Effect of Polyvinylpyrrolidone Content on Pure Titanium Injection Molding
Abstract
:1. Introduction
2. Materials and Methods
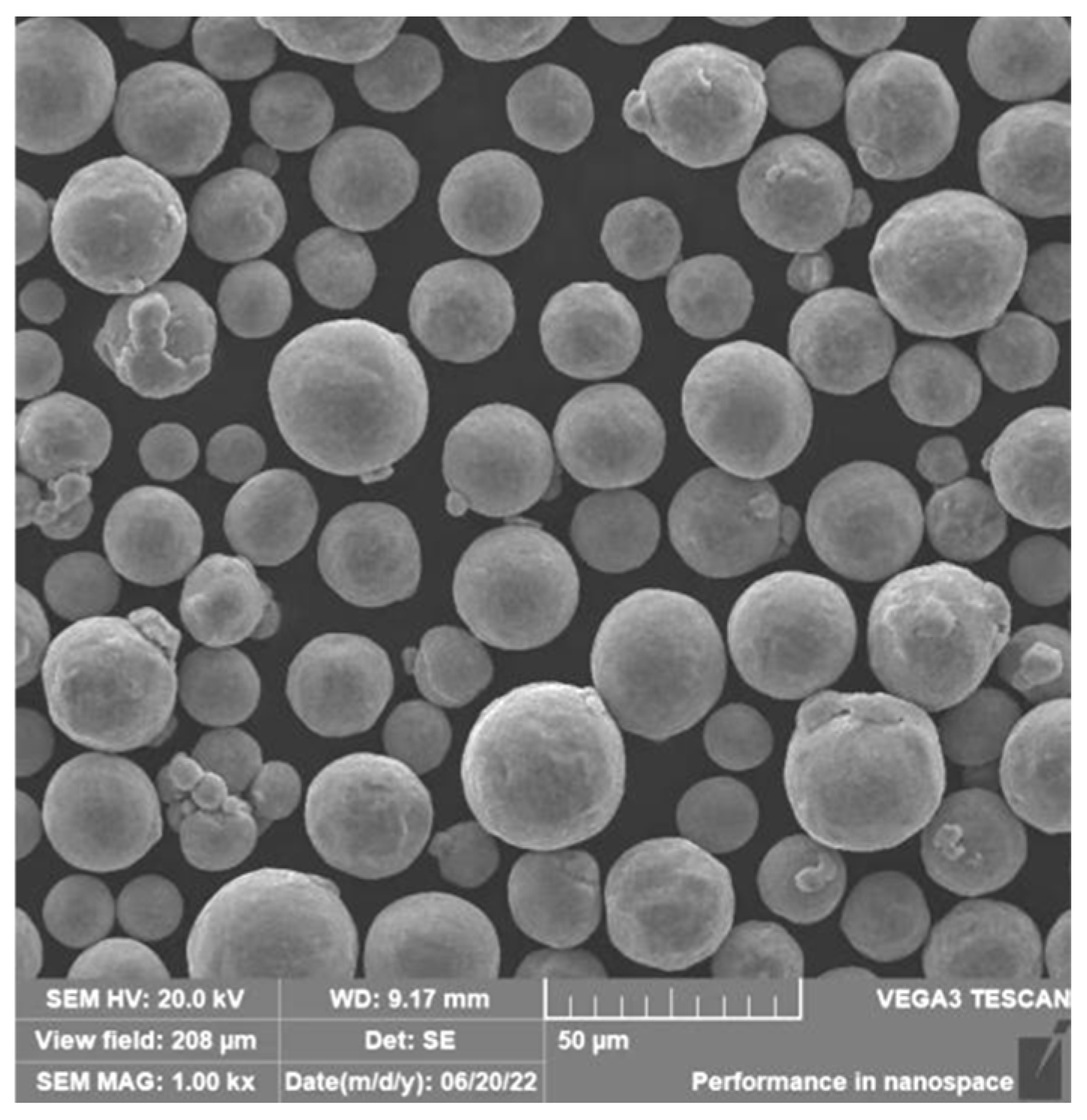
2.1. Experimental Materials
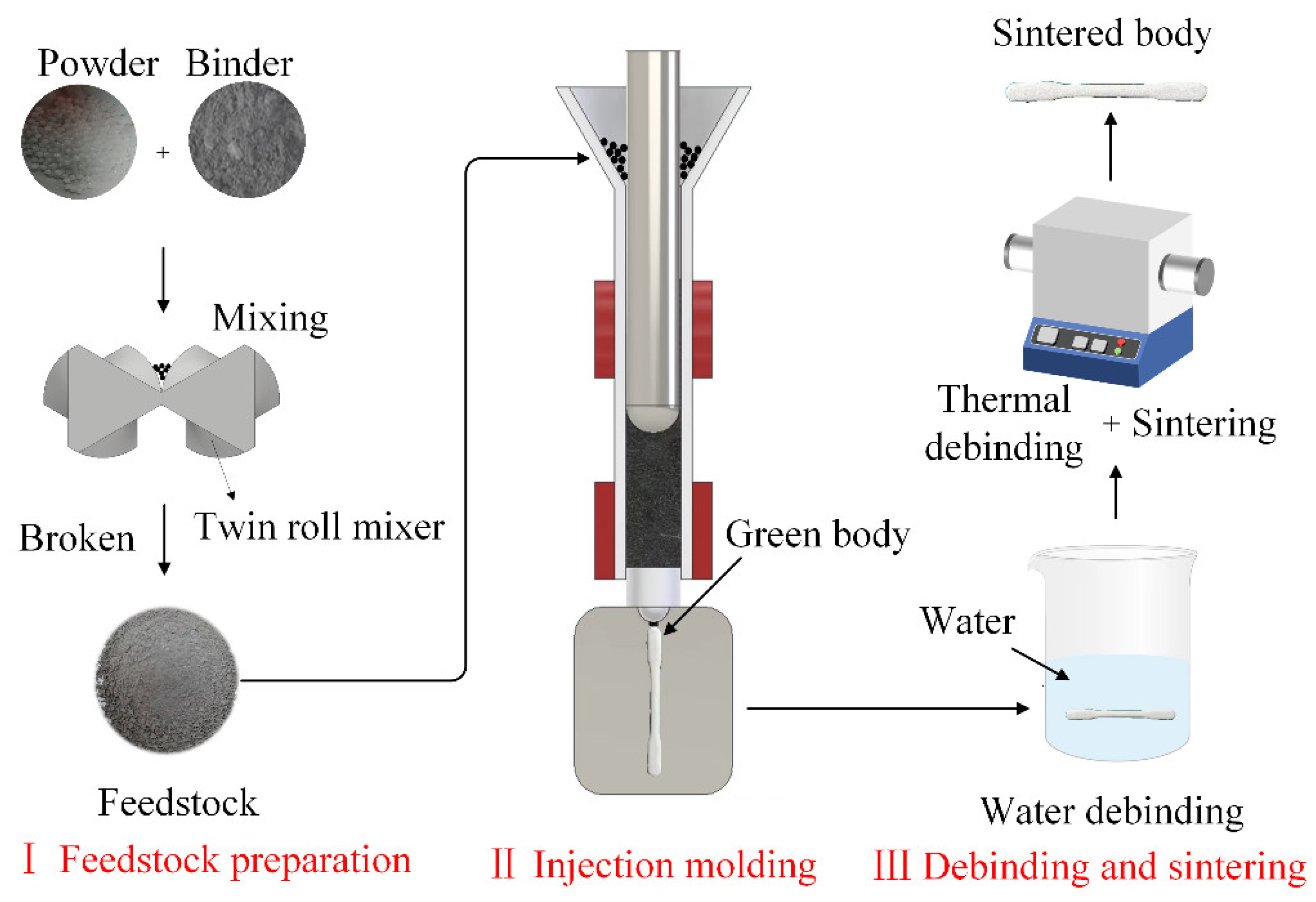
2.2. Experimental Methods
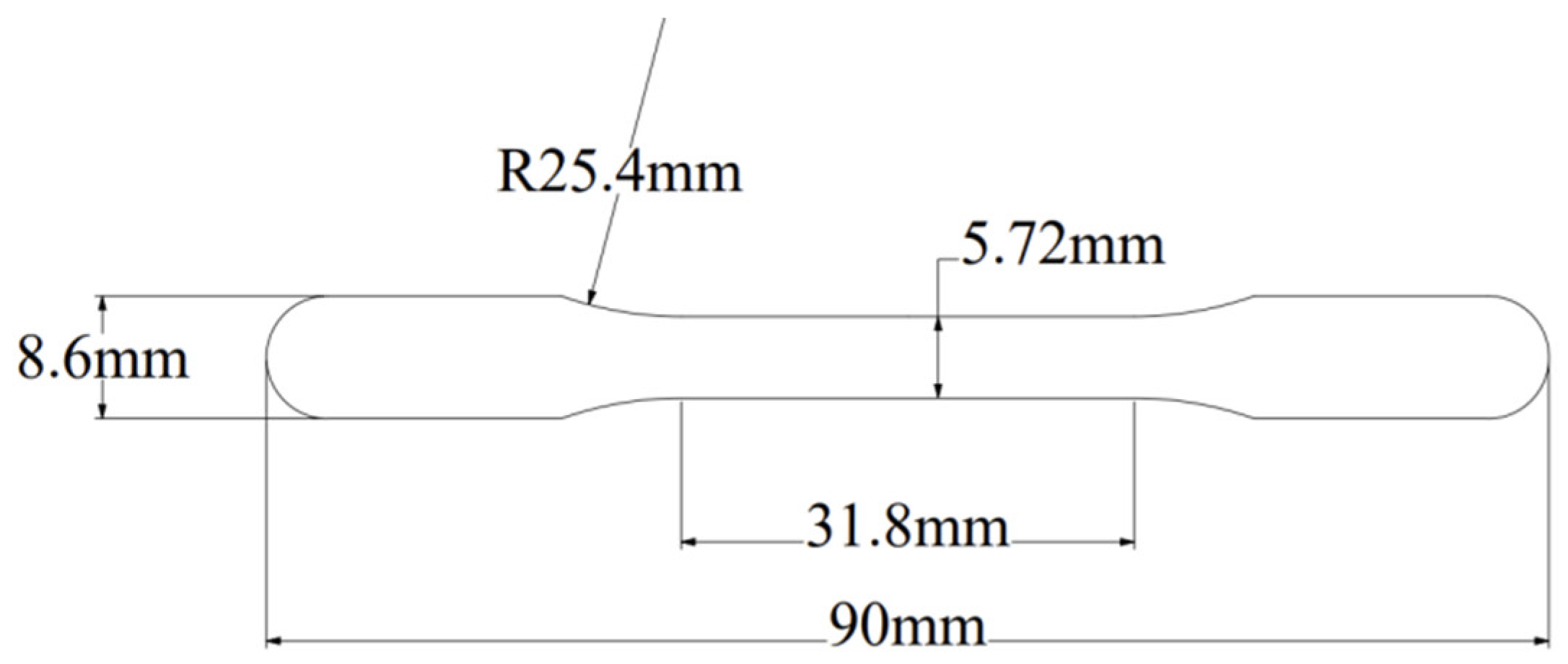
2.3. Characterization
3. Results and Discussion
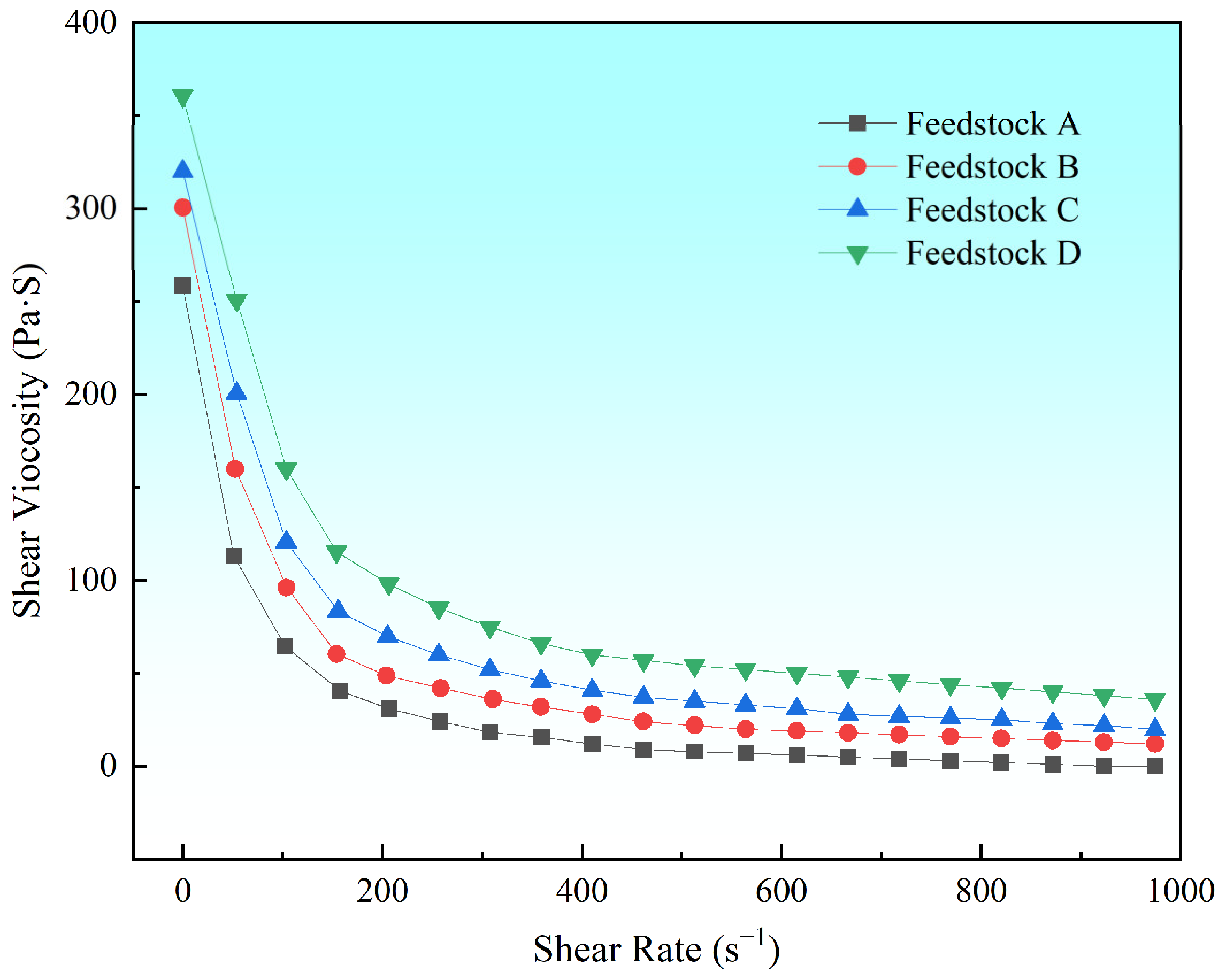
3.1. Rheological Properties
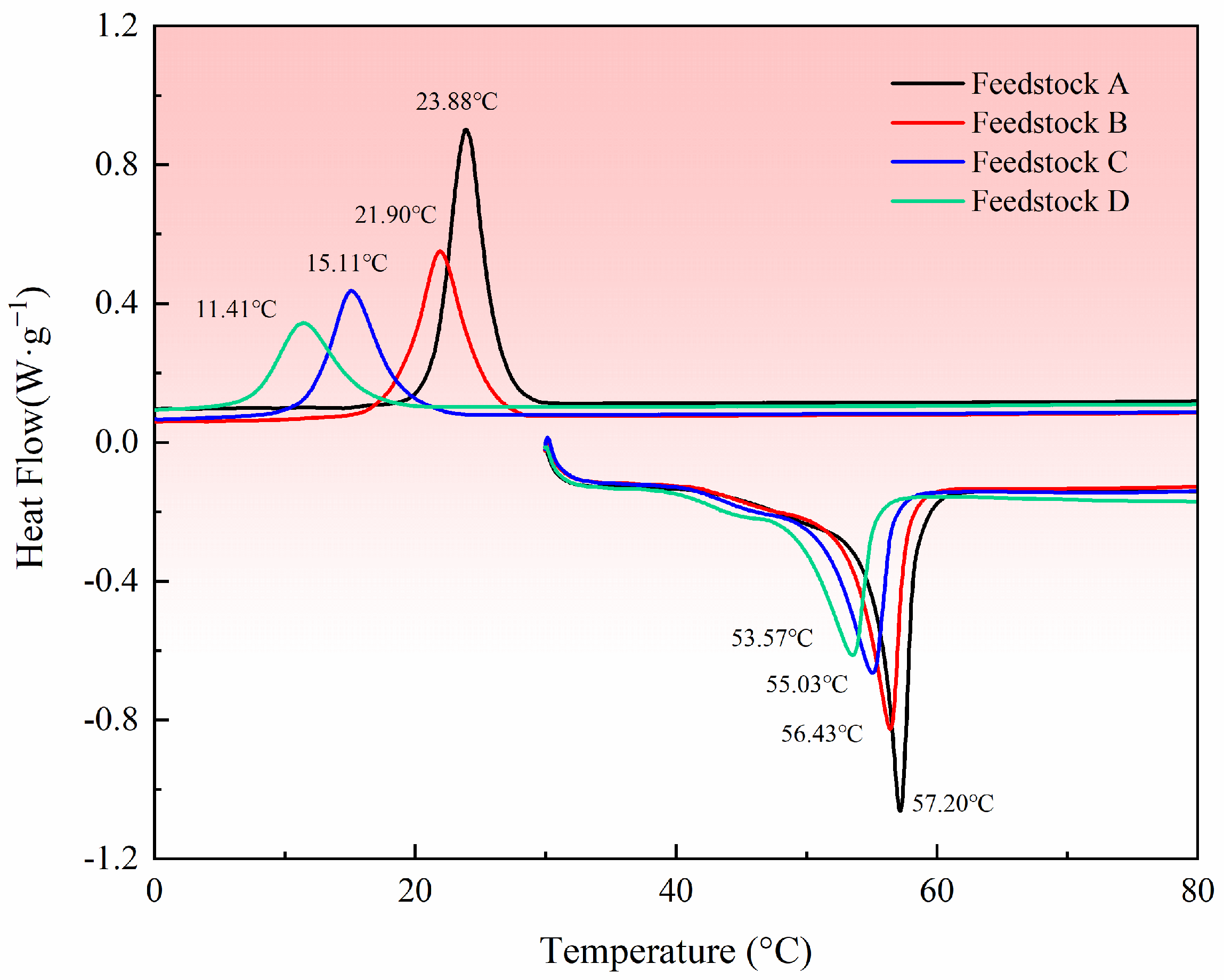
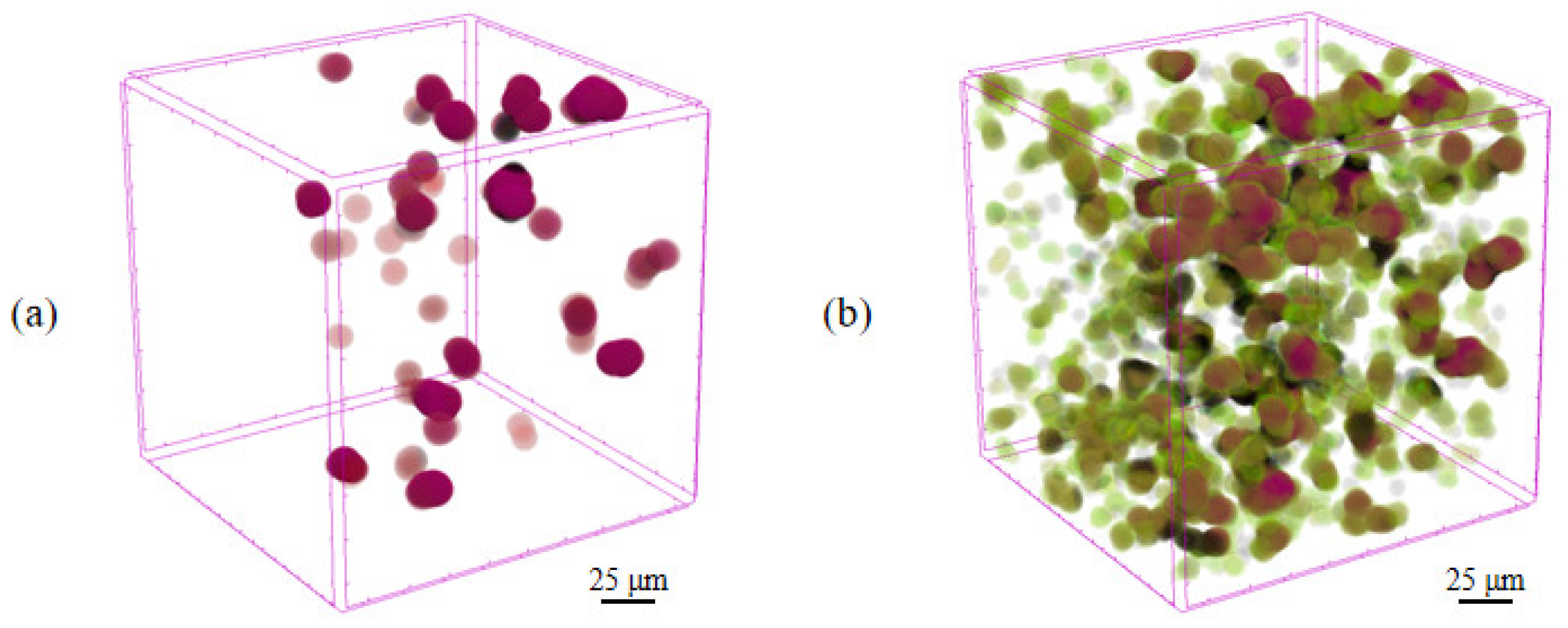
3.2. Inhibition of PEG Crystallization by PVP
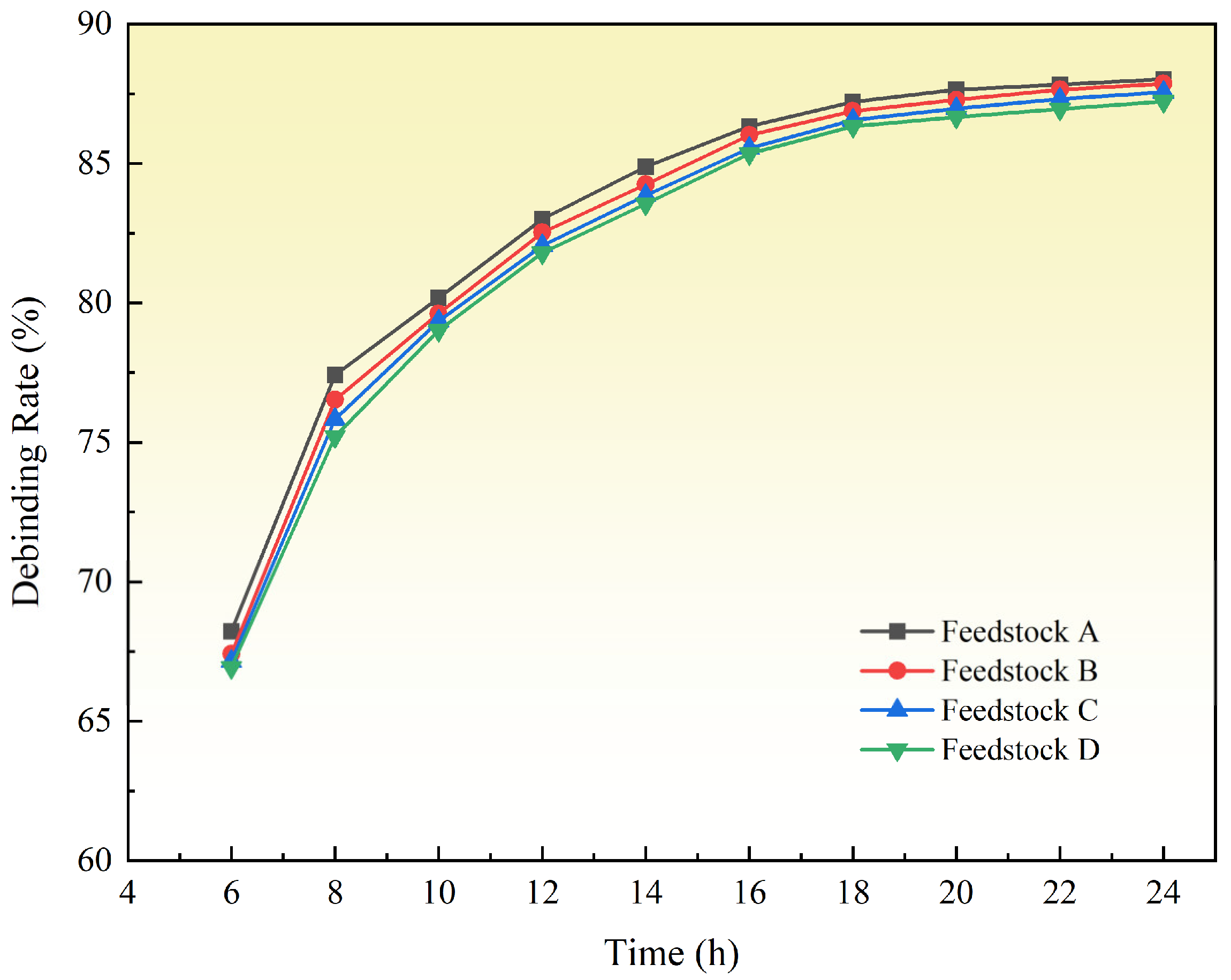
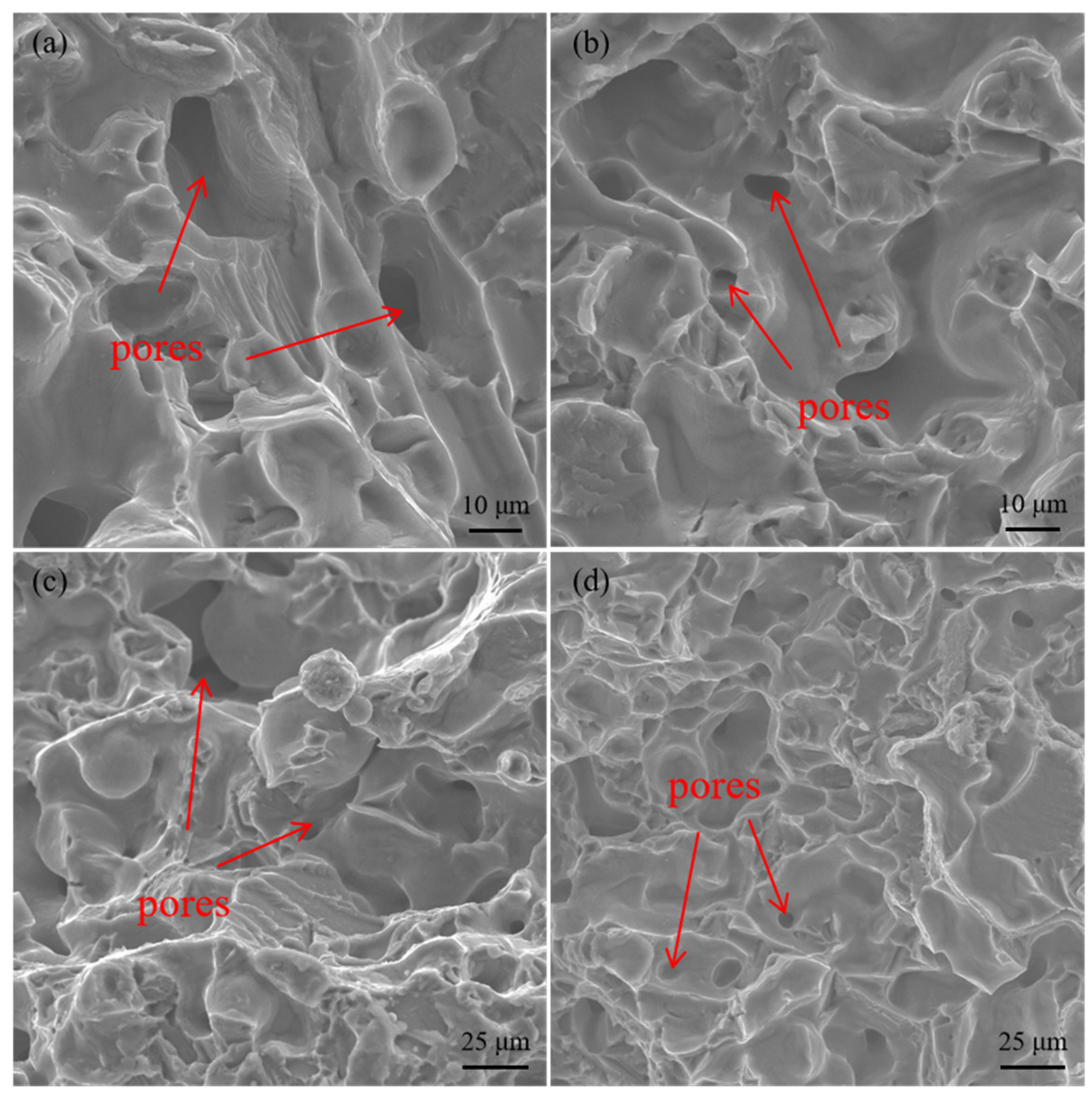
3.3. Effect of PVP Content on Surface Quality and Water Removal of Samples
3.4. Effect of PVP Content on Mechanical Properties of Samples
4. Conclusions
- (1)
- The addition of PVP effectively eliminated the voids caused by the inconsistent volume shrinkage, which exists due to the temperature difference. The large molecular weight of PVP compressed the crystallization space of PEG molecules and inhibited the crystallization of PEG molecules.
- (2)
- It was confirmed that the appropriate content of PVP is 20 wt.% PEG, ensuring defect-free samples after water debinding.
- (3)
- The novel binder system had good rheological properties and feedstock uniformity, resulting in overall good mechanical properties. It was revealed that the 20 wt.% PVP resulted in a density of 98.6%, ultimate tensile strength of 547.9 MPa and elongation of 12.53%, respectively, which meet ASTM F2989-13 Grade 3 quality.