2SiO5. J. Am. Ceram. Soc. 2014, 97, 1823–1828. [Google Scholar] [CrossRef]
- Iskhakova, L.D.; Ilyukhin, A.B.; Kutovoi, S.A.; Vlasov, V.I.; Zavartsev, Y.D.; Tarasov, V.F.; Eremina, R.M. The crystal structure of new quantum memory-storage material Sc1.368Y0.632SiO5. Acta Cryst. 2019, C75, 1202–1207. [Google Scholar] [CrossRef]
- Jobbitt, N.L.; Wells, J.P.R.; Reid, M.F.; Longdell, J.J. Raman heterodyne determination of the magnetic anisotropy for the ground and optically excited states of Y2SiO5 doped with Sm3+. Phys. Rev. B 2021, 103, 205114. [Google Scholar] [CrossRef]
- Buisson, G.; Michel, C. Serie isomorphe d’orthosilicates (T2SiO5) et d’orthogermanates (T2GeO5) de terres rares. Mat. Res. Bull. 1968, 3, 193–197. [Google Scholar] [CrossRef]
- Wanklyn, B.M.; Wondre, F.R.; Ansell, G.B.; Davison, W. New rare earth silicate crystals: Dy2MoSi2Al4O16, compounds in the systems R2O3-SiO2-PbO, and a new form of R2SiO5. J. Mater. Sci 1975, 10, 1494–1500. [Google Scholar] [CrossRef]
- Maqsood, A. Single crystal preparation of the rare earth oxyorthosilicates R2SiO5 (R = Er, Ho, Dy) by a flux method. J. Mater. Sci. Lett. 1984, 3, 65–67. [Google Scholar] [CrossRef]
- Novoselov, A.; Ogino, H.; Yoshikawa, A.; Nikl, M.; Pejchal, J.; Mares, J.; Beitlerova, A.; D’Ambrosio, C.; Fukuda, T. Study on crystal growth and luminescence properties of Pr-doped RE2SiO5 (RE = Y, Lu). J. Cryst. Growth 2006, 287, 309–312. [Google Scholar] [CrossRef]
- Xue, Z.; Chen, L.; Zhao, S.; Yang, F.; An, R.; Wang, L.; Sun, Y.Y.; Feng, H.; Ding, D. Enhancement of Scintillation Properties of LYSO:Ce Crystals by Al Codoping. Cryst. Growth Des. 2023, 23, 4562–4570. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, P.; Luo, L.; Guo, R.; Wang, Y. Structural and optical characterization of Gd2SiO5:Tb3+ crystal obtained by optical floating zone method. Optik 2021, 225, 165814. [Google Scholar] [CrossRef]
- Nair, H.S.; DeLazzer, T.; Reeder, T.; Sikorski, A.; Hester, G.; Ross, K.A. Crystal Growth of Quantum Magnets in the Rare-Earth Pyrosilicate Family R2Si2O7 (R = Yb, Er) Using the Optical Floating Zone Method. Crystals 2019, 9, 196. [Google Scholar] [CrossRef]
- Ciomaga Hatnean, M.; Petrenko, O.A.; Lees, M.R.; Orton, T.E.; Balakrishnan, G. Optical Floating Zone Crystal Growth of Rare-Earth Disilicates, R2Si2O7 (R = Er, Ho, and Tm). Cryst. Growth Des. 2020, 20, 6636–6648. [Google Scholar] [CrossRef]
- De La Fuente, G.F.; Black, L.R.; Andrauskas, D.M.; Verdún, H.R. Growth of Nd-doped rare earth silicates by the laser floating zone method. Solid State Ionics 1989, 32–33, 494–505. [Google Scholar] [CrossRef]
- Rey-García, F.; Sedrine, N.B.; Soares, M.R.; Fernandes, A.J.S.; Lopes, A.B.; Ferreira, N.M.; Monteiro, T.; Costa, F.M. Structural and optical characterization of Gd2SiO5 crystalline fibres obtained by laser floating zone. Opt. Mater. Express 2017, 7, 868–879. [Google Scholar] [CrossRef]
- Rey-García, F.; Rodrigues, J.; Fernandes, A.J.S.; Soares, M.R.; Monteiro, T.; Costa, F.M. (Lu0.3Gd0.7)2SiO5:Y3+ single crystals grown by the laser floating zone method: Structural and optical studies. CrystEngComm 2018, 20, 7386–7394. [Google Scholar] [CrossRef]
- Rey-García, F.; Sedrine, N.B.; Fernandes, A.; Monteiro, T.; Costa, F. Shifting Lu2SiO5 crystal to eutectic structure by laser floating zone. J. Eur. Ceram. Soc. 2018, 38, 2059–2067. [Google Scholar] [CrossRef]
- Rey-García, F.; Bao-Varela, C.; Costa, F.M. Laser floating zone: General overview focusing on the oxyorthosilicates growth. In Synthesis Methods and Crystallization; Marzouki, R., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 6. [Google Scholar] [CrossRef]
- Rey-García, F.; Fernandes, A.; Costa, F. Influence of Lu content on (LuxGd1-x)2SiO5 oxyorthosilicates grown by Laser Floating Zone: Structural studies and transparency. Mat. Res. Bull. 2019, 112, 413–419. [Google Scholar] [CrossRef]
- Ciomaga Hatnean, V.C.; Pui, A.; Simonov, A.; Ciomaga Hatnean, M.; Materials Discovery Laboratory, Department of Materials, ETH Zurich, Switzerland. 2023; manuscript in preparation.
