Abstract
DFT-D3 calculations based on structural X-ray diffraction data obtained for 3-oxo-camphorsulfonyl imine (1), camphorsulfonyl chloride (2) and seven camphor sulfonimines (O2SNC10H13NR, L1−L7), from which L2 (R=4-OHC6H4), L4 (R=4-ClC6H4) and L6 (R=3,5-(CH3)2C6H3) are synthesized and characterized in this work, provide information into the intra- and inter-molecular interactions with concomitant elucidation of the supramolecular arrangement of the compounds. The DFT-D3 calculations performed in small clusters of two or three molecular units reproduce the interactions observed via X-ray analyses, showing that, as a general trend, the structural arrangement of the molecules is driven by electronic rather than by packing parameters. In all compounds, the self-assembly of 3D structures involves the sulfonyl imine group (-NSO2) either to establish hydrogen bonds through oxygen atoms or non-classic oxygen–aliphatic hydrogen or non-bonding interactions (NBIs), which also involve sulfonyl oxygen atoms. Interestingly, the camphor sulfonimine compounds (L2, L3), having protic groups (R=C6H4X:X=OH, L2 or X=NH2, L3) at the aromatic imine substituents (=NR), present an extra π-π stacking, which is absent in the other compounds’ aromatic derivatives. The X-ray analysis shows that all the reported camphor sulfonimine compounds display the E configuration with respect to the imine substituent (R). The study of the redox behavior of the compounds by cyclic voltammetry enables insight into the solution properties of the compounds and the rationalization of the molecular interactions that stand in the solid and solution states. Camphor sulfonimine compounds (L) display appropriate binding atoms to coordinate transition metals. The results herein show that monodentate coordination through the nitrogen atom of the sulfonimine five-membered ring to the {Ag(NO3)} metal center is favored. When this imine nitrogen atom is not itself involved in the organic framework, DFT-D3 calculations show that the complexation does not affect the non-covalent interactions that are reproduced in the MOF structure.
1. Introduction
The molecular structure of coordination compounds is formed by organic and inorganic moieties that combine to form new metal–ligand interactions. The formation of the new compounds can involve a major change in the pre-existing structural arrangement of the ligand or the metal precursors, or be shaped through binding of one of them into the pre-organized structures of the other without a major reorganization.
In the case of complexes based on neutral ligands, covalent dative bonds are established involving the ligand heteroatom’s lone pair (e.g., N, O, S) without change to the metal oxidation state or modification of the ligand composition. This is the case with camphor sulfonimines (RNC10H13NSO2), which typically bind the metal through the nitrogen lone pair (=NSO2), forming dative bonds that, in some cases, are reinforced by Van der Waals interactions (non-bonding interactions, NBIs) involving the imine nitrogen atom (=NR) [1,2,3].
Pre-existing intermolecular NBIs in the organic or inorganic scaffolds can be maintained or be broken within the formation of the coordination compound. If the NBIs remain, the coordination compound eventually acquires a polymeric character. NBIs play a significant role in the structural, reactivity and selectivity of the coordination compounds [4,5] and are referred to as being the primary transport mechanism by which thermal conductivity is increased in cross-linked polymers [6].
In some cases, a structure/reactivity relationship can be established in complexes [7,8], allowing the prediction of properties based on the structure; e.g., biological and/or catalytic properties of camphor sulfonimine coordination compounds [3,9]. Unfortunately, the structural analysis of coordination compounds based on X-ray diffraction is sometimes impossible due to a lack of suitable crystals. However, if X-ray data on the organic moieties exist, it is possible to predict through computational calculations the structure of the derived coordination compounds. DFT-D3, by providing an approach to the treatment of London dispersion interactions, has been applied to systems depicting NCI. The methodology was extensively review by Goerigk, in particular for NCI energies and geometry optimizations [10].
2. Results and Discussion
Two new coordination compounds [Ag(NO3)L2] (C1: L6 and C2: L7) were synthesized (see Section 4) from the reaction of AgNO3 with camphor sulfonimines, 3,5-Me2C6H3C10H13SO2 (L6) and C6H5C10H13SO2 (L7), which enlarges the pool of [Ag(NO3)Ln] (n = 1, 2) camphor sulfonimine complexes with potential biological applications as anticancer or antimicrobial agents [3,11]. Unfortunately, the structure of these two camphor sulfonimine Ag(I) complexes, as with that of many others, could not be confirmed by X-ray diffraction analysis since no suitable crystals could be obtained. Therefore, calculations based on X-ray data obtained for the ligands can help to gain insight into the structural arrangement of the complexes. In one case, data obtained from X-rays showed that [Ag(NO3)L2] (R=NMe2) arranges as a calix (Scheme 1) [3].
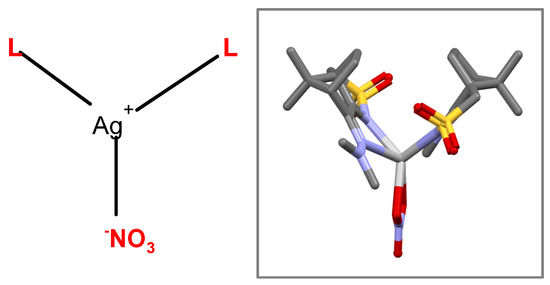
Scheme 1.
