Investigation of Supercapacitor Electrodes Based on MIL-101(Fe) Metal-Organic Framework: Evaluating Electrochemical Performance through Hydrothermal and Microwave-Assisted Synthesis
Abstract
:1. Introduction
2. Experimental Section
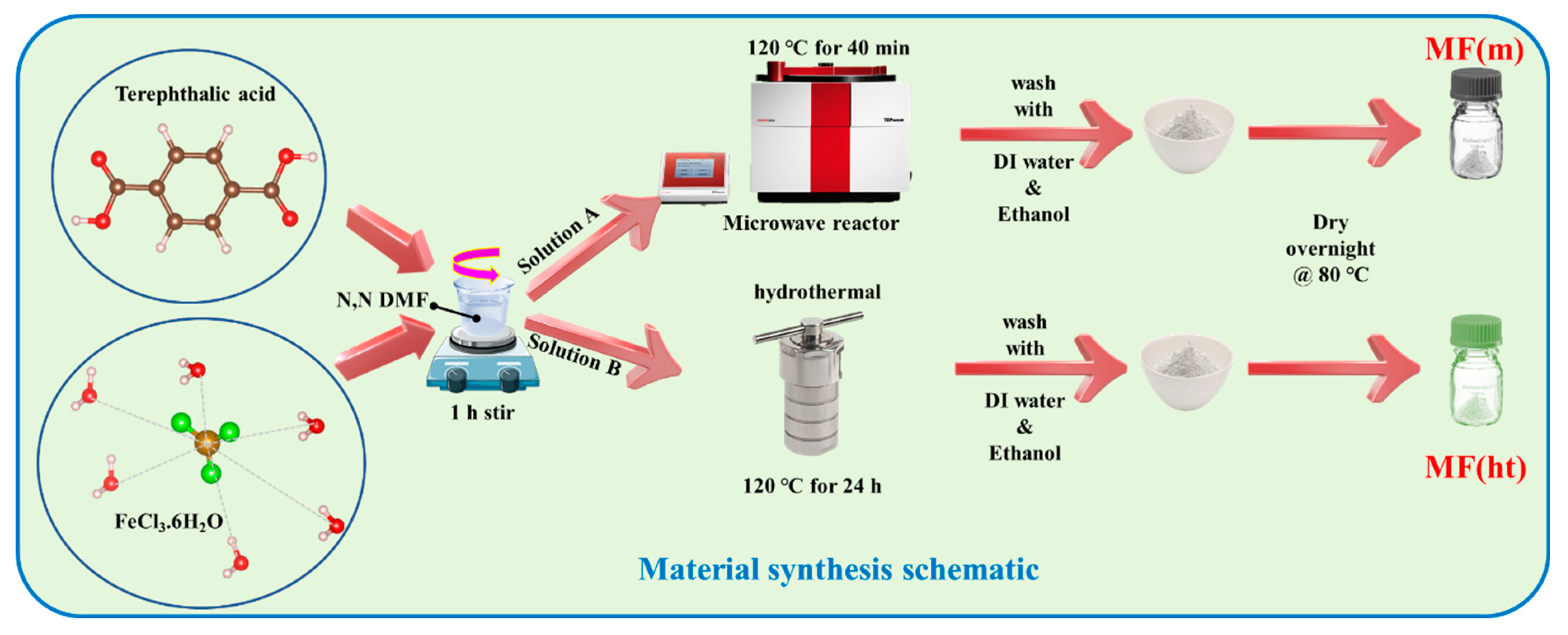
2.1. Material Synthesis
2.2. Characterization Techniques
2.3. Preparation of Electrodes
2.4. Electrochemical Characterizations
2.5. Symmetric Supercapacitor Preparation and Testing
3. Results and Discussion
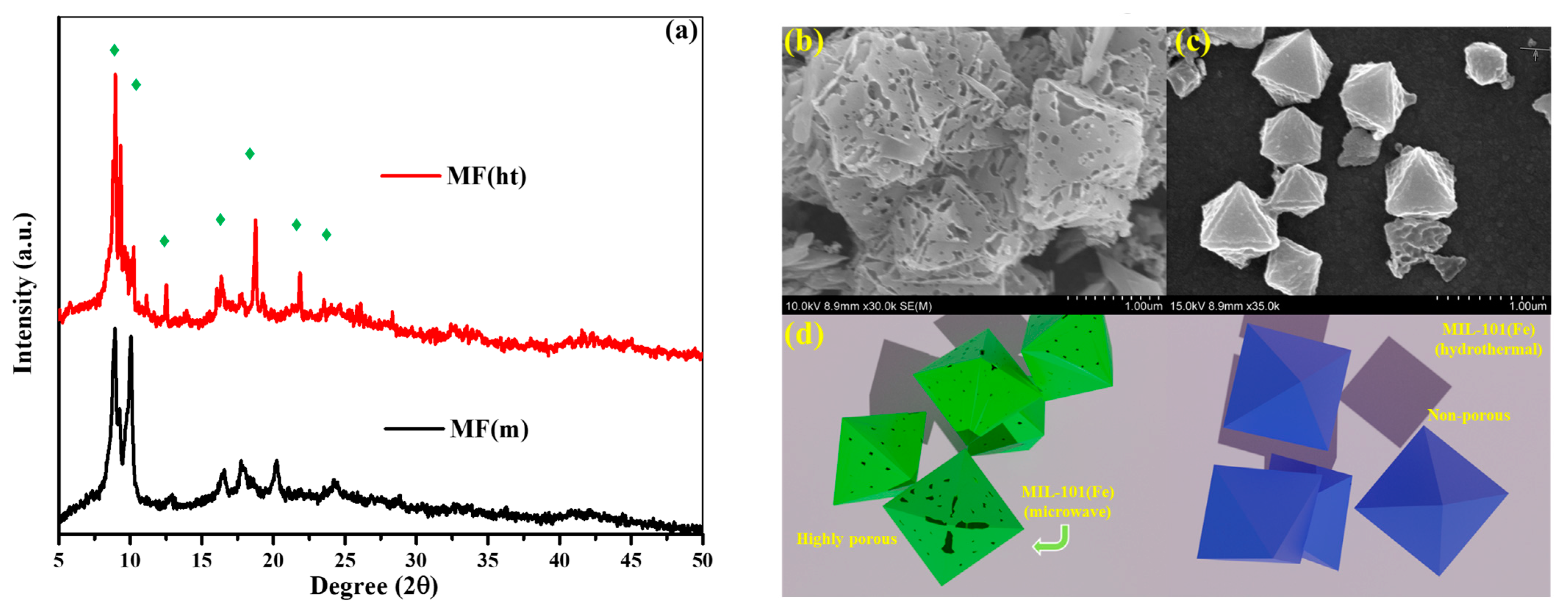
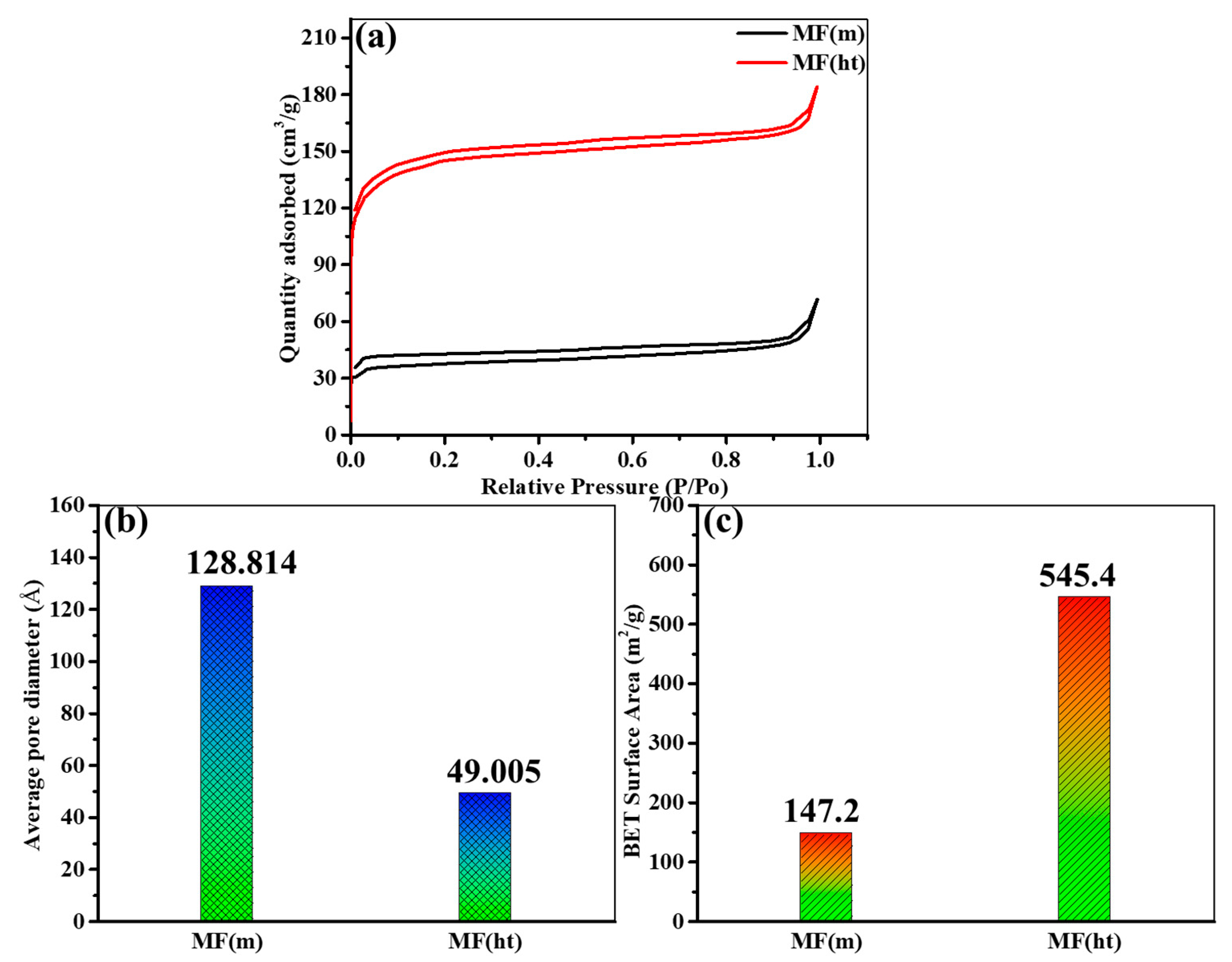
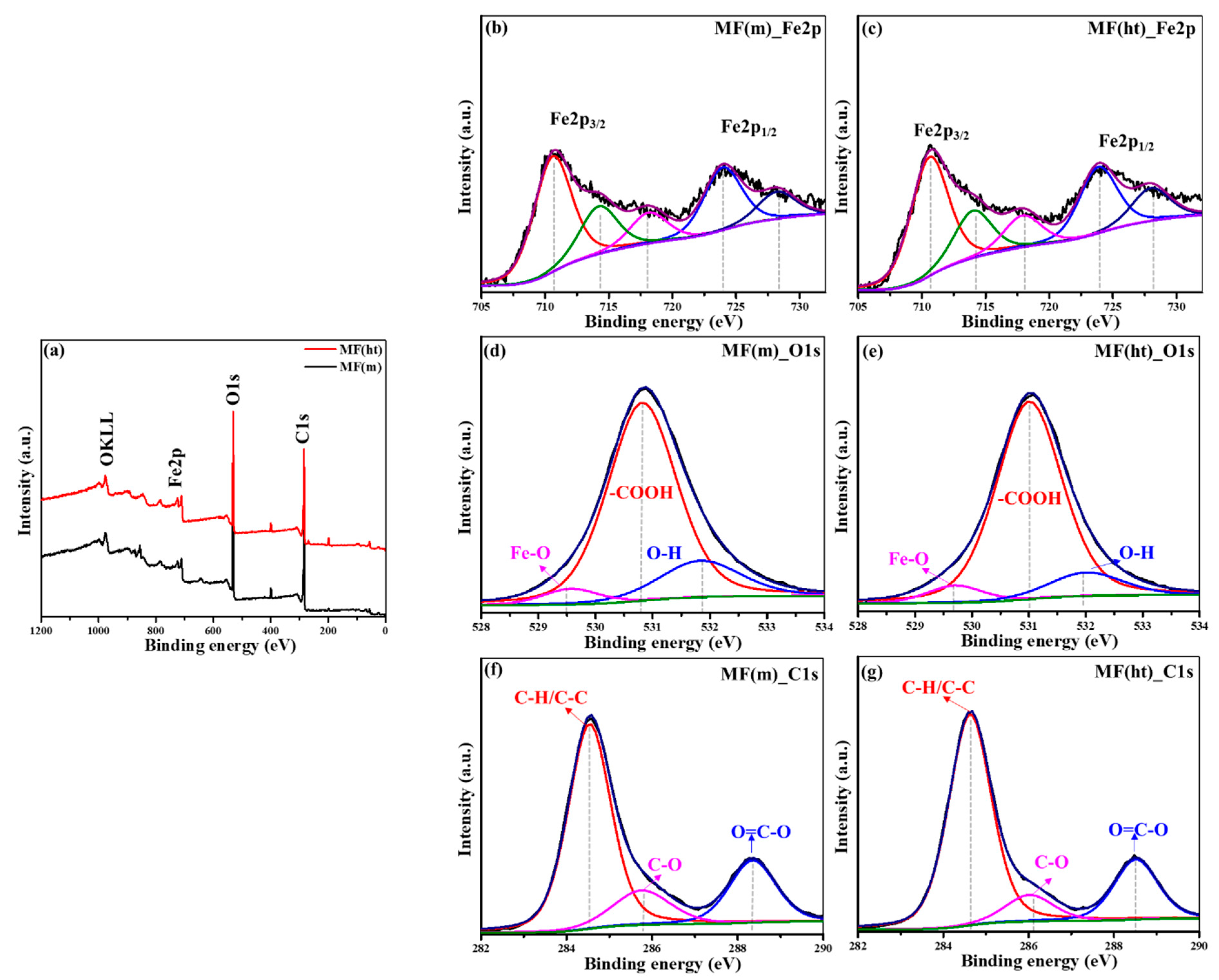
3.1. Morphological, Structural, and Chemical Characteristics
3.2. Supercapacitor Electrode Performance Evaluation
- (a.)
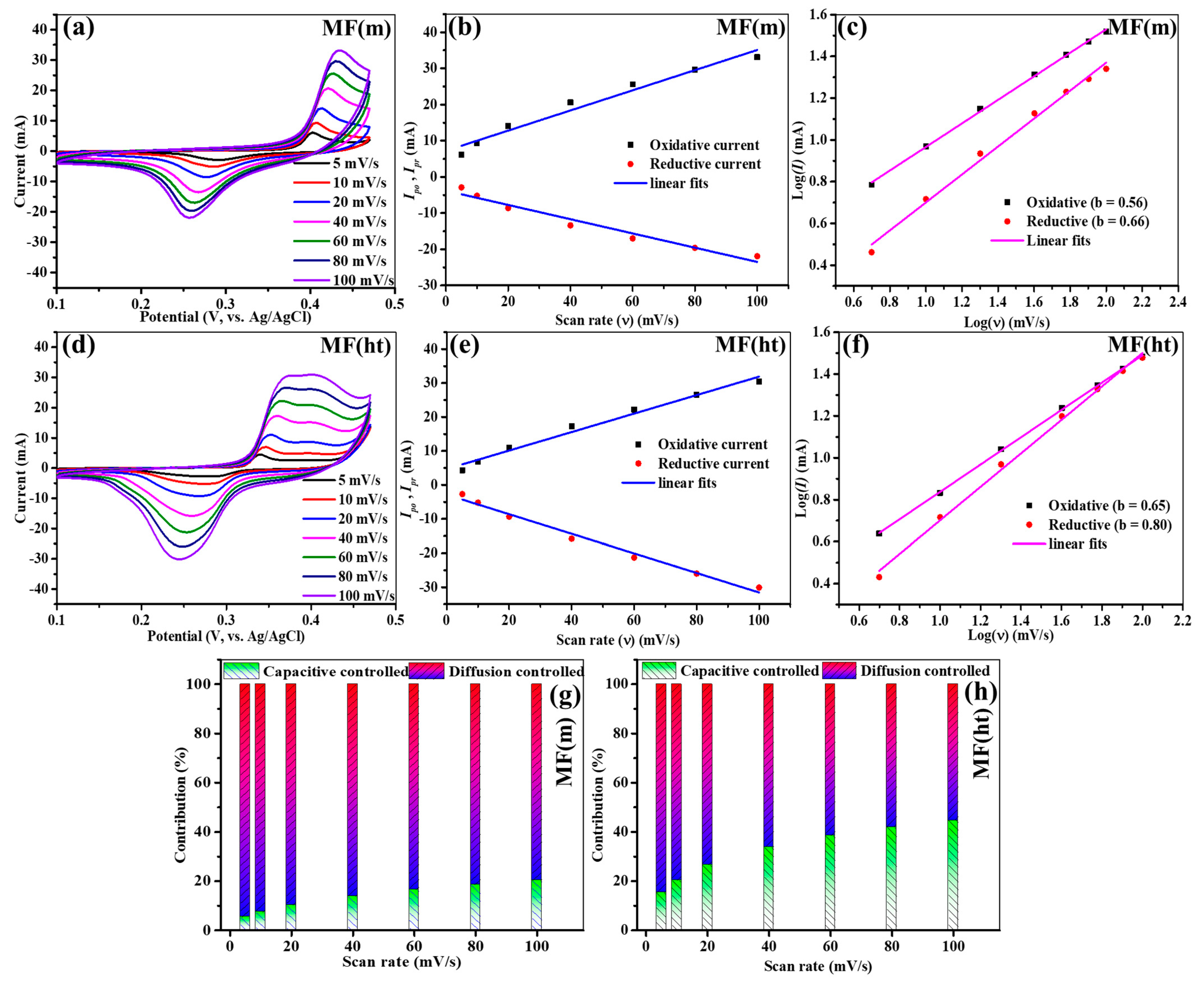
- Morphological Differences: The most prominent difference between the two synthesis methods lies in the resulting morphologies of the MIL-101(Fe) MOFs. MF(ht) exhibited a non-porous octahedral morphology, while MF(m) featured a highly porous structure. This distinction in morphology significantly influenced the electrochemical behavior of the electrodes.
- (b.)
- Charge Storage Mechanism: Through our electrochemical analysis, we found that both MF(ht) and MF(m) electrodes demonstrated characteristics closely aligned with a diffusion-controlled charge storage mechanism. This indicates that the charge storage in both electrodes is primarily governed by the kinetics of ion diffusion. However, the degree of dominance of the diffusion-controlled mechanism varied slightly between the two methods.
- (c.)
- Performance Discrepancies: Despite both synthesis methods showing diffusion-controlled charge storage, MF(ht) outperformed MF(m) in terms of electrochemical performance. MF(ht) exhibited higher specific capacitance, more extended charge–discharge durations, and better capacitance retention during extended cycling. These differences can be attributed to the non-porous octahedral morphology of MF(ht) and its superior surface area.
- (d.)
- Kinetics of Faradaic Reactions: Both MF(m) and MF(ht) electrodes displayed non-linear GCD profiles, indicating concurrent kinetics of faradaic oxidation and reduction. The prolonged GCD duration observed for MF(ht) is associated with its non-porous octahedral morphology and augmented surface area, which promotes charge storage.