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dehghan-Manshadi, A.; Bermingham, M.J.; Dargusch, M.S.; StJohn, D.; Qian, M. Metal injection moulding of titanium and titanium alloys: Challenges and recent development. Powder Technol. 2017, 319, 289–301. [Google Scholar] [CrossRef]
- Lee, S.W.; Ahn, S.; Whang, C.J. Effects of process parameters in plastic, metal, and ceramic injection molding processes. Korea-Aust. Rheol. J. 2011, 23, 127–138. [Google Scholar] [CrossRef]
- Huang, M.S.; Hsu, H.C. Effect of backbone polymer on properties of 316L stainless steel MIM compact. J. Mater. Process. Technol. 2009, 209, 5527–5535. [Google Scholar] [CrossRef]
- Hamidi, M.; Harun, W.S.W.; Samykano, M.; Ghani SA, C.; Ghazalli, Z.; Ahmad, F.; Sulong, A.B. A review of biocompatible metal injection moulding process parameters foor biomedical applications. Mater. Sci. Eng. C 2017, 78, 1263–1276. [Google Scholar] [CrossRef]
- Dehghan-Manshadi, A.; Yu, P.; Dargusch, M.; StJohn, D.; Qian, M. Metal injection moulding of surgical tools, biomaterials and medical devices: A review. Powder Technol. 2020, 364, 189–204. [Google Scholar] [CrossRef]
- Froes, F.H. Getting better: Big boost for titanium MIM prospects. Metal Powder Rep. 2006, 61, 20–23. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.F.; Tang, H.P.; Liu, C.T.; Liu, B.; Huang, B.Y. Design of powder metallurgy titanium alloys and composites. Mater. Sci. Eng. A 2006, 418, 25–35. [Google Scholar] [CrossRef]
- Askari, A.; Momeni, V. Rheological investigation and injection optimization of Fe-2Ni-2Cu feedstock for metal injection molding process. Mater. Chem. Phys. 2021, 271, 124926. [Google Scholar] [CrossRef]