The packing structure of this complex (Figure 1) shows that the silver atom occupies essentially the empty space (gray polyhedral in Figure 1) in the packing imposed by the non-bonding interactions (NBIs) established between the organic fragments. Such observation prompts the prediction of other MOFs through superimposition of Ag(I) metal centers in the camphor sulfonimines’ organic frameworks, whose structures are available from X-ray diffraction data and/or are calculated via DFT-D3.
2.1. Synthesis of Camphor Sulfonimines
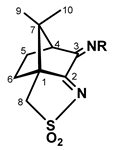
The camphor sulfonimine compounds (RNC10H13NSO2: L1, R=OH; L2, R=4-OHC6H4; L3, R=4-NH2C6H4; L4, R=4-ClC6H4; L5, R=4-CH3C6H4; L6, R=3,5-(CH3)2C6H3; L7, R=C6H5) were obtained by condensation (Scheme 2, right) of the convenient primary amine (H2NR) with (1S)-3-oxo-camphorsulfonyl imine (1). Precursor 1 was obtained from camphor sulfonic acid through a sequential process that involves the formation of compound 2 (Scheme 2, left) and ends on the oxidative annulation of a third ring to the bicyclic camphor precursor.
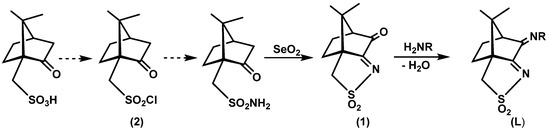
Scheme 2.
R=OH, L1; R=4-OHC6H4, L2; R=4-NH2C6H4, L3; R=4-ClC6H4, L4; R=4-CH3C6H4, L5; R=3,5-(CH3)2C6H3, L6; R= C6H5, L7.
With respect to the camphorimine species under study (L1−L7), four are new (L1, L2, L4 and L6) and were characterized by conventional analytical and spectroscopic techniques (FTIR, 1H NMR, 13C NMR and 2D NMR). The remaining compounds were just recrystallized to obtain single crystals. The structural arrangement of all compounds was then studied by X-ray diffraction analysis. Based on the X-ray data, the intra- and inter-molecular NBIs were predicted via DFT-D3. Therefore, the supramolecular arrangement of compounds 1 and 2 and camphor sulfonimines L1−L7 was elucidated.
2.2. Structural Arrangement Predicted via DFT-D3
Compounds 1 and 2
The X-ray data obtained for compound 1 indicate that a 3D helicoidal packing exists around the 31 crystallographic axes due to non-conventional oxygen∙∙∙hydrogen (aliphatic) NBIs (Figure 1a). DFT-D3 calculations, performed on a subset dimer of 1, confirm the observed NBIs and predict them to be even stronger than those experimentally observed via X-ray analysis, as illustrated by shorter distances (Figure 2b).
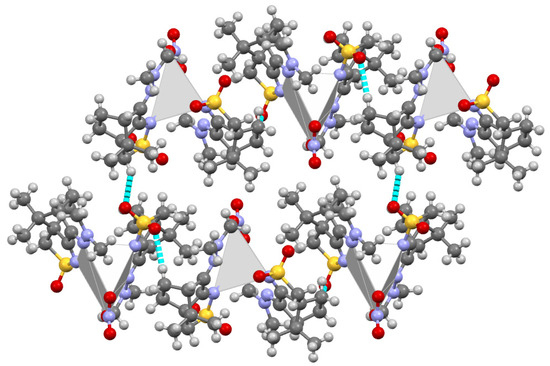
Figure 1.
MOF for [Ag(NO3)L2] (L=RNC10H13NSO2; R=NMe2). The gray polyhedral represents the volume occupied by the silver atom.
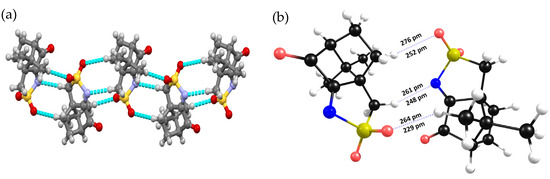
Figure 2.
(a) X-ray diffraction data showing the helicoidal packing for 1; (b) NBI lengths according to X-ray data (above) and DFT-D3 calculations on a subset dimer of 1.
For 2, calculations were based on the X-ray data reported by others [12]. The X-ray shows a zig-zag 1D structural arrangement due to chloride–oxygen intermolecular NBIs established by the pendant sulphonyl chloride (SO2Cl) groups in adjacent molecules (Figure 3). This type of cooperative intermolecular S-Cl∙∙∙O interaction was previously discussed [13] on the basis of the S-Cl∙∙∙O and S-O∙∙∙Cl angles (θ) and classified (Figure 3, insert) as type I (θ1 = θ2) or type II (θ1 = 180°, θ2 = 90°) [14].
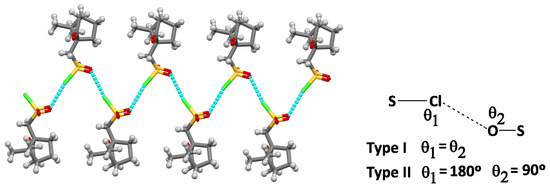
Figure 3.
Drawing evidencing the bi-dimensional intermolecular interactions in 2.