- (e.)
- Impedance Analysis: EIS analysis further substantiated the superior performance of the MF(ht) electrodes. The charge transfer resistance (R2) for MF(m) was notably higher than that of MF(ht), indicating less conducive ion motion in the highly porous MF(m) structure
- (f.)
- Pseudocapacitance: Both electrodes exhibited pseudocapacitance. However, the value for MF(ht) was higher than that of MF(m), reflecting the enhanced charge accumulation within the double layer at the solid–liquid interface of the former electrode.
- (g.)
- Extended Cycling Stability: MF(ht) demonstrated remarkable capacitance retention even after 5000 charge–discharge cycles, surpassing the performance of MF(m) in terms of capacitance retention.
3.3. Supercapacitor Two-Electrode Device Performance Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hussain, I.; Lamiel, C.; Sahoo, S.; Javed, M.S.; Ahmad, M.; Chen, X.; Gu, S.; Qin, N.; Assiri, M.A.; Zhang, K. Animal- and Human-Inspired Nanostructures as Supercapacitor Electrode Materials: A Review. Nano-Micro Lett. 2022, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Ullah, E.; Shah, M.Z.U.; Ahmad, S.A.; Sajjad, M.; Khan, S.; Alzahrani, F.M.; Yahya, A.E.; Eldin, S.M.; Akkinepally, B.; Shah, A.; et al. Hydrothermal assisted synthesis of hierarchical SnO2 micro flowers with CdO nanoparticles based membrane for energy storage applications. Chemosphere 2023, 321, 138004. [Google Scholar] [CrossRef]
- Tantis, I.; Talande, S.; Tzitzios, V.; Basina, G.; Shrivastav, V.; Bakandritsos, A.; Zboril, R. Non-van der Waals 2D Materials for Electrochemical Energy Storage. Adv. Funct. Mater. 2023, 321, 138004. [Google Scholar] [CrossRef]
- Shah, M.S.U.; Zuo, X.; Shah, A.; Al-Saeedi, S.I.; Alabbad, E.A.; Hou, H.; Ahmad, S.A.; Arif, M.; Sajjad, M.; Haq, T.U. CoSe nanoparticles supported NiSe2 nanoflowers cathode with improved energy storage performance for advanced hybrid supercapacitors. J. Energy Storage 2023, 65, 107267. [Google Scholar] [CrossRef]
- Sharma, M.; Adalati, R.; Kumar, A.; Mehta, M.; Chandra, R. Composite Assembling of Oxide-Based Optically Transparent Electrodes for High-Performance Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2022, 14, 26791–26802. [Google Scholar] [CrossRef] [PubMed]