- Basir, A.; Muhamad, N.; Sulong, A.B.; Jamadon, N.H.; Foudzi, F.M. Recent advances in processing of titanium and titanium alloys through metal injection molding for biomedical application: 2013–2022. Materials 2023, 16, 3991. [Google Scholar] [CrossRef]
- Momeni, V.; Zangi, H.; Alaei, M. Effect of thermal debinding and sintering parameters on the mechanical properties of 4605 MIM compact using the RSM. Adv. Mater. Process. Technol. 2021, 8, 3199–3214. [Google Scholar] [CrossRef]
- Abajo, C.; Jiménez-Morales, A.; Torralba, J.M. New processing route for ZrSiO4 by powder injection moulding using an eco-friendly binder system. Bol. Soc. Esp. Ceram. V 2015, 54, 93–100. [Google Scholar] [CrossRef]
- Ebel, T. Handbook of Metal Injection Molding, 2nd ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 431–460. [Google Scholar]
- Banerjee, S.; Joens, C.J. Debinding and Sintering of Metal Injection Molding (MIM) Components, 2nd ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 129–171. [Google Scholar]
- Wolff, M.; Schaper, J.; Suckert, M.; Dahms, M.; Feyerabend, F.; Ebel, T.; Willumeit-Römer, R.; Klassen, T. Metal injection molding (MIM) of Magnesium and its alloys. Metals 2016, 6, 118. [Google Scholar] [CrossRef]
- Wen, G.; Cao, P.; Gabbitas, B.; Zhang, D.; Edmonds, N. Development and design of binder system for titanium metal injection molding: An overview. Metall. Mater. Trans. A 2012, 44, 1530. [Google Scholar] [CrossRef]