As with 1, DFT-D3 calculations for 2 were carried out on a subset dimer of the structural arrangement found by X-ray diffraction analysis. Once more, the output data show good agreement between experimental and calculated values (Table 1), in agreement with the electrostatic directionality of the interactions.

Table 1.
Intermolecular interactions in 2 (distances pm; angles deg).
2.3. Camphor Sulfonimines (L1−L7)
X-ray data collected for the sulfonimine compounds L1 to L7 show different packing motives according to the imine substituent (R). In L1 (R=OH), the asymmetric unit is formed by two structurally different molecules, as shown in Figure 4 (center), since chirality prevents the existence of an inversion center.
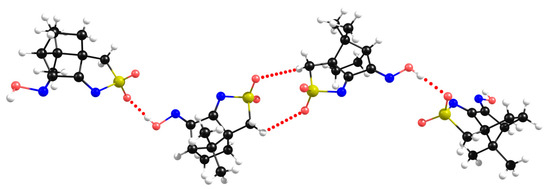
Figure 4.
X-ray packing for L1. The central dimer is the asymmetric unit composed by two different molecules.
The X-ray data show that at the “dimeric” asymmetric unit, the NBIs are type I (θ1 = θ2, i.e., S-O∙∙∙H=C-H∙∙∙O = 108°), while the NBIs established between neighboring asymmetric units (involving the sulfonimine oxygen atom and the hydrogen hydroxyl atom) are of type II (θ1 ≠ θ2, i.e., S-O∙∙∙H=109° and O-H∙∙∙O=171°). The DFT-D3 calculations corroborate the type II nature of this NBI (Figure 5, left), but concerning the asymmetric unit, DFT-D3 calculations predict a type II NBI (Figure 5, right), in contrast to those experimentally observed by X-ray diffraction. Such results indicate that NBI type I experimental observations are due to packing constraints rather than to electrostatic effects.
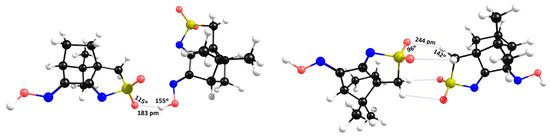
Figure 5.
Data obtained via DFT-D3 for NBIs in the hydroxyl (left) and central dimer (right) in L1.
Figure 4 and Figure 5 show that the asymmetric unit remains unchanged both experimentally and in DFT-D3 calculations, with the two nitrogen atoms not involved in NBIs and, thus, available for complexation with the metal center AgNO3. The introduction of the metal center in the calculations (Figure 6) confirms that an MOF can form through coordination of the cyclic imine nitrogen atom in the metal, keeping the 1D chain of the organic framework (OF).

Figure 6.
Prediction via DFT-D3 of the structural arrangement of [AgNO3L1].
Camphor sulfonimine compounds with aromatic substituents (R, Scheme 2) at the imine group (L2 to L7) display self-assembly behaviors quite different from L1 (R=OH).
A 1D assembly, which exists in L4 (R=4-ClC6H4), L5 (R=4-CH3C6H4), L6 (3,5-(CH3)2C6H4) and L7 (R=C6H5), is formed by non-conventional NBIs established between the nitrogen atom of the -SO2N- group and one of the hydrogen atoms of the methylene (CH2) group of the five-membered sulfonimine ring of a neighboring molecule. As a representative example, X-ray diffraction data are displayed for L7 (Figure 7, left). The results from the DFT-D3 calculations agree well with the experimentally obtained X-ray data (Figure 7, right). As with 1, the predicted NBI distances are shorter than those experimentally measured, pointing to stronger electronic interactions.
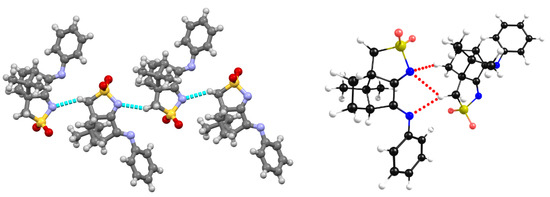
Figure 7.
Structural arrangement of L7: X-ray data (left) showing the 1D assembly through an N∙∙∙H-C NBI and DFT-D3-optimized structure (right).
In the aromatic substituted camphor sulfonimines (L4 to L7), the cyclic nitrogen imine atom of the 1D organic framework is involved in NBIs, which means that complexation would break down the self-assembly trends observed in the OF. So, MOFs are expected to have different frameworks from those observed in ligands L4 to L7.
For the camphor sulfonimines bearing a protic substituent (R=OH in L2 or R=NH2 in L3) at the -NR imine group (Scheme 2), the characteristics of the NBIs differ from those observed in L4−L7. In L2 and L3, the NBIs are of the stacking π-π type, established between aromatic carbon atoms of two distinct asymmetric units occupying the ortho positions relative to the heterogeneous (N or O) atoms, as depicted for L2 (Figure 8, left). In L2 and L3, NBIs involving the sulfonyl imine group (not observed for L4−L7) are established by a symmetry-related chain of molecules (Figure 8, left). These additional interactions were included in the DFT-D3 calculations (Figure 8, right) to obtain consistency between X-ray and calculated structures. Here again, as with L1, the two nitrogen imine atoms are free to complex the AgNO3 metal center to form an MOF mimicking the self-assembly characteristics of the corresponding OF.