- Akkinepally, B.; Reddy, I.N.; Lee, C.; Ko, T.J.; Rao, P.S.; Shim, J. Promising electrode material of Fe3O4 nanoparticles decorated on V2O5 nanobelts for high-performance symmetric supercapacitors. Ceram. Int. 2022, 49, 6280–6288. [Google Scholar] [CrossRef]
- Roy, N.; Barai, H.R.; Banerjee, A.N.; Kim, J.S.; Joo, S.W. Solvent-dependent structural and electrochemical properties of zinc cobaltite via a self-assembled mechanism for battery-type supercapacitors. Chem. Eng. Sci. 2023, 277, 118834. [Google Scholar] [CrossRef]
- Feng, T.; Liu, G.; Li, G.; Li, Y.; Liang, J.; Wang, K. Engineering iron-rich nanomaterials for supercapacitors. Chem. Eng. J. 2023, 473, 145045. [Google Scholar] [CrossRef]
- Roy, N.; Mangiri, R.; Reddy, G.P.; Manohar, A.; Chung, E.; Raju, B.D.P.; Reddy, G.R.; Joo, S.W. Carbon nanofiber-supported elongated square bipyramid-like MnWO4 composite electrodes for high-performance battery-type supercapacitors: Enhanced electrochemical performance via synergistic effect. J. Electroanal. Chem. 2023, 947, 117764. [Google Scholar] [CrossRef]
- Shaheen, I.; Hussain, I.; Zahra, T.; Javed, M.S.; Shah, S.S.A.; Khan, K.; Hanif, M.B.; Assiri, M.A.; Said, Z.; Arifeen, W.U.; et al. Recent advancements in metal oxides for energy storage materials: Design, classification, and electrodes configuration of supercapacitor. J. Energy Storage 2023, 72, 108719. [Google Scholar] [CrossRef]
- Wang, K.B.; Xun, Q.; Zhang, Q. Recent progress in metal-organic frameworks as active materials for supercapacitors. Energy Chem. 2020, 2, 100025. [Google Scholar] [CrossRef]
- Niu, L.; Wu, T.; Chen, M.; Yang, L.; Yang, J.; Wang, Z.; Kornyshev, A.A.; Jiang, H.; Bi, S.; Feng, G. Conductive Metal–Organic Frameworks for Supercapacitors. Adv. Mater. 2022, 34, e2200999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nai, J.; Yu, L.; Lou, X.W. Metal-Organic-Framework-Based Materials as Platforms for Renewable Energy and Environmental Applications. Joule 2017, 1, 77–107. [Google Scholar] [CrossRef]
- Hussain, I.; Iqbal, S.; Lamiel, C.; Alfantazi, A.; Zhang, K. Recent advances in oriented metal–organic frameworks for supercapacitive energy storage. J. Mater. Chem. A 2022, 10, 4475–4488. [Google Scholar] [CrossRef]