- Mos, Y.M.; Vermeulen, A.C.; Buisman, C.J.; Weijma, J. X-ray Diffraction of Iron Containing Samples: The Importance of a Suitable Configuration. Geomicrobiol. J. 2018, 35, 511–517. [Google Scholar] [CrossRef]
- Rao, S.G.; Shu, R.; Boyd, R.; Greczynski, G.; le Febvrier, A.; Eklund, P. Phase formation and structural evolution of multicomponent (CrFeCo)1-yNy films. Surf. Coat. Technol 2021, 412, 127059. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- CrysAlis PRO. Agilent; Agilent Technologies Ltd.: Yarnton, Oxfordshire, England, UK, 2014. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Koohpayeh, S.; Fort, D.; Bradshaw, A.; Abell, J. Thermal characterization of an optical floating zone furnace: A direct link with controllable growth parameters. J. Cryst. Growth 2009, 311, 2513–2518. [Google Scholar] [CrossRef]
- Koohpayeh, S.; Fort, D.; Abell, J. The optical floating zone technique: A review of experimental procedures with special reference to oxides. Prog. Cryst. Growth Charact. Mater. 2008, 54, 121–137. [Google Scholar] [CrossRef]
- Dabkowska, H.A.; Dabkowski, A.B. Crystal Growth of Oxides by Optical Floating Zone Technique. In Springer Handbook of Crystal Growth; Dhanaraj, G., Byrappa, K., Prasad, V., Dudley, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 367–391. [Google Scholar] [CrossRef]
- Pierre, P.D.S.S. A Note on the Melting Point of Titanium Dioxide. J. Am. Ceram. Soc. 1952, 35, 188. [Google Scholar] [CrossRef]
- Tian, Z.; Zheng, L.; Wang, J.; Wan, P.; Li, J.; Wang, J. Theoretical and experimental determination of the major thermo-mechanical properties of RE2SiO5 (RE = Tb, Dy, Ho, Er, Tm, Yb, Lu, and Y) for environmental and thermal barrier coating applications. J. Eur. Ceram. Soc. 2016, 36, 189–202. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Y.; Tian, Z.; Wang, J.; Wang, J. Theoretical exploration of the abnormal trend in lattice thermal conductivity for monosilicates RE2SiO5 (RE = Dy, Ho, Er, Tm, Yb and Lu). J. Eur. Ceram. Soc. 2018, 38, 3539–3546. [Google Scholar] [CrossRef]