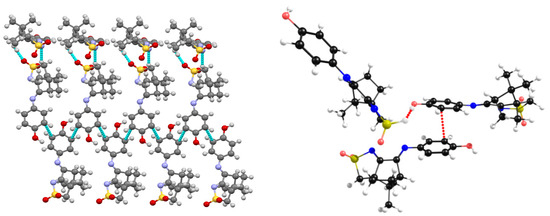
Figure 8.
X-ray data (left) showing the π-π stacking in L2; structure optimization made via DFT-D3 (right).
2.4. Redox Properties
Packing and electronic parameters have distinct effects on the properties of molecules. While electronic characteristics persist no matter the physical state (solid or solution), packing essentially drives solid-state properties.
The above results show that the structural arrangement in camphor sulfonimines (L2−L7) and in compounds 1 and 2 is essentially directed by electronic parameters. In contrast, packing plays an important role in the supramolecular arrangement of L1 by imposing a type I NBI geometry (see above).
In order to obtain insights as to whether a correlation exists between structural parameters and redox potentials, and to try to ascertain whether NBI interactions remain in a solution, the electrochemical behavior of the L1−L7 compounds was studied by cyclic voltammetry.
In acetonitrile, compounds L1−L7 display one irreversible anodic wave and one (L1, L2, L5, L6, L7) or two (L3, L4) cathodic waves (Figure 9). In the case of two cathodic waves, the higher potential wave (I) typically displays a higher intensity than the lower potential one (wave II). The reversibility of wave I decreases upon scanning at the potential of wave II, as shown (Figure 9, left). The loss of reversibility and emergence of anodic wave X is consistent with chemical reactivity being induced by electron transfer.

Figure 9.
Cyclic voltammograms obtained in Bu4NBF4/CH3CN at 200 mV/s: L4 (left)—full cathodic scan; L5 (right)—partial scan reversed after wave I.
The anodic and cathodic potentials measured for L1−L7 are displayed in Table 2.

Table 2.
Cyclic voltammetry data a for camphor sulfonimine compounds L1−L7.
The potential trend observed for wave I (Table 2) suggests that reduction involves the imine >C=N-R group. In fact, an excellent correlation exists (Figure 10, left) between the redox potential and the Hammett sigma parameter (σp) [15] which accounts for the electronic characteristics of the R group driving the cathodic process. Compounds L1 (R=OH) and L3 (4-NH2C6H4) are aside from the linear correlation (R2 = 0.987) established by the other compounds, showing that other than electronic parameters operate.
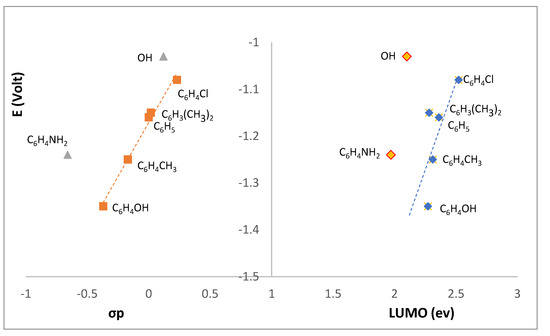
Figure 10.
Correlation between the reduction potential () of L1−L7 and the Hammett parameter (σp) (left) or LUMO (right).
With respect to L1, such behavior was somehow expected due to the non-aromatic character of the R group (unlike the R groups at L2−L7) and the distinct type of NBI (see DFT-D3 discussion, above) compared to the other compounds.
With respect to the relationship between and the LUMO (Figure 10, right), the energy calculations for L1 show that there is a decrease of 0.11 eV in the absolute value of the energy in the H-bonded dimer. Such an energy shift may account for the non-fitting of the potential (Figure 10, right), pointing to L1 keeping some dimer characteristics in the electrolyte solution.
L3 is the other compound that does not fit either the versus σp (Figure 10, left) or the versus LUMO (Figure 10, right) correlations. The two compounds (L1, R=OH and L3, R=C6H4NH2) have in common the protic character of the R imine substituent, which induces formation of H-bonded dimers (see above).
With respect to the anodic behavior, only L2, L3, L5 and L6 show oxidation processes. L2 and L3 display the lowest oxidation potentials, in agreement with the electron releasing characteristics of the aromatic R groups, as corroborated through linear correlation with Hammett σp parameters (Figure 11, left). A good correlation with the HOMO was found (Figure 11, right).
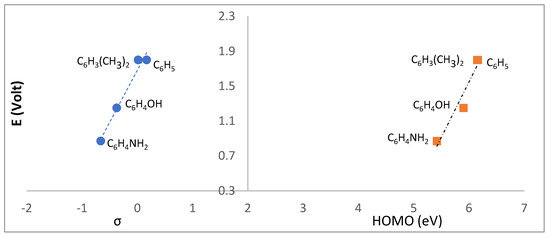
Figure 11.
Relationship between the Hammett parameters (σp) and the oxidation potentials measured for the camphor sulfonimines.
The calculated HOMO and LUMO contours for compound L7 show that the HOMO is mostly localized at the aromatic ring (all carbons), while the LUMO is localized at the camphor skeleton, with an important contribution from the two-, four- and six-ring carbon atoms (Figure 12).