- Nagaraju, G.; Santhoshkumar, P.; Sekhar, S.C.; Ramulu, B.; Nanthagopal, M.; Babu, P.S.; Lee, C.W.; Yu, J.S. Metal organic framework-derived MnO@carbon composites for highly durable Li-ion batteries and hybrid electrochemical cells. J. Power Sources 2022, 549, 232113. [Google Scholar] [CrossRef]
- Ramulu, B.; Mule, A.R.; Arbaz, S.J.; Yu, J.S. Design of high-mass loading metal–organic framework-based electrode materials with excellent redox activity for long-lasting electrochemical energy storage applications. Chem. Eng. J. 2023, 455, 140905. [Google Scholar] [CrossRef]
- Ghosh, T.K.K.; Rao, G.R. Design and synthesis of mixed-ligand architectured Zn-based coordination polymers for energy storage. Dalton Trans. 2023, 52, 5943–5955. [Google Scholar] [CrossRef]
- Krishnan, S.; Gupta, A.K.; Singh, M.K.; Guha, N.; Rai, D.K. Nitrogen-rich Cu-MOF decorated on reduced graphene oxide nanosheets for hybrid supercapacitor applications with enhanced cycling stability. Chem. Eng. J. 2022, 435, 135042. [Google Scholar] [CrossRef]
- Salunkhe, A.; Pawar, P.; Pagare, P.; Kadam, A.; Katkar, P.; Torane, A. MOF derived NiCo2O4 nanosheets for high performance asymmetric supercapacitor. J. Electroanal. Chem. 2023, 939, 117475. [Google Scholar] [CrossRef]
- Cao, W.; Xiong, C.; Chen, N.; Zhao, W.; Du, G.; Li, W.; Tang, L. Heterogeneous Mn-Ni(OH)2/NiO@C hierarchical porous nanosheets for high energy density hybrid supercapacitors. J. Alloys Compd. 2023, 934, 167790. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, Y.; Yan, Y.; Min, Y.; Fan, J.; Xu, Q.; Yun, H. (Metal-Organic Framework)-Polyaniline sandwich structure composites as novel hybrid electrode materials for high-performance supercapacitor. J. Power Sources 2016, 316, 176–182. [Google Scholar] [CrossRef]
- Cao, X.; Cui, L.; Liu, B.; Liu, Y.; Jia, D.; Yang, W.; Razal, J.M.; Liu, J. Reverse synthesis of star anise-like cobalt doped Cu-MOF/Cu2+1O hybrid materials based on a Cu(OH)2 precursor for high performance supercapacitors. J. Mater. Chem. A 2019, 7, 3815–3827. [Google Scholar] [CrossRef]
- Shao, D.; Wang, L.; Lu, B.; Guo, J.; Zhang, S.; Lu, Y. A high N content cobalt-based metal organic framework with nanorod structure for supercapacitor electrode material. J. Electroanal. Chem. 2019, 847, 113188. [Google Scholar] [CrossRef]
- Yang, J.; Ma, Z.; Gao, W.; Wei, M. Layered Structural Co-Based MOF with Conductive Network Frames as a New Supercapacitor Electrode. Chem.—A Eur. J. 2017, 23, 631–636. [Google Scholar] [CrossRef]