- Thavanayagam, G.; Swan, J.E. Aqueous debinding of polyvinyl butyral based binder system for titanium metal injection moulding. Powder Technol. 2018, 326, 402–410. [Google Scholar] [CrossRef]
- Momeni, V.; Askari, A.; Alaei, M.H.; Rahimi, A.H.; Nekouee, K.; Zangi, H. The effect of powder loading and binder system on the mechanical, rheological and microstructural properties of 4605 powder in MIM process. Trans. Indian. Inst. Met. 2019, 72, 1245–1254. [Google Scholar] [CrossRef]
- Momeni, V.; Zangi, H.; Allaei, M.H. Effect of polyproptlene as the backbone of MIM feedstock on the micro-structural phase constituents, mechanical and rheological properties of 4605 low alloy steel compacts. Powder. Metall. 2019, 63, 27–34. [Google Scholar] [CrossRef]
- Momeni, V.; Askari, A.; Allaei, M.H.; Zangi, H. Investigating the effect of stearic acid on the mechanical, rheological, and microstructural properties of AISI 4605 feedstock for metal injection molding process. Trans. Indian. Inst. Met. 2021, 74, 2161–2170. [Google Scholar] [CrossRef]
- Setasuwon, P.; Bunchavimonchet, A.; Danchaivijit, S. The effects of binder components in wax/oil systems for metal injection molding. J. Mater. Process. Technol. 2008, 196, 94–100. [Google Scholar] [CrossRef]
- Hnatkova, E.; Hausnerova, B.; Hales, A.; Jiranek, L.; Derguti, F.; Todd, I. Processing of MIM feedstock based on Inconel 718 powder and partially water-soluble binder varying in PEG molecular weight. Powder Technol. 2017, 322, 439–446. [Google Scholar] [CrossRef]