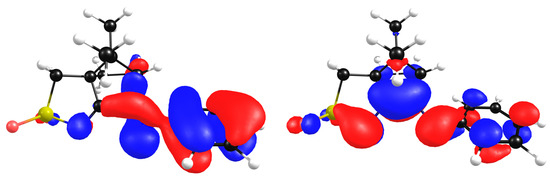
Figure 12.
Wavefunction contours for HOMO (left) and LUMO (right) for L7.
The electrochemical behavior of [Ag(NO3)L2] (C1, C2) complexes studied by cyclic voltammetry shows that a cathodic process exists at a potential ( = −1.18 V, C1 and = −1.17 V, C2) similar to that measured for organic precursors L6 and L7, respectively (Table 2). Such patterns indicate that the electronic properties of the sulfonimine ligands remain essentially identical in the complexes. As expected, the cyclic voltammograms of C1 and C2 display a cathodic wave attributed to the Ag(I)→Ag(0) reduction at a potential ( = 0.16 V) that does not differ much from that reported for related complexes [3].
The redox behavior of 1 was also studied by cyclic voltammetry, showing a quasi-reversible cathodic wave ( = −0.92 V; ipc/ipa = 1, Epa − Epc = 133 mV) but no anodic process. According to the cathodic potential value, the reduction of 1 is easier than for any of the sulfonimine compounds (L1−L7, Table 1). Therefore, sulfonimines are more electron rich than 1 and, consequently, better ligands for metal sites. In fact, no Ag(I) complex based on 1 could be experimentally obtained, in contrast with several complexes derived from sulfonimine ligands; for example, the new [Ag(NO3)L2] (C1 and C2) now reported (see Section 4).
3. Conclusions
DFT-D3 calculations based on X-ray data obtained for a series of camphor sulfonimine compounds corroborate that NBIs are responsible for their organic framework. For 2L−7L, the NBIs are essentially electrostatic, in contrast with 1L, where packing plays a relevant role in the solid-state arrangement observed via X-ray analysis.
The characteristics of the sulfonimine substituent (R) drives the NBIs established and directs the ability of the L compounds to bind the Ag(I) metal center forming MOFs, such as those detected for [Ag(NO3)(O2SNC10H13NNMe2)2], whose X-ray structure was formerly reported.
It was found that complexation of the metal center always occurs in a monodentate fashion through the imine nitrogen atom in the sulphonyl five-membered ring. It was predicted via DFT-D3 calculations that NBI interactions not involving this atom remain after complexation transfer to the MOF. The conjugation of the X-ray structures of the organic ligands with DFT-D3 calculations proved to be a powerful tool to predict the self-assembly trends of the corresponding MOF structures of the complexes.
The correlation between the redox potentials of 1L−7L (studied by cyclic voltammetry), the Hammett σ-parameter and calculated HOMO and LUMO energies are in excellent agreement with different types of NBIs being established by 1L and 2L compared to the other camphor sulfonimine compounds.
The results presented in this work are expected to enable the bottom-up design of new MOFs by probing, in silica, the incorporation of metal fragments in the OF of existing ligands.
4. Experimental Section
The camphor sulfonimines L1, L3, L5 and L7 were synthesized according to published procedures [16]. The complexes were synthesized under nitrogen using Schlenk and vacuum techniques. The FTIR spectra were obtained from KBr pellets using a JASCO FT/IR 4100 spectrometer. The NMR spectra (1H, 13C, DEPT, HSQC, and HMBC) were obtained from CDCl3, CD3CN or DMSO solutions using Bruker Avance II+ (300 or 400 MHz) spectrometers. The NMR chemical shifts are referred to TMS (δ = 0 ppm).
The redox properties were studied by cyclic voltammetry using a three-compartment cell equipped with Pt wire electrodes (work and secondary) and interfaced with VoltaLab PST050 equipment. The cyclic voltammograms were obtained from NBu4BF4/CH3CN (0.10 M) solutions used as electrolyte. The potentials were measured in Volts (±10 mV) versus SCE at 200 mV/s using [Fe(η5-C5H5)2]0/+ ( = 0.382 V; CH3CN) as an internal reference. The window of potential was established by scanning a fresh solution of the electrolyte to the limits.
4.1. Synthesis
4.1.1. Camphor Sulfonimines
(E)-7-(hydroxyimino)-8,8-dimethyl-4,5,6,7-tetrahydro-3H-3a,6-ethanobenzo[c] isothiazole 2,2-dioxide (L1)-Compound 1 (682 mg; 3 mmol) was dissolved in ethanol (10 mL) acidified with acetic acid (0.4 mL) and the mixture was stirred for ca. 15 min before the hydroxylamine (99 mg, 3 mmol) was added. The reaction was stopped after 24 h of stirring with the addition of 5 mL of water. The organic phase was then extracted with dichloromethane (3 × 5 mL) and dried over MgSO4 for a couple of hours. After filtration, the solvent was allowed to evaporate slowly in the exhaust hood. Yield 71%. Elem. Anal. (%) for C10H14N2O3S: Found: C, 49.8; N, 11.3; H, 6.0; S, 13.7. Calc.: C, 49.6; N, 11.6; H, 5.8; S, 13.2. FTIR (KBr, cm−1): 3367, 1668, 1633, 1327, 1158. 1H NMR (DMSO, δppm): 12.8 (s, 1H); 3.59, 3.36 (2d, J = 14.0 Hz, 2H); 3.25 (d, J = 4.2 Hz, 1H); 2.22–2.01 (m, 2H); 1.63–1.40 (m, 2H); 1.01 (s, 3H); 0.78 (s, 3H). 13C NMR (DMSO, δppm): 184.7, 155.3, 64.2, 49.0, 47.9, 47.1, 28.8, 23.6, 19.5, 18.1.