- Senthil, R.A.; Osman, S.; Pan, J.; Liu, X.; Wu, Y. Recent progress on porous carbon derived from Zn and Al based metal-organic frameworks as advanced materials for supercapacitor applications. J. Energy Storage 2021, 44, 103263. [Google Scholar] [CrossRef]
- Jiao, Y.; Pei, J.; Yan, C.; Chen, D.; Hu, Y.; Chen, G. Layered nickel metal–organic framework for high performance alkaline battery-supercapacitor hybrid devices. J. Mater. Chem. A 2016, 4, 13344–13351. [Google Scholar] [CrossRef]
- Deka, R.; Rajak, R.; Kumar, V.; Mobin, S.M. Effect of Electrolytic Cations on a 3D Cd-MOF for Supercapacitive Electrodes. Inorg. Chem. 2023, 62, 3084–3094. [Google Scholar] [CrossRef]
- Goswami, A.; Ghosh, D.; Pradhan, D.; Biradha, K. In Situ Grown Mn(II) MOF upon Nickel Foam Acts as a Robust Self-Supporting Bifunctional Electrode for Overall Water Splitting: A Bimetallic Synergistic Collaboration Strategy. ACS Appl. Mater. Interfaces 2022, 14, 29722–29734. [Google Scholar] [CrossRef]
- Kim, J.; Young, C.; Lee, J.; Heo, Y.-U.; Park, M.-S.; Hossain, S.A.; Yamauchi, Y.; Kim, J.H. Nanoarchitecture of MOF-derived nanoporous functional composites for hybrid supercapacitors. J. Mater. Chem. A 2017, 5, 15065–15072. [Google Scholar] [CrossRef]
- Subramanian, V.; Zhu, H.; Wei, B. Nanostructured MnO2: Hydrothermal synthesis and electrochemical properties as a supercapacitor electrode material. J. Power Sources 2006, 159, 361–364. [Google Scholar] [CrossRef]
- Lee, J.W.; Hall, A.S.; Kim, J.-D.; Mallouk, T.E. A Facile and Template-Free Hydrothermal Synthesis of Mn3O4 Nanorods on Graphene Sheets for Supercapacitor Electrodes with Long Cycle Stability. Chem. Mater. 2012, 24, 1158–1164. [Google Scholar] [CrossRef]
- Dong, Y.; Hu, T.; Pudukudy, M.; Su, H.; Jiang, L.; Shan, S.; Jia, Q. Influence of microwave-assisted synthesis on the structural and textural properties of mesoporous MIL-101(Fe) and NH2-MIL-101(Fe) for enhanced tetracycline adsorption. Mater. Chem. Phys. 2020, 251, 123060. [Google Scholar] [CrossRef]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Advances in Microwave Synthesis of Nanoporous Materials. Adv. Mater. 2021, 33, 2103477. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ma, R.; Fang, H.; Shi, H.; Liu, D. MIL-101(Fe)-Attached Graphene Oxide for High-Performance Supercapacitors with Sound Stability in Acid Electrolyte. Cryst. Growth Des. 2022, 22, 2997–3006. [Google Scholar] [CrossRef]