- Tian, Z.; Zheng, L.; Hu, W.; Sun, L.; Zhang, J.; Wang, J. Tunable properties of (HoxY1-x)2SiO5 as damage self-monitoring environmental/thermal barrier coating candidates. Sci. Rep. 2019, 9, 415. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, J.; Zhang, T.; Ren, X.; Hu, W.; Zheng, L.; Wang, J. Towards thermal barrier coating application for rare earth silicates RE2SiO5 (RE = La, Nd, Sm, Eu, and Gd). J. Eur. Ceram. Soc. 2019, 39, 1463–1476. [Google Scholar] [CrossRef]
- Zhong, X.; Zhu, T.; Niu, Y.; Zhou, H.; Zhang, L.; Zhang, X.; Li, Q.; Zheng, X. Effect of microstructure evolution and crystal structure on thermal properties for plasma-sprayed RE2SiO5 (RE = Gd, Y, Er) environmental barrier coatings. J. Mater. Sci. Technol. 2021, 85, 141–151. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, Z.; Zheng, L.; Ming, K.; Ren, X.; Wang, J.; Li, B. (Ho0.25Lu0.25Yb0.25Eu0.25)2SiO5 high-entropy ceramic with low thermal conductivity, tunable thermal expansion coefficient, and excellent resistance to CMAS corrosion. J. Adv. Ceram. 2022, 11, 1279–1293. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Cryst. 