(E)-7-((4-hydroxyphenyl)imino)-8,8-dimethyl-4,5,6,7-tetrahydro-3H-3a,6-methanobenzo[c]isothiazole 2,2-dioxide (L2)-3-oxo-camphorsulfonimide (1, 682 mg; 3 mmol) was dissolved in ethanol (10 mL) acidified with CH3COOH (0.4 mL). The mixture was stirred for 15 min before adding 4-aminophenol (329 mg; 3 mmol). A light brown compound was formed upon overnight stirring at 50 °C that was filtered off the solution and dried. Yield 81%. Elem. Anal. (%) for C16H18N2O3S·H2O: Found: C, 56.9; N, 8.0; H, 6.2; S, 11.1. Calc.: C, 57.1; N, 8.3; H, 6.0; S, 9.5. FTIR (KBr, cm−1): 3567, 1653, 1633, 1330, 1160. 1HNMR (CD3CN, δppm): 7.35 (s, 1H, OH), 7.00 (d, J = 7.8 Hz, 2H), 6.88 (d, J = 7.8 Hz, 2H), 3.48, 3.25 (2d, J = 13.9 Hz, 2H), 3.10 (d, J = 2.6 Hz, 1H), 2.34–2.23 (m, 2H), 1.89–1.75 (m, 2H), 1.06 (s, 3H), 0.84 (s, 3H). 13C NMR (CD3CN, δppm): 187.9, 166.0, 157.3, 141.9, 124.9, 116.7, 64.1, 52.6, 50.5, 47.8, 29.4, 24.4, 19.9, 18.3.
(E)-7-((4-chlorophenyl)imino)-8,8-dimethyl-4,5,6,7-tetrahydro-3H-3a,6-methanobenzo[c]isothiazole 2,2-dioxide (L4)-Compound 1 (682 mg; 3 mmol) was dissolved in acidified (acetic acid, 0.4 mL) ethanol (10 mL) and stirred for ca. 15 min. Upon addition of 4-chloroaniline (384 mg, 3 mmol) the reaction was stirred overnight at 40 °C. A yellow precipitate formed that was filtered off solution and dried. Yield 76%. Elem. Anal. (%) for C16H17ClN2O2S·1/5H2O: Found: C, 56.5; N, 8.1; H, 5.3; S, 10.1. Calc.: C, 56.5; N, 8.2; H, 5.2; S, 9.4. FTIR (KBr, cm−1): 1664, 1636, 1340, 1161. 1H NMR (CDCl3, δppm): 7.39 (d, J = 8.6 Hz, 2H); 6.93 (d, J = 8.6 Hz, 2H); 3.42, 3.21 (2d, J = 13.4 Hz, 2H); 2.99 (d, J = 8.6 Hz, 1H); 2.29–2.21 (m, 2H); 2.10–2.00 (m, 1H); 1.82–1.74 (m, 1H); 1.11 (s, 3H); 0.95 (s, 3H). 13C NMR (CDCl3, δppm): 185.0, 167.5, 147.2, 132.1, 129.4, 122.0, 62.7, 51.4, 50.0, 46.8, 28.4, 24.1, 20.0, 18.4.
(E)-7-((3,5-dimethylphenyl)imino)-8,8-dimethyl-4,5,6,7-tetrahydro-3H-3a,6-methanobenzo[c]isothiazole 2,2-dioxide (L6)-Compound 1 (454 mg; 2 mmol) was dissolved in ethanol (7 mL) acidified with acetic acid (0.2 mL) and the mixture stirred for ca. 15 min. Then, 3,5-dimethylaniline (0.25 mL, 2 mmol) was added and the reaction stirred for 24 h at RT. A brown precipitate formed that was filtered off the solution and dried. Yield 86%. Elem. Anal. (%) for C18H22N2O2S: Found: C, 65.3; N, 8.4; H, 6.7; S, 9.5. Calc.: C, 65.4; N, 8.5; H, 6.7; S, 9.7. FTIR (KBr, cm−1): 1665, 1636, 1339, 1161. 1H NMR (CDCl3, δppm): 6.87 (s, 1H); 6.56 (s, 2H); 3.39, 3.18 (2d, J = 13.4 Hz, 1H); 3.01 (d, J = 4.0 Hz, 1H); 2.34 (s, 6H); 2.24–2.19 (m, 2H); 2.05–1.98 (m, 1H); 1.82–1.75 (m, 1H); 1.09 (s, 3H); 0.94 (s. 3H). 13C NMR (CDCl3, δppm): 185.4, 166.5, 149.1, 139.1, 128.1, 118.2, 113.6, 62.9, 51.5, 50.1, 46.8, 28.6, 24.3, 21.5, 21.4, 20.1, 18.5.