- Barbosa, A.D.; Julião, D.; Fernandes, D.M.; Peixoto, A.F.; Freire, C.; de Castro, B.; Granadeiro, C.M.; Balula, S.S.; Cunha-Silva, L. Catalytic performance and electrochemical behaviour of Metal–organic frameworks: MIL-101(Fe) versus NH2-MIL-101(Fe). Polyhedron 2017, 127, 464–470. [Google Scholar] [CrossRef]
- Jarrah, A.; Farhadi, S. Encapsulation of K6P2W18O62 into magnetic nanoporous Fe3O4/MIL-101 (Fe) for highly enhanced removal of organic dyes. J. Solid State Chem. 2020, 285, 121264. [Google Scholar] [CrossRef]
- Hajiali, M.; Farhadian, M.; Tangestaninejad, S.; Khosravi, M. Synthesis and characterization of Bi2MoO6/MIL-101(Fe) as a novel composite with enhanced photocatalytic performance: Effect of water matrix and reaction mechanism. Adv. Powder Technol. 2022, 33, 103546. [Google Scholar] [CrossRef]
- Hajiali, M.; Farhadian, M.; Tangestaninejad, S. Novel ZnO nanorods/Bi2MoO6/MIL-101(Fe) heterostructure immobilized on FTO with boosting photocatalytic activity for tetracycline degradation: Reaction mechanism and toxicity assessment. Appl. Surf. Sci. 2022, 602, 154389. [Google Scholar] [CrossRef]
- Khan, N.A.; Lee, J.S.; Jeon, J.; Jun, C.-H.; Jhung, S.H. Phase-selective synthesis and phase-conversion of porous aluminum-benzenetricarboxylates with microwave irradiation. Microporous Mesoporous Mater. 2012, 152, 235–239. [Google Scholar] [CrossRef]
- Liu, N.; Tang, M.; Wu, J.; Tang, L.; Huang, W.; Li, Q.; Lei, J.; Zhang, X.; Wang, L. Boosting Visible-Light Photocatalytic Performance for CO2 Reduction via Hydroxylated Graphene Quantum Dots Sensitized MIL-101(Fe). Adv. Mater. Interfaces 2020, 7, 2000468. [Google Scholar] [CrossRef]
- Ramachandran, R.; Zhao, C.; Luo, D.; Wang, K.; Wang, F. Morphology-dependent electrochemical properties of cobalt-based metal organic frameworks for supercapacitor electrode materials. Electrochim. Acta 2018, 267, 170–180. [Google Scholar] [CrossRef]
- Li, X.; Guo, W.; Liu, Z.; Wang, R.; Liu, H. Fe-based MOFs for efficient adsorption and degradation of acid orange 7 in aqueous solution via persulfate activation. Appl. Surf. Sci. 2016, 369, 130–136. [Google Scholar] [CrossRef]