1969, B25, 925–946. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Cryst. 1991, B47, 192–197. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ciomaga Hatnean, M.; Decorse, C.; Lees, M.R.; Petrenko, O.A.; Balakrishnan, G. Zirconate Pyrochlore Frustrated Magnets: Crystal Growth by the Floating Zone Technique. Crystals 2016, 6, 79. [Google Scholar] [CrossRef]
- Sibille, R.; Lhotel, E.; Ciomaga Hatnean, M.; Nilsen, G.J.; Ehlers, G.; Cervellino, A.; Ressouche, E.; Frontzek, M.; Zaharko, O.; Pomjakushin, V.; et al. Coulomb spin liquid in anion-disordered pyrochlore Tb2Hf2O7. Nat. Commun. 2017, 8, 892. [Google Scholar] [CrossRef]
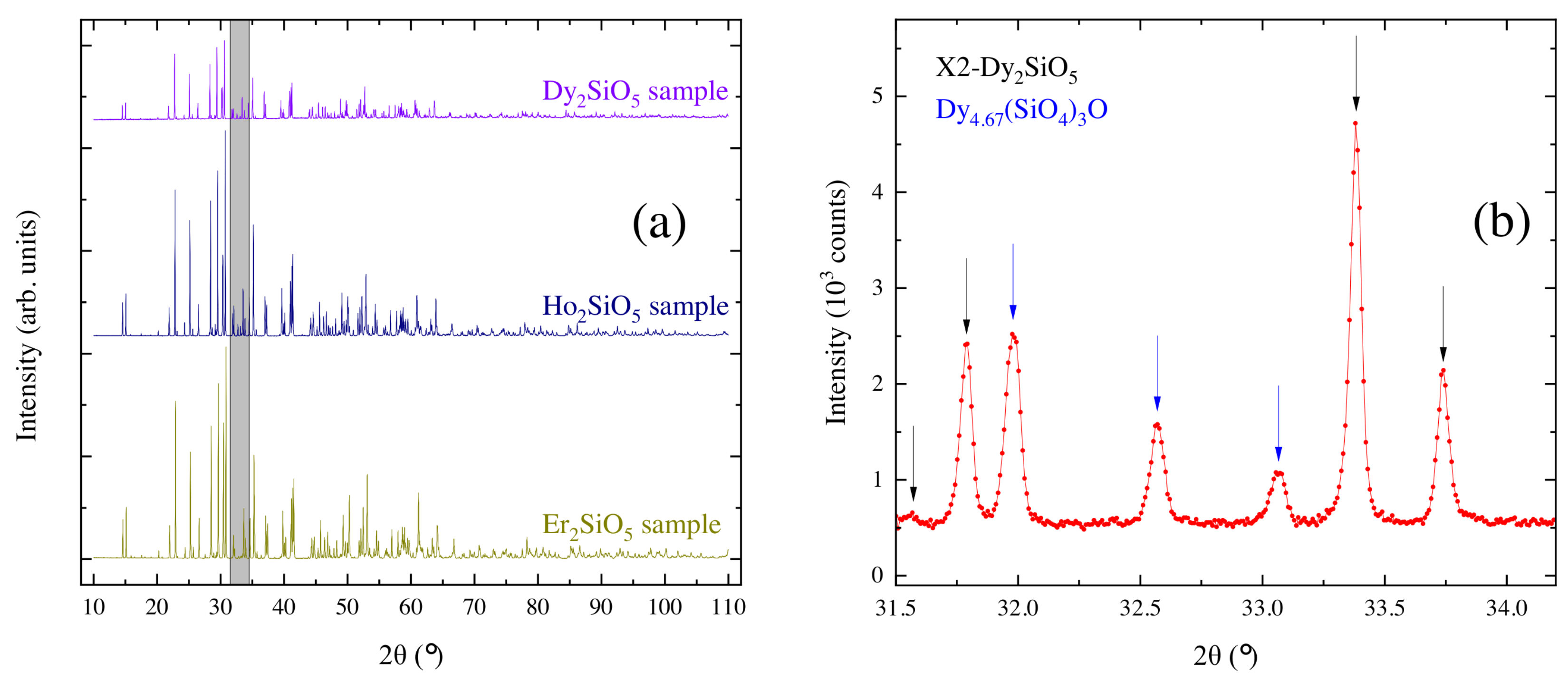
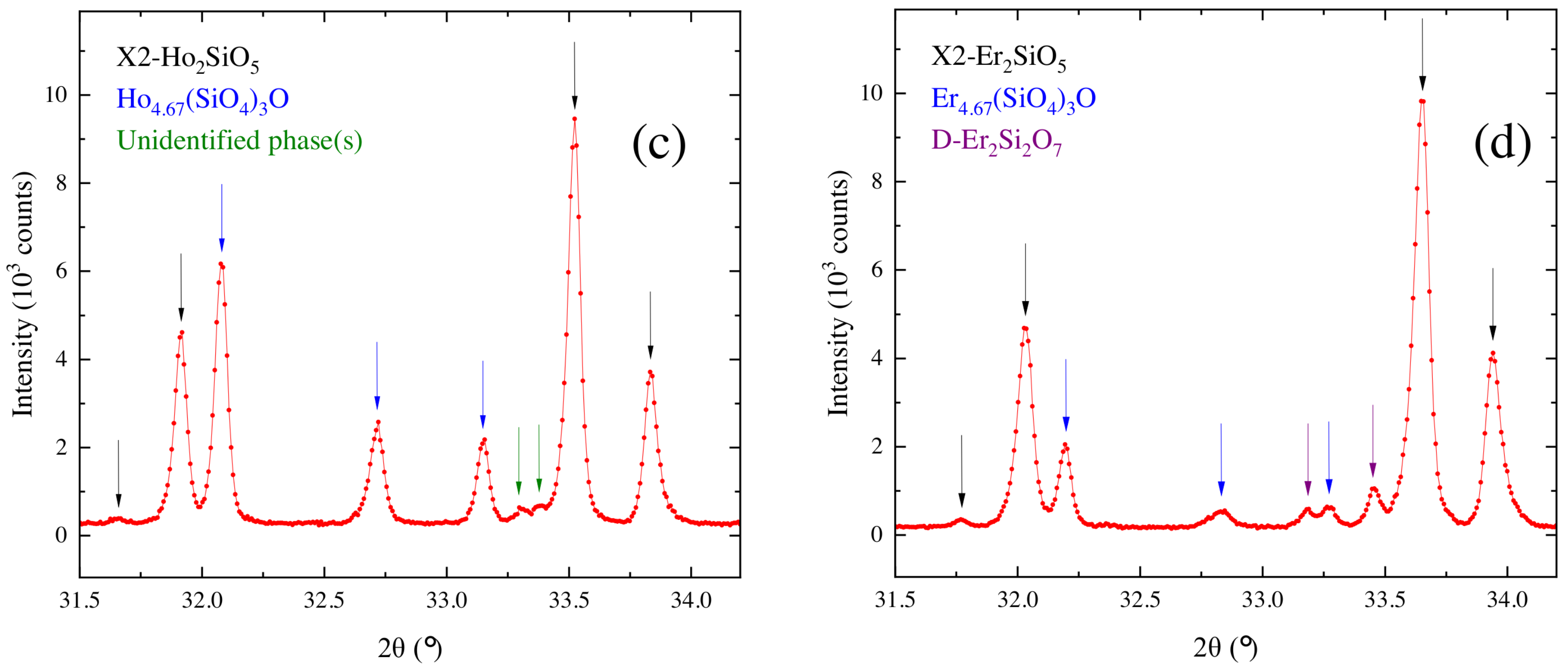
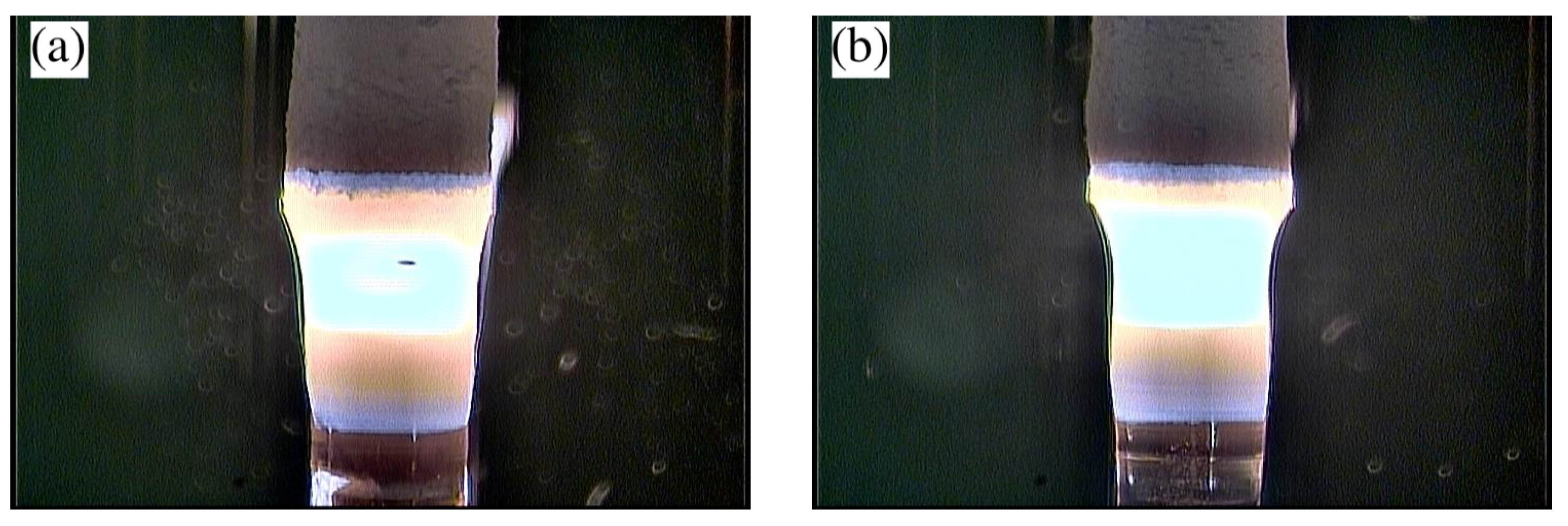

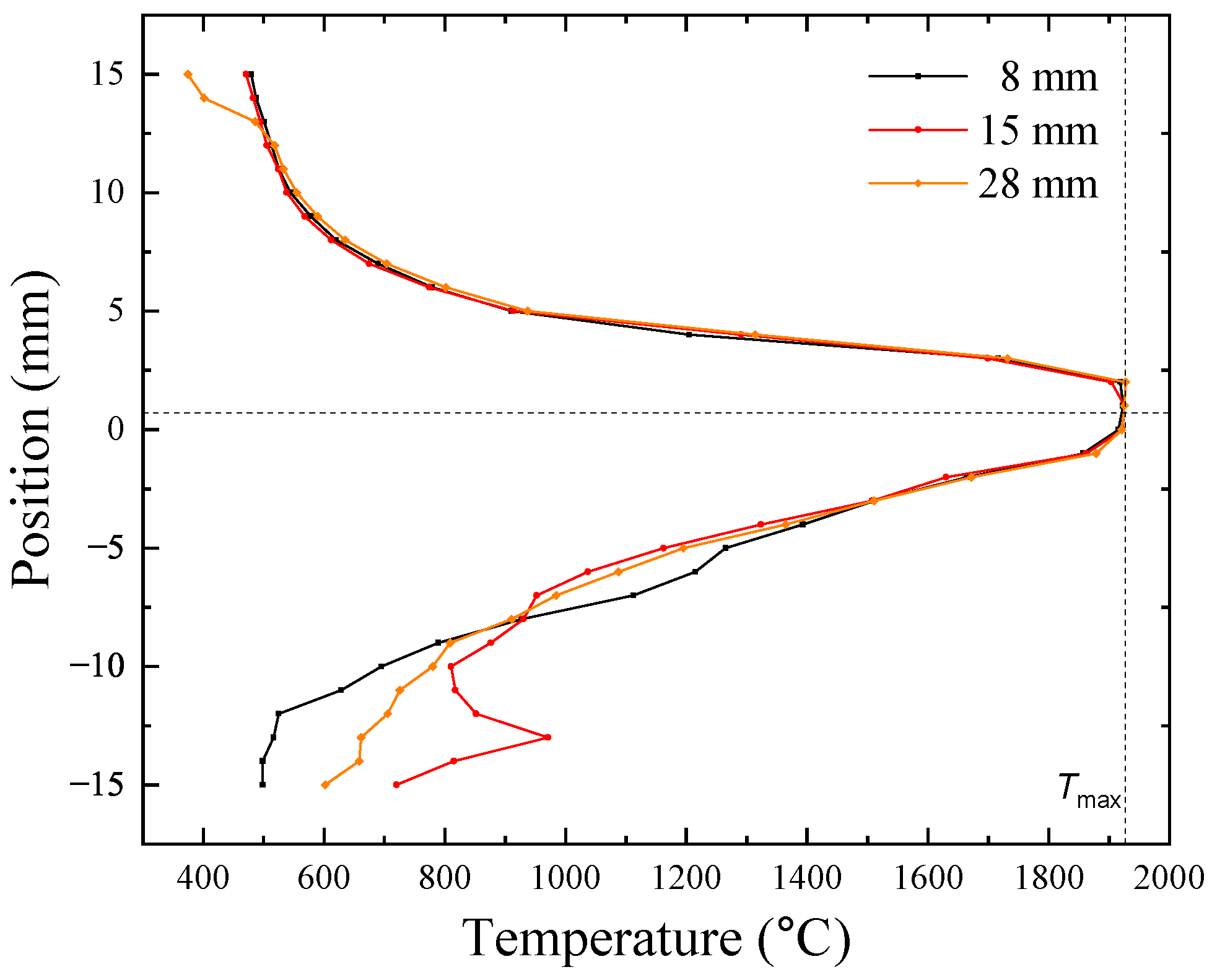

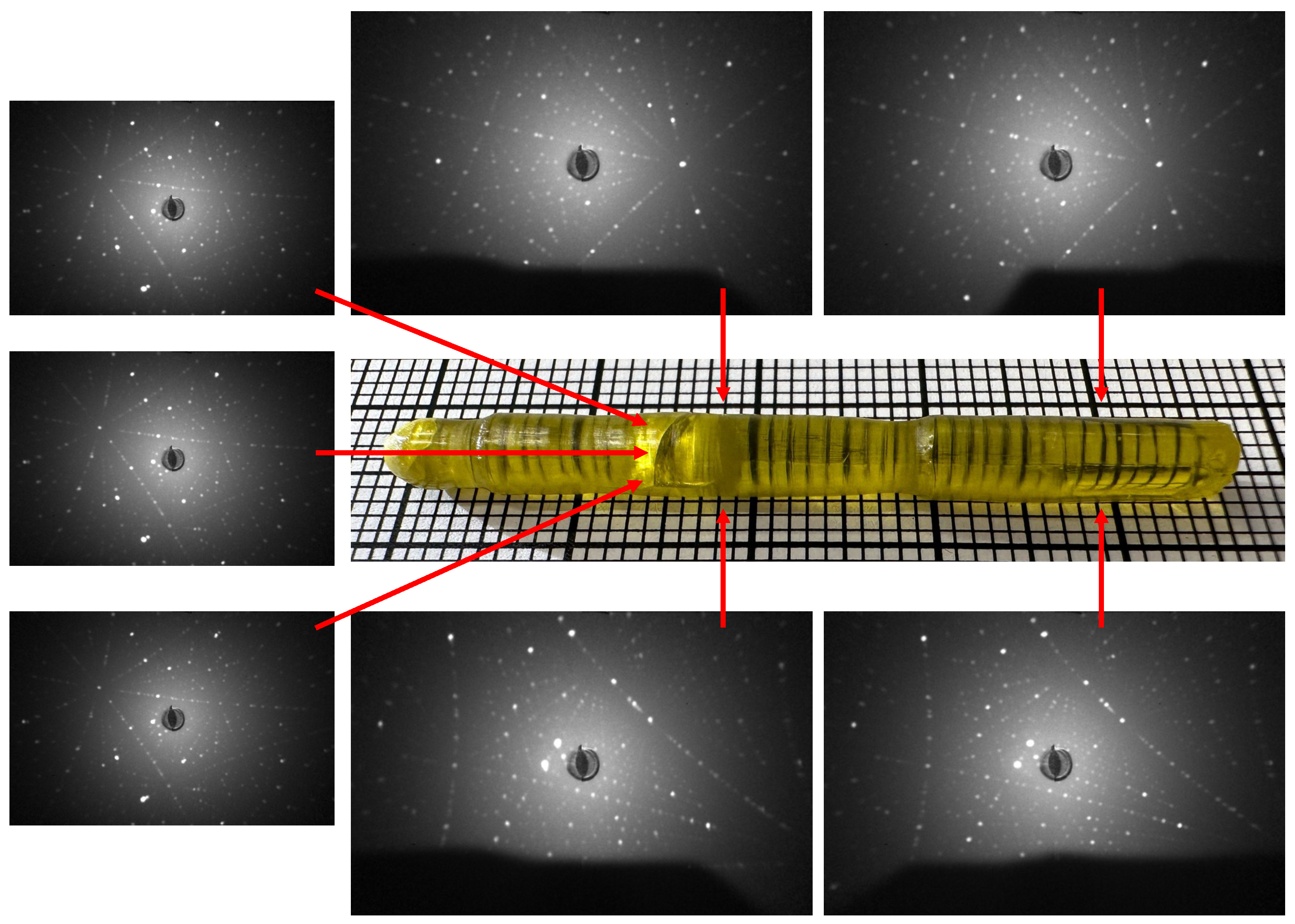

| Chemical Composition | Growth Rate (mm/h) | Gas Atmosphere/Pressure/Flow | Feed & Seed Rotation Rate (rpm) | Remarks |
|---|---|---|---|---|
| DySiO | 10 | air, ambient, 1 L/min | 10 | very fragile boule, with a few cracks |