4.1.2. Complexes (See Supplementary Material for FTIR and 1H and 13C NMR Spectra)
[Ag(NO3){(3,5-Me2C6H3)NC10H13NSO2}2] (C1)–AgNO3 (42.5 mg; 0.25 mmol) was added to a solution of L6 (165 mg; 0.50 mmol) in CH3CN (5 mL) and the mixture was stirred for 2 h. A light suspension formed that was filtered to separate silver residues. The filtered solution was evaporated and dried under vacuum to afford CB as a dark yellow compound. Yield 72%. Elemental analysis for AgC36H44N5O7S2·1/5HNO3 Exp.: C, 51.2; N, 8.4; H, 5.5; S, 8.8. Calc.: C, 51.3; N, 8.6; H, 5.3; S, 7.6. FTIR (KBr, cm−1): 1666, 1644, 1384, 1338, 1161. 1H NMR (400 MHz, CD3CN, δppm): 6.91 (s, 1H); 6.59 (s, 2H); 3.51, 3.28 (2d, J = 14 Hz, 2H); 2.96 (d, J = 4.8 Hz, 1H); 2.31 (s, 6H); 2.24–2.30 (m, 1H); 1.71–1.88 (m, 3H); 1.05 (s, 3H); 0.87 (s, 3H). 13C NMR (400 MHz, CD3CN, δppm): 186.5, 167.7, 149.2, 139.2, 127.6, 117.8, 63.2, 51.4, 49.7, 46.5, 28.0, 23.6, 20.3, 19.1, 17.1.
[Ag(NO3)(C6H5NC10H13NSO2)2] (C2)-Silver nitrate (42.5 mg; 0.25 mmol) was added to a solution of L7 (151 mg; 0.50 mmol) in CH3CN (3 mL) and the mixture was stirred for 2 h.
The suspension was then filtered to remove silver residues. Upon solvent evaporation and drying under vacuum, the compound was obtained as a yellow powder. Yield 62%. Elemental analysis for AgC32H36N5O7S2·H2O Exp.: C, 48.6; N, 9.0; H, 4.7; S, 7.6. Calc.: C, 48.5; N, 8.8; H, 4.8; S, 8.1. FTIR (KBr, cm−1): 1664, 1635, 1384, 1346, 1165. 1H NMR (400 MHz, DMSO, δppm): 7.46 (t, J = 15.7 Hz, 2H); 7.25 (t, J = 7.4 Hz, 1H); 7.00 (d, J = 8.4 Hz, 2H); 3.74, 3.52 (2d, J = 14 Hz, 2H); 2.88 (d, J = 4.8 Hz, 1H); 2.34–2.25 (m, 1H); 2.19–2.10 (m, 1H); 1.80–1.70 (m, 2H); 1.01 (s, 3H); 0.84 (s, 3H). 13C NMR (400 MHz, DMSO, δppm): 186.0, 167.8, 148.8, 129.3, 126.0, 120.1, 63.0, 51.0, 49.3, 46.3, 27.7, 23.4, 19.2, 17.5.
4.2. Computational Methods
All theoretical calculations were of the DFT type, carried out using GAMESS-US version R3 [17], using the implemented version of the B3LYP functional and a 6-31G** basis set for organic molecules and SBKJC basis set/ECP in metal complexes. DFT-D3 [18], with standard parametrization of GAMESS-US, was used in all computational work. Optimized geometries were confirmed to be minima by checking for absence of imaginary frequencies in the Hessian.
4.3. X-ray Diffraction Analysis
X-ray diffraction analysis (Bruker, Karlsruhe, Germany) was performed on mono crystals of 1,3 and L1 to L7. Data were collected on a Bruker AXS-KAPPA APEX II area detector apparatus using graphite-monochromatized Mo Kα (λ = 0.71073 Å) radiation and were corrected for Lorentz’s polarization and for empirical absorption. The structure was solved via direct methods using SHELX97 [19] and refined via full matrix least squares against F2 using SHELX97, all included in the suite of WinGX v1.70.01 programs for Windows [20]. Non-hydrogen atoms were refined anisotropically and H atoms were inserted in idealized positions and allowed to refine riding on the parent carbon atom. Crystal data and refinement parameters are summarized in Table 3. Illustrations of the molecular structures were made with Chemcraft [21] or Mercury [22].

Table 3.
Crystallographic data for 1 and L1 to L4, L6, L7.
CCDC 2252538 to 2252543 and CCDC 2252556 contain the supplementary crystallographic data for this paper, which can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 1 September 2023) (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge, CB2 1EZ, UK; fax: +44 1223 336033; or deposit@ccdc.cam.ac.uk).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst13101499/s1,1H, 13C and FTIR spectra are provided for coordination compounds C1 and C2. Figure S1: NMR 1H spectrum of compound C1; Figure S2: NMR 13C spectrum of compound C1; Figure S3: FTIR spectrum of compound C1; Figure S4: NMR 1H spectrum of compound C2; Figure S5: NMR 13C spectrum of compound C2; Figure S6: FTIR spectrum of compound C2.