- Zhu, M.; Xu, R.; Wang, X.; Zhang, J.L.Q.; Wei, J. MIL-101 (Fe) modified carbon paste electrode for the efficient simultaneous detection of hydroquinone and catechol. Int. J. Electrochem. Sci. 2021, 16, 211228. [Google Scholar] [CrossRef]
- Goswami, S.; Dillip, G.R.; Nandy, S.; Banerjee, A.N.; Pimentel, A.; Joo, S.W.; Martins, R.; Fortunato, E. Biowaste-derived carbon black applied to polyaniline-based high-performance supercapacitor microelectrodes: Sustainable materials for renewable energy applications. Electrochim. Acta 2019, 316, 202–218. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Nallapureddy, R.R.; Goli, H.R.; Banerjee, A.N.; Reddy, G.R.; Joo, S.W. Bioinspired tailoring of nanoarchitectured nickel sulfide@nickel permeated carbon composite as highly durable and redox chemistry enabled battery-type electrode for hybrid supercapacitors. J. Mater. Chem. A 2021, 9, 25208–25219. [Google Scholar] [CrossRef]
- Omar, F.S.; Numan, A.; Duraisamy, N.; Bashir, S.; Ramesh, K.; Ramesh, S. Ultrahigh capacitance of amorphous nickel phosphate for asymmetric supercapacitor applications. RSC Adv. 2016, 6, 76298–76306. [Google Scholar] [CrossRef]
- Ghaly, H.A.; El-Deen, A.G.; Souaya, E.R.; Allam, N.K. Asymmetric supercapacitors based on 3D graphene-wrapped V2O5 nanospheres and Fe3O4@3D graphene electrodes with high power and energy densities. Electrochim. Acta 2019, 310, 58–69. [Google Scholar] [CrossRef]
- Li, T.; He, P.; Dong, Y.; Chen, W.; Wang, T.; Gong, J.; Chen, W. Polyoxometalate-Based Metal-Organic Framework/Polypyrrole Composites toward Enhanced Supercapacitor Performance. Eur. J. Inorg. Chem. 2021, 2021, 2063–2069. [Google Scholar] [CrossRef]
- Ojha, M.; Wu, B.; Deepa, M. Cost-Effective MIL-53(Cr) Metal–Organic Framework-Based Supercapacitors Encompassing Fast-Ion (Li+/H+/Na+) Conductors. ACS Appl. Energy Mater. 2021, 4, 4729–4743. [Google Scholar] [CrossRef]
- Sundriyal, S.; Mishra, S.; Deep, A. Study of Manganese-1,4-Benzenedicarboxylate Metal Organic Framework Electrodes Based Solid State Symmetrical Supercapacitor. Energy Procedia 2019, 158, 5817–5824. [Google Scholar] [CrossRef]
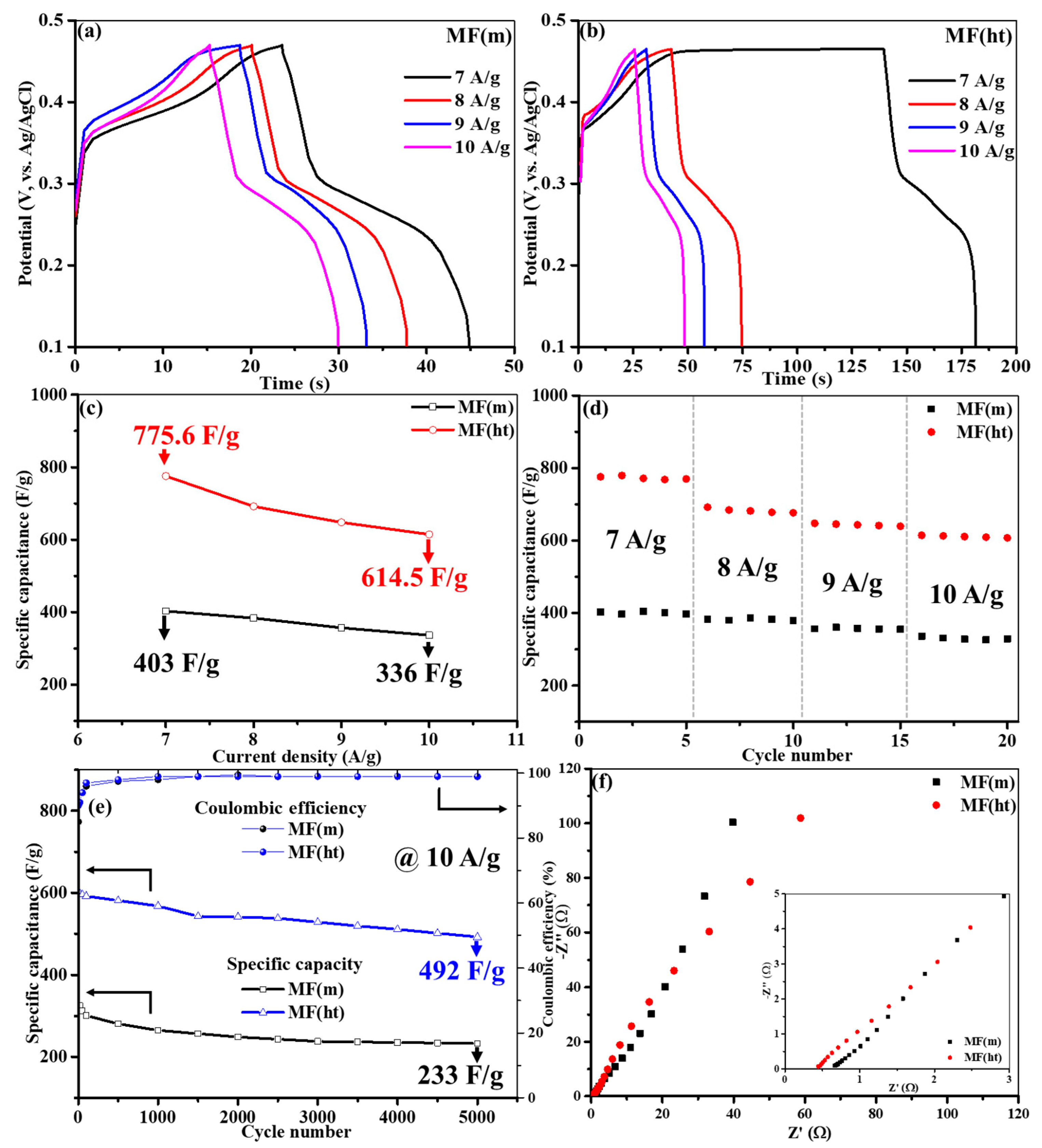

| Electrode | R1 (Ω) | R2 (Ω) | Q2 (mF·s(α−1)) | C3 (mF) |
|---|---|---|---|---|
| MF(m) | 0.63 | 39.4 | 3.8 | 5 |
| MF(ht) | 0.42 | 4.5 | 3.4 | 15 |