| 12 | Ar:O (80%:20%), 5 bars | 10 | robust boule, despite a few cracks * | |
| HoSiO | 10–15 | air, ambient, 1 L/min | 10 | robust boule, without cracks |
| 10 | air, ambient | 10 | robust boule, despite one crack | |
| 12 | Ar:O (80%:20%), 5 bars | 10 | robust boule, without cracks * | |
| ErSiO | 15 | air, ambient, 1 L/min | 10 | very fragile boule, with a lot of cracks |
| 8–15 | air, ambient | 10 | very fragile boule, with a lot of cracks | |
| 12 | Ar:O (80%:20%), 5 bars | 10 | less fragile boule, despite some cracks * | |
| 5 | Ar:O (80%:20%), 5 bars | 10 | less fragile boule, despite some cracks |
| Chemical Composition | Lattice Parameters | GOF | |||
|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | () | ||
| DySiO | 10.46578(2) | 6.75135(1) | 12.54282(2) | 102.7299(1) | 1.34 |
| HoSiO | 10.41830(1) | 6.72696(1) | 12.50449(2) | 102.6148(1) | 1.76 |
| ErSiO | 10.37192(1) | 6.70451(1) | 12.46547(2) | 102.5388(1) | 1.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciomaga Hatnean, V.C.; Pui, A.; Simonov, A.; Ciomaga Hatnean, M. Crystal Growth of the R2SiO5 Compounds (R = Dy, Ho, and Er) by the Floating Zone Method Using a Laser-Diode-Heated Furnace. Crystals 2023, 13, 1687. https://doi.org/10.3390/cryst13121687
Ciomaga Hatnean VC, Pui A, Simonov A, Ciomaga Hatnean M. Crystal Growth of the R2SiO5 Compounds (R = Dy, Ho, and Er) by the Floating Zone Method Using a Laser-Diode-Heated Furnace. Crystals. 2023; 13(12):1687. https://doi.org/10.3390/cryst13121687
Chicago/Turabian StyleCiomaga Hatnean, Vasile Cristian, Aurel Pui, Arkadiy Simonov, and Monica Ciomaga Hatnean. 2023. "Crystal Growth of the R2SiO5 Compounds (R = Dy, Ho, and Er) by the Floating Zone Method Using a Laser-Diode-Heated Furnace" Crystals 13, no. 12: 1687. https://doi.org/10.3390/cryst13121687
APA StyleCiomaga Hatnean, V. C., Pui, A., Simonov, A., & Ciomaga Hatnean, M. (2023). Crystal Growth of the R2SiO5 Compounds (R = Dy, Ho, and Er) by the Floating Zone Method Using a Laser-Diode-Heated Furnace. Crystals, 13(12), 1687. https://doi.org/10.3390/cryst13121687