Author Contributions
A.M.G.: X-ray diffraction analysis, DFT-D3 calculations, writing; M.F.N.N.d.C.: conceptualization, writing, revision; J.P.d.C.: Synthesis of compounds, FTIR, NMR analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FCT—Fundação para a Ciência e a Tecnologia through projects CQE (UIDB/00100/2020, UIDP/00100/2020). Institute of Molecular Sciences through project (LA/P/0056/2020). FCT—Fundação para a Ciência e a Tecnologia through PhD Grant to J.P.d.C. (UI/BD/152244/2021).
Acknowledgments
The Portuguese NMR IST–UL Centers for facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carvalho, M.F.N.N.; Consiglieri, A.C.; Duarte, M.T.; Galvão, A.M.; Pombeiro, A.J.L.; Herrmann, R. Transition metal complexes of (1S,2S,3S)-3-hidroxy-camphorsultam. Inorg. Chem. 1993, 32, 5160–5164. [Google Scholar] [CrossRef]
- Carvalho, M.F.N.N.; Costa, L.M.G.; Pombeiro, A.J.L.; Schier, A.; Scherer, W.; Harbi, S.K.; Verfürth, U.; Herrmann, R. Synthesis, structure and electrochemistry of palladium complexes with camphor-derived chiral ligands. Inorg. Chem. 1994, 33, 6270–6277. [Google Scholar] [CrossRef]
- Cardoso, J.M.S.; Correia, I.; Galvão, A.M.; Marques, F.; Carvalho, M.F.N.N. Synthesis and assessment of the cytotoxic and DNA binding properties of Ag(I) camphor sulphonylimine complexes. J. Inorg. Biochem. 2017, 166, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-N.; Lu, L.-Q.; Xiao, W.-J. Non-Bonding Interactions Enable the Selective Formation of Branched Products in Palladium-Catalyzed Allylic Substitution Reactions. Chem. Asian J. 2018, 13, 2174–2183. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gou, S.; Chen, Y.; Liu, Y. Potential new antitumor agents from an innovative combination of camphorato, a ramification of traditional Chinese medicine, with a platinum moiety. Bioorg. Med. Chem. Lett. 2005, 15, 3417–3422. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, V.; Coyle, E.J.; Sebeck, K.; Kieffer, J.; Pipe, K.P. Thermal Conductance in Cross-linked Polymers: Effects of Non-Bonding Interactions. J. Phys. Chem. B 2017, 121, 4600–4609. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.F.N.N.; Leite, S.; Costa, J.P.; Galvão, A.M.; Leitão, J.H. Ag(I) camphor complexes: Antimicrobial activity by design. J. Inorg. Biochem. 2019, 199, 110791. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.A.; Mendes, F.; Roseiro, A.P.S.; Santos, I.; Carvalho, M.F.N.N. Insight into the cytotoxic activity of polynuclear Cu(I) camphor complexes. Polyhedron 2015, 87, 215–219. [Google Scholar] [CrossRef]
- Fernandes, T.A.; Ferraria, A.M.; Galvão, A.M.; Rego, A.M.B.D.; Suárez, A.C.M.; Carvalho, M.F.N.N. Synthesis, characterization and study of properties of Zn(II) camphor derived complexes. J. Organometal. Chem. 2014, 760, 186–196. [Google Scholar] [CrossRef]
- Goerigk, L. A comprehensive overview of the DFT-D3 London-dispersion correction. Non-Covalent Interact. Quantum Chem. Phys. 2017, 195–219. [Google Scholar] [CrossRef]
- Leitão, J.H.; Sousa, S.A.; Leite, S.A.; Carvalho, M.F.N.N. Silver camphorimine complexes: Novel antibacterial compounds from old medicines. Antibiotics 2018, 7, 65. [Google Scholar] [CrossRef]
- Armstrong, H.E.; Lowry, T.M. Studies of the terpenes and allied compounds. The sulphonation of camphor. I. Camphorsulphonic acid (reychler): The formation of anhydramides. J. Chem. Soc. 1902, 81, 1441. [Google Scholar] [CrossRef]
- Liu, X.; McMillen, C.D.; Thrasher, J.S. Cooperative intermolecular S–Cl⋯O and F⋯F associations in the crystal packing of α,ω-di(sulfonyl chloride) perfluoroalkanes, ClSO2(CF2)nSO2Cl, where n = 4, 6. New J. Chem. 2018, 42, 10484–10488. [Google Scholar] [CrossRef]
- Mukherjee, A.; Desiraju, S.T.G.R. Halogen Bonds in Crystal Engineering: Like Hydrogen Bonds yet Different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Leo, A.; Taft, R.W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 1991, 97, 165–195. [Google Scholar] [CrossRef]
- Costa, J.P.; Sousa, S.A.; Galvão, A.M.; Mata, J.M.; Leitão, J.H.; Carvalho, M.F.N.N. Key Parameters on the Antibacterial Activity of Silver Camphor Complexes. Antibiotics 2021, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H.J. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELX-97- Programs for Crystal Structure Analysis (Release 97-2); Tammanstrasse 4, D-3400; Institüt für Anorganische Chemie der Universität: Göttingen, Germany, 1998. [Google Scholar]
- Farrugia, L.J. WINGX. J. Appl. Crystallogr. 1999, 32, 837. [Google Scholar] [CrossRef]
- Chemcraft-Graphical Software for Visualization of Quantum Chemistry Computations. Available online: https://www.chemcraftprog.com (accessed on 1 September 2023).
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. Wood J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).