- Hidalgo, J.; Abajo, C.; Jiménez-Morales, A.; Torralba, J.M. Effect of binder system on the low-pressure powder injection moulding of water-soluble zircon feedstocks. J. Eur. Ceram. Soc. 2013, 33, 3185–3194. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, D.; Zhao, Y. Development of synthesis and application of high molecular weight poly(methyl mechacrylate). Polymers 2022, 14, 2632. [Google Scholar] [CrossRef] [PubMed]
- Williams, B. Challenges for MIM titanium parts. Metal Powder Rep. 2003, 58, 30. [Google Scholar] [CrossRef]
- Hayat, M.D.; Zhang, H.; Karumbaiah, K.M.; Singh, H.; Xu, Y.; Zou, L.; Qu, X.; Cao, P. A novel PEG/PMMA based binder composition for void-free metal injection mouldingn of Ti conponents. Powder Technol. 2021, 382, 431–440. [Google Scholar] [CrossRef]
- Wen, J.; Liu, W.; Xie, Z.; Lou, C.; Yang, X. Effects of PVP incroporation on the properties of injection-molded high-performance ceramics with PEG-based binders. Ceram. Int. 2018, 44, 2718–2726. [Google Scholar] [CrossRef]
- Hausnerova, B.; Mukund, B.N.; Sanetrnik, D. Rheological properties of gas and water atomized 17-4PH stainless steel MIM feedstocks: Effect of powder shape and size. Powder Technol. 2017, 312, 152–158. [Google Scholar] [CrossRef]
- German, R.M. Progress in titanium metal powder injection molding. Materials 2013, 6, 3641–3662. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, Q.; Huang, C. Crystallization of Poly (ethylene glycol) in Poly (methyl methacrylate) Networks. Mater. Sci. 2013, 19, 147–151. [Google Scholar] [CrossRef]