| Electrodes | Operating Potential (V) | Electrolyte | Specific Capacitance (F·g−1) | Energy Density (Wh·kg−1) | Power Density (W·kg−1) | Capacitance Retention | Ref. |
|---|---|---|---|---|---|---|---|
| Fe3O4-V2O5 | 0–0.85 | 3 M KOH | 93 | 13 | 1530 | 84% after 5000 cycles | [6] |
| PW12@MIL-101/PPy-0.15 | 0–1 | 1 M Li2SO4 | 149 | 20.7 | 277.6 | 83.7% after 2000 cycles | [48] |
| BPC//MIL-53(Cr) ASCD | 0–1 | 1 M aqueous CSA | 70 | 9.7 | 1250 | 85% after 10,000 cycles | [49] |
| Mn-BDC MOF | 0–1.5 | PVA-1 M Na2SO4 | 64.5 | 4.3 | 171.6 | 98% after 2000 cycles | [50] |
| MIL-101(Fe) | 0–1.2 | 3 M KOH | 103 | 14.2 | 3780 | 63% after 5000 cycles | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkinepally, B.; Kumar, G.D.; Reddy, I.N.; Rao, H.J.; Nagajyothi, P.C.; Alothman, A.A.; Alqahtani, K.N.; Hassan, A.M.; Javed, M.S.; Shim, J. Investigation of Supercapacitor Electrodes Based on MIL-101(Fe) Metal-Organic Framework: Evaluating Electrochemical Performance through Hydrothermal and Microwave-Assisted Synthesis. Crystals 2023, 13, 1547. https://doi.org/10.3390/cryst13111547
Akkinepally B, Kumar GD, Reddy IN, Rao HJ, Nagajyothi PC, Alothman AA, Alqahtani KN, Hassan AM, Javed MS, Shim J. Investigation of Supercapacitor Electrodes Based on MIL-101(Fe) Metal-Organic Framework: Evaluating Electrochemical Performance through Hydrothermal and Microwave-Assisted Synthesis. Crystals. 2023; 13(11):1547. https://doi.org/10.3390/cryst13111547
Chicago/Turabian StyleAkkinepally, Bhargav, Gara Dheeraj Kumar, I. Neelakanta Reddy, H. Jeevan Rao, Patnamsetty Chidanandha Nagajyothi, Asma A. Alothman, Khadraa N. Alqahtani, Ahmed M. Hassan, Muhammad Sufyan Javed, and Jaesool Shim. 2023. "Investigation of Supercapacitor Electrodes Based on MIL-101(Fe) Metal-Organic Framework: Evaluating Electrochemical Performance through Hydrothermal and Microwave-Assisted Synthesis" Crystals 13, no. 11: 1547. https://doi.org/10.3390/cryst13111547
APA StyleAkkinepally, B., Kumar, G. D., Reddy, I. N., Rao, H. J., Nagajyothi, P. C., Alothman, A. A., Alqahtani, K. N., Hassan, A. M., Javed, M. S., & Shim, J. (2023). Investigation of Supercapacitor Electrodes Based on MIL-101(Fe) Metal-Organic Framework: Evaluating Electrochemical Performance through Hydrothermal and Microwave-Assisted Synthesis. Crystals, 13(11), 1547. https://doi.org/10.3390/cryst13111547