- Feldstein, M.M.; Roos, A.; Chevallier, C.; Creton, C.; Dormidontova, E.E. Relation of glass transition temperature to the hydrogen bonding degree and energy in poly(N-vinyl pyrrolidone) blends with hydroxyl-containing plasticizers: 3. Analysis of two glass transition temperatures featured for PVP solutions in liquid poly(ethylene glycol). Polymer 2003, 44, 1819–1834. [Google Scholar]
- Sidambe, A.T.; Figureueroa, I.A.; Hamilton, H.G.C.; Todd, I. Metal injection moulding of CP-Ti components for biomedical applications. J. Mater. Process. Technol. 2012, 212, 1591–1597. [Google Scholar] [CrossRef]
- Li, D.; Hou, H.; Tan, Z.; Lee, K. Metal injection molding of pure molybdenum. Adv. Powder. Technol. 2009, 20, 480–487. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.; Sun, J.; Wu, X.; Zhang, J.; Kuang, F.; Lu, X. Metal injection molding of high-permance Ti composites using hydride-dehydride (HDH) powder. J. Manuf. Process. 2023, 89, 328–337. [Google Scholar] [CrossRef]
- Omar, M.A.; Ibrahim, R.; Sidik, M.I.; Mustapha, M.; Mohamad, M. Rapid debinding of 316L stainless steel injection moulded component. J. Mater. Process. Technol. 2003, 140, 397–400. [Google Scholar] [CrossRef]
- Krauss, V.A.; Oliveira, A.A.M.; Klein, A.N.; Al-Qureshi, H.; Fredel, M. A model for PEG removal from alumina injection moulded parts by solvent debinding. J. Mater. Process. Technol. 2017, 182, 268–273. [Google Scholar] [CrossRef]
- ASTM F2989-13; Standard Specification for Metal Injection Molded Unalloyed Titanium Components for Surgical Implant Applications. ASTM International: West Conshohocken, PA, USA, 2021.

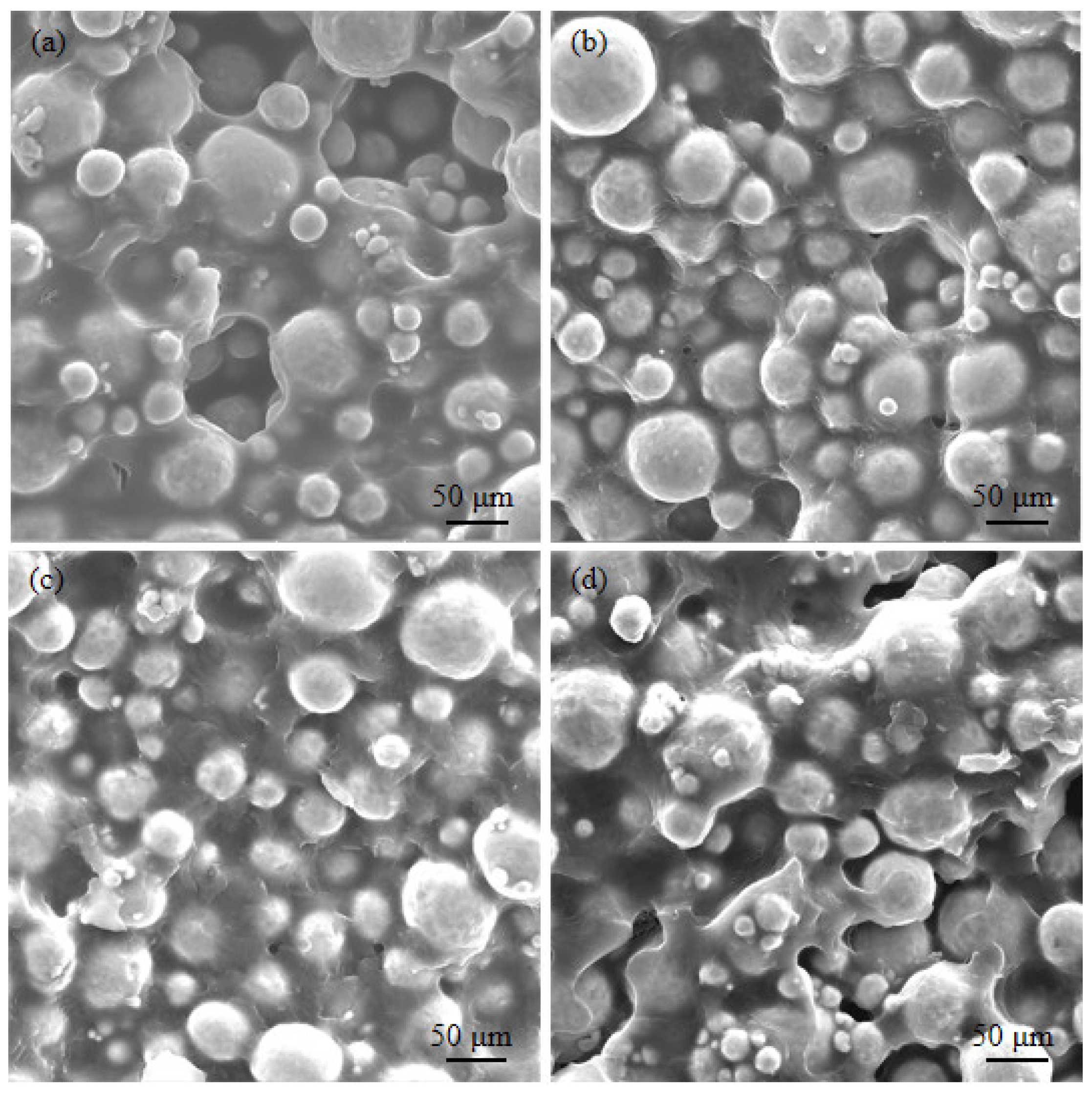
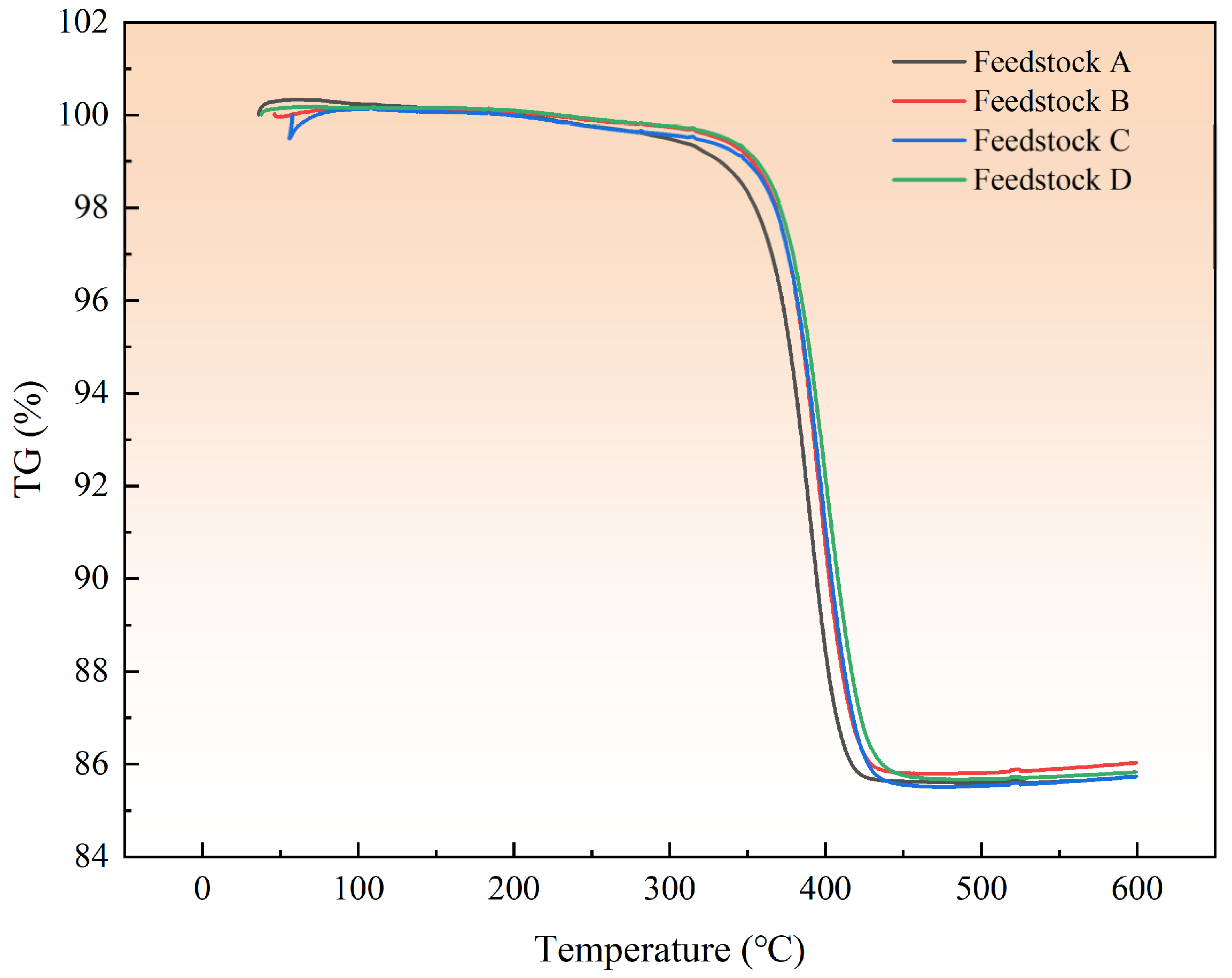


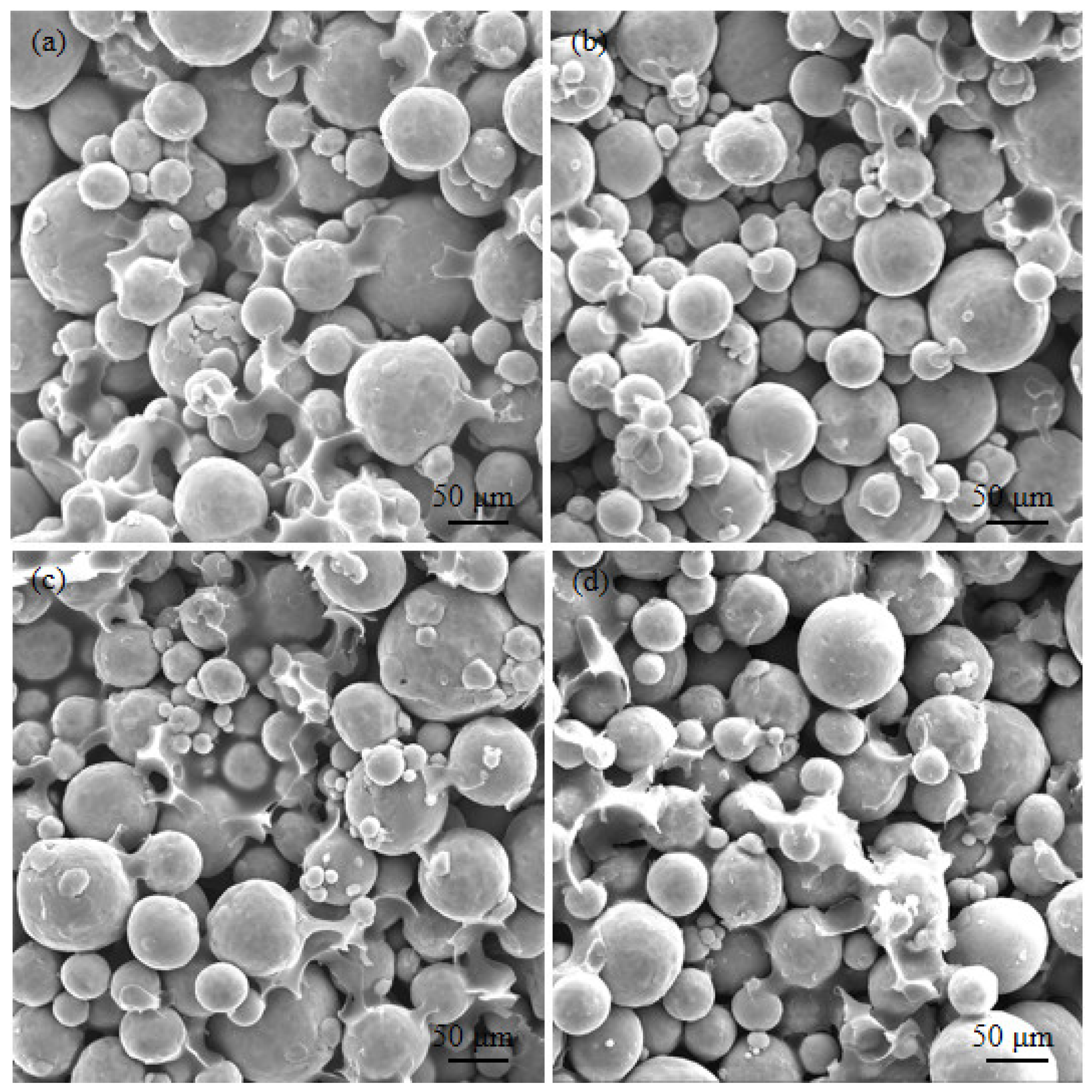


| Element (%) | Ti | N | O | H | Fe | C |
|---|---|---|---|---|---|---|
| Content (wt.%) | Bal. | 0.007 | 0.095 | 0.002 | 0.18 | 0.01 |
| Heat Absorption (J/g) | Enthalpy of Crystallization (J/g) | |
|---|---|---|
| Feedstock A | −28.47 | 25.32 |
| Feedstock B | −20.58 | 16.04 |
| Feedstock C | −16.35 | 11.17 |
| Feedstock D | −15.62 | 8.85 |
| Ultimate Tensile Strength (MPa) | Elongation (%) | Relative Density (%) | Content of Impurity (%) | |||
|---|---|---|---|---|---|---|
| C | O | N | ||||
| Feedstock A | 386.4 | 8.83 | 97.8 | 0.03 | 0.22 | 0.01 |
| Feedstock B | 499.0 | 11.49 | 98.6 | 0.05 | 0.25 | 0.02 |
| Feedstock C | 547.9 | 12.53 | 98.3 | 0.06 | 0.28 | 0.02 |
| Feedstock D | 355.3 | 7.78 | 97.5 | 0.09 | 0.32 | 0.03 |
| Grade 3 ASTM F2989-13 [36] | 495 (min) | 10 (min) | ~98–99 | 0.08 (max) | 0.30 (max) | 0.05 (max) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Li, L.; Li, C.; Sun, Y.; Hayat, M.D.; Li, Y.; Chen, G.; Yuan, Z.; Wang, X. Effect of Polyvinylpyrrolidone Content on Pure Titanium Injection Molding. Crystals 2023, 13, 1563. https://doi.org/10.3390/cryst13111563
Zhang W, Li L, Li C, Sun Y, Hayat MD, Li Y, Chen G, Yuan Z, Wang X. Effect of Polyvinylpyrrolidone Content on Pure Titanium Injection Molding. Crystals. 2023; 13(11):1563. https://doi.org/10.3390/cryst13111563
Chicago/Turabian StyleZhang, Weichen, Lu Li, Chuanyong Li, Yanhua Sun, Muhammad Dilawer Hayat, Yugeng Li, Gang Chen, Zhentao Yuan, and Xiao Wang. 2023. "Effect of Polyvinylpyrrolidone Content on Pure Titanium Injection Molding" Crystals 13, no. 11: 1563. https://doi.org/10.3390/cryst13111563
APA StyleZhang, W., Li, L., Li, C., Sun, Y., Hayat, M. D., Li, Y., Chen, G., Yuan, Z., & Wang, X. (2023). Effect of Polyvinylpyrrolidone Content on Pure Titanium Injection Molding. Crystals, 13(11), 1563. https://doi.org/10.3390/cryst13111563






